Abstract
Background and Aims
Aluminium (Al) toxicity is one of the most severe limitations to crop production in acid soils. Inhibition of root elongation is the primary symptom of Al toxicity. However, the underlying basis of the process is unclear. Considering the multiple physiological and biochemical functions of pectin in plants, possible involvement of homogalacturonan (HG), one of the pectic polysaccharide domains, was examined in connection with root growth inhibition induced by Al.
Methods
An immunolabelling technique with antibodies specific to HG epitopes (JIM5, unesterified residues flanked by methylesterifed residues; JIM7, methyl-esterified residues flanked by unesterified residues) was used to visualize the distribution of different types of HG in cell walls of root apices of two maize cultivars differing in Al resistance.
Key Results
In the absence of Al, the JIM5 epitope was present around the cell wall with higher fluorescence intensity at cell corners lining the intercellular spaces, and the JIM7 epitope was present throughout the cell wall. However, treatment with 50 µm Al for 3 h produced 10 % root growth inhibition in both cultivars and caused the disappearance of fluorescence in the middle lamella of both epitopes. Prolonged Al treatment (24 h) with 50 % root growth inhibition in ‘B73’, an Al-sensitive cultivar, resulted in faint and irregular distribution of both epitopes. In ‘Nongda3138’, an Al-resistant cultivar, the distribution of HG epitopes was also restricted to the lining of intercellular spaces when a 50 % inhibition to root growth was induced by Al (100 µm Al, 9 h). Altered distribution of both epitopes was also observed when of roots were exposed to 50 µm LaCl3 for 24 h, resulting in 40 % inhibition of root growth.
Conclusions
Changes in HG distribution and root growth inhibition were highly correlated, indicating that Al-induced perturbed distribution of HG epitopes is possibly involved in Al-induced inhibition of root growth in maize.
Key words: Al toxicity, cell wall, homogalacturnonan, immunofluorescence, methylesterification, pectin
INTRODUCTION
Aluminium (Al) toxicity is one of the most severe limitations to crop production in acid soils around the world (Kochian, 1995; Matsumoto, 2000; Ma, 2007). Al primarily inhibits root elongation, along with uptake of water and nutrients, which ultimately results in the loss of production. However, the primary causes leading to the inhibition of root elongation are still unknown. One of the major controversies stems from the discrepancy as to whether Al toxicity results from apoplastic or symplastic lesions. When roots are exposed to Al, the cell wall is the first site in contact with Al. Therefore, it is unlikely that Al toxicity occurs from symplastic lesions without influencing cell wall functions. Several lines of evidence support apoplastic lesions as primary factors in Al toxicity. First, cell walls are the major sites of Al accumulation. For example, in giant algal cells of Chara coralline, up to 99·9 % of the total cellular Al accumulated in the cell wall (Taylor et al., 2000) and almost 90 % of the total Al was associated with cell walls of cultured tobacco cells (Chang et al., 1999). Secondly, binding of Al to cell walls affects important properties like decreasing extensibility (Tabuchi and Matsumoto, 2001; Ma et al., 2004). Furthermore, Al also causes changes in cell wall polysaccharides. For example, Al treatment resulted in the accumulation of pectin and hemicellulose in both monocots (Tabuchi and Matsumoto, 2001; Eticha et al., 2005; Yang et al., 2008) and dicots (Van et al., 1994). Therefore, the cell wall is one of the major targets of Al toxicity and plays a very important role in Al-induced inhibition of root elongation.
Pectin is a major component of primary cell walls of all land plants and plays many important roles in plant growth and development including cell expansion (Willats et al., 2001a; Pelloux et al., 2007). Al stress has been demonstrated to increase cell wall pectin content in a number of plant species (Van et al., 1994; Eticha et al., 2005; Yang et al., 2008), which implies the possible involvement of pectin in Al toxicity. Binding of Al to pectin may hamper the binding of newly synthesized polysaccharides to cell wall materials and result in disordered deposition of pectin and reduced cell elongation. Furthermore, not only pectin content but also structural features which are mainly modified by pectin methylesterases (PME) are involved in its physiological and biochemical functions. For example, Wen et al. (1999) showed that the partial inhibition of PME by antisense RNA reduced root elongation in transgenic pea hairy roots. Therefore, it will be interesting to investigate whether the pectin structural features are changed or not during the Al-induced root growth inhibition process.
PMEs play a major role in pectin remodelling in muro and belong to large multigene families. But overall changes in PME activity do not reflect specific structural features of pectin. Immunocytochemical approaches with anti-pectin antibody probes provided a powerful way to further our understanding of the structure/function relationships of pectin and Al (Yang et al., 2008). This method was also adopted to investigate the relationship between cadmium-induced inhibition of flax hypocotyl and changes in pectin structural features (Douchiche et al., 2007). In the present study, changes in homogalacturonan (HG) structures, one of the major pectic polysaccharide domains, were investigated during the course of Al-induced root growth inhibition in both Al-resistant and Al-sensitive maize cultivars. Evidence is provided that disorganized distribution of HG epitopes in the cell wall is associated with Al-induced root growth inhibition in maize.
MATERIALS AND METHODS
Plant material and growth conditions
Seeds of two maize (Zea mays L.) cultivars, ‘Nongda3138’ (Al resistant) and ‘B73’ (Al sensitive) were soaked in de-ionized water for 30 h and then transferred to an incubator at 25 °C for germination in the dark for 2 d. Germinated seeds were transferred to a plastic net tray floating in a plastic container filled with 5 L of 0·5 mm CaCl2 solution at pH 4·5. The solution was renewed daily. The seedlings were grown for 3 d in the growth chamber with a 14-h/26 °C day and a 10-h/23 °C night regime, a light intensity of 250 µmol photon m−2 s−1 and a relative humidity of 70 %.
Al treatments and growth measurements
A compartmental hydroponic screening system was adopted to measure the effect of Al on root elongation (Yang et al., 2005). In brief, uniform-sized seedlings were transplanted to 18-mL opaque glass test tubes containing 17 mL of treatment solution. The seedlings were planted one to each tube. Al resistance was examined by measuring root elongation of primary roots of the 3-d-old seedlings, after they were grown in 0·5 mm CaCl2 solution, pH 4·5, containing 0 or 50 µm AlCl3. Root length was measured with a ruler before and after treatments (3 h or 24 h). In another case, roots were exposed to 0·5 mm CaCl2 solution (pH 4·5) containing 0 or 100 µm Al for 9 h.
Lanthanum (La) treatment
The protocol for La treatment was the same as used for Al treatments. In brief, 3-d-old seedlings were subjected to a solution of 0·5 mm CaCl2 (pH 4·5) containing 0 or 50 µm LaCl3. Root length was measured with a ruler before and after 24-h treatments.
Monoclonal antibodies
Rat monoclonal antibodies, JIM5 and JIM7, were used. They are kindly donated by Prof. Knox from the University of Leeds. JIM5 is an antibody that specifically recognizes a relatively low-methylesterified HG epitope, and JIM7 is an antibody that specifically recognizes relatively a high-methylesterified HG epitope. Detailed information on the generation of the antibodies and the epitopes recognized are given in Clausen et al. (2003).
Immunolabelling of HG epitopes
After treatment, roots were hand-sectioned from 2 mm behind the apex and directly collected into a fixation solution containing 4 % paraformaldehyde in 50 mm PIPES (1,4-piperazine-diethanesulphonic acid), 5 mm MgSO4 and 5 mm EGTA (ethylene glycol bis(β-amino-ethylether)-N,N,N′,N′-tetraacetic acid), pH 6·9. After 1 h of fixation at room temperature, the samples were washed repeatedly with phosphate-buffered saline (PBS) and blocked with 0·2 % BSA in PBS for 30 min. Then the samples were incubated with the monoclonal antibodies JIM5 and JIM7, diluted 1 : 10 in PBS containing 0·2 % BSA, for 2 h. Subsequently, the samples were washed three times in PBS and incubated with goat anti-rat IgG (whole molecule) FITC conjugate, diluted 1 : 50 in PBS containing 0·2 % BSA for 2 h at 37 °C. Then samples were washed briefly with PBS three times and mounted on glass slides and examined under the laser scanning system LSM 510 confocal microscope (Zeiss).
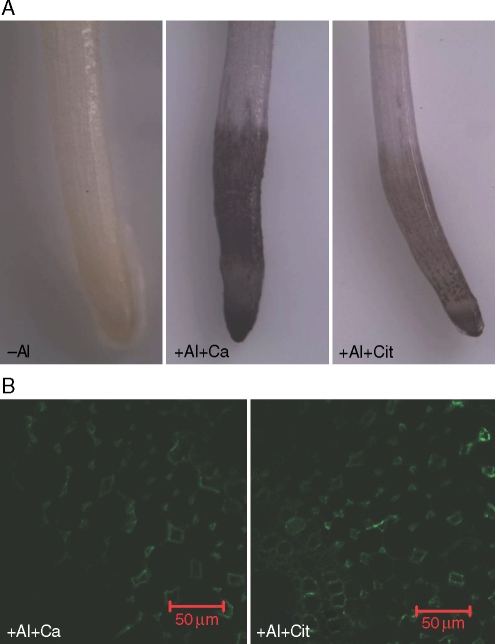
Control samples (treated with secondary antibody instead of primary antibody) were examined and, as no fluorescence was observed, these images are not shown. To verify whether the presence of Al interferes with immunofluorescence labelling, a desorption experiment with 1 mm citric acid in 0·5 mm CaCl2 solution (pH 4·5; see Fig. 6; +Al + Cit) or with 0·5 mm CaCl2 solution (pH 4·5; see Fig. 6; +Al + Ca) was conducted with seedlings treated with 100 µm Al for 9 h. Roots were placed in desorption solution which was replaced once during a 30-min desorption period. After being washed with de-ionized water three times, roots were used for haematoxylin staining according to Polle et al. (1978). In a parallel experiment, roots were used for immunolabelling of JIM7-recognized HG epitopes as described above.
Fig. 6.
Effect of citric acid desorption on haematoxylin staining (A) and JIM7-recognized HG epitope immunofluorescence labelling (B). Three-day-old seedlings (‘Nongda3138’) were subjected to 0·5 mm CaCl2 solution (pH 4·5) either with 0 (–Al) or 100 µm Al for 9 h. After treatment, roots were washed with 0·5 mm CaCl2 solution at pH 4·5 (+Al + Ca) or with 1 mm citric acid in 0·5 mm CaCl2 solution at pH 4·5 (+Al + Cit) for 30 min. After desorption, roots were used for haematoxylin staining and immunolabelling. The images are representative of at least ten replicate roots for each treatment. Scale bars = 50 µm.
Experimental design and images analysis
Ten seedlings were used per treatment, and about 30 sections were made from ten replications per treatment. Experiments were independently repeated twice, and the data shown are representative of two independent biological replicates. Photoshop 7·0 (Adobe Systems) was used to compile the fluorescence images.
RESULTS
Al impacts on root growth
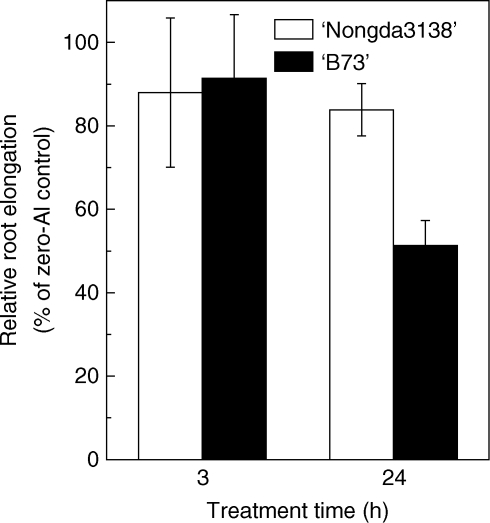
The primary symptom of Al toxicity is the inhibition of root elongation. In the present study, relative root elongation was used to indicate the effect of Al on root growth. Root elongation was inhibited by about 10 % after a 3-h treatment with 50 µm Al in both varieties of maize. After 24-h exposure, root elongation was inhibited by 50 % in ‘B73’; however, root elongation in ‘Nongda3138’ was not further inhibited by prolonged exposure (Fig. 1), indicating that ‘Nongda3138’ is resistant to Al compared with ‘B73’, which is sensitive to Al (Piñeros et al., 2005).
Fig. 1.
Effect of Al on root elongation of two maize cultivars ‘B73’ (Al sensitive) and ‘Nongda3138’ (Al resistant). Three-day-old seedlings were subjected to 0·5 mm CaCl2 solution (pH 4·5) with 0 or 50 µm Al. Root lengths were measured before or after Al treatment with a ruler. Relative root elongation was defined as root elongation with Al/root elongation without Al (× 100). Values are means ± s.d. (n = 10).
Al impacts on JIM5- and JIM7-recognized HG epitopes in ‘B73’
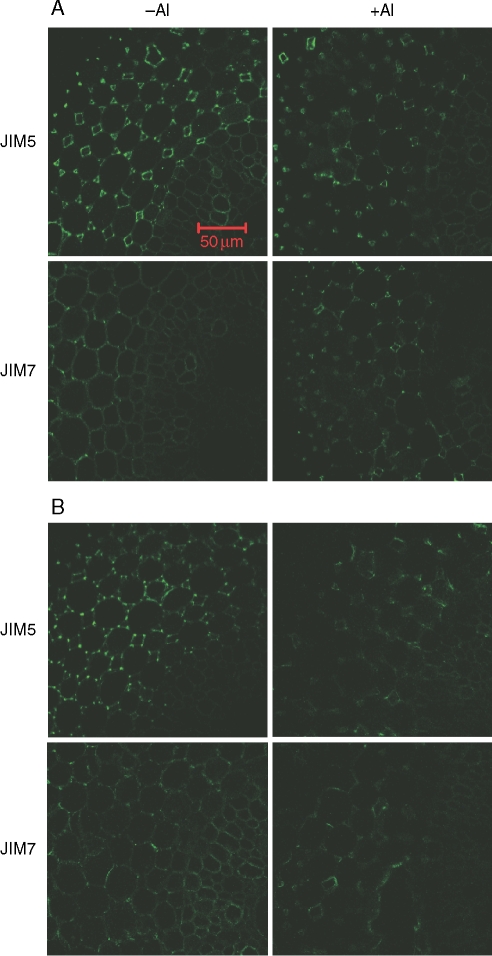
JIM5 (relatively low methyl-esterified epitopes) mainly stained HG in the cortex of ‘B73’ maize primary roots, and was bound throughout the cell walls, especially in the intercellular spaces of the root apex in the absence of Al. After 3 h of Al treatment, the fluorescence appears to be restricted to the lining of intercellular spaces and becomes faint in the middle lamella which is the HG-rich intercellular material cementing together the primary walls of adjacent plant cells (Fig. 2A). However, after 24-h exposure with root elongation inhibition of 50 %, the fluorescence becomes faint with irregular distribution around cell walls (Fig. 2B).
Fig. 2.
Immunofluorescence labelling with JIM5 and JIM7 of hand-cut transverse sections of maize (‘B73’, Al sensitive) root apex subjected to 0·5 mm CaCl2 solution (pH 4·5) either with 0 or 50 µm Al for 3 h (A) or 24 h (B). Root sections were taken 2 mm behind the apex. Representative images from at least ten replicate roots for each treatment. Scale bar = 50 µm, for all images.
JIM7 (relatively high methyl-esterified epitopes) localized HG not only in the cell walls of cortical cells in maize primary roots, but also in vascular tissues. Similar to JIM5, JIM7 stained throughout the cell walls in the root apex in the absence of Al. After 3-h exposure to 50 µm Al, the fluorescence was confined to the lining of intercellular spaces, and the fluorescence in the middle lamella disappeared (Fig. 2A). The fluorescence also became faint and had an irregular distribution after 24-h exposure to 50 µm Al (Fig. 2B).
Al impacts on JIM5- and JIM7-recognized HG epitopes in ‘Nongda3138’
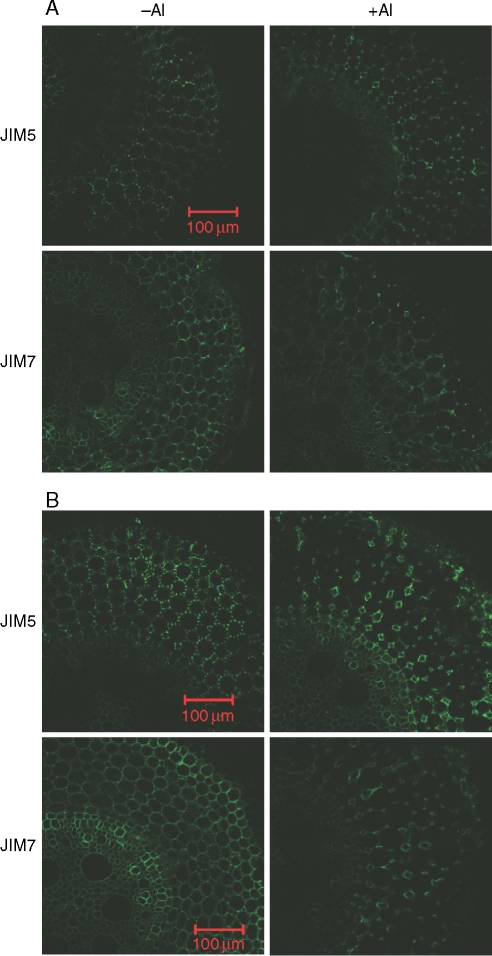
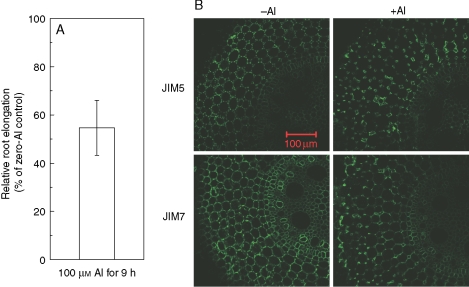
In the absence of Al, the distribution of JIM5- and JIM7-recognized HG epitopes in ‘Nongda3138’ was similar to that in ‘B73’ (compare Fig. 2 with Fig. 3). After roots were treated with 50 µm Al for 3 h, fluorescence of JIM5- and JIM7-recognized HG epitopes was located in the lining of intercellular spaces and disappeared from the middle lamella (Fig. 3A). With prolonged treatment of roots with 50 µm Al for 24 h, such restricted distribution became more evident (Fig. 3B). When ‘Nongda3138’ was exposed to 100 µm Al for 9 h, root elongation was inhibited by about 50 % (Fig. 4A), which was similar to that in ‘B73’ with 24-h exposure of 50 µm Al (Fig. 1).This caused a change in the distribution of both JIM5- and JIM7-recognized HG epitopes similar to that found in roots treated with 50 µm Al for 24 h (compare Fig 3B with Fig. 4B).
Fig. 3.
Immunofluorescence labelling with JIM5 and JIM7 of hand-cut transverse sections of maize (‘Nongda3138’, Al resistant) root apex subjected to 0·5 mm CaCl2 solution (pH 4·5) either with 0 or 50 µm Al for 3 h (A) or 24 h (B). Root sections were taken 2 mm behind the apex. Representative images from at least ten replicate roots for each treatment. Scale bars = 100 µm, for all images.
Fig. 4.
Al-induced root elongation inhibition and changes of HGA epitopes distributuion in ‘Nongda3138’, an Al-resistant cultivar. Three-day-old seedlings were subjected to 0·5 mm CaCl2 solution (pH 4·5) either with 0 or 100 µm Al for 9 h. (A) Relative root elongation was defined as root elongation with Al/root elongation without Al (× 100) and the root length was measured before and after the treatment. Values are means ± s.d. (n = 10). (B) Immunofluorescence labelling with JIM5 and JIM7 of hand-cut transverse sections of root apex after treatment. Root sections were taken 2 mm behind the apex. The images are representative of at least ten replicate roots for each treatment. Scale bar = 100 µm, for all images.
Impacts of La on root growth and distribution of HG epitopes
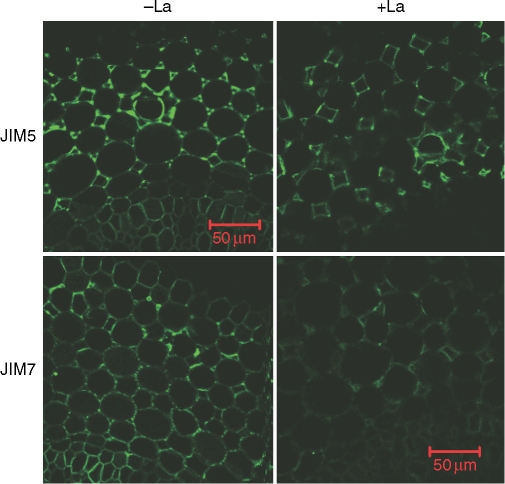
Root elongation of ‘Nongda3138’ was inhibited by 40 % when roots were exposed to 50 µm La for 24 h (data not shown). The distribution of JIM7-recognized HG epitope after La treatment was also altered (Fig. 5), similar to that which occurred in Al treatment (Figs 3 and 4).
Fig. 5.
Immunofluorescence labelling with JIM5 and JIM7 of hand-cut transverse sections of maize ( ‘Nongda3138’, Al-resistant) root apex subjected to 0·5 mm CaCl2 solution (pH 4·5) either with 0 or 50 µm LaCl3 for 24 h. Root sections were taken from 2 mm behind the apex. The images are representative of at least ten replicate roots for each treatment. Scale bars = 50 µm, for all images.
Effect of citrate desorption on the distribution of HG epitope
Haematoxylin staining has been used widely to detect Al accumulation in roots visually (Polle et al., 1978). Seedling roots of ‘Nongda3138’ treated with 100 µm Al for 9 h were heavily stained with haematoxylin (Fig. 6A; +Al + Ca). After the roots were washed with 1 mm citric acid for 30 min, the staining intensity was significantly reduced (Fig. 6A; +Al + Cit), indicating that Al has been removed from the root apex. Roots without Al treatment were not stained by haematoxylin (Fig. 6A; –Al). However, there was no difference in the distribution of JIM7-recognized HG epitope between +Al + Ca and +Al + Cit treatments (Fig. 6B), suggesting that Al bound to cell walls does not interfere with immunolabelling.
DISCUSSION
Pectic polysaccharides are proposed to influence a range of cell wall properties such as cell expansion, cell development, intercellular adhesion and defence mechanisms (Willats et al., 2001b; Pelloux et al., 2007; Mohnen, 2008). Several studies demonstrated that Al accumulated in the cell wall is mainly bound to pectin. For example, Chang et al. (1999) found about 72–82 % of the total cellular Al was associated with pectin in cultured tobacco cells. Schmohl and Horst (2000) and Horst et al. (1999) found that increased pectin content resulted in higher Al accumulation and lower Al resistance in maize suspension cells and roots. However, the underlying basis of how the bound Al inhibits cell elongation is still unknown. In the present study, the anti-HG monoclonal antibodies were employed to illustrate changes of HG distribution during the course of Al-induced root growth inhibition. The inhibition of root elongation of ‘B73’, an Al-sensitive cultivar, was well correlated with changed HG distribution in a time-dependent manner (Figs 1–3). In ‘Nongda3138’, Al-induced change in HG distribution was more evident with increasing external Al concentrations (Figs 4 and 5). Furthermore, such changes were also related to root growth inhibition (Figs 1, 4 and 5). These results imply that Al-induced changes of HG epitope distribution are possibly involved in Al-induced root growth inhibition. When root growth was inhibited, similar impacts between La and Al on the distribution of both epitopes further confirmed that the disorganized distribution is related to root growth inhibition (Fig. 5). Although it is not possible to provide direct evidence to relate such changes with inhibition of root elongation, several lines of evidence provide clues to the present hypothesis. For example, it is well known that plant cells adhere to each other by contact across the middle lamella at HG-rich regions of the cell wall developed from cell plates formed at cytokinesis. Al-induced irregular distribution of HG epitopes in the middle lamella will potentially affect the cell separation process. Dumville and Fry (2000) reported that pectin-derived oligogalacturonides generated by pectinolytic cleavage are involved in signalling processes during development and in the defence response to plant pathogens. Al-induced changes of HG distribution structure will influence the ability of pectinase and pectolyase to cleave pectin and may also be the cause of Al-induced inhibition of the apoplastic bypass flow of solutes (Sivaguru et al., 2006), since pectin plays an important role in determining the porosity of cell walls (Carpita and McCann, 2000). Therefore, we thought that these influences are very likely to play an important role in Al-induced root inhibition of elongation in maize.
It is interesting to note that, after Al treatment, pectin was more concentrated in the intercellular sites as both JIM5-stained low-methyl-ester HG and JIM7-stained high-methyl-ester HG showed higher fluorescence intensity (Figs 2–4). Intercellular sites play an important role in cell adhesion through Ca2+ cross-linking (Carptia and McCann, 2000). Intercellular sites also bear greater tension, and conductivity of mechanical stresses throughout a plant tissue depends greatly on the cell corners and intervening edges (Ryden et al., 2003). It has been speculated that pectin degradation is a prerequisite to cell wall loosening and cell expansion, the enzymatic degradation of pectin being reduced by gelation with Ca and Al in vitro (Wehr et al., 2003). Thus, it can be deduced that more Al or Ca ions will be bound to HG in intercellular sites, which in turn will reduce pectin degradation, and finally lead to inhibition of cell wall expansion and root elongation.
In the present study, since a simple Ca solution was used as the basal treatment, the possibility remains that the altered distribution of HG epitopes observed under Al stress may be the result of Al-induced boron (B) deficiency. However, since both JIM5- and JIM7-recognized epitopes showed the same distribution patterns with time spent under –Al treatment (e.g. –Al treatments in Fig. 1A, B), the B content stored in seeds was sufficient for normal plant root growth. It was also found that supplementation of B (5 µm) cannot alleviate Al-induced root growth inhibition (data not shown), further confirming that the observed altered distribution of HG epitopes is the result of Al toxicity but not B deficiency. Corrales et al. (2008) also found that supplying B (from 4 to 32 µm) cannot alleviate Al-induced root growth inhibition in maize. However, since adequate B (16 µm) supply alleviated Al-induced cell death (Corrales et al., 2008), the role of B in alleviating the Al-induced alteration of pectin structure deserved further research.
Here, symplasmic lesions of Al toxicity are not questioned. The fact that Al mainly accumulated in the root apoplast does not necessarily imply that primary Al targets are restricted to the cell walls. In fact, several studies have demonstrated that Al also enters cytosol very quickly (Silva et al., 2000; Taylor et al., 2000). There is also ample experimental evidence of Al interfering with cytoplasmic constituents. Therefore, it is difficult to identify the primary mechanism of Al toxicity and it is very likely that Al may target multiple sites simultaneously, not just one site (Zheng and Yang, 2005; Ma, 2007). On the other hand, given the existence of the cell wall–plasma membrane–cytoskeleton continuum (Horst et al., 1999), Al does not need to cross the plasma membrane to exert its toxic effects on plant root growth.
In conclusion, the strong correlation between Al-induced root elongation inhibition and changes in HG epitopes indicated that the disorganized distribution of HG epitopes is one of the possible mechanisms responsible for Al-induced root elongation inhibition.
ACKNOWLEDGEMENTS
We think Professor J. Paul Knox (Centre for Plant Science, University of Leeds, UK) for kindly donating the monoclonal antibodies specific for pectins (JIM5 and JIM7), and Professor Leon V. Kochian (Department of Plant Biology, Cornell University, New York) for kindly donating the maize seeds of ‘B73’. This work was financially supported by the Natural Science Foundation of China (Grant Nos. 30830076, 30821140538), and China Postdoctoral Science Special Foundation.
LITERATURE CITED
- Carpita N, McCann M. The cell wall. In: Buchanan B, Gruissem W, Jones R., editors. Biochemistry and molecular biology of plants. Rockville, MD: American Society of Plant Physiologists; 2000. pp. 52–108. [Google Scholar]
- Chang Y-C, Yamamoto Y, Matsumoto H. Accumulation of aluminium in the cell wall pectin in cultured tobacco (Nicotiana tabacum L.) cells treated with a combination of aluminium and iron. Plant, Cell & Environment. 1999;22:1009–1017. [Google Scholar]
- Clausen MH, Willats WGT, Knox JP. Synthetic methyl hexagalacturonate hapten inhibitors of anti homogalacturonan monoclonal antibodies LM7, JIM5 and JIM7. Carbohydrate Research. 2003;338:1791–1800. doi: 10.1016/s0008-6215(03)00272-6. [DOI] [PubMed] [Google Scholar]
- Corrales I, Poschenrieder C, Barceló J. Boron-induced amelioration of aluminium toxicity in a monocot and a dicot species. Journal of Plant Physiology. 2008;165:540–513. doi: 10.1016/j.jplph.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Douchiche O, Rihouey C, Schaumann A, Driouich A, Morvan C. Cadmium-induced alterations of the structural features of pectins in flax hypocotyl. Planta. 2007;225:1301–1312. doi: 10.1007/s00425-006-0425-7. [DOI] [PubMed] [Google Scholar]
- Dumville JC, Fry SC. Uronic acid-containing oligosaccharins: their biosynthesis, degradation and signaling roles in non-diseased plant tissues. Plant Physiology and Biochemistry. 2000;38:125–140. [Google Scholar]
- Eticha D, Stass A, Horst WJ. Cell-wall pectin and its degree of methylation in the maize root-apex: significance for genotypic differences in aluminium resistance. Plant, Cell & Environment. 2005;28:1410–1420. [Google Scholar]
- Horst WJ, Schmohl N, Kollmeier M, Baluska F, Sivaguru M. Does aluminium affect root growth of maize through interaction with the cell wall-plasma membrane-cytoskeleton continuum? Plant and Soil. 1999;215:163–174. [Google Scholar]
- Kochian LV. Cellular mechanisms of aluminum toxicity and resistance in plants. Annual Review of Plant Physiology and Plant Molecular Biology. 1995;46:237–260. [Google Scholar]
- Ma JF. Syndrome of aluminum toxicity and diversity of aluminum resistance in higher plants. International Review of Cytology. 2007;246:225–252. doi: 10.1016/S0074-7696(07)64005-4. [DOI] [PubMed] [Google Scholar]
- Ma JF, Shen R, Nagao S, Tanimoto E. Aluminum targets elongating cells by reducing cell wall extensibility in wheat roots. Plant and Cell Physiology. 2004;45:583–589. doi: 10.1093/pcp/pch060. [DOI] [PubMed] [Google Scholar]
- Matsumoto H. Cell biology of aluminum toxicity and tolerance in higher plants. International Review of Cytology. 2000;200:1–46. doi: 10.1016/s0074-7696(00)00001-2. [DOI] [PubMed] [Google Scholar]
- Mohnen D. Pectin structure and biosynthesis. Current Opinion in Plant Biology. 2008;11:266–277. doi: 10.1016/j.pbi.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Pelloux J, Rustérucci C, Mellerowicz EJ. New insights into pectin methylesterase structure and function. Trends in Plant Science. 2007;12:267–277. doi: 10.1016/j.tplants.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Piñeros MA, Shaff JE, Manslank HS, Alves VMC, Kochian LV. Aluminum resistance in maize cannot be solely explained by root organic acid exudation: a comparative physiological study. Plant Physiology. 2005;137:231–241. doi: 10.1104/pp.104.047357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polle E, Konzack CF, Littrik JA. Visual detection of aluminium tolerance levels in wheat by hematoxylin staining of seedling roots. Crop Science. 1978;18:823–827. [Google Scholar]
- Ryden P, Sugimoto-Shirasu K, Smith AC, Findlay K, Reiter WD, McCann MC. Tensile properties of Arabidopsis cell walls depends on both a xyloglucan cross-linked microfibrillar network and rhamnogalacturonan II-borate complexes. Plant Physiology. 2003;132:1033–1040. doi: 10.1104/pp.103.021873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmohl N, Horst WJ. Cell wall pectin content modulates aluminium sensitivity of Zea mays (L.) cells grown in suspension culture. Plant, Cell & Environment. 2000;23:735–742. [Google Scholar]
- Silva IR, Smyth TJ, Moxley DF, Carter TE, Allen NS, Rufty TW. Aluminum accumulation at nuclei of cells in the root tip: fluorescence detection using lumogallion and confocal laser scanning microscopy. Plant Physiology. 2000;123:543–552. doi: 10.1104/pp.123.2.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaguru M, Horst WJ, Eticha D, Matusmoto H. Aluminum inhibits apoplastic flow of high-molecular weight solutes in root apices of Zea mays L. Journal of Plant Nutrition and Soil Science. 2006;169:679–690. [Google Scholar]
- Tabuchi A, Matsumoto H. Changes in cell-wall properties of wheat (Triticum aestivum) roots during aluminium-induced growth inhibition. Physiologia Plantarum. 2001;112:353–358. doi: 10.1034/j.1399-3054.2001.1120308.x. [DOI] [PubMed] [Google Scholar]
- Taylor GJ, McDonald-Stephens JL, Hunter DB, et al. Direct measurement of aluminum uptake and distribution in single cells of Chara coralline. Plant Physiology. 2000;123:987–996. doi: 10.1104/pp.123.3.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van HL, Kuraishi S, Sakurai N. Aluminum-induced rapid root inhibition and changes in cell-wall components of squash seedlings. Plant Physiology. 1994;106:971–976. doi: 10.1104/pp.106.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehr JB, Menzies NW, Blamey FPC. Model studies on the role of citrate, malate and pectin esterification on the enzymatic degradation of Al- and Ca-pectate gels: possible implications for Al-tolerance. Plant Physiology and Biochemistry. 2003;41:1007–1010. [Google Scholar]
- Wen F, Zhu Y, Hawes MC. Effect of pectin methylesterase gene expression on pea root development. The Plant Cell. 1999;11:1129–1140. doi: 10.1105/tpc.11.6.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willats WGT, McCartney L, Mackie W, Knox JP. Pectin: cell biology and prospects for functional analysis. Plant Molecular Biology. 2001;a 47:9–27. [PubMed] [Google Scholar]
- Willats WGT, Orfila C, Limberg G, et al. Modulation of the degree and pattern of methyl-esterification of pectin homogalacturonan in plant cell walls: implications for pectin methyl esterase action, matrix properties, and cell adhension. Journal of Biological Chemistry. 2001;b 276:19404–19413. doi: 10.1074/jbc.M011242200. [DOI] [PubMed] [Google Scholar]
- Yang JL, Zheng SJ, He YF, Tang C, Zhou GD. Genotypic differences among plant species in response to aluminum stress. Journal of Plant Nutrition. 2005;28:949–961. [Google Scholar]
- Yang JL, Li YY, Zhang YJ, et al. Cell wall polysaccharides are specifically involved in the exclusion of aluminum from the rice root apex. Plant Physiology. 2008;146:602–611. doi: 10.1104/pp.107.111989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng SJ, Yang JL. Target sites of aluminum phytotoxicity. Biologia Plantarum. 2005;49:321–331. [Google Scholar]