Abstract
In this issue of Blood, Yokoyama and colleagues demonstrate in vitro differentiation of hESCs into mature neutrophils with functional capabilities (chemotaxis, phagocytosis, microbicidal oxidase activity, and bacterial killing) approaching or equal to that of normal peripheral blood neutrophils.
Despite the promise of human embryonic stem cells (hESCs) for producing therapeutically relevant hematopoietic cells, there have been few concrete demonstrations of the capacity of hESCs to produce functional mature hematopoietic progeny. The majority of hematopoietic differentiation studies on hESCs have focused on cell morphology, surface marker or gene expression, colony formation, or repopulation potential in nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mouse transplants. But they have not assessed whether the mature cells are capable of the full range of functions at the level of their normal peripheral blood hematopoietic counterparts. In this issue of Blood, Yokoyama et al,1 in addition to a recent publication by Saeki et al, 2 offer the first demonstrations of functional mature neutrophils being obtained from hESCs following differentiation in vitro. Notably, Yokoyama et al describe a method of highly directed terminal differentiation from immature hESCs into final cultures containing 70% to 80% mature neutrophils (stabs and polymorphonuclear cells) and 10% to 20% granulocytic metamyelocytes, with the remainder consisting of other myelomonocytic lineage cells. Yokoyama et al provide the first demonstration that these hESC-derived mature neutrophils are capable of similar levels of superoxide production, phagocytosis, bactericidal activity, and chemotaxis functions as peripheral blood neutrophils obtained from healthy human subjects.
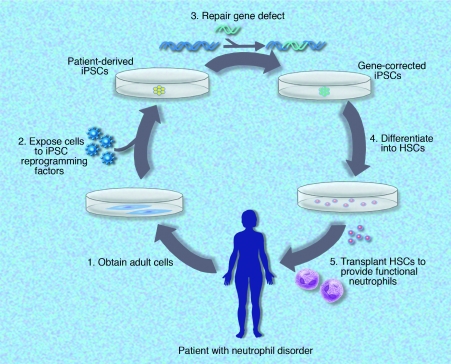
Treatment of genetic neutrophil disorders using patient-derived induced pluripotent stem cells (iPSCs) capable of producing functional neutrophils. Mature cells (fibroblasts or other cell types) could be obtained from the patient and reprogrammed into iPSCs using defined reprogramming factors. Following gene correction or repair, iPSCs could be expanded and differentiated in vitro using the Yokoyama et al or another similar protocol, modified to provide hematopoietic stem cells that are capable of producing functional neutrophils after autologous transplantation. Professional illustration by Marie Dauenheimer.
The ability to obtain functional hematopoietic progeny from hESCs is a major step toward the eventual use of hESCs or other pluripotent stem cells for the treatment of hematopoietic disorders. However, there are still major hurdles before that goal can be achieved. First, this differentiation protocol results in a relatively low (1.7-fold) increase in the number of neutrophils obtained relative to the initial number of hESCs, while the protocol described by Saeki et al2 resulted in roughly equal numbers of mature neutrophils as input hESCs. As such, the effective scaling-up of the differentiation procedure to obtain sufficient cell numbers for a therapeutic benefit remains a very formidable issue. In addition, while transplant of terminally differentiated mature hematopoietic cells could allow for short-term clinical benefit, the ability to engraft cells differentiated to a hematopoietic stem cell stage could allow for therapeutic long-term hematopoietic repopulation. While a few studies have demonstrated hematopoietic engraftment in NOD/SCID mice of apparent hematopoietic stem cells derived from in vitro differentiation of hESCs,3–5 normal functionality of the mature progeny of those engrafting cells has not yet been demonstrated. Consequently, a differentiation protocol that produces both efficient hematopoietic stem cell engraftment and functional progeny has yet to be conclusively demonstrated. Also, of critical importance for safety, undifferentiated hESCs have the potential to form teratomas. Therefore, differentiation cultures that result in even a small number of residual undifferentiated cells may pose a tumor risk in a transplantation setting.
Combined with the establishment of hematopoietic differentiation protocols for hESCs, the recent breakthroughs in creating induced pluripotent stem cells (iPSCs) also hold great potential for the treatment of neutrophil disorders and other hematopoietic diseases by providing highly expandable patient-specific stem cells. Human iPSCs can be derived from terminally differentiated cells by the introduction of combinations of exogenous transcription factor genes including OCT3/4, SOX2, c-MYC, KLF-4, NANOG, and LIN28, delivered using integrating gamma-retrovirus6 or lentivirus7 gene transfer vectors, or using nonintegrating episomal vectors for transient gene transfer.8 Typically skin fibroblasts have been used for this purpose, but a recent paper in this journal demonstrated that peripheral blood cells could also be used as a starting point.9
The Yokoyama et al hESC differentiation protocol provides a road map to determine whether human iPSCs may also produce functional hematopoietic progeny using a similar protocol. A number of investigators have noted that various hESC lines differ in their capacity for efficient differentiation into particular mature lineages including hematopoietic lineages. Saeki et al indicate that of the 3 hESC lines developed at Kyoto University that they examined (KhES-1, KhES-2, and KhES-3), only the KhES-3 line demonstrated efficient hematopoietic differentiation.2 These subtle differences in the in vitro differentiation capability of different hESC lines require further investigation. It is of note that Yokoyama et al also used the KhES-3 line for their studies. As with hESCs, it is likely that various iPSCs cloned lines derived even from the same patient also may demonstrate differences in their capacity for hematopoietic differentiation in vitro. Nonetheless, if human iPSCs can likewise give rise to functional hematopoietic progeny, it raises the possibility of developing patient-customized stem cells for autologous transplants to treat hematopoietic diseases in cases in which matched donor transplants are not available. For diseases arising from genetic defects, this process could involve obtaining mature cells from the patient, then using reprogramming factors to create iPSCs. Due to their capacity for prolonged (potentially indefinite) proliferation in an undifferentiated state, these iPSCs would be an attractive target for gene correction or repair, with subsequent cellular expansion to provide a larger pool of corrected cells than is achievable with current hematopoietic stem cell gene therapy. In vitro differentiation of gene-corrected iPSCs could then provide hematopoietic stem cells for autologous transplantation, resulting in long-term repopulation with functional progeny to treat the hematopoietic disorder following a theoretical schema (see figure).
Footnotes
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■
REFERENCES
- 1.Yokoyama Y, Suzuki T, Sakata-Yanagimoto M, et al. Derivation of functional mature neutrophils from human embryonic stem cells. Blood. 2009;113:6584–6592. doi: 10.1182/blood-2008-06-160838. [DOI] [PubMed] [Google Scholar]
- 2.Saeki K, Saeki K, Nakahara M, et al. A feeder-free and efficient production of functional neutrophils from human embryonic stem cells. Stem Cells. 2009;27:59–67. doi: 10.1634/stemcells.2007-0980. [DOI] [PubMed] [Google Scholar]
- 3.Wang L, Menendez P, Shojaei F, et al. Generation of hematopoietic repopulating cells from human embryonic stem cells independent of ectopic HOXB4 expression. J Exp Med. 2005;201:1603–1614. doi: 10.1084/jem.20041888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tian X, Woll PS, Morris JK, et al. Hematopoietic engraftment of human embryonic stem cell-derived cells is regulated by recipient innate immunity. Stem Cells. 2006;24:1370–1380. doi: 10.1634/stemcells.2005-0340. [DOI] [PubMed] [Google Scholar]
- 5.Ledran MH, Krassowska A, Armstrong L, et al. Efficient hematopoietic differentiation of human embryonic stem cells on stromal cells derived from hematopoietic niches. Cell Stem Cell. 2008;3:85–98. doi: 10.1016/j.stem.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 7.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 8.Yu J, Hu K, Smuga-Otto K, et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324:797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loh Y-H, Agarwal S, Park I-H, et al. Generation of induced pluripotent stem cells from human blood. Blood. 2009;113:5476–5479. doi: 10.1182/blood-2009-02-204800. [DOI] [PMC free article] [PubMed] [Google Scholar]