Abstract
Background
Mannans are hemicellulosic polysaccharides in the plant primary cell wall with two major physiological roles: as storage polysaccharides that provide energy for the growing seedling; and as structural components of the hemicellulose–cellulose network with a similar function to xyloglucans. Endo-β-mannanases are hydrolytic enzymes that cleave the mannan backbone. They are active during seed germination and during processes of growth or senescence. The recent discovery that endo-β-mannanase LeMAN4a from ripe tomato fruit also has mannan transglycosylase activity requires the role of endo-β-mannanases to be reinterpreted.
Aims
In this review, the role of endo-β-mannanases as mannan endotransglycosylase/hydrolases (MTHs) in remodelling the plant cell wall is considered by analogy to the role of xyloglucan endotransglucosylase/hydrolases (XTHs). The current understanding of the reaction mechanism of these enzymes, their three-dimensional protein structure, their substrates and their genes are reported.
Future outlook
There are likely to be more endohydrolases within the plant cell wall that can carry out hydrolysis and transglycosylation reactions. The challenge will be to demonstrate that the transglycosylation activities shown in vitro also exist in vivo and to validate a role for transglycosylation reactions during the growth and development of the plant cell wall.
Key words: Cell wall, endo-β-mannanase, endohydrolase, mannan, endotransglycosylase
MANNANS: CINDERELLA OF THE PRIMARY CELL WALL?
The polysaccharides of the primary cell wall are cellulose, hemicelluloses such as xyloglucans, mannans and glucuronoarabinoxylans, and pectic polymers. How these polysaccharides interact is still a matter of debate. Hemicelluloses are thought to coat cellulose fibrils and to cross-link them, either directly or via a pectin layer (for a review see Cosgrove, 2000). The resulting hemicellulose–cellulose framework is the major load-bearing structure in the primary cell wall. Of the hemicelluloses, the structure and role of xyloglucan has been most characterized. Xyloglucans bind to cellulose via hydrogen bonding, and because they are long-chain polysaccharides they can theoretically span microfibrils, thereby acting as tethers. Through this tethering, a xyloglucan–cellulose network is created that restricts turgor-driven cell expansion (McCann et al., 1990).
Compared with xyloglucans, little is known about the role of mannans in the cell wall. In cell-wall models mannans are seldom mentioned or they are referred to as ‘other polysaccharides’. However, mannans are more structurally diverse than the xyloglucans, which are comparatively homogeneous polysaccharides made of a glucan backbone substituted with xylosyl side chains that are occasionally extended with galactose or fucosyl–galactose residues. Mannans fall into four categories based on their backbone structure and presence of galactose side chains (Table 1).
Table 1.
Chemical structure and function of mannans in plants (based on review from Matheson, 1990)
| Polysaccharide | Backbone | Backbone substitution | Function | Features |
|---|---|---|---|---|
| Pure mannan | (1 → 4)-β-d-mannose residues | None | •Storage polysaccharide | •DP* 100–2500 |
| •Structural in some algae | •Soluble in boiling water†; gradually precipitates on cooling | |||
| •In thickened endosperm walls | ||||
| Galactomannan | (1 → 4)-β-d-mannose residues | Single (1 → 6)-α-d-galactose residues; non-regular distribution | •Storage polysaccharide in legume seeds | •Viscous |
| •Water-soluble† | ||||
| •DP 1000–10000 | ||||
| Glucomannan | (1 → 4)-β-d-glucose and mannose residues; non-regular distribution; partially acetylated | none | •Storage polysaccharide in monocot seeds, and in bulbs and tubers | •DP 100–5000 |
| •Structural mostly in wood | •Viscous | |||
| •Water-soluble† | ||||
| •In idioblasts | ||||
| Galactoglucomannan (GGM) | (1 → 4)-β-d-glucose and mannose residues; non-regular distribution | Single or double (1 → 6)-α-d-galactose residues on mannose or glucose residues; non-regular distribution | •Storage polysaccharide in, for example, Asparagus seeds | •DP 100–200 (kiwifruit) |
| •Structural in dicot primary cell walls and wood | •Can contain small amounts of xylose and arabinose |
* DP = degree of polymerization, an indication of polysaccharide chain length.
† Water at approximately neutral pH.
The structural diversity of mannans allows for a wide range of physicochemical properties, which in turn contributes to their in-planta functionality. Pure mannan is insoluble in cold water at neutral pH. When some of the mannose residues are replaced by glucose residues, as in the glucomannans, or substituted with galactose, as in the galactomannans, the water-solubility of the polymers increases. The pattern of mannose and glucose residues is often random, and the degree of substitution as well as the distribution of the galactosyl substituents varies markedly. Additionally, partial acetylation of some residues can occur. Reviews on the structure and occurrence of plant mannans can be found in Matheson (1990), Buckeridge et al. (2000) and Moreira and Filho (2008).
Plant cell-wall mannans function as storage and structural polysaccharides. As storage polysaccharides deposited inside the primary cell wall, pure mannans, galactomannans (both in seeds) and glucomannans (in bulbs or tubers) are most common. They are degraded and metabolized by the growing embryo or shoot. In seeds, this process occurs at a definite period after imbibition. Also, galactomannan protects the developing axis from fluctuations in water balance because of its hydrophilic nature (for reviews see Matheson, 1990; Buckeridge et al., 2000).
Glucomannans, galactoglucomannans (GGMs) and pure mannans are structural polysaccharides. Pure mannans can exist in crystalline and amorphous form (Chanzy et al., 1984), and are present as microfibrils in certain algae, replacing cellulose as the principal skeletal component (Mackie and Preston, 1968). Glucomannans or GGMs are ubiquitous in small amounts in the primary cell walls of dicotyledonous plants. The structural role of glucomannan and its interaction with cellulose resembles that of xyloglucan. This similarity has been shown in experiments using composite materials: artificial cell walls made by bacteria releasing cellulose fibrils into their growth medium. In these composite materials, glucomannans form cross-links with cellulose and reduce the crystallinity of the native bacterial cellulose microfibrils analagous to xyloglucans, introducing properties of flexibility and toughness (Whitney et al., 1998). Glucomannans are also found coating cellulose microfibrils and as interstitial material between the fibrils in coleoptiles from Zea mays (Carpita et al., 2001).
Less is known about the role of GGMs in the primary cell wall. GGMs can only be solubilized from the primary cell wall by extraction with strong alkali, and will bind to paper in an aqueous environment. Glucomannans, pure mannans and galactomannans do not bind to paper (Schröder et al., 2004). This indicates a close association of GGMs with either cellulose microfibrils or another insoluble cell-wall polymer, most likely through hydrogen bonding. GGMs are much shorter polysaccharides (20–40 kDa, Schröder et al., 2001) than glucomannans and are probably unable to create a network with cellulose similar to xyloglucans or glucomannans (Whitney et al., 2006).
In Arabidopsis, mannans are present throughout the plant but are more abundant in flowers, siliques and stems (Liepman et al., 2007). In Arabidopsis stems, mannans are found primarily in xylem parenchyma and in epidermis cells, especially the thickened outer epidermal walls (Handford et al., 2003). Maize coleoptile epidermal cells also have higher levels of mannans than mesophyll cells (Carpita et al., 2001). In seeds of lettuce (Lactuca sativa) and tomato (Solanum lycopersicum), it is thought that mannan increases the hardness of the endosperm (Gong et al., 2005). The highest levels of mannans are present in secondary walls of wood. In differentiating secondary cell walls of conifer tracheids, glucomannan coats cellulose microfibrils at night when water pressure is high, but not during the day when transpiration and hence water pressure is low (Hosoo et al., 2002). Mannans are therefore structurally important in thickened walls and may play a role in determining firmness and flexibility of a tissue. In fruit parenchyma cell walls, however, mannans are only present in minor amounts.
LeMAN4a – THE FIRST ENZYME IDENTIFIED THAT EXHIBITS BOTH ENDO-β-MANNANASE AND MANNAN TRANSGLYCOSYLASE ACTIVITY
To account for the loosening of the hemicellulose–cellulose network during growth-related cell expansion, and restoration of its original strength when growth has ceased, Albersheim (1976) postulated the need for enzymes acting in transglycosylase mode, i.e. cutting a hemicellulose chain and attaching the newly created chain end to another similar chain, thereby restoring the original strength of the cell wall. An enzyme that used the hemicellulose xyloglucan as its substrate was subsequently identified, and named xyloglucan endotransglucosylase/hydrolase (XTH) (Farkaš et al., 1992; Fry et al., 1992; Nishitani and Tominaga, 1992).
As mannans seem to have an analogous structural role in the primary cell wall to that of xyloglucan, we proposed that there should be an enzyme with mannan transglycosylase activity acting on mannans (just as XTH acts on xyloglucans), cutting and re-joining these polysaccharides in a transglycosylation reaction. In the search for mannan transglycosylase activity, a modified xyloglucan endotransglucosylase (XET) assay (Fry et al., 1992) was developed, where GGM and GGM-derived tritiated oligosaccharides were used as substrates. Mannan transglycosylase activity was subsequently detected in flowers, fruits and seedlings in a range of species, with the highest activity detected in flowers of kiwifruit species Actinidia deliciosa and A. eriantha, and in ripening tomato fruit (Schröder et al., 2004). Tomato and apple (Malus × domestica) flowers as well as lettuce and pea (Pisum sativum) seedlings also showed mannan transglycosylase activity. In ripe tomato fruit, the enzyme possessing mannan transglycosylase activity was purified and identified (Schröder et al., 2006) as the hydrolase endo-β-mannanase encoded by LeMAN4a (Carrington et al., 2002).
Endo-β-mannanases hydrolyse mannans by splitting their backbone at (1 → 4)-β-mannose residues even in the presence of glucose residues (as in glucomannans and GGMs) or galactosyl side chains attached to either mannose or glucose residues (galactomannans and GGMs). The same substrate promiscuity was observed for the mannan transglycosylase activity of LeMAN4a from tomato. GGM, glucomannan, galactomannan and pure mannan from ivory nut were used as the polysaccharide (‘donor’) substrates and transglycosylated with GGM-, galactomannan- or mannan-derived oligosaccharide (‘acceptor’) substrates, without any preferences. The only requirement for the polysaccharide substrates was a backbone containing β-(1 → 4)-linked mannose residues (Schröder et al., 2004). In the absence of oligosaccharides, the mannan substrates were completely degraded to oligosaccharides (our unpublished data).
LeMAN4a is the first (and currently the only) enzyme identified that exhibits both endo-β-mannanase and mannan transglycosylase activity. We proposed that endo-β-mannanases that possess these dual enzyme activities should be renamed as mannan transglycosylase/hydrolase (MTH), in accordance with the nomenclature established for xyloglucan endotransglucosylase/hydrolase (XTH; Rose et al., 2002).
WHAT IS THE ROLE FOR MANNAN TRANSGLYCOSYLASE ACTIVITY IN REMODELLING THE CELL WALL?
Compared with XTHs, the research on MTHs is still in its infancy. XTH enzymes have been comprehensively analysed in terms of expression profiles and functional analysis, and the large XTH family of genes and proteins has disclosed a wide range of versatile physiological roles for individual XTH enzymes in various plants (for a review see Rose et al., 2002; http://www.cazy.org/fam/GH16.html). In particular, the XET activity of XTH has been extensively characterized. The transglycosylase activity has been shown to be involved in modification of the xyloglucan–cellulose network during growth by breaking and rejoining existing xyloglucans, and to be involved in grafting newly synthesized xyloglucans to existing ones (Thompson and Fry, 2001), thereby restoring and refining the network. In contrast, the hydrolase activity of XTH has not been completely identified and defined, and to date only a few XTHs have shown xyloglucan endohydrolytic activity in vitro, for example XTH in nasturtium seeds (Edwards et al., 1986) and kiwifruit (Schröder et al., 1998).
Like XTHs, endo-β-mannanases have also been comprehensively analysed in terms of expression profiles and functional analysis, and are also present as large families of genes encoding multiple proteins and isoforms (http://www.cazy.org/fam/GH5.html; and see next section). However, to date, the role of these enzymes has only been considered with respect to their hydrolase activity (for reviews see Gong and Bewley 2007; Moreira and Filho, 2008). If endo-β-mannanases also possess transglycosylase activity, what role could MTH enzymes play in modifying structural cell-wall mannans during processes of growth or senescence? Unfortunately, there are few data, as only the product of the LeMAN4a gene in ripe tomato has been characterized for both transglycosylase and hydrolase activity in vitro. However, there are some developmental changes that suggest MTHs can reorganize structural mannans in the cell wall through transglycosylation. There is high mannan transglycosylase activity in tightly closed Actinidia flower buds, when ovule formation begins and petals first begin to expand inside the bud, and also during flower opening, when petals, stamens and styles elongate. In fully open flowers, when elongation of flower parts has ceased and flower senescence has begun, no activity was detected (Schröder et al., 2004).
Mannan transglycosylase activity may also be important during seedling growth. In tomato seeds, LeMAN3 mRNA expression peaks 72–96 h after imbibition is completed (Gong and Bewley, 2007). Constitutive over-expression of this gene in tomato seeds led to suppression of LeMAN2 mRNA, which in the wild-type is exclusively expressed in the endosperm cap early in germination. This in turn led to a delay in germination (Belotserkovsky et al., 2007). LeMAN3 was not readily able to replace LeMAN2 in the degradation of the thick cell walls of the micropylar area to allow radicle emergence, although mannan hydrolase activity was detected in vitro. In tomato seed germination, it may be possible that the LeMAN3 enzyme is acting mainly as a transglycosylase and not as a hydrolase. Preliminary results have shown that high mannan transglycosylase activity is present in 5-d-old tomato seedlings, where LeMAN3 is highly expressed (our unpublished data).
The only endo-β-mannanases with proven mannan transglycosylase activity are the products of the LeMAN4a gene and a mutated form of this gene, LeMAN4i, in which a 2-nt deletion leads to a truncation of the protein at the C-terminus (Bourgault and Bewley, 2002). The gene product of LeMAN4i is inactive in tomato extracts (Banik et al., 2001), but shows both transglycosylase and hydrolase function as a recombinant protein in vitro, albeit at much lower activities than LeMAN4a. LeMAN4a is a potent and promiscuous transglycosylase and hydrolase in vitro. In the presence of mannan oligosaccharides, it carries out transglycosylation reactions, but in their absence, mannan substrates are hydrolysed (Schröder et al., 2007). In vivo, during tomato ripening, its role is not resolved. When extracted and assayed in vitro, mannan hydrolase and mannan transglycosylase activity drastically increase once the fruit turn to the orange stage (Bewley et al., 2000; Schröder et al., 2004). This suggests a role for MTH in tomato softening but is it acting as a hydrolase or as a transglycosylase?
Fruit ripening is typically accompanied by a reduction in the molecular weight of cell-wall polysaccharides through hydrolytic enzyme action, which leads to weakening of cell walls and softening of the fruit (reviewed in Brummell, 2006). However, investigations on crude tomato cell-wall fractions showed no loss of mannose over the softening period, and mannose-containing cell-wall fractions showed no change in composition or size of polysaccharides (Seymour et al., 1990). More recently, the two native polysaccharide substrates for MTH isolated from ripe tomato (Schröder et al., 2007) have also been shown not to change in size over the softening period (our unpublished data). Together, these observations are consistent with MTH acting as a transglycosylase during softening given that, in this mode, unlike hydrolysis, no net change of molecular weight of polysaccharides occurs (Fry et al., 1992). In vitro, in the absence of oligosaccharides, however, MTH hydrolysed both native substrates to smaller fragments (our unpublished data).
How can this contradiction be explained? Conditions in the cell wall are difficult to mimic in vitro, e.g. availability of water, proximity and availability of substrates to the enzyme, accessibility of the enzyme to its substrate, and the ionic milieu. Moreover, in the cell wall the mannan substrates can be insoluble, whereas in the in-vitro assays, solubilized substrates are used. These differences may determine whether hydrolysis or transglycosylation takes place. For example, in vitro, XET activity is influenced by a variety of ions (Takeda and Fry, 2004), and mannan hydrolase activity is almost completely suppressed in the presence of ∼0·7–0·8m NaCl, whereas the transglycosylase activity is not influenced under these conditions (Schröder et al., 2006).
For both MTH and XTH, in vitro assays may not always reflect what is happening in the plant. For example, nasturtium XTH (TmXTH1) shows both transglycosylase and hydrolase activities when assayed in vitro (depending on the substrates it is given); however, in planta the enzyme acts only as a hydrolase to degrade storage xyloglucan (Edwards et al., 1986; Farkaš et al., 1992; Fanutti et al., 1993). For MTH, a similar situation may exist. LeMAN4a exhibits both transglycosylase and hydrolase activity in in-vitro assays – but which activity is preferred in a particular tissue will require empirical testing and validation in planta.
USING COMPARATIVE GENOMICS STUDIES TO DETERMINE ENDO-β-MANNANASE FUNCTION
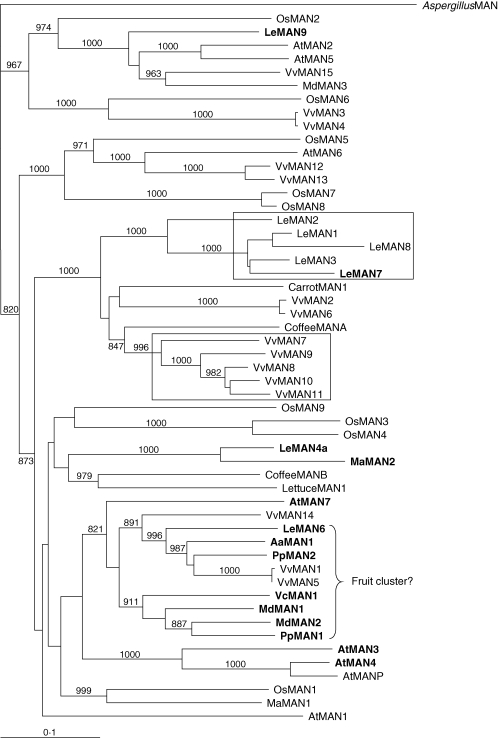
Like many other cell-wall hydrolases, endo-β-mannanases are found as large gene families encoding multiple proteins and isoforms. Eight, nine and 11 endo-β-mannanase genes have been identified in the genomes of Arabidopsis thaliana, rice (Oryza sativa) and poplar (Populus trichocarpa), respectively (Yuan et al., 2007), and 15 endo-β-mannanase genes in the grape (Vitis vinifera) genome (F-IPCfG, 2007). Figure 1 shows a phylogenetic tree of endo-β-mannanases that uses Arabidopsis and rice sequences as a framework for comparison of sequences from grape, tomato, apple and kiwifruit. Previous studies have shown that the existence of endo-β-mannanases pre-dates the divergence of angiosperm and gymnosperm lineages, but diversification occurred after the divergence of plants from microbes, fungi and animals (Yuan et al., 2007). There is also evidence for relatively recent duplication of endo-β-mannanase genes within some species, e.g. the VvMAN7-11 cluster in grape (boxed in Fig. 1).
Fig. 1.
Phylogenetic comparison of endo-β-mannanase amino acid sequences. Trees were constructed with PHYLIP and visualized in TREEVIEW (v.1·6·6). Confidence values for groupings in trees were obtained using BOOTSTRAP N-J TREE using 1000 bootstrap trials. Sequences are from Arabidopsis thaliana [AtMAN1-7, AtMANP], Oryza sativa [OsMAN1-9], Coffea arabica [CoffeeMANA, CoffeeMANB], Lactuca sativa [LettuceMAN1], Aspergillus sp. [AspergillusMAN; Yuan et al., 2007], Solanum lycopersicum [LeMAN1-4a (Gong and Bewley, 2007), LeMAN6 (SGNU316912), LeMAN7 (SGNU316863), LeMAN8 (SGNU335864), LeMAN9 (SGNU318129) (www.sgn.cornell.edu)], Actinidia arguta [AaMAN1 (accession number FJ194533)], Daucus carota [CarrotMAN1 (AAN34823)], Malus × domestica [MdMAN1 (FJ194534), MdMAN2 (FJ194535), MdMAN3 (FJ194536)], Prunus persica [PpMAN1 (ABV32547), PpMAN2 (ABV32548)], Musa acuminata [MaMAN1 (ABF69949), MaMAN2 (Zhuang et al., 2006)], Vaccinium corymbosum [VcMAN1 (FJ194537)], Vitis vinifera [VvMAN1 (CAN70632), VvMAN2 (CAN71995), VvMAN3 (CAN77937), VvMAN4 (CAO21138), VvMAN5 (CAO22814), VvMAN6 (CAO44560), VvMAN7 (CAO44561), VvMAN8 (CAO44562), VvMAN9 (CAO44563), VvMAN10 (CAO44564), VvMAN11 (CAO44565), VvMAN12 (CAO48888), VvMAN13 (CAO48890), VvMAN14 (CAO611260) and VvMAN15 (CAO69748)]. Sequences shown in bold type are expressed in fruit. The boxed sequences appear to result from recent gene duplications.
The expression of endo-β-mannanases has been recently reviewed in tomato (Gong and Bewley, 2007) and in Arabidopsis and rice (Yuan et al., 2007). Individual endo-β-mannanases can be expressed in multiple tissues (e.g. AtMAN1; Yuan et al., 2007), or in more tissue-specific and developmentally regulated sites (e.g. LeMAN2; Nonogaki et al., 2000). Few endo-β-mannanases have been well characterized in fruit. Expression of LeMAN4a increases markedly at the breaker stage and remains high through red and over-ripe stages (Carrington et al., 2002). MaMAN2 from banana (Musa acuminata) shows a similar expression profile (Zhuang et al., 2006). There are many uncharacterized endo-β-mannanase expressed sequence tags (ESTs) expressed in fruit, including a major cluster of ESTs obtained from tomato, apple, peach (Prunus persica), kiwifruit and blueberry (Vaccinium corymbosum; Fig. 1). Endo-β-mannanase genes are expressed and translated in developing or green mature tomato fruit, but interestingly neither hydrolase nor transglycosylase activity has been detected in these tissues (Bewley et al., 2000; Schröder et al., 2006).
What are the prospects for identifying endo-β-mannanases with mannan transglycosylase activity using a phylogenetic approach given that only one endo-β-mannanase with proven mannan transglycosylase activity has been identified? Phylogenetic trees of XTH enzymes show a division of sequences into three main clades (Campbell and Braam, 1999). Enzymes in Group 1 and 2 were thought to exhibit predominantly XET and not xyloglucan endohydrolase (XEH) activity, whilst enzymes in Group 3 would act predominantly as hydrolases. Saladie et al. (2006) tried to define a sequence–enzyme–action relationship for XTH using recombinant enzymes in different groups, but the position in a phylogenetic clade did not predict any preference for hydrolysis or transglycosylation. These results suggest that using the complete amino acid sequence for phylogenetic comparisons may not predict enzyme function and that other approaches, for example a focus on the catalytic site of the enzyme, may be more informative.
The enzymic mechanism that lies behind endohydrolytic and transglycosylation reactions has been investigated in some detail (see also Bourgault et al., 2005). The enzymes utilize a V-shaped groove into which the polysaccharide slides, and which is lined with sub-sites containing aromatic amino acids to align and bind the substrate. An acid/base and a nucleophile amino acid catalyse the polysaccharide cleavage. After cleavage, the ‘donor’ part remains covalently attached to the nucleophile, whereas the other part leaves the active site. The reaction can now go in different directions: hydrolysis or transglycosylation. For hydrolysis, the acid/base de-protonates a water molecule. The nucleophile regenerates and the donor part is released. For transglycosylation, however, instead of water another sugar chain slides into the groove. The sugar residue attached to the nucleophile is transferred on to this sugar chain (which can be an oligosaccharide or a polysaccharide), leading to chain elongation rather than degradation through hydrolysis. Both the water molecule and the incoming sugar chain are called acceptors. The three-dimensional (3-D) structure of the active site is very similar in XTHs and LeMAN4a (Johansson et al., 2004; Bourgault et al., 2005) and in other endohydrolases. They not only share the V-shaped groove, but the sub-sites in the groove that bind the sugar residues, and the catalytic amino acid residues acid/base and nucleophile are arranged in a similar way.
An XTH from nasturtium seeds, which mainly acts as a hydrolase, was modelled on to poplar XTH, which mainly acts as a transglycosylase. A deletion mutation in the nasturtium XTH created an enzyme that was structurally similar to the poplar XTH and which had an increased transglycosylation to hydrolysis ratio (Baumann et al., 2007). A similar strategy has been used to model the 3-D structure of LeMAN4a on to a fungal endo-β-mannanase (Bourgault et al., 2005). Although they share many site residues and structural motifs, a significant difference is in the loop containing phenylalanine-138 (F138). Whereas in the fungal mannanase F138 is part of the active site, in LeMAN4a it is not. The consequence of this structural alteration is currently not known, but it may enable the enzyme to carry out transglycosylation reactions involving polysaccharides by reducing the enzyme's affinity for water, or increasing its affinity for mannans.
FUTURE OUTLOOK
The formation of glycosidic bonds is an energy-requiring process. Within the cell, transferases in the Golgi apparatus use activated sugars to synthesize polysaccharide chains de novo, which are secreted into the cell wall. The cell wall itself does not contain the machinery for making activated sugars or the transferases for creating glycosidic bonds with them. Apart from self-assembly, insertion of newly synthesized polysaccharides into existing frameworks or restoration of covalent bonds between cell-wall polysaccharides can only be achieved via transglycoslation reactions in which the energy released through breaking of a (sugar–sugar) glycosidic bond is preserved and used to create a new bond.
Strohmeier et al. (2004) predicted that XTHs from Poaceae (e.g. barley, rice) could carry out hetero-transglycosylation reactions involving xyloglucan and mixed-linkage (1 → 3, 1 → 4)-β-glucan (MLG) or arabinoxylan, polysaccharides highly abundant in grasses. Indeed, an XTH isoform from barley seedlings has been found that catalyses the formation of covalent linkages between cellulose, MLG and xyloglucan through transglycosylation reactions in vitro (Hrmova et al., 2007). Ait-Mohand and Farkaš (2006) have also shown such a hetero-transglycosylation activity in nasturtium extracts, but whether the enzyme responsible is an XTH has not been determined. In Equisetum and Charophyta, Fry et al. (2008) also found an enzyme activity that transglycosylates MLG with xyloglucan-derived oligosaccharides. Although the Equisetum protein has not been identified, it has been suggested that the MLG:XET activity was not due to XTH but to another enzyme.
The ability of XTH to hetero-transglycosylate may explain the large number of XTH genes in Poaceae and their high expression, despite xyloglucan being only a minor hemicellulose in grass cell walls. A similar situation is found for mannans and MTH in Poaceae cell walls. Here, mannose levels are even lower than in dicotyledonous walls. Yet in rice, nine endo-β-mannanase genes have been identified (Yuan et al., 2007). In rice and in barley, these genes have been shown to produce active enzymes (Wang et al., 2005; Hrmova et al., 2006). However, for determination of mannan hydrolase activity in vitro, only commercial substrates have been used, shedding no light on the native substrates of MTH in Poaceae cell walls, and also whether MTHs in Poaceae are able to hetero-transglycosylate.
To date, no transglycosylase reactions involving pectic polysaccharides and pectin-derived oligosaccharides have been identified (García-Romera and Fry, 1994). Endopolygalacturonases, the enzymes that are the most likely candidates to carry out such a reaction, belong to the set of ‘inverting’ glycosylhydrolases (as opposed to ‘retaining’ glycosylhydrolases such as mannanases, XTHs and xylanases). Owing to a difference in the reaction mechanism, where the anomerism of the product (α or β) is opposite to that of the substrate, the inverting enzymes are not able to carry out transglycosylase reactions, but hydrolysis only. Pectin hydrolysis occurs extensively during fruit softening, but there may be no need for transglycosylation of pectin in cell walls. Non-branched or lightly branched pectins are comparatively easy to extract from the cell wall using water, chelators or weak alkaline solutions such as Na2CO3, indicating they are held in the cell wall by Ca2+ or weak covalent bonds such as ester linkages. One can speculate that if the pectin network has to be modified during development, the calcium-pectate gels in the cell wall may be broken easily by a change in pH or ion concentration.
In contrast, hemicellulose–cellulose networks are strong, with xyloglucan and mannans attached to cellulose fibrils by hydrogen bonding. In vitro, these bonds can only be broken by use of solvents such as guanidinium thiocyanate or KOH at high molarity. In planta, the interruption of hydrogen bonds between cellulose and xyloglucan or mannans is carried out by expansin proteins (McQueen-Mason and Cosgrove, 1994), whereas transglycosylation reactions are thought to be needed for integration of newly synthesized polysaccharides into a growing cell wall, re-arrangement of polysaccharides during development, and the creation of cross-links between different polysaccharides. Therefore, if there are more hydrolases acting as transglycosylases in the plant cell wall, one would predict that they are probably acting on hemicelluloses, or on the neutral galactan or arabinan side chains of pectin. It is only a matter of time before additional endohydrolases such as galactanase, arabinase or xylanase are shown to have transglycosylase activity in vitro. The challenge will be to elucidate their mode of action in planta.
ACKNOWLEDGEMENTS
We thank David Brummell and Erin O'Donoghue for reviewing the manuscript. This project was funded by FoRST (C06X0202, C06X0403), New Zealand.
LITERATURE CITED
- Ait-Mohand F, Farkaš V. Screening for hetero-transglycosylating activities in extracts from nasturtium (Tropaeolum majus) Carbohydrate Research. 2006;341:577–581. doi: 10.1016/j.carres.2006.01.018. [DOI] [PubMed] [Google Scholar]
- Albersheim P. The primary cell wall. In: Bonner J, Varner JE., editors. Plant biochemistry. 3rd edn. New York: Academic Press; 1976. pp. 225–274. [Google Scholar]
- Banik M, Bourgault R, Bewley JD. Endo-β-mannanase is present in an inactive form in ripening tomato fruits of the cultivar Walter. Journal of Experimental Botany. 2001;52:105–111. [PubMed] [Google Scholar]
- Baumann MJ, Eklöf JM, Michel G, et al. Structural evidence for the evolution of xyloglucanase activity from xyloglucan endo-transglycosylases: biological implications for cell wall metabolism. Plant Cell. 2007;19:1947–1963. doi: 10.1105/tpc.107.051391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belotserkovsky H, Berger Y, Shahar R, Wolf S. Specific role of LeMAN2 in the control of seed germination exposed by overexpression of the LeMAN3 gene in tomato plants. Planta. 2007;227:199–209. doi: 10.1007/s00425-007-0607-y. [DOI] [PubMed] [Google Scholar]
- Bewley JD, Banik M, Bourgault R, Feurtado JA, Toorop P, Hilhorst HWM. Endo-β-mannanase activity increases in the skin and outer pericarp of tomato fruits during ripening. Journal of Experimental Botany. 2000;51:529–538. doi: 10.1093/jexbot/51.344.529. [DOI] [PubMed] [Google Scholar]
- Bourgault R, Bewley JD. Variation in its C-terminal amino acids determines whether endo-β-mannanase is active or inactive during tomato fruit ripening. Plant Physiology. 2002;130:1254–1262. doi: 10.1104/pp.011890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgault R, Oakley AJ, Bewley JD, Wilce MCJ. Three-dimensional structure of 1,4-β-d-mannan mannanohydrolase from tomato fruit. Protein Science. 2005;14:1233–1241. doi: 10.1110/ps.041260905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummell DA. Cell wall disassembly in ripening fruit. Functional Plant Biology. 2006;33:103–119. doi: 10.1071/FP05234. [DOI] [PubMed] [Google Scholar]
- Buckeridge MS, Pessoa dos Santos H, Tiné MAS. Mobilisation of storage cell wall polysaccharides in seeds. Plant Physiology and Biochemistry. 2000;38:141–156. [Google Scholar]
- Campbell P, Braam J. Xyloglucan endotransglycosylases: diversity of genes, enzymes and potential wall-modifying functions. Trends in Plant Science. 1999;4:361–366. doi: 10.1016/s1360-1385(99)01468-5. [DOI] [PubMed] [Google Scholar]
- Carpita NC, Defernez MD, Findlay K, et al. Cell wall architecture of the elongating maize coleoptile. Plant Physiology. 2001;127:551–565. [PMC free article] [PubMed] [Google Scholar]
- Carrington CMS, Vendrell M, Domĩnguez-Puigjaner E. Characterisation of an endo-1,4-β-mannanase LeMAN4a expressed in ripening tomato fruit. Plant Science. 2002;163:599–606. [Google Scholar]
- Chanzy HD, Grosrenaud A, Vuong R, Mackie W. The crystalline polymorphism of mannan in plant cell walls and after recrystallisation. Planta. 1984;161:320–329. doi: 10.1007/BF00398722. [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ. Expansive growth of plant cell walls. Plant Physiology and Biochemistry. 2000;38:109–124. doi: 10.1016/s0981-9428(00)00164-9. [DOI] [PubMed] [Google Scholar]
- Edwards M, Dea ICM, Bulpin PV, Reid JSG. Purification and properties of a novel xyloglucan-specific endo-1 → 4-β-d-glucanase from germinated nasturtium seeds Tropaeolum majus L. Journal of Biological Chemistry. 1986;261:9489–9494. [PubMed] [Google Scholar]
- Fanutti C, Gidley MJ, Reid JSG. Action of a pure xyloglucan endo-transglycosylase formerly called xyloglucan-specific endo-1 → 4-β-d-glucanase from the cotyledons of germinated nasturtium seeds. Plant Journal. 1993;3:691–700. doi: 10.1046/j.1365-313x.1993.03050691.x. [DOI] [PubMed] [Google Scholar]
- Farkaš V, Sulova Z, Stratilova E, Hanna R, Maclachlan G. Cleavage of xyloglucan by nasturtium seed xyloglucanase and transglycosylation to xyloglucan subunit oligosaccharides. Archives of Biochemistry and Biophysics. 1992;298:365–370. doi: 10.1016/0003-9861(92)90423-t. [DOI] [PubMed] [Google Scholar]
- F-IPCfG. The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature. 2007;449:463–467. doi: 10.1038/nature06148. [DOI] [PubMed] [Google Scholar]
- Fry SC, Smith RC, Renwick KF, Martin DJ, Hodge SK, Matthews KJ. Xyloglucan endotransglycosylase, a new cell wall-loosening enzyme activity from plants. Biochemical Journal. 1992;282:821–828. doi: 10.1042/bj2820821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry SC, Mohler KE, Nesselrode BHWA, Franková L. Mixed-linkage β-glucan: xyloglucan endotransglucosylase, a novel wall-remodelling enzyme from Equisetum (horsetails) and charophytic algae. Plant Journal. 2008;55:240–252. doi: 10.1111/j.1365-313X.2008.03504.x. [DOI] [PubMed] [Google Scholar]
- García-Romera I, Fry SC. Absence of transglycosylation with oligogalacturonides in plant cells. Phytochemistry. 1994;35:67–72. [Google Scholar]
- Gong XM, Bewley JD. Sorting out the LeMANs: endo-β-mannanase genes and their encoded proteins in tomato. Seed Science Research. 2007;17:143–154. [Google Scholar]
- Gong XM, Bassel GW, Wang A, Greenwood JS, Bewley JD. The emergence of embryos from hard seeds is related to the structure of the cell walls of the micropyar endosperm, and not to endo-β-mannanase activity. Annals of Botany. 2005;96:1165–1173. doi: 10.1093/aob/mci269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handford MG, Baldwin TC, Goubet F, et al. Localisation and characterisation of cell wall mannan polysaccharides in Arabidopsis thaliana. Planta. 2003;218:27–36. doi: 10.1007/s00425-003-1073-9. [DOI] [PubMed] [Google Scholar]
- Hosoo Y, Yoshida M, Imai T, Okuyama T. Diurnal difference in the amount of immunogold-labeled glucomannans detected with field emission scanning electron microscopy at the innermost surface of developing secondary walls of differentiating conifer tracheids. Planta. 2002;215:1006–1012. doi: 10.1007/s00425-002-0824-3. [DOI] [PubMed] [Google Scholar]
- Hrmova M, Burton RA, Biely P, Lahnstein J, Fincher GB. Hydrolysis of (1,4)-β-d-mannans in barley (Hordeum vulgare) is mediated by the concerted action of (1,4)-β-d-mannan endohydrolase and β-d-mannosidase. Biochemical Journal. 2006;399:77–90. doi: 10.1042/BJ20060170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrmova M, Farkaš V, Lahnstein J, Fincher GB. A barley xyloglucan xyloglucosyl transferase covalently links xyloglucan, cellulosic substrates, and (1,3;1,4)-β-d-glucans. The Journal of Biological Chemistry. 2007;282:12951–12962. doi: 10.1074/jbc.M611487200. [DOI] [PubMed] [Google Scholar]
- Johansson P, Brumer H, III, Baumann MJ, et al. Crystal structures of a poplar xyloglucan endotransglycosylase reveal details of transglycosylation acceptor binding. Plant Cell. 2004;16:874–886. doi: 10.1105/tpc.020065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liepman AH, Nairn CJ, Willats WGT, Sørensen I, Roberts AW, Keegstra K. Functional genomic analysis supports conservation of function among cellulose synthase-like A gene family members and suggests diverse roles of mannans in plants. Plant Physiology. 2007;143:1881–1893. doi: 10.1104/pp.106.093989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie W, Preston RD. The occurrence of mannan microfibrils in the green algae Codium fragile and Acetabularia crenulata. Planta. 1968;79:249–253. doi: 10.1007/BF00396031. [DOI] [PubMed] [Google Scholar]
- Matheson NK. Mannose-based polysaccharides. Methods in Plant Biochemistry. 1990;2:371–413. [Google Scholar]
- McCann MC, Wells B, Roberts K. Direct visualisation of cross-links in the primary plant cell wall. Journal of Cell Science. 1990;96:323–334. [Google Scholar]
- McQueen-Mason SJ, Cosgrove DJ. Disruption of hydrogen bonding between plant cell wall polymers by proteins that induce wall extension. Proceedings of the National Academy of Science of the USA. 1994;91:6574–6578. doi: 10.1073/pnas.91.14.6574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira LRS, Filho EXF. An overview of mannan structre and mannan-degrading enzyme systems. Applied Microbiology and Biotechnology. 2008;79:165–178. doi: 10.1007/s00253-008-1423-4. [DOI] [PubMed] [Google Scholar]
- Nishitani K, Tominaga R. Endo-xyloglucan transferase, a novel class of glycosyltransferase that catalyzes transfer of a segment of xyloglucan molecule to another xyloglucan molecule. Journal of Biological Chemistry. 1992;267:21058–21064. [PubMed] [Google Scholar]
- Nonogaki H, Gee OH, Bradford KJ. A germination-specific endo-β-mannanase gene is expressed in the micropylar endosperm cap of tomato seeds. Plant Physiology. 2000;123:1235–1246. doi: 10.1104/pp.123.4.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JKC, Braam J, Fry SC, Nishitani K. The XTH family of enzymes involved in xyloglucan endotransglucosylation and endohydrolysis: current perspectives and a unifying nomenclature. Plant Cell Physiology. 2002;43:1421–1435. doi: 10.1093/pcp/pcf171. [DOI] [PubMed] [Google Scholar]
- Saladie M, Rose JKC, Cosgrove DJ, Catalá C. Characterisation of a new xyloglucan endotransglucosylase/hydrolase (XTH) from ripening tomato fruit and implications for the diverse modes of enzymic action. Plant Journal. 2006;47:282–295. doi: 10.1111/j.1365-313X.2006.02784.x. [DOI] [PubMed] [Google Scholar]
- Schröder R, Atkinson RG, Langenkämper G, Redgwell RJ. Biochemical and molecular characterisation of xyloglucan endotransglycosylase from ripe kiwifruit. Planta. 1998;204:242–251. doi: 10.1007/s004250050253. [DOI] [PubMed] [Google Scholar]
- Schröder R, Nicolas P, Vincent SJF, Fischer M, Reymond S, Redgwell RJ. Purification and characterisation of a galactoglucomannan from ripe kiwifruit Actinidia deliciosa. Carbohydrate Research. 2001;331:291–306. doi: 10.1016/s0008-6215(01)00046-5. [DOI] [PubMed] [Google Scholar]
- Schröder R, Wegrzyn TF, Bolitho KM, Redgwell RJ. Mannan transglycosylase: a novel enzyme activity in cell walls of higher plants. Planta. 2004;219:590–600. doi: 10.1007/s00425-004-1274-x. [DOI] [PubMed] [Google Scholar]
- Schröder R, Wegrzyn TF, Sharma NN, Atkinson RG. LeMAN4 endo-β-mannanase from ripe tomato fruit has dual enzyme activity and can act as a mannan transglycosylase and hydrolase. Planta. 2006;224:1091–1102. doi: 10.1007/s00425-006-0286-0. [DOI] [PubMed] [Google Scholar]
- Schröder R, Sharma NN, Redgwell RJ, Atkinson RG. Mannan transglycosylase/ hydrolase (MTH): a cell wall enzyme in search of a function. Mitteilungen der Bundesforschungsanstalt für Forst- und Holzwirtschaft, Hamburg; Proceedings of the 2nd New Zealand–German Cell Wall Symposium; Hamburg, Germany. 2007. Nr. 223, August ISSN 0368-8798. [Google Scholar]
- Seymour GB, Colquhoun IJ, DuPont MS, Parsley KR, Selvendran RR. Composition and structural features of cell wall polysaccharides from tomato fruits. Phytochemistry. 1990;29:725–731. [Google Scholar]
- Strohmeier M, Hrmova M, Fischer M, Harvey AJ, Fincher GB, Pleiss J. Molecular modelling of family GH16 glycoside hydrolases: potential roles for xyloglucan transglucosylases/hydrolases in cell wall modification in the Poaceae. Protein Science. 2004;13:3200–3213. doi: 10.1110/ps.04828404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda T, Fry SC. Control of xyloglucan endotransglycosylase activity by salts and ionic polymers. Planta. 2004;219:722–732. doi: 10.1007/s00425-004-1267-9. [DOI] [PubMed] [Google Scholar]
- Thompson JE, Fry SC. Restructuring of wall-bound xyloglucan by transglycosylation in living plant cells. Plant Journal. 2001;26:23–34. doi: 10.1046/j.1365-313x.2001.01005.x. [DOI] [PubMed] [Google Scholar]
- Wang A, Wang X, Ren Y, Gong X, Bewley JD. Endo-β-mannanase activities in rice grains during and following germination, and the influence of gibberellin and abscissic acid. Seed Science Research. 2005;15:219–227. [Google Scholar]
- Whitney SEC, Brigham JE, Darke AH, Reid JSG, Gidley MJ. Structural aspects of the interaction of mannan-based polysaccharides with bacterial cellulose. Carbohydrate Research. 1998;307:299–309. [Google Scholar]
- Whitney SEC, Wilson E, Webster J, Bacic A, Reid JSG, Gidley MJ. Effects of structural variation in xyloglucan poymers on interactions with bacterial cellulose. American Journal of Botany. 2006;93:1402–1414. doi: 10.3732/ajb.93.10.1402. [DOI] [PubMed] [Google Scholar]
- Yuan JS, Yang X, Lai J, Lin H, Cheng ZM, Nonogaki H, Chen F. The endo-beta-mannanase gene families in Arabidopsis, rice, and poplar. Functional and Integrative Genomics. 2007;7:1–16. doi: 10.1007/s10142-006-0034-3. [DOI] [PubMed] [Google Scholar]
- Zhuang J-P, Su J, Chen W-X. Molecular cloning and characterization of fruit softening related gene β-mannanase from banana fruit. Agricultural Sciences in China. 2006;5:277–283. [Google Scholar]