Abstract
Background and Aims
Cost–benefit models predict that carnivory can increase the rate of photosynthesis (AN) by leaves of carnivorous plants as a result of increased nitrogen absorption from prey. However, the cost of carnivory includes decreased AN and increased respiration rates (RD) of trapping organs. The principal aim of the present study was to assess the costs and benefits of carnivory in the pitcher plant Nepenthes talangensis, leaves of which are composed of a lamina and a pitcher trap, in response to feeding with beetle larvae.
Methods
Pitchers of Nepenthes grown at 200 µmol m−2 s−1 photosynthetically active radiation (PAR) were fed with insect larvae for 2 months, and the effects on the photosynthetic processes were then assessed by simultaneous measurements of gas exchange and chlorophyll fluorescence of laminae and pitchers, which were correlated with nitrogen, carbon and total chlorophyll concentrations.
Key Results
AN and maximum (Fv/Fm) and effective quantum yield of photosystem II (ΦPSII) were greater in the fed than unfed laminae but not in the fed compared with unfed pitchers. Respiration rate was not significantly affected in fed compared with unfed plants. The unfed plants had greater non-photochemical quenching (NPQ) of chlorophyll fluorescence. Higher NPQ in unfed lamina did not compensate for their lower ΦPSII, resulting in lower photochemical quenching (QP) and thus higher excitation pressure on PSII. Biomass and nitrogen and chlorophyll concentration also increased as a result of feeding. The cost of carnivory was shown by lower AN and ΦPSII in pitchers than in laminae, but RD depended on whether it was expressed on a dry weight or a surface area basis. Correlation between nitrogen and AN in the pitchers was not found. Cost–benefit analysis showed a large beneficial effect on photosynthesis from feeding as light intensity increased from 200 to 1000 µmol m−2 s−1 PAR after which it did not increase further. All fed plants began to flower.
Conclusion
Feeding pitchers with insect larvae increases AN of leaf laminae, due to higher nutrient acquisition, with strong correlation with nitrogen concentration, but AN of pitchers does not increase, despite increased nitrogen concentration in their tissue. Increased AN improves growth and reproduction and is likely to increase the competitive advantage of carnivorous over non-carnivorous plants in nutrient-poor habitats.
Key words: carnivorous plants, chlorophyll fluorescence, Nepenthes talangensis, nitrogen, pitcher plant, photosynthetic rate, photosystem II, respiration rate
INTRODUCTION
Carnivorous pitcher plants of the genus Nepenthes are largely found in south-east Asia, principally Borneo, Sumatra, Java and peninsular Malaysia, with scattered populations in India, Sri Lanka, Australia, New Caledonia, Madagascar and the Seychelles. Nepenthes leaves are differentiated into a photosynthetically active lamina and a pitcher trap, which has evolved to attract, trap and digest prey. The pitchers usually consist of different structural and functional zones: lid, peristome, and upper waxy and lower glandular zones within the pitcher (Clarke, 1997, 2001).
Carnivorous plants grow terrestrially in sunny, nutrient-poor and permanently moist habitats. Cost–benefit models of carnivory predict that in a well-lit environment the nutritional benefits gained from captured prey exceed the costs of modifying leaves into photosynthetically inefficient traps (Givnish et al., 1984). Generally, costs of carnivory include energetic demands for growth of traps and their function: either increased rate of dark respiration (RD) as a result of extra energy requirements for attracting, capturing and digesting the prey, or decreased photosynthetic rate (AN) as a result of leaf adaptation for carnivory, or both. Three potential benefits resulting from increased mineral absorption from prey have been proposed. First, carnivory may increase a plant's rate of photosynthesis (AN) through improved nutrient supply, particularly nitrogen status, although other nutrients (principally phosphate and potassium) may be important. Second, carnivory may results in an increased seed production through improved mineral acquisition; and third, carnivory may replace autotrophy partly by heterotrophy (Givnish et al., 1984). Givnish et al. (1984) considered the second benefit as a part of the first, as increased AN should lead to increased seed production. Several authors have dismissed the third benefit, as experimental findings suggest that carnivorous plants do not obtain substantial amounts of carbon from prey and carnivory could not replace autotrophy at low light intensity (Chandler and Anderson, 1976). However, Rischer et al. (2002) found that Nepenthes incorporated carbon from carnivory into organic substances, which raises a question about the importance of facultative heterotrophy. The growth of some species of carnivorous plants is partly dependent on organic carbon uptake from prey, as revealed by increased growth without increasing AN (Adamec, 1997, 2008).
With regard to the costs of carnivory, it has been observed that photosynthetic rates of traps are lower than those of leaves (Knight, 1992; Adamec, 2006; Pavlovič et al., 2007). With regard to the benefits, around 30 studies have tested whether the growth of carnivorous plants is enhanced by carnivory. Ellison (2006) concluded that there is a significant positive effect (P = 0·02) of adding prey on plant growth among different carnivorous genera, supporting the hypothesis that there is a benefit to carnivory. However, he pointed out that this is only indirect evidence, because the cost–benefit model expresses benefits in terms of photosynthetic rates, not in terms of growth. Only three studies have examined the effect of prey capture on AN of terrestrial carnivorous plants directly, and these gave very different results. Convincing evidence that prey availability increased absolute AN in Sarracenia has been provided by Farnsworth and Ellison (2008). However, Méndez and Karlsson (1999) and Wakefield et al. (2005) did not find any changes in AN in Pinguicula vulgaris and Sarracenia purpurea in response to the capture of prey.
Either of two possible results can be expected from feeding prey to carnivorous plants. First, fed plants will have greater biomass but nitrogen (N) concentration (mg N g−1 d. wt) will not be affected, despite total N (mg) per plant increasing. This was observed by Moran and Moran (1998) in Nepenthes rafflesiana. In extreme cases, N concentrations per unit dry matter might even decrease due to dilution by increased growth (Karlsson and Carlsson, 1984; Adamec, 2008). Because N concentration is positively correlated with AN in carnivorous plants (Ellison and Farnsworth, 2005; Pavlovič et al., 2007), it can be expected that AN will not be enhanced, as found by Méndez and Karlsson (1999) in Pinguicula vulgaris. In this case, the cost–benefit model must be considered in terms of growth rate partly due to direct uptake of organic compounds from prey. The second possibility is that fed plants will have higher leaf N concentrations and, therefore, higher AN as predicted by Givnish et al. (1984). This was found by Farnsworth and Ellison (2008) in Sarracenia.
The principal aim of the present study was to assess the costs and benefits of carnivory in Nepenthes, which provides a good experimental model for the study of the cost–benefit model of carnivory because the leaves are divided into photosynthetically active laminae and a pitcher trap. We assumed that increased photosynthesis is the most important benefit of carnivory, and tested the hypothesis that prey availability would result in increased AN, photosystem II (PSII) efficiency, biomass, and nutrient and chlorophyll concentrations and that the cost of carnivory would include increased RD and decreased AN and PSII efficiency in the pitcher sensu stricto according to the Givnish hypothesis (Givnish et al., 1984). Tests were made on the pitcher plant Nepenthes talangensis, a rare, endemic species from Sumatra. This is the first detailed study of photosynthesis in a carnivorous plant, with and without experimental addition of prey, using simultaneous measurements of gas exchange and chlorophyll fluorescence by the saturation pulse method.
MATERIALS AND METHODS
Plant material and culture conditions
The pitcher plant Nepenthes talangensis Nerz and Wistuba (1994) grows in mossy forest and stunted upper mountain forest near the summit of Gunung Talang (1800–2500 m alt.) in Sumatra (Nerz and Wistuba, 1994; Clarke, 2001). Its pitchers are light green to yellow in colour with red spots, lack a waxy zone (glandular region covers entire inner surface) and the pitcher fluid is extremely viscous (Clarke, 2001). Five-year-old plants, propagated from seeds, were 20–30 cm tall, with three or four pitchers up to 5 cm long. During the experiments, ten plants were grown under controlled conditions in a growth chamber with a photoperiod of 12 h dark/12 h light [200 µmol m−2 s−1 photosynthetically active radiation (PAR), day/night temperatures of 25/17 °C and high humidity (80–100 %)]. They were grown in a Sphagnum/perlite/bark/moss mixture substrate. To prevent entry of prey into pitchers they were plugged, without damaging them, with wads of cotton wool moistened in distilled water. Any newly opened pitchers during experiments were treated in the same way. The wads were removed from five plants after 6 months. The remaining five plants served as unfed controls. The fed plants were supplied with one live meal worm (Tenebrio molitor) for each pitcher each week for 8 weeks (total 2·46 ± 0·05 g f. wt worms per plant over 8 weeks, N concentration in worms = 8·7 %, 78 mg N per plant over 8 weeks). Fed plants were also able to catch natural prey, mostly sciarid flies, but the contribution of these to the nutrition of fed plants was negligible (<2 % of a worm's weight).
Simultaneous measurement of CO2 assimilation and chlorophyll fluorescence
To assess whether feeding enhanced photosynthetic efficiency, we analysed five youngest fully developed laminae (one per plant) with un-formed pitchers that had developed during the 8-week feeding period (‘young lamina without pitcher’), and five older laminae carrying the pitcher (separated into ‘older lamina with pitcher’ and ‘pitcher’), which had developed before the feeding experiment had started. Rates of photosynthesis (AN) and chlorophyll fluorescence were measured simultaneously with a CIRAS-2 (PP-Systems, Hitchin, UK) and a fluorcam FC 1000-LC (Photon Systems Instruments, Brno, Czech Republic) attached to an infrared gas analyser. Prior to measurements, the plants were dark-adapted overnight to achieve fully relaxed non-photochemical quenching (NPQ). Thereafter, the middle part of the lamina and the lid in the case of the pitcher (2·5 cm2) were enclosed in the leaf cuvette (PLC6, PP-Systems). Once stabilization (15 min) was achieved the respiration rate (RD) was recorded. Then the chlorophyll fluorescence was measured. Minimal fluorescence (F0) and then maximal fluorescence (Fm) were measured using a saturation pulse (4000 µmol m−2 s−1 PAR, 800-ms duration): maximal quantum yield of PSII (Fv/Fm) was calculated as Fm – F0/Fm. An induction curve of 15 min duration was then obtained by switching on an actinic light of 200 µmol m−2 s−1 PAR. For analysis of the quenching mechanism, ten saturation pulses were triggered. Simultaneously, stable AN was recorded at a CO2 concentration of 360 µmol mol−1, leaf temperature 23 ± 1 °C, relative air humidity 65–70 % and water vapour deficit 700–1000 Pa. Effective quantum yield of photosystem II (ΦPSII), photochemical quenching (QP) and NPQ were calculated (Maxwell and Johnson, 2000). The saturation irradiance (1200 µmol m−2 s−1 PAR) was applied for 15 min to allow adaptation, and light response curves were determined. The light intensity was decreased stepwise with irradiation periods of 3 min and subsequent saturation pulses were applied until 40 µmol m−2 s−1 PAR was reached. The apparent quantum yield of CO2 fixation (ΦCO2) was determined as the slope of the light response curve between 40 and 150 µmol m−2 s−1 PAR (Farquhar et al., 1980). Light response curves of AN, ΦPSII and NPQ were recorded simultaneously. All measurements were taken between 0900 and 1200 h.
Chlorophyll, nitrogen and carbon determination
The leaves from five fed and unfed plants were removed. Parts of the leaves were dried at 70 °C for 5 days to determine percentage dry weight. Chlorophyll concentrations were determined on the same types of leaves on which AN had been measured. Samples of leaves from young laminae without pitchers, older laminae carrying pitchers and pitchers themselves were ground in a mortar and pestle with small amount of sand and extracted with 80 % (v/v) chilled acetone with MgCO3 to avoid acidification and phaeophytinization of pigments. The samples were centrifuged at 8000g for 5 min at 4 °C. Chlorophyll a + b (chl a + b) in supernatant were determined spectrophotometrically (Jenway 6400, London, UK): chl a at 663·2 nm, chl b at 646·8 nm. Chlorophyll concentration (mg L−1) was calculated according to Lichtenthaler (1987) and re-expressed as mg chl a + b g−1 d. wt.
Leaf tissues from photosynthetic measurements were dried at 70 °C for 5 days and N and C were determined using an EA 1108 CHN analyser (Fisons Instruments, Milan, Italy). Nepenthes pitchers were washed using distilled water before drying and analysing to avoid contamination with nitrogen from prey. After N determination, photosynthetic nitrogen use efficiency (PNUE) was calculated for each type of leaf as: PNUE (μmol CO2 mol N−1 s−1) = ANmax (μmol CO2 g−1 d. wt s−1)/N (mol N g−1 d. wt).
Statistical analysis
Prior to statistical tests, data were analysed for normality and homogeneity of variance. When non-homogeneity was present, a t-test was employed with the appropriate corrected degrees of freedom. To evaluate the significance of the data between fed and unfed plants [leaf dry weight, RD, ANmax, stomatal conductance (gs), Fv/Fm, ΦPSII, ΦCO2, QP, NPQ, C, N, PNUE, chl a + b, chl a/b] a t-test was used. Paired data (comparison between the lamina and the pitcher within the same old leaf carrying the pitcher) were statistically evaluated by a two-tailed paired t-test. The results are expressed as the mean of five replicated measurements. ANCOVA (StatistiXL ver. 1.7 for Microsoft Excel) was used to test the homogeneity of slopes of the relationships between AN and N content for lamina and pitcher.
RESULTS
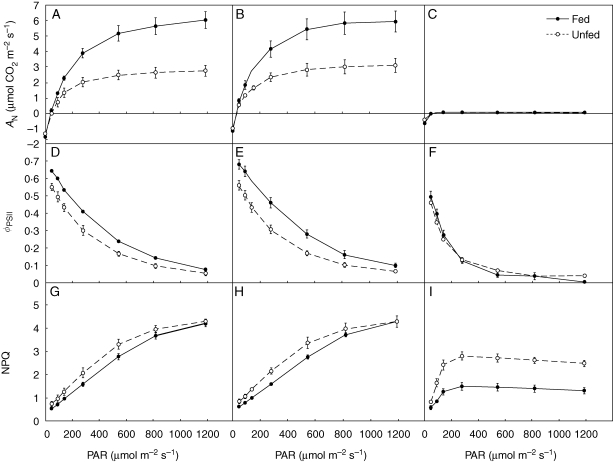
Feeding the pitchers of Nepenthes with beetle larvae increased the dark and light reactions of photosynthesis. In the laminae, AN increased almost linearly with increasing irradiance at irradiances less than about 160 µmol photon m−2 s−1 PAR and reached saturation under an irradiance of about 1000 µmol photon m−2 s−1 PAR (Fig. 1A, B). The AN of the young fed lamina without pitcher was significantly higher than the unfed control (Table 1). The ANmax of laminae from unfed plants was about 50 % that of fed lamina at saturating irradiance (Fig. 1A). Consistent with this, effective quantum yield of PSII (ΦPSII) and apparent quantum yield of CO2 fixation (ΦCO2) were also higher in young laminae from plants that had been fed (Table 1). ΦPSII decreased with increasing irradiance (Fig. 1D). Fed laminae had Fv/Fm values of about 0·800, significantly greater than those of laminae from unfed plants. The primary electron acceptor from PSII (plastoquinone A, QA) was more reduced in unfed plants, based on the higher value of QP in fed plants (Table 1). NPQ increased with increasing irradiance: the higher NPQ in laminae from unfed plants suggests greater heat dissipation via the xanthophyll cycle (Fig. 1G). Chlorophyll concentrations and chlorophyll a/b ratios were greater in fed plants than in unfed plants (Tables 1 and 2), indicating an increased proportion of light-harvesting complexes (LHC II) to reaction centres (RC II) in PSII in unfed plants. This is consistent with higher values of F0 in unfed plants (data not shown). Lamina dry weight, nitrogen concentration and PNUE were significantly higher in fed plants. Respiration rate was not significantly different, but there was a trend towards slightly greater RD in fed plants in all tissues studied (Table 1).
Fig. 1.
Rate of net photosynthesis (AN; A–C), effective quantum yield of PSII (ΦPSII; D–F) and non-photochemical quenching (NPQ; G–I) in response to irradiance in young lamina without pitcher (A, D, G), older lamina (B, E, H) and pitcher (C, F, I). Fed and unfed plants as indicated, values are means ± s.e. PAR, photosynthetically active radiation.
Table 1.
Leaf biomass, chlorophyll fluorescence, gas exchange, chlorophyll, nitrogen and carbon concentration, and photosynthetic nitrogen use efficiency in young lamina without pitcher
| Parameter | Unfed | Fed |
|---|---|---|
| Leaf dry weight (mg) | 104·5 ± 13·7 | 162·0 ± 12·2* |
| RD (μmol CO2 m−2 s−1) | 1·30 ± 0·16 | 1·58 ± 0·15ns |
| RD (nmol CO2 g−1 d. wt s−1) | 8·9 ± 2·2 | 11·5 ± 2·2ns |
| ANmax (μmol CO2 m−2 s−1) | 2·8 ± 0·2 | 6·0 ± 0·4** |
| ANmax (nmol CO2 g−1 d. wt s−1) | 16·9 ± 0·60 | 46·1 ± 7·6** |
| gs (mmol m−2 s−1) | 62·1 ± 4·5 | 152·0 ± 8·2** |
| Fv/Fm | 0·758 ± 0·011 | 0·800 ± 0·003* |
| ΦPSII | 0·41 ± 0·02 | 0·52 ± 0·01** |
| ΦCO2 (mol CO2 mol quanta−1) | 0·014 ± 0·001 | 0·023 ± 0·002** |
| QP | 0·71 ± 0·01 | 0·77 ± 0·01** |
| NPQ | 1·28 ± 0·13 | 0·84 ± 0·06* |
| C (mg g−1 d. wt) | 459·8 ± 2·4 | 468·4 ± 3·1* |
| N (mg g−1 d. wt) | 10·6 ± 1·0 | 18·6 ± 2·2* |
| PNUE (μmol CO2 mol N−1 s−1) | 23·7 ± 1·6 | 32·5 ± 2·4* |
| Chl a + b (mg g−1 d. wt) | 1·21 ± 0·11 | 2·72 ± 0·22** |
| Chl a/b | 1·91 ± 0·3 | 2·15 ± 0·03** |
See Appendix for definitions. Values shown are means ± s.e., n = 5. Significantly different values (t-test) are indicated: * P < 0·05, ** P < 0·01; ns, non-significant differences.
Table 2.
Leaf biomass, chlorophyll fluorescence, gas exchange, chlorophyll, nitrogen and carbon concentration, and photosynthetic nitrogen use efficiency in old lamina carrying the pitcher
| Lamina |
Pitcher |
|||
|---|---|---|---|---|
| Parameter | Unfed | Fed | Unfed | Fed |
| Leaf dry weight (mg) | 104·7 ± 15·8 | 120·5 ± 19·6ns | 141·3 ± 5·2†† | 199·5 ± 47·8ns †† |
| RD (μmol CO2 m−2 s−1) | 0·97 ± 0·02 | 1·15 ± 0·06ns | 0·43 ± 0·02†† | 0·60 ± 0·1ns †† |
| RD (nmol CO2 g−1 d. wt s−1) | 5·8 ± 0·5 | 7·0 ± 0·21ns | 6·5 ± 0·4†† | 9·6 ± 1·7 ns †† |
| ANmax (μmol CO2 m−2 s−1) | 3·1 ± 0·3 | 6·0 ± 0·5** | 0·10 ± 0·04†† | 0·15 ± 0·03ns †† |
| ANmax (nmol CO2 g−1 d. wt s−1) | 19·4 ± 2·0 | 37·8 ± 5·4* | 2·0 ± 0·9†† | 3·5 ± 1·4ns †† |
| gs (mmol m−2 s−1) | 57·0 ± 6·4 | 92·7 ± 4·6** | 37·7 ± 4·4†† | 31·3 ± 1·6ns †† |
| Fv/Fm | 0·770 ± 0·010 | 0·823 ± 0·011* | 0·715 ± 0·012† | 0·740 ± 0·008ns †† |
| ΦPSII | 0·41 ± 0·02 | 0·59 ± 0·02** | 0·18 ± 0·01†† | 0·16 ± 0·01ns †† |
| ΦCO2 (mol CO2 mol quanta−1) | 0·011 ± 0·002 | 0·021 ± 0·001** | 0·001 ± 0·000†† | 0·001 ± 0·000ns †† |
| QP | 0·73 ± 0·02 | 0·82 ± 0·01** | 0·34 ± 0·04†† | 0·25 ± 0·03ns †† |
| NPQ | 1·64 ± 0·14 | 0·79 ± 0·03** | 1·66 ± 0·14ns | 0·68 ± 0·14** ns |
| C (mg g−1 d. wt) | 464·2 ± 1·9 | 477·5 ± 5·8* | 457·1 ± 0·9†† | 462·2 ± 1·2* †† |
| N (mg g−1 d. wt) | 11·3 ± 0·4 | 20·0 ± 2·8* | 7·8 ± 0·2†† | 17·0 ± 1·5** † |
| PNUE (μmol CO2 mol N−1 s−1) | 20·7 ± 1·5 | 24·8 ± 4·1ns | 4·4 ± 1·2†† | 2·7 ± 0·8ns †† |
| Chl a + b (mg g−1 d. wt) | 1·30 ± 0·01 | 2·51 ± 0·34** | 0·93 ± 0·09†† | 1·02 ± 0·05ns †† |
| Chl a/b | 1·94 ± 0·02 | 2·15 ± 0·06** †† | 0·96 ± 0·04 | 1·45 ± 0·07** †† |
See Appendix for definitions. Values shown are means ± s.e., n = 5. Comparisons were made (t-test) between fed and unfed lamina or pitcher at *P < 0·05, **P < 0·01, and between fed pitcher and fed lamina or unfed pitcher and unfed lamina respectively at †P < 0·05, ††P < 0·01 (paired t-test); ns, non-significant differences.
Differences in measured photosynthetic characteristics between fed and unfed plants in the older laminae carrying the pitcher were similar to those of young laminae except their dry weight (Table 2, Fig. 1B, E, H). This is not surprising given that the older laminae were fully developed before the feeding experiment started, whereas the young laminae were developing during the feeding experiment. There were no significant differences in PNUE between the older laminae of fed and unfed plants.
In the pitchers, AN was very low and increased linearly with increasing irradiance at irradiances less than about 50 µmol photon m−2 s−1 PAR and reached saturation only at 100 µmol photon m−2 s−1 (Fig. 1C). In contrast to laminae, there were no statistical differences between pitchers of fed and unfed plants in ANmax, Fv/Fm, ΦPSII, ΦCO2, QP, gs or chlorophyll concentration (Table 2). However, unfed pitchers had higher NPQ than fed pitchers and lower nitrogen concentrations, similar to the pattern in young and old laminae. Almost all photosynthetic parameters were significantly lower in pitchers than in laminae (Table 2). The primary acceptor of PSII, QA, was maintained at more than 70 % oxidized in the lamina, but in the pitcher it was only 25–35 % oxidized. NPQ was similar in pitchers and laminae, but was strongly dependent on whether the plants were fed or unfed. However, at higher irradiance, laminae had higher NPQ (Fig. 1H). The NPQ was saturated at 300 µmol photon m−2 s−1 PAR in the pitcher (Fig. 1I). Stomatal conductance, PNUE, and nitrogen, carbon and chlorophyll concentrations were also significantly lower in the pitcher. The RD per unit surface area was higher in the lamina. By contrast, RD per unit mass was higher in the pitcher (Table 2).
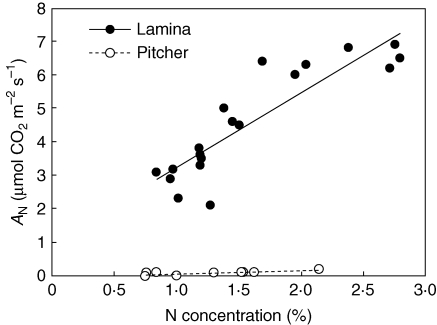
Figure 2 summarizes the relationship between nitrogen concentration and AN in lamina and trap separately. It is obvious that there is a strong correlation between nitrogen concentration and AN in laminae (P < 0·01) but not in pitchers (P = 0·06). Furthermore, the relationships do not have similar slopes (P = 0·013).
Fig. 2.
Net photosynthetic rate (AN) in relation to leaf nitrogen concentration in lamina and pitcher, as indicated. The lines have different slopes (P = 0·013); no relationship was found between AN and N in the pitcher (P = 0·06) but a significant relationship was found in the lamina (P < 0·01).
One year after the feeding experiment all fed plants, but no unfed plants, had begun to flower.
DISCUSSION
The effects of feeding pitchers with beetle larvae on the photosynthetic activity of the pitcher plant Nepenthes talangensis was investigated, using simultaneous measurement of gas exchange and chlorophyll fluorescence, and relating them to nitrogen and chlorophyll content of the laminae and pitchers. Nepenthes is a good experimental genus for studying the cost–benefit model of carnivory with leaves composed of photosynthetically active laminae and a pitcher trap. The laminae of fed N. talangensis had a greater N concentration as a result of nitrogen absorption from prey (Tables 1 and 2). In their natural environment, Nepenthes species are N-limited, and have evolved the pitcher to assist in their uptake of N (Osunkoya et al., 2007). The average N acquired from insects is high: 61·5, 53·8 and 68·1 % of the total for N. mirabilis, N. rafflesiana and N. albomarginata, respectively (Schulze et al., 1997; Moran et al., 2001). Chlorophyll concentration, AN and maximum and effective quantum yield of PSII were higher in fed plants. Two consequences of this are (1) an increase in biomass of new formed laminae and (2) flowering of plants after feeding (Table 1). Because about 50–80 % of foliar N is incorporated in photosynthetic proteins (Evans, 1989), we suggest that the lower AN of unfed plants is due to lower N and Rubisco concentrations and thus lower capacity for CO2 fixation. The smaller AN was accompanied by a smaller stomatal conductance (gs) in unfed compared with fed plants but intercellular CO2 concentration (Ci) was statistically unchanged (data not shown). This indicates that reduced AN was due to reduced carboxylation efficiency rather than to stomatal limitation. Lower ΦPSII is a secondary consequence of impaired CO2 assimilation. When carbon fixation is inhibited, ΦPSII is often down-regulated to match the reduced requirement for electrons and to minimize the production of reactive oxygen species (Golding and Johnson, 2003). Maximum quantum yield of PSII of dark-adapted leaves, which is proportional to the quantum yield of O2 evolution, was slightly lower in unfed plants, reflecting that potentional quantum yields for photochemistry in PSII were also negatively affected in prey-deprived plants (Tables 1 and 2). When nutrient stress restricts carboxylation, even moderate light may become excessive and may result in destructive photo-oxidative reactions. In the first line of defence against photo-oxidation, xanthophylls transform excessive excitation energy to heat, measured as NPQ of chlorophyll fluorescence (Krause and Jahns, 2004). In laminae, NPQ values were higher in unfed plants as a consequence of less light energy being used in photochemistry and through greater heat dissipation by the xanthophyll cycle. This suggests that increased thermal dissipation by the xanthophyll cycle slightly compensates for the lower ΦPSII in unfed lamina, but probably not sufficiently. The decline of ΦPSII in unfed laminae was not offset by thermal dissipation, leading to a lower QP and higher excitation pressure on PSII and thus higher susceptibility to photoinhibition in unfed plants. The unfed N. talangensis plants exhibited similar symptoms to plants under nitrogen stress. Huang et al. (2004) found lower AN, gs, Fv/Fm, ΦPSII, QP, chl and chl a/b in nitrogen-deprived rice.
All photosynthetic parameters were significantly lower in pitchers than in laminae (Table 2). Nepenthes pitchers have digestive functions and the absence of a positive correlation between N and AN (Table 2, Figs 1 and 2) suggests that factors other than N limit AN. Pavlovič et al. (2007) suggest high diffusional resistance for CO2 uptake in the Nepenthes pitcher due to very low stomatal density and compact mesophyll. Low PNUE in the pitchers found in this and in our previous study (Pavlovič et al., 2007) indicates either high resistance for CO2 uptake or increased allocation of N to structural materials rather than to photosynthetic machinery (Osunkoya et al., 2007, 2008). Lower QP (Table 2) and saturation of NPQ at relatively low irradiance in the pitchers (Fig 1I) result in increase excitation pressure on PSII and higher susceptibility to photoinhibition. This may explain the reduced longevity of pitchers relative to laminae, which is well documented (Osunkoya et al., 2008). All the above demonstrate a strong adaptation of pitchers to the carnivorous, but not to the assimilation, function.
The only study to date that has quantified the effects of nutrient stress in Nepenthes is that of Moran and Moran (1998), who examined foliar reflectance in nutrient-starved N. rafflesiana. They observed no significant differences in root or leaf N concentration, which is inconsistent with the present results. However, N content (concentration × biomass) was lower in their prey-deprived plants. They suggested that increased growth upon feeding is a primary adaptation because under conditions of resource limitation, plants are able to maintain critical foliar nutrient concentrations by a reduction in growth rate.
Increased AN after feeding was found by Farnsworth and Ellison (2008) in the genus Sarracenia. The well-fed plants had slightly higher foliar N concentration, chlorophyll content and Fv/Fm. However, these differences were only found in young unfed Sarracenia leaves that were produced subsequent to feeding. Wakefield et al. (2005) found no changes of AN measured on fed leaves of Sarracenia purpurea. This agrees with the findings of Butler and Ellison (2007), who demonstrated that nutrients captured by older pitchers are rapidly translocated to newly formed leaves. In contrast, we found enhanced AN in older lamina carrying the fed pitcher, although the pitcher itself did not increase in photosynthetic efficiency (Fig. 2C, F). The results of Schulze et al. (1997) show that not only young developing leaves carrying closed pitchers obtain a high portion of N from captured prey, but also that older fully developed leaves carrying open pitchers also obtain more than 50 % of their nitrogen from prey in Nepenthes. Ellison and Gotelli (2002) showed an increase in AN following addition of inorganic nitrogen to Sarracenia purpurea, but this response resulted from plants producing non-carnivorous phyllodes, which are more efficient in photosynthesis than the carnivorous pitcher. S. purpurea produces trapless phyllodes only during drought, under shade or with increased nutrient availability. In contrast to Nepenthes, Sarracenia does not have leaves that are differentiated into a photosynthetically active lamina and a pitcher trap, but usually have only a rosette of pitchers that must function in both photosynthesis and prey capture to achieve positive carbon gain, and their photosynthetic efficiency is closer to Nepenthes lamina than to the Nepenthes pitcher (Pavlovič et al., 2007).
The rate of photosynthesis was not increased as a result of prey capture in the carnivorous butterwort Pinguicula vulgaris (Méndez and Karlsson, 1999). However, supplementary feeding in situ increased both rosette size and reproduction, through an increase in flowering frequency and seed production (Thorén and Karlsson, 1998). Méndez and Karlsson (1999) also concluded that the benefits from capturing prey are larger in reproductive terms than in terms of photosynthesis. We also found accelerated flowering in fed Nepenthes plants, but propose that this is an indirect effect of increased AN rather than a direct effect of feeding. Adamec (2008) observed two different responses to feeding in the aquatic carnivorous plants Utricularia australis and Aldrovanda vesiculosa. Both species, when fed, produced longer shoots and had smaller N concentrations in comparison with control plants. Photosynthetic rate was higher in fed Aldrovanda but lower in fed Utricularia. It was suggested that tissue N in fed plants was diluted by growth processes much more than in unfed controls, so the main physiological effect of catching prey was not based on enhancement of AN, as was suggested by Givnish et al. (1984), but on providing N and P (and probably C) for essential growth processes. The present study results contrasted with this, as there was a positive correlation between N and AN in lamina (Fig. 2), in agreement with the study of Ellison and Farnsworth (2005), but not of Wakefield et al. (2005). The contradictory results concerning feeding experiments discussed above might lie in genotypic differences among plant species.
The Givnish model considers not only the ability of carnivory to enhance AN, but also the costs associated with carnivory. The costs include a reduced AN and higher RD. The first was confirmed in this and in our previous study of N. alata and N. mirabilis (Pavlovič et al., 2007), but the second is probably species-specific. Different results were obtained here depending on the units of measurements. Area-based RD was higher in the lamina, and mass-based RD was higher in the pitcher. This discrepancy is due to different leaf mass area (LMA was higher in lamina) of these two distinct organs. Differences in leaf thickness between lamina and pitcher are well documented in six Nepenthes species (Osunkoya et al., 2007). It appears that the result is influenced more by leaf structure than by specialization for carnivorous or photosynthetic function. Reduced AN and higher RD was found in Utricularia bladder. Photosynthetic rate in leaves of six aquatic Utricularia species exceed that in bladders seven- to ten-fold and RD of bladders was 75–200 % greater than in leaves (Adamec, 2006). The high RD of bladders in Utricularia is consistent with specific amino acid changes (Leu113, Ser114 replaced by Cys113, Cys114) in Utricularia cytochrome c-oxidase, the rate-limiting enzyme in the respiratory cycle, which accelerates the rate of respiration. These amino acid changes were not confirmed for Nepenthes (Jobson et al., 2004).
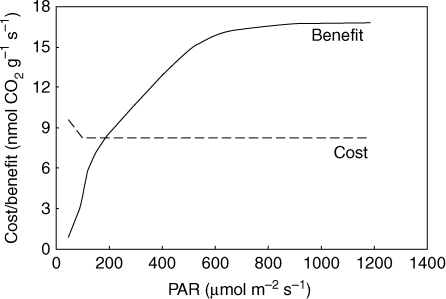
According to Givnish, there is a trade-off between photosynthetic costs and benefits that could lead to the evolution of carnivory. Enhancement of AN resulting from the addition of nutrients as a result of carnivory should be more rapid in high-light than in shady environments (Givnish et al., 1984; Ellison and Gotelli, 2001). However, convincing evidence for this is lacking. From the present data we can calculate the benefit of carnivory in terms of AN and RD as a difference between fed and unfed lamina [(AN fed lamina – RD fed lamina)] – [(AN unfed lamina – RD unfed lamina)]. The cost of carnivory was calculated as [RD pitcher – AN pitcher]. The result is summarized in Fig. 3. As expected, the benefit from carnivory is higher with increasing irradiance and exceeds the cost at approx. 200 µmol m−2 s−1 PAR. At low light intensity the light is more limiting to photosynthesis than nutrient acquisition (the benefit is lower than the cost) and carnivory does not pay. It is known that Nepenthes stop producing pitchers and thus decrease the cost of carnivory when they grow at low light intensity (usually below 50 µmol m−2 s−1 PAR, our personal observations), in general support of the analysis.
Fig. 3.
Trade-off between cost and benefit calculated from gas exchange experiments in older leaves carrying the pitcher at different irradiance. Note that the benefit of carnivory exceeds its own cost with increasing irradiance.
In conclusion, despite contradictory results in this area of research, the present results clearly demonstrate the positive effects of experimental carnivory on increasing the chlorophyll and nitrogen contents of laminae and stimulating the rate of photosynthesis and utilization of absorbed light energy for photochemistry in the carnivorous plant N. talangensis. In contrast to the laminae, the pitchers are unable to increase AN in response to feeding, suggesting that they are highly specialized for nutrient acquisition. The ability of the laminae to increase biomass and photosynthetic efficiency in response to prey capture may provide a competitive advantage for these plants over non-carnivorous plants in sunny and nutrient-poor habitats. Moreover, one year after the feeding experiment all fed plants, but no unfed plants, started to flower, confirming that carnivory may also increase reproductive success in nutrient-poor habitats, probably indirectly via increased AN.
ACKNOWLEDGEMENTS
This work was supported by grant VEGA 1/0040/09. We thank Zelené údolí (Czech Republic) for providing the N. talangensis plants for our experiments and Professor Philip White for language corrections.
APPENDIX
Abbreviations used in the text.
- AN
Net photosynthetic rate
- ANmax
Maximal net photosynthetic rate at saturation irradiance
- Fv/Fm
Maximal quantum yield of PSII
- gs
Stomatal conductance
- LHC II
Light harvesting complex of PSII
- LMA
Leaf mass area
- NPQ
Non-photochemical quenching coefficient
- PAR
Photosynthetically active radiation
- PNUE
Photosynthetic nitrogen use efficiency
- PSI
Photosystem I
- PSII
Photosystem II
- QA
Plastochinone A
- QP
Photochemical quenching coefficient
- RC II
Reaction centre of PSII
- RD
Dark respiration rate
- ΦCO2
Apparent quantum yield of CO2 assimilation
- ΦPSII
Effective quantum yield of PSII
LITERATURE CITED
- Adamec L. Mineral nutrition of carnivorous plants – a review. Botanical Review. 1997;63:273–299. [Google Scholar]
- Adamec L. Respiration and photosynthesis of bladders and leaves of aquatic Utricularia species. Plant Biology. 2006;8:765–769. doi: 10.1055/s-2006-924540. [DOI] [PubMed] [Google Scholar]
- Adamec L. The influence of prey capture on photosynthetic rate in two aquatic carnivorous plant species. Aquatic Botany. 2008;89:66–70. [Google Scholar]
- Butler JL, Ellison AM. Nitrogen cycling dynamics in the carnivorous northern pitcher plant, Sarracenia purpurea. Functional Ecology. 2007;21:835–843. [Google Scholar]
- Chandler GE, Anderson JW. Studies on the nutrition and growth of Drosera species with reference to the carnivorous habit. New Phytologist. 1976;76:129–141. [Google Scholar]
- Clarke C. Nepenthes of Borneo. Kota Kinabalu, Malaysia: Natural History Publication; 1997. [Google Scholar]
- Clarke C. Nepenthes of Sumatra and Peninsular Malaysia. Kota Kinabalu, Malaysia: Natural History Publication; 2001. [Google Scholar]
- Ellison AM. Nutrient limitation and stoichiometry of carnivorous plants. Plant Biology. 2006;8:740–747. doi: 10.1055/s-2006-923956. [DOI] [PubMed] [Google Scholar]
- Ellison AM, Farnsworth EJ. The cost of carnivory for Darlingtonia californica (Sarraceniaceae): evidence from relationships among leaf traits. American Journal of Botany. 2005;92:1085–1093. doi: 10.3732/ajb.92.7.1085. [DOI] [PubMed] [Google Scholar]
- Ellison AM, Gotelli NJ. Evolutionary ecology of carnivorous plants. Trends in Ecology and Evolution. 2001;16:623–629. [Google Scholar]
- Ellison AM, Gotelli NJ. Nitrogen availability alters the expression of carnivory in the northern pitcher plant, Sarracenia purpurea. Proceedings of the National Academy of Sciences of the USA. 2002;99:4409–4412. doi: 10.1073/pnas.022057199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JR. Photosynthesis and nitrogen relationships in the leaves of C3 plants. Oecologia. 1989;78:9–19. doi: 10.1007/BF00377192. [DOI] [PubMed] [Google Scholar]
- Farnsworth EJ, Ellison AM. Prey availability directly affects physiology, growth, nutrient allocation and scaling relationships among leaf traits in 10 carnivorous plant species. Journal of Ecology. 2008;96:213–221. [Google Scholar]
- Farquhar GD, von Caemmerer S, Berry JA. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta. 1980;149:78–90. doi: 10.1007/BF00386231. [DOI] [PubMed] [Google Scholar]
- Givnish TJ, Burkhardt EL, Happel RE, Weintraub JD. Carnivory in the bromeliad Brocchinia reducta with a cost/benefit model for the general restriction of carnivorous plants to sunny, moist, nutrient poor habitats. American Naturalist. 1984;124:479–497. [Google Scholar]
- Golding AJ, Johnson GN. Downregulation of linear and activation of cyclic electron transport during drought. Planta. 2003;218:107–114. doi: 10.1007/s00425-003-1077-5. [DOI] [PubMed] [Google Scholar]
- Huang ZA, Jiang DA, Yang Y, Sun JW, Jin SH. Effect of nitrogen deficiency on gas exchange, chlorophyll fluorescence and antioxidant enzymes in leaves of rice plants. Photosynthetica. 2004;42:357–364. [Google Scholar]
- Jobson RW, Nielsen R, Laakkonen L, Wikström M, Albert VA. Adaptive evolution of cytochrome c oxidase: infrastructure for a carnivorous plant radiation. Proceedings of the National Academy of Sciences of the USA. 2004;99:4409–4412. doi: 10.1073/pnas.0408092101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson PS, Carlsson B. Why does Pinguicula vulgaris L. trap insects? New Phytologist. 1984;97:25–30. [Google Scholar]
- Knight SE. Costs of carnivory in the common bladderwort, Utricularia macrorhiza. Oecologia. 1992;89:348–355. doi: 10.1007/BF00317412. [DOI] [PubMed] [Google Scholar]
- Krause GH, Jahns P. Non-photochemical energy dissipation determined by chlorophyll fluorescence quenching: characterisation and function. In: Papageorgiou GC, Govindjee, editors. Chlorophyll a fluorescence: a signature of photosynthesis. Dordrecht: Springer; 2004. pp. 463–495. [Google Scholar]
- Lichtenthaler HK. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods in Enzymology. 1987;148:350–382. [Google Scholar]
- Maxwell K, Johnson GN. Chlorophyll fluorescence – a practical guide. Journal of Experimental Botany. 2000;51:659–668. doi: 10.1093/jxb/51.345.659. [DOI] [PubMed] [Google Scholar]
- Méndez M, Karlsson PS. Costs and benefits of carnivory in plants: insights from photosynthetic performance of four carnivorous plants in a subarctic environment. Oikos. 1999;86:105–112. [Google Scholar]
- Moran JA, Moran AJ. Foliar reflectance and vector analysis reveal nutrient stress in prey-deprived pitcher (Nepenthes rafflesiana) International Journal of Plant Science. 1998;159:996–1001. [Google Scholar]
- Moran JA, Merbach MA, Livingstone NJ, Clarke CM, Booth WE. Termite prey specialization in the pitcher plant Nepenthes albomarginata – evidence from stable isotope analysis. Annals of Botany. 2001;88:307–311. [Google Scholar]
- Nerz J, Wistuba A. Five new taxa of Nepenthes (Nepenthaceae) from north and west Sumatra. Carnivorous Plant Newsletter. 1994;23:101–114. [Google Scholar]
- Osunkoya OO, Daud SD, Di-Giusto B, Wimmer FL, Holige TM. Construction costs and physico-chemical properties of the assimilatory organs of Nepenthes species in northern Borneo. Annals of Botany. 2007;99:895–906. doi: 10.1093/aob/mcm023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osunkoya OO, Daud SD, Wimmer FL. Longevity, lignin content and construction cost of the assimilatory organs of Nepenthes species. Annals of Botany. 2008;102:845–853. doi: 10.1093/aob/mcn162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovič A, Masarovičová E, Hudák J. Carnivorous syndrome in Asian pitcher plants of the genus Nepenthes. Annals of Botany. 2007;100:527–536. doi: 10.1093/aob/mcm145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rischer H, Hamm A, Bringmann G. Nepenthes insignis uses a C2-portion of the carbon skeleton of l-alanine acquired via its carnivorous organs, to built up the allelochemical plumbagin. Phytochemistry. 2002;59:603–609. doi: 10.1016/s0031-9422(02)00003-1. [DOI] [PubMed] [Google Scholar]
- Schulze W, Schulze ED, Pate JS, Gillinson AN. The nitrogen supply from soils and insects during growth of the pitcher plants Nepenthes mirabilis, Cephalotus follicularis and Darlingtonia californica. Oecologia. 1997;112:464–471. doi: 10.1007/s004420050333. [DOI] [PubMed] [Google Scholar]
- Thorén ML, Karlsson PS. Effects of supplementary feeding on growth and reproduction of three carnivorous plant species in a subarctic environment. Journal of Ecology. 1998;86:501–510. [Google Scholar]
- Wakefield AE, Gotelli NJ, Wittman SE, Ellison AM. Prey addition alters nutrient stoichiometry of the carnivorous plant Sarracenia purpurea. Ecology. 2005;86:1737–1743. [Google Scholar]