Abstract
Background and Aims
Molybdenum (Mo) is an essential trace element for higher plants. It has been shown that application of Mo enhances the cold resistance of winter wheat. In order to improve our understanding of the molecular mechanisms of cold resistance arising from application of Mo in winter wheat, investigations were made regarding the transcription of cold-responsive (COR) genes in abscisic acid (ABA)-dependent and ABA-independent pathways in winter wheat regulated by Mo application under low-temperature stress.
Methods
Two cultivars of winter wheat (Triticum aestivum), Mo-efficient cultivar ‘97003’ and Mo-inefficient cultivar ‘97014’, were grown in control (−Mo) and Mo fertilizer (+Mo) treatments for 40 d at 15/12 °C (day/night), and the temperature was then reduced to 5/2 °C (day/night) to create low-temperature stress. Aldehyde oxidase (AO) activities, ABA contents, the transcripts of basic leucine zipper (bZIP)-type transcription factor (TF) genes, ABA-dependent COR genes, CBF/DREB transcription factor genes and ABA-independent COR genes were investigated at 0, 3, 6 and 48 h post cold stress.
Key Results
Mo application significantly increased AO activity, ABA levels, and expression of bZIP-type TF genes (Wlip19 and Wabi5) and ABA-dependent COR genes (Wrab15, Wrab17, Wrab18 and Wrab19). Mo application increased expression levels of CBF/DREB transcription factor genes (TaCBF and Wcbf2-1) and ABA-independent COR genes (Wcs120, Wcs19, Wcor14 and Wcor15) after 3 and 6 h exposure to low temperature.
Conclusions
Mo might regulate the expression of ABA-dependent COR genes through the pathway: Mo → AO → ABA → bZIP → ABA-dependent COR genes in winter wheat. The response of the ABA-dependent pathway to Mo was prior to that of the ABA-independent pathway. Similarities and differences between the Mo-efficient and Mo-inefficient wheat cultivars in response to Mo under cold stress are discussed.
Key words: Molybdenum, cold resistance, cold responsive gene, low-temperature stress, ABA-dependent pathway, ABA-independent pathway, aldehyde oxidase
INTRODUCTION
Molybdenum (Mo) is an essential element for higher plants and plays a vital role in many physiological and biochemical processes. More than 40 Mo-enzymes catalysing diverse redox reactions have been found in all organisms. However, only four of these enzymes have been found in plants (Schwarz and Mendel, 2006), namely nitrate reductase (NR), aldehyde oxidase (AO), xanthine dehydrogenase (XDH) and sulfite oxidase (SO; a list of abbreviations is given in Table 1). These Mo-enzymes participate in diverse metabolic processes, such as nitrate assimilation, phytohormone synthesis, purine catabolism and sulfite detoxification in plants (Mendel and Hansch, 2002). Among them, AO has been shown to catalyse the final steps in the conversion of indole-3-acetaldehyde to indole-3-abscisic acid (IAA), and the oxidation of abscisic aldehyde to abscisic acid (ABA; Kaiser et al., 2005). Mutations in either the AO apoprotein or enzymes involved in Mo-cofactor (Moco) biosynthesis and Moco activation (sulfuration) disrupt ABA synthesis (Sagi et al., 2002; Schwarz, 2005). A low ABA level results in a wilty appearance on plants as a result of excessive transpiration, loss of stomatal control, altered seed dormancy and impaired defence responses to environmental stress (Mendel and Hansch, 2002; Kaiser et al., 2005).
Table 1.
List of main abbreviations used in the text
| COR | Cold-responsive or cold-regulated |
| NR | Nitrate reductase |
| AO | Aldehyde oxidase |
| XDH | Xanthine dehydrogenase |
| SO | Sulfite oxidase |
| TF | Transcription factor |
| CBF | C-repeat binding factor |
| bZIP | Basic leucine zipper |
Wheat has been regarded as being insensitive crop to Mo deficiency, and thus few studies were reported until 1954 (Mulder, 1954). It was reported that approximately 446 million hectares of arable land was Mo-deficient in China. Mo deficiency in soil is becoming a limiting factor for crop production in many provinces of China, such as Shanxi, Shandong, Jiangxi, Jiangsu and Sichuan (Hu et al., 2002). Wang et al. (1990) found that Mo deficiency was a major factor causing leaf-yellowing and tiller death on wheat in winter, and low-temperature stress accelerated the development of Mo-deficiency symptoms. Further studies showed that application of Mo improved the cold-resistance of winter wheat (Wang et al., 1995). Vankova-Radeva et al. (1997) and Li et al. (2001) also found that Mo application resulted in the increase of frost tolerance of winter wheat grown in acidic soil. Further research showed that Mo application affected the lipid composition of winter wheat leaves (Yaneva et al., 1995) and increased the activities of Mo-containing enzymes (Yaneva et al., 1996; Vunkova-Radeva et al., 2003) and antioxidative enzymes (Sun et al., 2006c). Moreover, nitrogen-containing compounds (Hu et al., 2002), chlorophyll biosynthesis and photosynthetic characteristics (Yu et al., 2006; Sun et al., 2006a) were also found to be closely related to Mo application in winter wheat under low-temperature stress. These studies mainly focused on the physiological basis of cold resistance enhanced by Mo application in wheat, but little is known about its molecular mechanism.
Low temperature is one of the most common adverse environmental factors affecting the growth of winter wheat in central China. Various mechanisms have been suggested to account for chilling injury or freezing tolerance in plants (Lee et al., 1999). In the past decade, many reports have investigated the molecular mechanisms of low-temperature signal transduction and cold acclimation. A number of cold-responsive (COR) genes have been identified and characterized from both dicotyledonous and monocotyledonous plants (Sharma et al., 2005). Genetic analysis indicated that COR gene expression is mediated by both ABA-dependent and ABA-independent pathways (Thomashow, 1999; Shinozaki and Yamaguchi-Shinozaki, 2000; Sharma et al., 2005). In the ABA-dependent pathway, endogenous ABA might activate basic leucine zipper (bZIP) transcription factors, and then regulate ABA-dependent COR genes through ABA-responsive elements (ABREs; Uno et al., 2000; Xiong et al., 2002), whereas in the ABA-independent pathway, low temperature triggers the expression of the CBF (C-repeat binding factor) family of transcription factors (TFs), which in turn activate downstream COR genes that confer or enhance freezing tolerance in plants (Thomashow, 1999). The ABA-dependent COR genes in wheat include Wrab15 (Kobayashi et al., 2004), Wrab17 (Tsuda et al., 2000; Kobayashi et al., 2008c), Wrab18 (Kobayashi et al., 2004) and Wrab19 (Tsuda et al., 2000; Egawa et al., 2006), whereas the ABA-independent COR genes include Wcs19 (Fowler et al., 2001), Wcor14 (Tsvetanov et al., 2000) and Wcor15 (Takumi et al., 2003).
Molybdenum regulates ABA biosynthesis via AO, and the phytohormone ABA is involved in mediating expression of COR genes. In order to improve our understanding of the molecular mechanisms of cold resistance enhanced by Mo application in winter wheat, the present study focuses on the effects of Mo application on COR gene transcription in winter wheat, and, in particular, compares the differential responses of ABA-dependent and ABA-independent pathways in COR gene expression to Mo under low-temperature stress.
MATERIALS AND METHODS
Plant preparation and sample collection
Two cultivars of winter wheat (Triticum aestivum), Mo-efficient cultivar ‘97003’ and Mo-inefficient cultivar ‘97014’, which differ in Mo uptake and distribution (Yu et al., 2002), were grown in Mo-deficient yellow-brown soil in a controlled-climate growth chamber for 40 d at 15/12 °C (day/night) with a 14-h photoperiod at a light intensity of 400 µmol m−2 s−1 and 70 % air relative humidity. The properties of the soil were: Tamm reagents-extractable Mo 0·100 mg kg−1, pH 5·02 (H2O; water/soil = 1 : 1), organic matter 17·8 g kg−1, alkaline hydrolysable nitrogen 80·5 mg kg−1, Olsen-extractable phosphate 6·77 mg kg−1 and exchangeable potassium 121·3 mg kg−1. Plants were irrigated daily with distilled water. The following chemicals were added to the soil as fertilizers: (NH4)2SO4 1 g kg−1 soil, KH2PO4 1 g kg−1 soil and KCl 1 g kg−1 soil; all chemicals were of analytical grade. The treatments were designed as control (−Mo, without addition of Mo) and Mo fertilization (+Mo, Mo 0·15 mg·kg−1 soil using molybdate [(NH4)6Mo7O24.4H2O]). The temperature of the growth chamber was reduced to 5/2 °C (day/night) for cold stress after 40 d growth from germination. The first fully expanded leaves from −Mo and +Mo treatments were collected after 0, 3, 6 and 48 h of cold stress, frozen in liquid nitrogen and then stored at –80 °C for further analysis. Four biological replicates were prepared for each treatment.
Tissue extraction and analysis of AO activity
AO activities were assayed according to Sagi et al. (1999) with some modification. Frozen leaf samples (1 g) were homogenized with ice-cold extraction medium containing 50 mm Tris-HCl (pH 6·8), 10 % (v/v) glycerin, 1 mm EDTA, 1 mm dithiothreitol (DTT), 5 mm flavin adenine dinucleotide and 3 % (w/v) polyvinylpolypyrrolidone (PVPP). The ratio of tissue to extraction buffer was 1:3 (w/v). The homogenized plant material was centrifuged at 27 000 g, 4 °C for 15 min. Ammonium sulfate was added to the supernatant to 60 % saturation. The resulting mixture was stirred for 30 min and then centrifuged at 15 000 g for 15 min. The precipitate was dissolved in a small volume of 50 mm K-phosphate buffer (pH 7·8) and desalted on Sephadex G-25 (Pharmacia) columns equilibrated with the same buffer. AO activity was assayed by monitoring the decrease of absorbance at 600 nm in a UV 2100 spectrophotometer (Shimadzu, Kyoto, Japan) using 2,6-dichloroindorphenol (DCIP) as an electron donor. The reaction mixture (3 mL) contained 100 µL of the enzyme extract, 200 mm Tris-HCl buffer (pH 7·4), 0·02 % DCIP, 1 mm phenazine methosulphate and 2 mm indole-3-aldehyde. AO activity was expressed as nmol−1 DCIP mg−1 protein min−1. Soluble proteins in the assays were measured (Bradford, 1976) using crystalline bovine serum albumin as a reference.
Total RNA extraction
Frozen leaves (200 mg) were ground in liquid nitrogen with a mortar and pestle. Total RNA was isolated from leaves by using Trizol reagent (Invitrogen, Carlsbad, CA, USA). Extracted total RNA was quantified with a UV 2100 spectrophotometer (Shimadzu). RNA quality was assessed by running 2 µg of the total RNA on a 1·2 % agarose gel. The total RNA was stored at –80 °C.
cDNA synthesis and real-time PCR
Total RNA samples were treated with DNase and then reverse transcribed with a First-Strand cDNA synthesis Kit (Shinegene, Shanghai, China). The cDNA was used as a template for the real-time PCR reaction with an FTC2000 fluorescent quantitative PCR detection system (Funglyn, Toronto, Canada) using SYBR green for detection of the product at the end of each amplification cycle (Karsai et al., 2002). Real time-PCR was carried out according to Van Riet et al. (2006) using a one-step RT-PCR kit (Shinegene). The thermal profile was as follows: 4 min denaturation at 94 °C, and then 35 cycles of 20 s at 94 °C, 25 s at 60 °C and 30 s at 72 °C. The gene-specific forward and reverse primers and a 1 : 3 dilution of the cDNA were added to the SYBR Green PCR master mix (Applied Biosystems, Foster City, CA, USA). Primers for the genes of interest were designed by Primer 5 with wheat gene sequences from GenBank. The wheat actin gene was used as a reference for all genes of interest. Primers designed for the genes of interest and reference genes are detailed in Table 2. A dissociation curve was also set up at the end of the 35 cycles in order to ensure that only one product was amplified for each gene. Real-time PCR experiments were conducted on the three biological replicates, with two technical replicates for each sample.
Table 2.
Sequences of primers used for real-time PCR amplification
| Gene | Primer type | Primer sequence 5′ to 3′ |
|---|---|---|
| Wabi5 | Forward | AACCAATGCCGTACTCGTTC |
| Reverse | TCTGCCTGTTTCCTCACCA | |
| Wlip19 | Forward | GACCGAGCTGACCAAGGTG |
| Reverse | TTGGCTCAGAACTGGAACG | |
| Wrab17 | Forward | GAAAAGCGAGGCTGTCACGA |
| Reverse | CTGTAGCGGCACCCACCATA | |
| Wrab19 | Forward | CCGCACCGAGGAGAAGACC |
| Reverse | ACCCATGCCCAGCGTGTT | |
| Wrab18 | Forward | AGGCCCGCACTGAGGAGAA |
| Reverse | GGCGGTGTTGGTGTTGTCG | |
| Wrab15 | Forward | CTGCTGTATCCTCTGTATGCGTC |
| Reverse | CTTCCTGAGCTGCTCCCTGA | |
| Wcbf2-1 | Forward | GGGCGGACCAAGTTTAAGGA |
| Reverse | TCTCGGCGGTGGTGAAGGT | |
| TaCBF | Forward | GGACCAAGTTCAGGGAGACGC |
| Reverse | GTCCATGCCGCCAAACCA | |
| Wcor14 | Forward | GGCTTCTTCTTCCGTGCTG |
| Reverse | CCCCTTCCGAGACCTTGTC | |
| Wcs19 | Forward | CGAGTTGAAGAAGGGCGTG |
| Reverse | GGCGACTTTGTCCGTGATG | |
| Wcs120 | Forward | GCCACGGAGATCACCAGC |
| Reverse | GTGTCCCAGTGCCAGTCG | |
| Wcor15 | Forward | ACGACGCTGCGGATGCTAC |
| Reverse | CCTTGTCCGTGATGCCCTGT | |
| actin | Forward | ACTGGGATGACATGGGGAA |
| Reverse | ACCGCTGGCATACAAGGAC |
ABA determination
ABA content was measured using high-performance liquid chromatography (HPLC) as follows. The methods for extraction and purification of ABA were as described by Nayyar et al. (2005) with some modifications. Leaf samples [2·0 ± 0·01 g fresh weight (f. wt)] were homogenized in ice-cold extraction medium (80 % methanol, 10 mg L−1 butylated hydroxytoluene) at a 1 : 10 ratio of sample (g f. wt) to extraction medium (mL). The homogenate was then further extracted in the dark at 4 °C for 15 h. After centrifugation at 5000 g for 10 min, the supernatant was removed, the pellet was re-extracted twice more and the supernatants were pooled, dried in vacuum and dissolved in 8·0 mL 0·1 mol L−1 ammonium acetate (pH 9·0). After thawing, the extract was centrifuged at 27 000 g for 20 min. For purification, the supernatant from the centrifugation step was applied to a preconditioned column combination of PVPP (Sigma, St Louis, MO, USA), DEAE-Sephadex G-25 (Whatman, Maidstone, UK) and ChromosepC18 column (C18 Sep-Pak cartridge, Waters, Milford, MA, USA). Finally, the hormone fraction was collected in 50 % methanol for HPLC (Agilent Technologies, Waldborn, Germany) analysis (Wang et al., 2008).
Determination of plant Mo concentration
Plant samples were ground, carbonized and ashed. Mo was determined by using polarographic catalytic wave analysis with a JP-2 oscilloscope polarograph according to Wan et al. (1988).
Determination of freezing tolerance
The freezing tolerance of leaves was estimated using the electrolyte leakage method as described by Gray et al. (1997) and Ndong et al. (2002) with slight modifications. Leaves from −Mo and +Mo treatments were thoroughly washed with deionized water and cut into 1-cm sections. Leaf segments were wrapped in moist cheesecloth. Each sample was first covered with an ice chip and equilibrated at –1 °C for 1 h to initiate freezing, then placed in a series of controlled-freezing baths. Temperature in the baths was adjusted by use of freezing-alcohol. The temperature inside the bath boxes was, respectively, –3, –6, –9, –12, –15, –18 and –21 °C. At various temperatures, samples were kept for 2 h, and slowly thawed before adding 10 mL of ice-cold deionized water. Samples were then vacuum-infiltrated, shaken and warmed to room temperature, and the conductivity of the leachate was measured with a conductivity meter. Percentage ion leakage was calculated as the ratio of the conductivity before and after boiling. Percentage ion leakage was plotted versus temperature, and a classic logistic function was fitted to the data using the SPSS v13·0 software non-linear regression package (SPSS, Chicago, IL, USA). Freezing tolerance (LT50) was expressed as the temperature which caused 50 % ion leakage.
Statistical analysis
Data are presented as the averages of three or four replicates. Results were analysed by GLM with Duncan multiple comparison using SAS v6·12 (SAS Institute, Cary, NC, USA). All statistically significant differences were tested at P < 0·05.
RESULTS
Mo concentrations in leaves of winter wheat
In wheat leaves of the +Mo treatment, Mo concentrations were significantly higher than those in the −Mo treatment across all the time courses of low-temperature stress in two cultivars (Table 3). In the −Mo treatment, Mo concentrations in the Mo-efficient cultivar ‘97003’ were significantly higher than those in the Mo-inefficient cultivar ‘97014’ (Table 3), showing the greater ability of cultivar ‘97003’ to take up Mo from Mo-deficient soil. Mo concentration was not affected by the different time courses of low-temperature stress.
Table 3.
Mo contents in leaves of Mo-deficient (−Mo) and Mo-fertilized (+Mo) winter wheat during low temperature stress (μg g−1)
| Period of low-temperature stress (h) |
|||||
|---|---|---|---|---|---|
| Cultivar | Treatment | 0 | 3 | 6 | 48 |
| ‘97003’ | −Mo | 0·035 ± 0·02b | 0·033 ± 0·02b | 0·037 ± 0·03b | 0·035 ± 0·01b |
| +Mo | 0·049 ± 0·04a | 0·053 ± 0·03a | 0·052 ± 0·04a | 0·048 ± 0·03a | |
| ‘97014’ | −Mo | 0·025 ± 0·02c | 0·027 ± 0·03c | 0·024 ± 0·03c | 0·028 ± 0·04c |
| +Mo | 0·047 ± 0·05a | 0·049 ± 0·02a | 0·051 ± 0·05a | 0·050 ± 0·02a | |
−Mo and +Mo treatments represent plants fertilized with 0 and 0·15 mg Mo [(NH4)6Mo7O24.4H2O] per kg soil, respectively. Different letters in a column indicate significant differences at P < 0·01 as determined by ANOVA followed by Duncan's test.
Effects of Mo on freezing tolerance in winter wheat
Freezing tolerance was determined by measuring the lethal temperature of 50 % of the leaf tissues (LT50, also called index of freezing injury) by ion leakage (Flint et al., 1967). LT50 values in +Mo treatments were lower than those in −Mo treatments at all times in the two cultivars (Table 4), suggesting that Mo application increased the freezing tolerance of winter wheat. In −Mo treatments, the LT50 values in cultivar ‘97014’ were all higher than those in cultivar ‘97003’ (Table 4), indicating a greater decrease of the freezing tolerance of cultivar ‘97014’ under Mo-deficient conditions.
Table 4.
LT50 ( °C) in leaves of Mo-efficient and Mo-inefficient winter wheat cultivars under low-temperature stress
| Period of low-temperature stress (h) |
|||||
|---|---|---|---|---|---|
| Cultivar | Treatment | 0 | 3 | 6 | 48 |
| ‘97003’ | −Mo | −5·8 ± 0·1b | −6·4 ± 0·3b | −7·5 ± 0·2b | −8·2 ± 0·3b |
| +Mo | −6·5 ± 0·3c | −7·6 ± 0·2c | −8·7 ± 0·3c | −9·5 ± 0·4c | |
| ‘97014’ | −Mo | −5·0 ± 0·1a | −5·4 ± 0·1a | −6·5 ± 0. 2a | −7·2 ± 0·2a |
| +Mo | −6·8 ± 0·2c | −7·5 ± 0·3c | −9·1 ± 0·7c | −9·9 ± 0·3c | |
−Mo and +Mo treatments represent plants fertilized with 0 and 0·15 mg Mo [(NH4)6Mo7O24.4H2O] per kg soil, respectively. Different letters in a column indicate significant differences at P < 0·01 as determined by ANOVA followed by Duncan's test.
Effects of Mo on AO activities
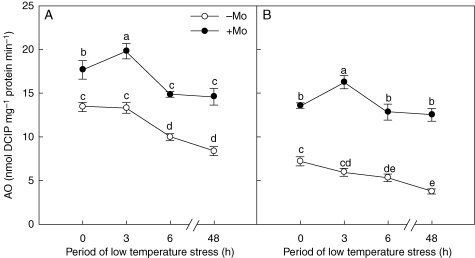
Application of Mo resulted in a substantial increase of AO activity in the two cultivars (Fig. 1). With sufficient Mo supply, AO activity significantly increased at 3 h post low-temperature stress, and then decreased sharply until 6 h post stress. Under Mo-deficiency conditions, however, AO activity decreased continuously (Fig. 1). Compared with those in control plants, AO activities in the leaves of Mo-treated plants in cultivar ‘97003’ increased by about 32, 49, 49 and 74 % after 0, 3, 6 and 48 h of low-temperature stress, respectively; the increases seen in cultivar ‘97014’ were even higher at 88, 173, 140 and 232 %, respectively. The results suggested that the AO activity of the Mo-inefficient cultivar was more dependent on Mo supply than that of the Mo-efficient cultivar (Fig. 1).
Fig. 1.
Effects of molybdenum on aldehyde oxidase (AO) activities in Mo-efficient winter wheat cultivar ‘97003’ (A) and Mo-inefficient winter wheat cultivar ‘97014’ (B) under low-temperature stress. −Mo and +Mo treatments represent plants fertilized with 0 and 0·15 mg Mo [(NH4)6Mo7O24.4H2O] per kg soil, respectively. Error bars represent s.e. from n = 4 experiments. Different letters indicate significant differences at P < 0·05 as determined by ANOVA followed by Duncan's test.
Effects of Mo on ABA levels
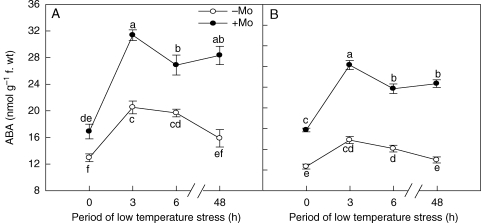
Mo application also significantly increased the ABA content in the leaves of the two winter wheat cultivars after low-temperature stress (Fig. 2). ABA concentrations in leaves of wheat increased rapidly and reached a maximum after 3 h of exposure to chilling, and then decreased slightly with the continuation of low-temperature stress. As compared with controls, +Mo treatment induced a clear increase of ABA content in the leaves of cultivars ‘97003’ and ‘97014’, with rates of increase being several times higher in the latter (Fig. 2A, B), which also indicated that application of Mo fertilizer caused cultivar ‘97014’ to produce more endogenous ABA in its leaf tissue than cultivar ‘97003’.
Fig. 2.
Effects of molybdenum on ABA contents in Mo-efficient winter wheat cultivar ‘97003’ (A) and Mo-inefficient winter wheat cultivar ‘97014’ (B) under low-temperature stress. −Mo and +Mo treatments represent plants fertilized with 0 and 0·15 mg Mo [(NH4)6Mo7O24.4H2O] per kg soil, respectively. Error bars represent s.e. from n = 4 experiments. Different letters indicate significant differences at P < 0·05 as determined by ANOVA followed by Duncan's test.
Effects of Mo on the expression of the bZIP genes
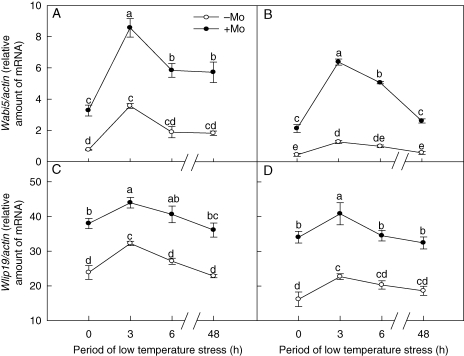
Both Wlip19 and Wabi5 are bZIP-type TF genes (Kobayashi et al., 2006; Ishibashi et al., 2007). Mo application significantly increased the mRNA levels of Wabi5 (Fig. 3A, B) and Wlip9 (Fig. 3C, D) in the leaves of both wheat cultivars after 0, 3, 6 and 48 h of low-temperature stress. The expression of Wabi5 and Wlip9 reached a maximum after 3 h exposure to low temperature, after which a further temperature decrease resulted in a reduction in expression of these genes. There was a clear difference in gene expression between cultivar ‘97003’ and ‘97014’. This difference was similar to the results seen for AO activity and ABA concentration; the average rates of increase of the transcripts of Wlip19 and Wabi5 (at 0, 3, 6, 48 h of low-temperature stress) in +Mo treatment in cultivar ‘97003’ were 50 and 224 %, and those in cultivar ‘97014’ were 84 and 464 %, respectively (Fig. 3). The results also indicated that Mo fertilizer induced a greater increase of Wlip19 and Wabi5 transcripts in cultivar ‘97014’ than that in cultivar ‘97003’.
Fig. 3.
Effects of molybdenum on expression of the bZIP genes (Wlip19 and Wabi5) in Mo-efficient winter wheat cultivar ‘97003’ (A, C) and Mo-inefficient winter wheat cultivar ‘97014’ (B, D) under low temperature stress. −Mo and +Mo treatments represent plants fertilized with 0 and 0·15 mg Mo [(NH4)6Mo7O24.4H2O] per kg soil, respectively. Error bars represent s.e. from n = 6 experiments. Different letters indicate significant differences at P < 0·05 as determined by ANOVA followed by Duncan's test.
Effects of Mo on expression of ABA-dependent COR genes
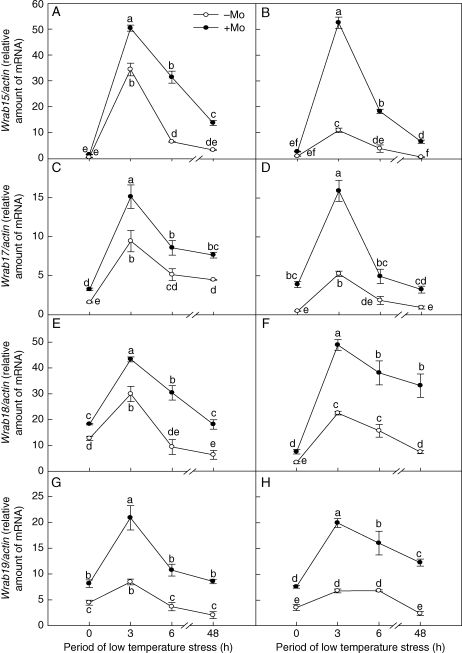
Wrab15, Wrab17, Wrab18 and Wrab19 belong to the ABA-dependent COR genes. Before low-temperature stress, expression levels of Wrab17 (Fig. 4C, D), Wrab18 (Fig. 4E, F) and Wrab19 (Fig. 4G, H) in the leaves of Mo-treated plants were significantly higher than those in control plants; the expression level of Wrab15 in Mo-treated plants was not significantly different from that in control plants (Fig. 4A, B). After low-temperature stress, expression of the four genes (Wrab15, Wrab17, Wrab18 and Wrab19) in Mo-treated plants was significantly higher than that in control plants. With the prolongation of low-temperature stress, the expression level of ABA-dependent COR genes for both Mo-deficient and Mo-fertilized treatments increased rapidly in the first 3 h of exposure, and then decreased gradually. The average rates of increase of the transcripts of Wrab15, Wrab17, Wrab18 and Wrab19 (at 0, 3, 6, 48 h of low-temperature stress) exhibited a similar tendency as the expression levels of bZIP TFs for each cultivar: 256, 77, 124 and 186 % in cultivar ‘97003’, and 660, 340, 186 and 212 % in cultivar ‘97014’, respectively. The increased rates of ABA-dependent COR gene expression in cultivar ‘97014’ were significantly higher than those in cultivar ‘97003’ with Mo application under low-temperature stress.
Fig. 4.
Effects of molybdenum on expression of the ABA-dependent COR genes (Wrab15, Wrab17, Wrab18 and Wrab19) in Mo-efficient winter wheat cultivar ‘97003’ (A, C, E, G) and Mo-inefficient winter wheat cultivar ‘97014’ (B, D, F, H) under low-temperature stress. −Mo and +Mo treatments represent plants fertilized with 0 and 0·15 mg Mo [(NH4)6Mo7O24.4H2O] per kg soil, respectively. Error bars represent s.e. from n = 6 experiments. Different letters indicate significant differences at P < 0·05 as determined by ANOVA followed by Duncan's test.
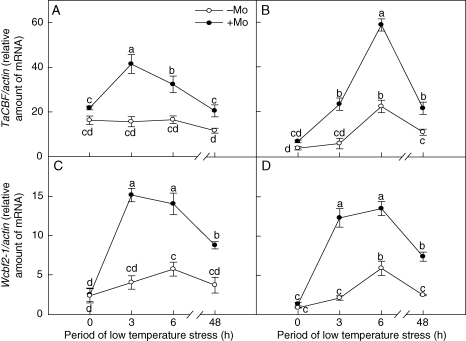
Effects of Mo on expression of the CBF/DREB TF genes
TaCBF (Fig. 5A, B) and Wcbf2-1 (Fig. 5C, D) belong to CBF/DREB TF genes (Kume et al., 2005). There was no significant difference between the −Mo and +Mo treatments in the expression of CBF/DREB TF genes (TaCBF and Wcbf2-1) before low-temperature stress (0 h). However, 3–48 h of low-temperature stress significantly up-regulated the expression of CBF/DREB TF genes (TaCBF and Wcbf2-1) in the leaves from the +Mo treatment. With extended exposure to low-temperature stress, the expression levels of CBF/DREB TF genes in both Mo-deficient and Mo-fertilized treatments increased rapidly and reached a maximum at 6 h of low-temperature stress, but then decreased at 48 h (Fig. 5A–D).
Fig. 5.
Effects of molybdenum on the expression of the CBF/DREB transcription factor genes (TaCBF and Wcbf2-1) in Mo-efficient winter wheat cultivar ‘97003’ (A, C) and Mo-inefficient winter wheat cultivar ‘97014’ (B, D) under low-temperature stress. −Mo and +Mo treatments represent plants fertilized with 0 and 0·15 mg Mo [(NH4)6Mo7O24.4H2O] per kg soil, respectively. Error bars represent s.e. from n = 6 experiments. Different letters indicate significant differences at P < 0·05 as determined by ANOVA followed by Duncan's test.
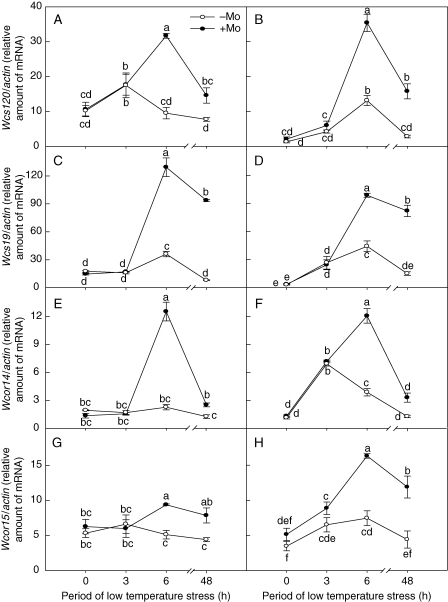
Effects of Mo on expression of the ABA-independent COR genes under low-temperature stress
Wcs120 (Ouellet et al., 1998), Wcs19 (Fowler et al., 2001), Wcor14 (Tsvetanov et al., 2000) and Wcor15 (Takumi et al., 2003) belong to the ABA-independent COR family of genes. There was no significant difference between the control and Mo-fertilized treatments in the expression of these genes at 0 and 3 h of exposure to low temperature. However, at 6 and 48 h of low-temperature stress, ABA-independent COR gene expression levels in winter wheat were significantly up-regulated in the Mo-fertilized treatment (Fig. 6A–H). Similarly, with continued low-temperature stress, ABA-independent COR gens expression levels for both Mo-deficient and Mo-fertilized plants reached a maximum at 6 h of exposure and then decreased at 48 h (Fig. 6A–H).
Fig. 6.
Effects of molybdenum on the expression of the ABA-independent COR genes (Wcs120, Wcs19, Wcor14 and Wcor15) in Mo-efficient winter wheat cultivar ‘97003’ (A, C, E, G) and Mo-inefficient winter wheat cultivar ‘97014’ (B, D, F, H) under low-temperature stress. −Mo and +Mo treatments represent plants fertilized with 0 and 0·15 mg Mo [(NH4)6Mo7O24.4H2O] per kg soil, respectively. Error bars represent s.e. from n = 6 experiments. Different letters indicate significant differences at P < 0·05 as determined by ANOVA followed by Duncan's test.
DISCUSSION
Mo might regulate the ABA-dependent pathway of COR gene expression in winter wheat under low-temperature stress
As previously mentioned, AO catalyses the last step of ABA, and IAA synthesis has been verified in many plants such as Arabidopsis thaliana (Akaba et al., 1999; Seo et al., 2000), maize (Katalin Barabas et al., 2000), tomato (Min et al., 2000) and pea (Zdunek-Zastocka, 2008); however, few data have been reported in wheat. In the present study, Mo application significantly increased AO activities and ABA concentrations of leaves of winter wheat. This indicates that Mo has a close relationship with ABA biosynthesis. ABA plays a critical role in the ABA-dependent signal pathway. Many reports have shown that ABA might activate bZIP transcription factors, and then regulate ABA-dependent COR genes through ABREs in Arabidopsis (Uno et al., 2000; Xiong et al., 2002). Recently, a number of bZIP-type genes and ABA-dependent COR genes have been isolated and characterized in wheat and related species (Kobayashi et al., 2004; Ishibashi et al., 2007). ABA regulated ABA-dependent COR genes via expression of bZIP genes, which has also been reported in wheat (Kobayashi et al., 2008a, b). Wlip19 and Wabi5 are bZIP-type TF genes in wheat (Kobayashi et al., 2006). Wrab15, Wrab17, Wrab18 and Wrab19 all belong to the ABA-dependent COR genes of wheat. ABA levels, expression levels of bZIP-type TF genes (Wlip19 and Wabi5) and ABA-dependent COR genes (Wrab15, Wrab17, Wrab18, and Wrab19) were all significantly increased in Mo-fertilized winter wheat at 0, 3, 6, 48 h of low-temperature stress. The results also showed that AO activity, ABA concentration, expression levels of bZIP TFs and ABA-dependent COR genes changed in a synchronous manner. They reached a maximum at 3 h of low-temperature stress, and then decreased slightly both in Mo-deficient and in Mo-fertilized winter wheat after prolonged exposure to low-temperature stress (Fig. 4). These results suggest that Mo regulates COR gene expression in winter wheat from the ABA-dependent signal pathway: Mo → AO → ABA → bZIP → ABA-dependent COR genes.
The four ABA-dependent COR genes encoded different stress-inducible polypeptides or proteins. Wrab15 putatively encoded a polypeptide of 130-amino-acid residues, which showed a high level of identity with a stress-inducible protein, HVA22, in barley (Shen et al., 1993, 2001). Wrab17 encoded an acidic and hydrophobic protein, which showed high homology (a mean of 84 % identity) with a barley gibberellic acid (GA3)-inducible protein, ES2A, and several other group-3 LEA/RAB proteins (Tsuda et al., 2000). WRAB18 was hydrophilic and showed 80·4 % amino-acid identity with WRAB19 (Kobayashi et al., 2004). Wrab17, WRAB18 and WRAB19 were homologous to other cereal RAB proteins, which belongs to the group 3 LEA protein family (Dure, 1993). Group 3 LEA proteins generally correlated well with desiccation tolerance in young seedlings (Baker et al., 1988), as well as salt tolerance (Moons et al., 1997) and freezing tolerance (Ndong et al., 2002). Transcripts of Wrab15, Wrab17, Wrab18 and Wrab19 in Mo-fertilized winter wheat increased significantly under low-temperature stress (Fig. 4), which may increase the expression of stress-inducible proteins (e.g. a homologue of HVA22) or group 3 LEA proteins, and then enhance the cold resistance of winter wheat.
Mo affects the ABA-independent pathway of COR gene expression in winter wheat under low-temperature stress
In the ABA-independent pathway, low temperature triggers the expression of the CBF family of TFs, which in turn activate many downstream COR genes that confer or enhance the freezing tolerance of plants (Thomashow, 1999; Shinozaki and Yamaguchi-Shinozaki, 2000). TaCBF and Wcbf2-1 belong to the CBF/DREB TF genes in wheat. The deduced polypeptides of the wheat TaCBF showed high degrees of identity to Arabidopsis CBF1/DREB1B within AP2/EREBP DNA-binding domains. The amino-acid sequence of WCBF2-1 AP2 domain showed perfect identity with those of TaCBF. Wcs120 (Ouellet et al., 1998), Wcs19 (Fowler et al., 2001), Wcor14 (Tsvetanov et al., 2000) and Wcor15 (Takumi et al., 2003) are ABA-independent COR genes, which contain the CRT/DRE sequence. Here no significant difference was found between Mo-deficient and Mo-fertilized treatment in the expression of CBF/DREB TF genes (TaCBF and Wcbf2-1) and ABA-independent COR genes (Wcs120, Wcs19, Wcor14 and Wcor15) before low-temperature stress. Expression of CBF/DREB TF genes (TaCBF and Wcbf2-1) in Mo-fertilized winter wheat was significantly up-regulated as compared with the Mo-deficient treatment, after 3 h of low-temperature stress, and the transcripts of ABA-independent COR genes (Wcs120, Wcs19, Wcor14 and Wcor15) in Mo-fertilized winter wheat was significantly increased after 6 h of low-temperature stress (Figs 5 and 6). This suggests that after low-temperature stress Mo first affects expression of CBF/DREB TF genes (TaCBF and Wcbf2-1), which in turn activate the expression of ABA-independent COR genes (Wcs120, Wcs19, Wcor14 and Wcor15).
The wheat gene Wcs120 was shown to be cold-inducible in both monocotyledonous and dicotyledonous transgenic plants (Ouellet et al., 1998). Wheat possesses a small family of Cor genes including Wcs19 (Fowler et al., 2001), Wcor14 (Tsvetanov et al., 2000) and Wcor15 (Takumi et al., 2003), all of which encode chloroplast-targeted COR proteins analogous to the Arabidopsis protein COR15a (Lin and Thomashow, 1992; Thomashow, 1999). Constitutive expression of COR15a enhances the freezing tolerance of chloroplasts and reduces freezing-induced damage to photosystem II in non-acclimated plants (Artus et al., 1996). Recent research showed that the WCOR15 and WCOR14 proteins, which were induced by low-temperature stress, were transported into chloroplasts and accumulated in the chloroplast stromal compartments (Shimamura et al., 2006). The deduced proteins of WCS19 possess chloroplast leader peptides that are highly homologous to wheat WCOR14 and WCOR15 (Takumi et al., 2003). It appears that these proteins encoded by ABA-independent COR genes have similar function. It is thus suggested that expression of the wheat COR genes, whose protein products are transported into chloroplasts, are regulated through the same or at least partly overlapped signal transduction pathways (Takumi et al., 2003). The present experiments show that the transcripts of ABA-independent COR genes (Wcs120, Wcs19, Wcor14 and Wcor15) in Mo-fertilized winter wheat were several times greater than those in −Mo treatment after 6 h of low-temperature stress (Fig. 6). Rapid increases in the expression level of these COR genes and accumulation of the COR proteins in Mo-fertilized winter wheat may help to maintain the activity of the photosynthetic apparatus through regulation of the redox state in chloroplasts (Takumi et al., 2003). Recent research also found that the biosynthesis of chlorophyll was inhibited and the net photosynthetic rate (Pn) decreased in Mo-deficient winter wheat under low-temperature stress (Sun et al., 2006a; Yu et al., 2006). These results suggest that Mo enhances freezing tolerance and photosynthesis through regulation of ABA-independent COR gene expression.
Response of the ABA-dependent pathway to Mo was prior to that of the ABA-independent pathway
Prior to low-temperature stress, Mo application increased AO activity, ABA concentration, and expression levels of all the bZIP genes and the majority of the ABA-dependent COR genes. After low-temperature stress, AO activity, ABA concentration, expression levels of all the bZIP genes and the ABA-dependent COR genes in Mo-treated plants were all higher than those in Mo-deficient plants (Figs 1–4). The results indicated that Mo regulated the ABA-dependent pathway and increased expression levels of ABA-dependent COR genes at all times. However, results regarding the ABA-independent pathway were quite different; no significant difference in the expression levels of CBF genes and ABA-independent COR genes was observed between control and Mo-treated plants for each cultivar before low-temperature stress. Mo application increased the expression levels of CBF genes and ABA-independent COR genes until 3 and 6 h of low-temperature stress, respectively (Figs 5 and 6). This suggests that the ABA-dependent pathway in COR gene expression was more sensitive to Mo deficiency as compared with the ABA-independent pathway. This may be due to the fact that Mo can directly regulate ABA biosynthesis through AO, and then trigger the ABA →̇bZIP →̇ABA-dependent COR gene expression pathway (Milborrow, 2001; Seo and Koshiba, 2002). We can infer that the response of the ABA-dependent pathway to Mo occurs prior to that of the ABA-independent pathway.
Recent genetic evidence suggests that the ABA-independent and ABA-dependent pathways are not completely independent, but instead have extensive interactions in controlling gene expression under abiotic stress. Thus, it is not yet certain whether ABA-independent COR gene expression is completely independent of ABA (Thomashow, 1999; Xiong et al., 2002). Whether Mo affects ABA-independent COR gene expression through interactions of the two pathways needs further investigation.
Similarity and differences of the Mo-efficient and Mo-inefficient wheat cultivars in response to Mo under cold stress
The response of these two wheat cultivars (‘97003’ and ‘97014’) to Mo was similar under low-temperature stress with a few exceptions. Mo application increased AO activity, ABA content, expression levels of bZIP TFs and ABA-dependent COR genes in both cultivars under low-temperature stress. Mo fertilizer also significantly up-regulated expression levels of CBF/DREB TF genes and ABA-independent COR genes after 3 and 6 h of low-temperature stress, respectively, for both cultivars. The similarity of the responses of these two wheat cultivars verified that the ABA-dependent and ABA-independent pathway of COR gene expression was affected by Mo under low-temperature stress.
On the other hand, the Mo-efficient cultivar ‘97003’ and Mo-inefficient cultivar ‘97014’ displayed differences in the rates of increase of AO activity, ABA content and CBF/bZIP/COR gene expression. Compared with control plants, AO activities in Mo-treated plants in cultivar ‘97003’ increased by about 32, 49, 49 and 74 % after 0, 3, 6 and 48 h of low-temperature stress, respectively; those in cultivar ‘97014’ were even higher at 88, 173, 140 and 232 %, respectively (Fig. 1). Similarly, the rates of increase of ABA content in cultivar ‘97003’ were 31, 53, 36, 79 at 0, 3, 6, 48 h of low-temperature stress, and those in cultivar ‘97014’ were 151, 148, 142 and 248 %, respectively (Fig. 2). There was a clear difference in bZIP gene expression between cultivar ‘97003’ and ‘97014’. The average rates of increase of the transcripts of Wlip19 and Wabi5 (at 0, 3, 6, 48 h of low-temperature stress) in +Mo treatment in cultivar ‘97003’ were 50 and 224 %, and those in cultivar ‘97014’ were 84 and 464 %, respectively (Fig. 3). The average rates of increase of the transcripts of Wrab15, Wrab17, Wrab18 and Wrab19 (at 0, 3, 6 and 48 h of low-temperature stress) exhibited a similar tendency as the expression levels of bZIP genes for each cultivar: 256, 77, 124 and 186 % in cultivar ‘97003’, and 660, 340, 186 and 212 % in cultivar ‘97014’ (Fig. 4). The rates of increase of AO activity, ABA concentration, and the transcripts of bZIP genes and ABA-dependent COR genes in the leaves of Mo-treated plants in cultivar ‘97014’ were higher than those in cultivar ‘97003’. Differences in these parameters between the −Mo and +Mo treatment in ‘97014’ was greater than those in ‘97003’. These differences were also verified by phenotypic characteristics and other physiological parameters. After 48 h of low-temperature stress (see Supplementary Data, Fig. S1, available online), Mo-deficiency symptoms such as leaf etiolation were more obvious in cultivar ‘97014’. The Mo-efficient winter wheat cultivar ‘97003’ had a yield that was 90 % more and the Mo-inefficient winter wheat cultivar ‘97014’ 50 % less under Mo-deficient conditions when compared with the Mo fertilizer treatment (Yu et al., 1999, 2002). Previous studies also showed that net photosynthetic rate (Pn) and activities of antioxidative enzymes (SOD, CAT, POD, APX) in the leaves of Mo-deficient plants in cultivar ‘97014’ decreased more than those in cultivar ‘97003’ under low-temperature stress (Sun et al., 2006a, b). The difference between cultivar ‘97003’ and cultivar ‘97014’ was also confirmed by LT50 values. Differences in LT50 between −Mo and +Mo treatment in cultivar ‘97014’ were greater than that in ‘97003’( Table 4). These results all revealed a differential response to Mo between these two cultivars.
SUPPLEMENTARY DATA
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (30671232) and the Program for New Century Excellent Talents, Ministry of Education, China (NCET-04-0731). We thank Dr Wei Wenxue (Institute of Subtropical Agriculture, The Chinese Academy of Sciences) and Dr Liu Renhu (Rothamsted Research, UK) for critical reading and helpful comments on the manuscript.
LITERATURE CITED
- Akaba S, Seo M, Dohmae N, et al. Production of homo- and hetero-dimeric isozymes from two aldehyde oxidase genes of Arabidopsis thaliana. J Biochem. 1999;126:395–401. doi: 10.1093/oxfordjournals.jbchem.a022463. [DOI] [PubMed] [Google Scholar]
- Artus NN, Uemura M, Steponkus PL, Gilmour SJ, Lin C, Thomashow MF. Constitutive expression of the cold-regulated Arabidopsis thaliana COR15a gene affects both chloroplast and protoplast freezing tolerance. Proceedings of the National Academy of Sciences. 1996;93:13404–13409. doi: 10.1073/pnas.93.23.13404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker J, Steele C, Dure L. Sequence and characterization of 6ww Lea proteins and their genes from cotton. Plant Molecular Biology. 1988;11:277–291. doi: 10.1007/BF00027385. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Dure L. A repeating 11-mer amino acid motif and plant desiccation. Plant Journal. 1993;3:363–369. doi: 10.1046/j.1365-313x.1993.t01-19-00999.x. [DOI] [PubMed] [Google Scholar]
- Egawa C, Kobayashi F, Ishibashi M, Nakamura T, Nakamura C, Takumi S. Differential regulation of transcript accumulation and alternative splicing of a DREB2 homolog under abiotic stress conditions in common wheat. Genes & Genetic Systems. 2006;81:77–91. doi: 10.1266/ggs.81.77. [DOI] [PubMed] [Google Scholar]
- Flint H, Boyce B, Beattie D. Index of injury is a useful expression of freezing injury to plant tissues as determined by the electrolytic method. Cananadian Journal of Plant Science. 1967;47:229–230. [Google Scholar]
- Fowler DB, Breton G, Limin AE, Mahfoozi S, Sarhan F. Photoperiod and temperature interactions tegulate low-temperature-induced gene expression in barley. Plant Physiology. 2001;127:1676–1681. [PMC free article] [PubMed] [Google Scholar]
- Gray G, Chauvin L, Sarhan F, Huner N. Cold acclimation and freezing tolerance (a complex interaction of light and temperature) American Society of Plant Biology. 1997;114:467–474. doi: 10.1104/pp.114.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C, Wang Y, Wei W. Effect of molybdenum applications on concentrations of free amino acids in winter wheat at different growth stages. Journal of Plant Nutrition. 2002;25:1487–1499. [Google Scholar]
- Ishibashi M, Kobayashi F, Nakamura J, Murai K, Takumi S. Variation of freezing tolerance, Cor/Lea gene expression and vernalization requirement in Japanese common wheat. Plant Breeding. 2007;126:464–469. [Google Scholar]
- Kaiser BN, Gridley KL, Ngaire B. The role of molybdenum in agricultural plant production. Annals of Botany. 2005;96:745–754. doi: 10.1093/aob/mci226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsai A, Muller S, Platz S, Hauser MT. Evaluation of a homemade SYBR Green I reaction mixture for real-time PCR quantification of gene expression. BioTechniques. 2002;32:790–796. doi: 10.2144/02324st05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katalin Barabas N, Omarov RT, Erdei L, Herman Lips S. Distribution of the Mo-enzymes aldehyde oxidase, xanthine dehydrogenase and nitrate reductase in maize (Zea mays L.) nodal roots as affected by nitrogen and salinity. Plant Science. 2000;155:49–58. doi: 10.1016/s0168-9452(00)00199-0. [DOI] [PubMed] [Google Scholar]
- Kobayashi F, Takumi S, Nakata M, Ohno R, Nakamura T, Nakamura C. Comparative study of the expression profiles of the Cor/Lea gene family in two wheat cultivars with contrasting levels of freezing tolerance. Physiologia Plantarum. 2004;120:585–594. doi: 10.1111/j.0031-9317.2004.0293.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi F, Takumi S, Egawa C, Ishibashi M, Nakamura C. Expression patterns of low temperature responsive genes in a dominant ABA-less-sensitive mutant line of common wheat. Physiologia Plantarum. 2006;127:612–623. [Google Scholar]
- Kobayashi F, Maeta E, Terashima A, Kawaura K, Ogihara Y, Takumi S. Development of abiotic stress tolerance via bZIP-type transcription factor LIP19 in common wheat. Journal of Experimental Botany. 2008;a 59:891–905. doi: 10.1093/jxb/ern014. [DOI] [PubMed] [Google Scholar]
- Kobayashi F, Maeta E, Terashima A, Takumi S. Positive role of a wheat HvABI5 ortholog in abiotic stress response of seedlings. Physiologia Plantarum. 2008;b 134:74–86. doi: 10.1111/j.1399-3054.2008.01107.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi F, Takumi S, Nakamura C. Increased freezing tolerance in an ABA-hypersensitive mutant of common wheat. Journal of Plant Physiology. 2008;c 165:224–232. doi: 10.1016/j.jplph.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Kume S, Kobayashi F, Ishibashi M, Ohno R, Nakamura C, Takumi S. Differential and coordinated expression of Cbf and Cor/Lea genes during long-term cold acclimation in two wheat cultivars showing distinct levels of freezing tolerance. Genes & Genetic Systems. 2005;80:185–197. doi: 10.1266/ggs.80.185. [DOI] [PubMed] [Google Scholar]
- Lee H, Xiong L, Ishitani M, Stevenson B, Zhu JK. Cold-regulated gene expression and freezing tolerance in an Arabidopsis thaliana mutant. The Plant Journal. 1999;17:301–308. doi: 10.1046/j.1365-313x.1999.00375.x. [DOI] [PubMed] [Google Scholar]
- Li W, Wang Z, Mi G, Han X, Zhang F. Molybdenum deficiency in winter wheat seedlings as enhanced by freezing temperature. Journal of Plant Nutrition. 2001;24:1195–1203. [Google Scholar]
- Lin C, Thomashow M. DNA sequence analysis of a complementary DNA for cold-regulated Arabidopsis gene cor15 and characterization of the COR 15 polypeptide. Plant Physiology. 1992;99:519–525. doi: 10.1104/pp.99.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendel RR, Hansch R. Molybdoenzymes and molybdenum cofactor in plants. Journal of Experimental Botany. 2002;53:1689–1698. doi: 10.1093/jxb/erf038. [DOI] [PubMed] [Google Scholar]
- Milborrow BV. The pathway of biosynthesis of abscisic acid in vascular plants: a review of the present state of knowledge of ABA biosynthesis. Journal of Experimental Botany. 2001;52:1145–1164. [PubMed] [Google Scholar]
- Min X, Okada K, Brockmann B, Koshiba T, Kamiya Y. Molecular cloning and expression patterns of three putative functional aldehyde oxidase genes and isolation of two aldehyde oxidase pseudogenes in tomato. Biochimica et Biophysica Acta. 2000;1493:337–341. doi: 10.1016/s0167-4781(00)00190-1. [DOI] [PubMed] [Google Scholar]
- Moons A, Prinsen E, Bauw G, Van Montagu M. Antagonistic effects of abscisic acid and jasmonates on salt stress-inducible transcripts in rice roots. The Plant Cell. 1997;9:2243–2259. doi: 10.1105/tpc.9.12.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder EG. Molybdenum in relation to growth of higher plants and microorganisms. Plant Soil. 1954;5:368–415. [Google Scholar]
- Nayyar H, Bains T, Kumar S. Low temperature induced floral abortion in chickpea: relationship to abscisic acid and cryoprotectants in reproductive organs. Environmental and Experimental Botany. 2005;53:39–47. [Google Scholar]
- Ndong C, Danyluk J, Wilson KE, Pocock T, Huner NPA, Sarhan F. Cold-regulated cereal chloroplast late embryogenesis abundant-like proteins. Molecular characterization and functional analyses. Plant Physiology. 2002;129:1368–1381. doi: 10.1104/pp.001925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellet F, Vazquez-Tello A, Sarhan F. The wheat wcs120 promoter is cold-inducible in both monocotyledonous and dicotyledonous species. FEBS Letters. 1998;423:324–328. doi: 10.1016/s0014-5793(98)00116-1. [DOI] [PubMed] [Google Scholar]
- Sagi M, Fluhr R, Lips SH. Aldehyde oxidase and xanthine dehydrogenase in a flacca tomato mutant with deficient abscisic acid and wilty phenotype. Plant Physiology. 1999;120:571–578. doi: 10.1104/pp.120.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagi M, Scazzocchio C, Fluhr R. The absence of molybdenum cofactor sulfuration is the primary cause of the flacca phenotype in tomato plants. The Plant Journal. 2002;31:305–317. doi: 10.1046/j.1365-313x.2002.01363.x. [DOI] [PubMed] [Google Scholar]
- Schwarz G. Molybdenum cofactor biosynthesis and deficiency. Cellular and Molecular Life Sciences (CMLS) 2005;62:2792–2810. doi: 10.1007/s00018-005-5269-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz G, Mendel RR. Molybdenum cofactor biosynthesis and molybdenum enzymes. Annual Review of Plant Biology. 2006;57:623–647. doi: 10.1146/annurev.arplant.57.032905.105437. [DOI] [PubMed] [Google Scholar]
- Seo M, Koshiba T. Complex regulation of ABA biosynthesis in plants. Trends in Plant Science. 2002;7:41–48. doi: 10.1016/s1360-1385(01)02187-2. [DOI] [PubMed] [Google Scholar]
- Seo M, Peeters AJ, Koiwai H, et al. The Arabidopsis aldehyde oxidase 3 (AAO3) gene product catalyzes the final step in abscisic acid biosynthesis in leaves. Proceedings of the National Academy of Sciences USA. 2000;97:12908–12913. doi: 10.1073/pnas.220426197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P, Sharma N, Deswal R. The molecular biology of the low-temperature response in plants. BioEssays. 2005;27:1048–1059. doi: 10.1002/bies.20307. [DOI] [PubMed] [Google Scholar]
- Shen Q, Uknes SJ, Ho TH. Hormone response complex in a novel abscisic acid and cycloheximide-inducible barley gene. Journal of Biological Chemistry. 1993;268:23652–23660. [PubMed] [Google Scholar]
- Shen Q, Chen CN, Brands A, Pan SM, Tuan-Hua DH. The stress- and abscisic acid-induced barley gene HVA22: developmental regulation and homologues in diverse organisms. Plant Molecular Biology. 2001;45:327–340. doi: 10.1023/a:1006460231978. [DOI] [PubMed] [Google Scholar]
- Shimamura C, Ohno R, Nakamura C, Takumi S. Improvement of freezing tolerance in tobacco plants expressing a cold-responsive and chloroplast-targeting protein WCOR15 of wheat. Journal of Plant Physiology. 2006;163:213–219. doi: 10.1016/j.jplph.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Shinozaki K, Yamaguchi-Shinozaki K. Molecular responses to dehydration and low temperature: differences and cross-talk between two stress signaling pathways. Current Opinion in Plant Biology. 2000;3:217–223. [PubMed] [Google Scholar]
- Sun X, Hu C, Tan Q, Gan Q. Effects of molybdenum on photosynthetic characteristics in winter wheat under low temperature stress. Acta Agronomica Sinica. 2006;a 32:1418–1422. [Google Scholar]
- Sun X, Qilin T, Hu C, Gan Q, Yi C. Effects of molybdenum on antioxidative enzymes in winter wheat under low temperature stress. Agricultural Sciences in China. 2006;b 39:952–959. [Google Scholar]
- Sun XC, Hu CX, Tan QL. Effects of molybdenum on antioxidative defense system and membrane lipid peroxidation in winter wheat under low temperature stress. Journal of Plant Physiology and Molecular Biology. 2006;c 32:175–182. [PubMed] [Google Scholar]
- Takumi S, Koike A, Nakata M, Kume S, Ohno R, Nakamura C. Cold-specific and light-stimulated expression of a wheat (Triticum aestivum L.) Cor gene Wcor15 encoding a chloroplast-targeted protein. Journal of Experimental Botany. 2003;54:2265–2274. doi: 10.1093/jxb/erg247. [DOI] [PubMed] [Google Scholar]
- Thomashow MF. Plant cold acclimation: freezing tolerance genes and regulatory mechanisms. Annual Reviews in Plant Physiology and Plant Molecular Biology. 1999;50:571–599. doi: 10.1146/annurev.arplant.50.1.571. [DOI] [PubMed] [Google Scholar]
- Tsuda K, Tsvetanov S, Takumi S, Mori N, Atanassov A, Nakamura C. New members of a cold-responsive group-3 Lea/Rab-related Cor gene family from common wheat (Triticum aestivum L.) Genes & Genetic Systems. 2000;75:179–188. doi: 10.1266/ggs.75.179. [DOI] [PubMed] [Google Scholar]
- Tsvetanov S, Ohno R, Tsuda K, et al. A cold-responsive wheat (Triticum aestivum L.) gene wcor14 identified in a winter-hardy cultivar Mironovska 808. Genes & Genetic Systems. 2000;75:49–57. doi: 10.1266/ggs.75.49. [DOI] [PubMed] [Google Scholar]
- Uno Y, Furihata T, Abe H, Yoshida R, Shinozaki K, Yamaguchi-Shinozaki K. Arabidopsis basic leucine zipper transcription factors involved in an abscisic acid-dependent signal transduction pathway under drought and high-salinity conditions. Proceedings of the National Academy of Sciences. 2000;97:11632–11637. doi: 10.1073/pnas.190309197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Riet L, Nagaraj V, Van den Ende W, Clerens S, Wiemken A, Van Laere A. Purification, cloning and functional characterization of a fructan 6-exohydrolase from wheat (Triticum aestivum L.) Journal of Experimental Botany. 2006;57:213–223. doi: 10.1093/jxb/erj031. [DOI] [PubMed] [Google Scholar]
- Vankova-Radeva RV, Yaneva IA, Baidanova VD, Lvov NP, Karasev GS, Trunova TI. Molybdenum-induced increase in frost tolerance of winter wheat grown on acidic soil. Russian Journal of Plant Physiology. 1997;44:204–209. [Google Scholar]
- Vunkova-Radeva R, Yaneva I, Strumin P. Mo-containing enzymes responses to low temperature stress of winter wheat grown on acid soil. Bulgarian Journal of Plant Physiology. 2003;14:382–383. [Google Scholar]
- Wan YX, Liu XD, Li ZY. Determination of soil available molybdenum and plant molybdenum by polarographic catalytic wave analysis. Soil Science Reports China. 1988;1:43–46. [Google Scholar]
- Wang C, Yang A, Yin H, Zhang J. Influence of water stress on endogenous hormone contents and cell damage of maize seedlings. Journal of Integrative Plant Biology. 2008;50:427–434. doi: 10.1111/j.1774-7909.2008.00638.x. [DOI] [PubMed] [Google Scholar]
- Wang YH, Wei WX, Tan QL. A study on molybdenum deficiency and molybdenum application of winter wheat in yellow-brown soil of Hubei province. Soil Fertility. 1995;8:24–28. (in Chinese). [Google Scholar]
- Wang Z, Wang Y, Min Z. A primary study on mechanism of yield increased by molybdenum fertilzer in winter wheat. Soil Fertility. 1990;3:29–31. (in Chinese) [Google Scholar]
- Xiong L, Schumaker KS, Zhu JK. Cell signaling during cold, drought, and salt stress. Plant Cell. 2002;14:165–183. doi: 10.1105/tpc.000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaneva I, Vunkova-Radeva R, Stefanov K, Tsenov A, Petrova T, Petkov G. Changes in lipid composition of winter wheat leaves under low temperature stress: effect of molybdenum supply. Biologia Plantarum. 1995;37:561–566. [Google Scholar]
- Yaneva I, Mack G, VunkovaRadeva R, Tischner R. Changes in nitrate reductase activity and the protective effect of molybdenum during cold stress in winter wheat grown on acid soil. Journal of Pant Physiology. 1996;149:211–216. [Google Scholar]
- Yu M, Hu C, Wang Y. Influences of seed molybdenum and molybdenum application on nitrate reductase activity, shoot dry matter, and grain yields of winter wheat cultivars. Journal of Plant Nutrition. 1999;22:1433–1441. [Google Scholar]
- Yu M, Hu CX, Wang YH. Molybdenum efficiency in winter wheat cultivars as related to molybdenum uptake and distribution. Plant and Soil. 2002;245:287–293. [Google Scholar]
- Yu M, Hu C, Wang Y. Effects of molybdenum on the intermediates of chlorophyll biosynthesis in winter wheat cultivars under low temperature. Agricultural Sciences in China. 2006;5:670–677. [Google Scholar]
- Zdunek-Zastocka E. Molecular cloning, characterization and expression analysis of three aldehyde oxidase genes from Pisum sativum L. Plant Physiology and Biochemistry. 2008;46:19–28. doi: 10.1016/j.plaphy.2007.09.011. [DOI] [PubMed] [Google Scholar]