Abstract
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare clonal blood disorder that manifests with hemolytic anemia, bone marrow failure, and thrombosis. Many of the clinical manifestations of the disease result from complement-mediated intravascular hemolysis. Allogeneic bone marrow transplantation is the only curative therapy for PNH. Eculizumab, a monoclonal antibody that blocks terminal complement activation, is highly effective in reducing hemolysis, improving quality of life, and reducing the risk for thrombosis in PNH patients. Insights into the relevance of detecting PNH cells in PNH and other bone marrow failure disorders are highlighted, and indications for treating PNH patients with bone marrow transplantation and eculizumab are explored.
Introduction
Paroxysmal nocturnal hemoglobinuria (PNH) is a clonal hematopoietic stem cell disease that can present with bone marrow failure, hemolytic anemia, smooth muscle dystonias, and thrombosis.1,2 PNH can arise de novo or in the setting of aplastic anemia (AA).3 The disease originates from a multipotent hematopoietic stem cell that acquires a mutation of the PIG-A gene.4,5 Expansion and differentiation of the PIG-A mutant stem cell lead to clinical manifestations of the disease. The PIG-A gene product is required for the biosynthesis of glycophosphatidylinositol anchors, a glycolipid moiety that attaches dozens of proteins to the plasma membrane of cells. Consequently, the PNH stem cell and all of its progeny have a reduction or absence of glycosyl phosphatidylinositol (GPI)–anchored proteins. Two of these proteins, CD55 and CD59, are complement regulatory proteins; the absence of these proteins is fundamental to the pathophysiology of the disease.6,7 CD55 inhibits C3 convertases and CD59 blocks formation of the membrane attack complex (MAC) by inhibiting incorporation of C9 into the MAC. The loss of complement regulatory proteins renders PNH erythrocytes susceptible to both intravascular and extravascular hemolysis, but it is the intravascular hemolysis that contributes to much of the morbidity and mortality from the disease.8 Intravascular hemolysis releases free hemoglobin into the plasma. Free plasma hemoglobin scavenges nitric oxide and depletion of nitric oxide at the tissue level contributes to numerous PNH manifestations, including esophageal spasm, male erectile dysfunction, renal insufficiency, and thrombosis. The natural history of PNH is highly variable, ranging from indolent to life-threatening.9–12 The median survival is 10 to 15 years, but with a wide range. Thrombosis is the leading cause of death, but others may die of complications of bone marrow failure, renal failure, myelodysplastic syndrome, and leukemia.
How to recognize PNH
Patients with classic PNH have signs and symptoms of intravascular hemolysis. These patients tend to have a normocellular to hypercellular bone marrow with erythroid hyperplasia, an elevated reticulocyte count, a large population of PNH cells (usually > 60% PNH granulocytes) and a lactic dehydrogenase (LDH) that is 2 to 10 times the upper limit of normal. Hemoglobinuria, smooth muscle dystonias (eg, esophageal spasm and erectile dysfunction), severe fatigue, and thrombosis are common in patients with classic PNH.
An expanded PNH clone is also found in up to 70% of patients with acquired AA demonstrating a pathophysiologic link between these disorders.13–15 In contrast to patients with classic PNH, these patients typically have a lower percentage of PNH cells. Acquired AA is an autoimmune disorder, where the target of the immune attack is primitive CD34+ bone marrow progenitors.16,17 Typically, fewer than 10% GPIAP–deficient granulocytes are detected in AA patients at diagnosis, but occasional patients may have larger clones.13 DNA sequencing of the GPI-AP–deficient cells from AA patients reveals clonal PIG-A gene mutations.18 Although most AA patients exhibit no signs or symptoms of PNH early in the natural history of their disease when the PNH clone size is small, many, but not all, will experience further expansion of the PIG-A mutant clone and progress to classic PNH.
How to diagnose PNH
Patients with a Coombs-negative hemolytic anemia, AA, refractory anemia, and unexplained thrombosis in conjunction with cytopenias or hemolysis should be screened for PNH. The original assays to detect PNH erythrocytes included the Ham test,19 the sucrose hemolysis test,20 and the complement lysis assay.21 These erythrocyte-based assays do not reliably quantitate the percentage of PNH cells and can be falsely negative in patients who have received red cell transfusions; thus, I no longer use these assays. Most laboratories now use flow cytometric evaluation of specific GPI-anchored proteins because of its high sensitivity and specificity.22,23 CD59 is most commonly assessed because it is expressed on all hematopoietic lineages; CD55 is also commonly evaluated. It is noteworthy that rare congenital deficiencies of CD59 and CD55 may lead to a false-positive test for PNH if only one monoclonal antibody is used.24,25 Ideally, at least 2 different monoclonal antibodies, directed against 2 different GPI-anchored proteins, on at least 2 different cell lineages should be used to diagnose a patient with PNH. Solely screening red cells for PNH can lead to falsely negative tests, especially in the setting of a recent hemolytic episode or a recent blood transfusion. Because granulocytes and monocytes have a short half-life and are not affected by blood transfusions, the percentage of PNH cells in these lineages best reflects the size of the PNH clone.
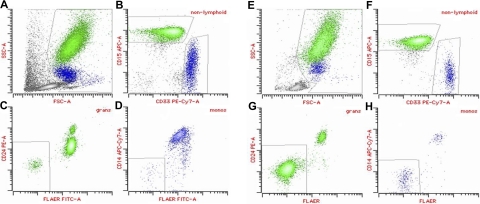
A fluorescein-labeled proaerolysin variant (FLAER) is increasingly being used as a flow cytometric assay to diagnose PNH (Figure 1).26 Aerolysin, the principal virulence factor of the bacterium Aeromonas hydrophila,27,28 is secreted as an inert protoxin, proaerolysin, that binds selectively and with high affinity to the GPI anchor.29 FLAER binds to the GPI anchor without forming channels and gives a more accurate assessment of the GPI anchor deficit in PNH than anti-CD59 or anti-CD55.26 Because the GPI anchor is the major determinant for binding FLAER, it allows for the direct assessment of GPI anchor expression on most cell lineages. Red cells and platelets are notable exceptions; this may be because both normal and PNH red cells express large amounts of glycophorin, a protein shown to bind aerolysin weakly.
Figure 1.
Multiparameter flow cytometry analysis of peripheral blood in PNH. (A-D) Aplastic anemia patient with small (2%) PNH clone; (E-H) classic PNH patient. (A,E) Forward scatter (FSC)/side scatter (SSC) display showing initial gate to exclude lymphocytes and debris. (B,F) Granulocytes (green) are identified as bright CD15 and low CD33, whereas monocytes (blue) are bright CD33 and low CD15. (C,G) Population of GPI anchor protein–deficient granulocytes showing lack of staining with both anti-CD24 and FLAER. (D,H) Population of GPI anchor protein–deficient monocytes showing lack of staining with both anti-CD14 and FLAER.
What percentage of PNH cells are relevant?
PIG-A mutant blood cells are readily detected in the blood and bone marrow of healthy control subjects at a frequency of approximately 1 in 50 000 (0.002%).30–32 Meticulous molecular and statistical analysis reveals that, unlike PIG-A mutations in PNH, most, if not all, of these mutations appear to arise from colony-forming cells and not multipotent hematopoietic stem cells.30,33 Thus, the reason why healthy controls have PIG-A mutations but never develop PNH is that these mutations arise in cells that lack self-renewal capacity.
GPI-AP-deficient cells (usually < 1% of granulocytes) have also been reported in patients with myelodysplastic syndrome (MDS),14,34 but sequencing of the PIG-A gene to establish clonality has not been performed in many of these studies. MDS patients reported to possess PNH cells tend to be classified as refractory anemia and often have the following characteristics: a hypocellular marrow, human leukocyte antigen (HLA)-DR15 positivity, normal cytogenetics, moderate to severe thrombocytopenia, and a high likelihood of response to immunosuppressive therapy.34 It is possible that many of these patients have moderate AA rather than hypoplastic MDS (hMDS). Indeed, it is quite common for classic PNH to evolve from acquired AA, but well documented cases of PNH arising from MDS are virtually nonexistent. Although no single test has been completely reliable in distinguishing between AA and hMDS, very low CD34 counts (< 0.1% CD34+ cells) are more common in (and may even be diagnostic of) AA.35 Regardless of whether small PNH clones in the setting of bone marrow failure are classified as refractory anemia, hMDS, or AA, they probably represent bone marrow failure that is immune mediated. Immunosuppressive therapy is probably the most effective therapy in these patients. Moreover, avoiding marrow-suppressive agents, such as 5-azacytidine, decitabine, or lenolidamide, in these patients is probably prudent because they can exacerbate the cytopenias in AA.
How do I treat patients with classic PNH?
Regardless of how one classifies patients with small PNH clones (eg, AA/PNH, MDS/PNH, or subclinical PNH), these patients do not have to be, and should not be, treated unless the patient is symptomatic from the PNH. If these patients do require therapy, it should target the underlying bone marrow failure disorder rather than the asymptomatic PNH clone. PNH clones less than 10% rarely require clinical intervention; nevertheless, these patients should be monitored closely because expansion of the clone may occur. I usually monitor granulocyte and erythrocyte clone size every 6 to 12 months in these patients. For patients with classic PNH, allogeneic bone marrow transplantation (BMT) and complement inhibition with eculizumab are the only proven effective therapies. Corticosteroids can improve hemoglobin levels and reduce hemolysis in some PNH patients, but the long-term toxicity and limited efficacy limit my enthusiasm for these agents.
Inhibition of terminal complement activation
Eculizumab is a humanized monoclonal antibody against C5 that inhibits terminal complement activation (Figure 2).36 Because C5 is common to all pathways of complement activation, blockade at this point aborts progression of the cascade regardless of the stimuli. Moreover, prevention of C5 cleavage blocks the generation of the potent proinflammatory and cell lytic molecules C5a and C5b-9, respectively (Figure 3).37 Importantly, C5 blockade preserves the critical immunoprotective and immunoregulatory functions of upstream components that culminate in C3b-mediated opsonization and immune complex clearance. In 2007, the US Food and Drug Administration approved eculizumab for use in PNH based on its efficacy in two phase 3 clinical trials.38,39 Eculizumab is highly effective in reducing intravascular hemolysis in PNH; it does not stop extravascular hemolysis, and it does not treat bone marrow failure. Thus, eculizumab is most effective in patients with classic PNH. Treatment with eculizumab decreases or eliminates the need for blood transfusions, improves quality of life, and reduces the risk of thrombosis.38–40 Two weeks before starting therapy, all patients should be vaccinated against Neisseria meningitides because inhibition of complement at C5 increases the risk for developing infections with encapsulated organisms, particularly N meningitides and N gonorrhoeae. Eculizumab is administered intravenously at a dose of 600 mg weekly for the first 4 weeks, then 900 mg biweekly starting on week 5. Eculizumab is safe and well tolerated but must be continued indefinitely because it does not treat the underlying cause of the disease. The most common side effect, headache, occurs in approximately 50% of patients, after the first dose or two, but rarely occurs thereafter. Neisserial sepsis is the most serious complication of eculizumab therapy; thus, it is imperative to remind patients that they have a 0.5% yearly risk of acquiring neisserial sepsis even after vaccination. Moreover, patients should be revaccinated against N meningitidis every 3 to 5 years after starting treatment and should be instructed to seek immediate medical attention if they develop signs or symptoms of neisserial infection.
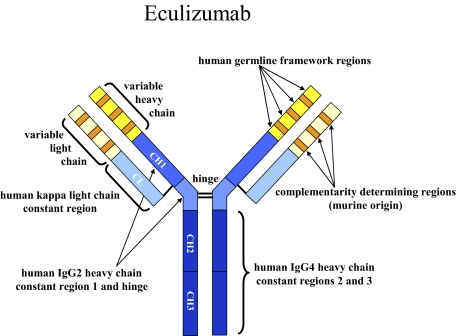
Figure 2.
Structure of eculizumab. Eculizumab was engineered to reduce immunogenicity and eliminate effector function. Human IgG2 and IgG4 heavy-chain sequences were combined to form a hybrid constant region that is unable to bind Fc receptors or to activate the complement cascade. Eculizumab exhibits high affinity for human C5, effectively blocking its cleavage and downstream proinflammatory and cell lytic properties. Reprinted from Rother et al36 with permission.
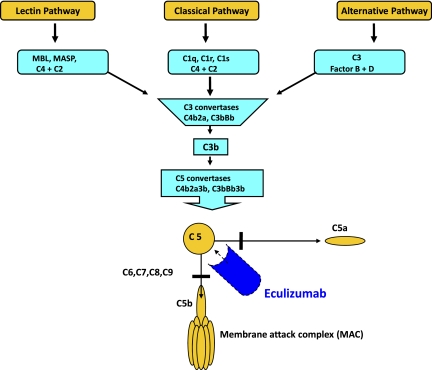
Figure 3.
Overview of the complement cascade. Classic, alternative, and lectin pathways converge at the point of C3 activation. The lytic pathway is initiated with the formation of C5 convertase and leads to the assembly of the C5, C6, C7, C8, (n) C9 membrane attack complex. Eculizumab is a monoclonal antibody that binds to C5, thereby preventing the formation of C5a and C5b. C5b is the initiating component of the MAC. Reprinted from Brodsky37 with permission.
Indications for therapy
There are no widely accepted evidence-based indications for treatment of PNH. In classic PNH, I recommend eculizumab for patients with disabling fatigue, thromboses, transfusion dependence, frequent pain paroxysms, renal insufficiency, or other end-organ complications from disease. Watchful waiting is appropriate for asymptomatic patients or those with mild symptoms. In patients with AA/PNH, therapy should be directed toward the underlying bone marrow failure with careful monitoring of the PNH clone using flow cytometry. Patients who meet criteria for severe AA should be managed with either allogeneic BMT or immunosuppressive therapy depending on the age of the patient and the availability of a suitable HLA-matched sibling donor.41,42
Monitoring patients on eculizumab
Most patients notice symptomatic improvement within hours to days after the first dose of eculizumab. I monitor patients with a complete blood count, reticulocyte count, LDH, and biochemical profile weekly for the first 4 weeks and then at least monthly thereafter. The LDH usually returns to normal or near normal within days to weeks after starting eculizumab (Figure 4); however, the reticulocyte count usually remains elevated and the hemoglobin response is highly variable. The reticulocyte count often remains elevated because most PNH patients on eculizumab continue to have some extravascular hemolysis. PNH erythrocytes frequently have increased deposition of C3 because of CD55 deficiency, and these cells are prematurely removed by the spleen.43 The hemoglobin response is largely dependent on the degree of extravascular hemolysis and the amount of underlying bone marrow failure. In classic PNH patients who are transfusion-dependent, a marked decrease in red cell transfusions is observed in virtually all patients, with more than 50% achieving transfusion independence.39,40,44 Breakthrough intravascular hemolysis and a return of PNH symptoms occur in less than 2% of PNH patients treated with eculizumab. This typically occurs a day or two before the next scheduled dose and is accompanied a spike in the LDH level. If this occurs on a regular basis, the interval between dosing can be shortened to 12 or 13 days or the dose of eculizumab can be increased to 1200 mg every 14 days. It is also important to recognize that increased complement activation that accompanies infections (eg, influenza or viral gastroenteritis) or trauma can also result in transient breakthrough hemolysis. These single episodes of breakthrough hemolysis do not require a change in dosing.
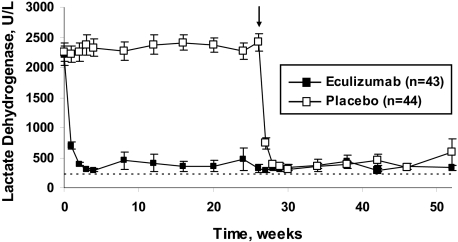
Figure 4.
Reduction in intravascular hemolysis during treatment with eculizumab. Mean levels of lactate dehydrogenase reflect the degree of hemolysis from baseline to week 52. The dashed line represents the upper limit of the normal range for lactate dehydrogenase (normal range, 103-223 U/L). In eculizumab-treated patients, the mean level of lactate dehydrogenase was rapidly reduced to just above the upper limit of the normal range. In the placebo group, the mean level of lactate dehydrogenase remained highly elevated. The arrow represents the transition of placebo-treated patients to eculizumab treatment in the phase 3 extension study, at which time levels of lactate dehydrogenase rapidly reduced to near normal values. Reprinted from Rother et al36 with permission.
Bone marrow transplantation
Bone marrow transplantation (BMT) is still the only curative therapy for PNH but is associated with significant morbidity and mortality. The International Bone Marrow Transplant Registry reported a 2-year survival probability of 56% in 48 recipients of HLA-identical sibling transplantations between 1978 and 1995.45 The median age was 28 years. The majority of the deaths in this study occurred within 1 year of transplantation. One of 7 recipients of alternative donor allogeneic transplants reported to the International Bone Marrow Transplant Registry during this period was alive 5 years after transplantation. The European Blood and Marrow Transplant group reported a 5-year survival rate of 70% after allogeneic BMT for PNH; however, only 54% met criteria for classic PNH.46 The median age in the study was 30 years. Graft failure occurred in 6% of patients, and acute and chronic graft-versus-host disease occurred in 15% and 20% of patients, respectively. Both nonmyeloablative syngeneic BMT and nonmyeloablative stem cell transplantations from HLA-matched or HLA-haploidentical donors have been successfully performed in PNH patients.47–50 Interestingly, the latter approach, but not the former, appears to cure the disease, suggesting that there is an important “graft-versus-PNH” effect with bone marrow transplantation. Now that an effective, nontransplantation therapy is available to treat PNH, the use of allogeneic BMT has decreased. At this point, I recommend allogeneic BMT only for PNH patients with life-threatening cytopenias or, possibly, the rare patient with disabling hemolysis or thrombosis that is not controlled with eculizumab.
How do I treat/prevent thrombosis in PNH?
Thrombosis is the leading cause of death from PNH and should be treated promptly with anticoagulation and sometimes thrombolytic therapy51 depending on the location of the thrombus. However, anticoagulation is only partially effective in preventing thrombosis in PNH; thus, I view thrombosis as an absolute indication for initiating treatment with eculizumab. A more controversial issue is whether PNH patients not taking eculizumab should receive prophylactic anticoagulation52 and whether patients on eculizumab therapy who have had a prior thrombus need to remain on anticoagulation. Prophylactic anticoagulation has never been proven to prevent thrombosis in PNH patients and is often dangerous given the low platelet counts that are observed in many PNH patients.11,40 Thus, in patients who do not meet my criteria for eculizumab therapy, I do not initiate prophylactic anticoagulation. Possible exceptions include patients with persistently elevated d-dimer levels, the pregnant PNH patient, and patients in the perioperative period. Discontinuing anticoagulation in patients on eculizumab with a previous thrombosis is even more controversial, and there are insufficient data to make strong recommendations.
How do I manage the pregnant patient with PNH?
Pregnant women with PNH have an even greater need for folate and iron supplementation because of the intravascular hemolysis. In some cases, PNH patients may require intravenous iron during pregnancy. Pregnancy and oral birth control pills also increase the risk for thrombosis in PNH. Fatal events have been reported in both the mother and fetus, but there are no prospective clinical trials that accurately define the risk.53 Anticoagulation with low molecular weight heparin is recommended as long as there is no contraindication to full anticoagulation. I administer a dose of 1 mg/kg subcutaneously every 12 hours when pregnancy is confirmed in a PNH patient with a large PNH clone. Depending on the rate and degree of weight gain, it may be necessary to monitor anti–factor Xa levels. It is often necessary to switch to unfractionated heparin around the time of delivery if a cesarean section is planned. Furthermore, anticoagulation should be continued during the puerperal period. Eculizumab is listed as a category C pharmaceutical; however, given the potential benefit of eculizumab in reducing thrombosis in PNH, an important question is whether this drug can be safely administered in pregnancy. The design of eculizumab with a hybrid Fc portion with IgG2 and IgG4 components is designed to abolish any effector mechanisms of the antibody (Figure 2). Because IgG2 isotypes do not cross the placenta, there should be little impact on the fetus. Indeed, there is a single case report where eculizumab was administered to a pregnant PNH patient in week 30 of her pregnancy.54 The fraternal twins, a baby boy and a baby girl, were delivered by cesarean section. No complications were reported; however, further experience with eculizumab is necessary to determine its safety in pregnancy.
Conclusions
In conclusion, tremendous advances have occurred over the past 2 decades in defining the molecular and cellular biology of PNH. These findings have been translated into novel diagnostic reagents and highly effective targeted therapy. Eculizumab is the only Food and Drug Administration–approved drug for the treatment of PNH. Thus, when to initiate therapy rather than what therapy to initiate is often the most important clinical question. Given that eculizumab is expensive, does not eradicate the PNH clone, and must be given lifelong, it is best reserved for the symptomatic patient with a large percentage of PNH cells or the PNH patient with thrombosis irrespective of the PNH clone size. Nevertheless, important clinical questions remain. Additional experience with eculizumab should clarify the indications for initiating therapy, whether or not prophylactic anticoagulation is necessary, and whether it is safe to use this drug during pregnancy.
Acknowledgments
The author thanks Dr Richard J. Jones for his thoughtful review of this manuscript and Dr Michael J. Borowitz for helpful discussion.
This work was supported by the National Institutes of Health (Bethesda, MD; grant CA70970).
Authorship
Contribution: R.A.B. wrote this manuscript.
Conflict-of-interest disclosure: R.A.B. serves on the international advisory board for Alexion Pharmaceuticals and declares no other competing financial interests.
Correspondence: Robert A. Brodsky, Johns Hopkins University School of Medicine, Ross Research Bldg, Rm 1025, 720 Rutland Ave, Baltimore, MD 21205-2196; e-mail: brodsro@jhmi.edu.
References
- 1.Brodsky RA. Narrative review. Paroxysmal nocturnal hemoglobinuria: the physiology of complement-related hemolytic anemia. Ann Intern Med. 2008;148:587–595. doi: 10.7326/0003-4819-148-8-200804150-00003. [DOI] [PubMed] [Google Scholar]
- 2.Parker CJ. Historical aspects of paroxysmal nocturnal haemoglobinuria: “defining the disease.”. Br J Haematol. 2002;117:3–22. doi: 10.1046/j.1365-2141.2002.03374.x. [DOI] [PubMed] [Google Scholar]
- 3.Parker C, Omine M, Richards S, et al. Diagnosis and management of paroxysmal nocturnal hemoglobinuria. Blood. 2005;106:3699–3709. doi: 10.1182/blood-2005-04-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miyata T, Takeda J, Iida Y, et al. The cloning of PIG-A, a component in the early step of GPI-anchor biosynthesis. Science. 1993;259:1318–1320. doi: 10.1126/science.7680492. [DOI] [PubMed] [Google Scholar]
- 5.Miyata T, Yamada N, Iida Y, et al. Abnormalities of PIG-A transcripts in granulocytes from patients with paroxysmal nocturnal hemoglobinuria. N Engl J Med. 1994;330:249–255. doi: 10.1056/NEJM199401273300404. [DOI] [PubMed] [Google Scholar]
- 6.Medof ME, Kinoshita T, Nussenzweig V. Inhibition of complement activation on the surface of cells after incorporation of decay-accelerating factor (DAF) into their membranes. J Exp Med. 1984;160:1558–1578. doi: 10.1084/jem.160.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rollins SA, Sims PJ. The complement-inhibitory activity of CD59 resides in its capacity to block incorporation of C9 into membrane C5b-9. J Immunol. 1990;144:3478–3483. [PubMed] [Google Scholar]
- 8.Rother RP, Bell L, Hillmen P, Gladwin MT. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA. 2005;293:1653–1662. doi: 10.1001/jama.293.13.1653. [DOI] [PubMed] [Google Scholar]
- 9.Hillmen P, Lewis SM, Bessler M, Luzzatto L, Dacie JV. Natural history of paroxysmal nocturnal hemoglobinuria. N Engl J Med. 1995;333:1253–1258. doi: 10.1056/NEJM199511093331904. [DOI] [PubMed] [Google Scholar]
- 10.de Latour RP, Mary JY, Salanoubat C, et al. Paroxysmal nocturnal hemoglobinuria: natural history of disease subcategories. Blood. 2008;112:3099–3106. doi: 10.1182/blood-2008-01-133918. [DOI] [PubMed] [Google Scholar]
- 11.Moyo VM, Mukhina GL, Garrett ES, Brodsky RA. Natural history of paroxysmal nocturnal hemoglobinuria using modern diagnostic assays. Br J Haematol. 2004;126:133–138. doi: 10.1111/j.1365-2141.2004.04992.x. [DOI] [PubMed] [Google Scholar]
- 12.Nishimura J, Kanakura Y, Ware RE, et al. Clinical course and flow cytometric analysis of paroxysmal nocturnal hemoglobinuria in the United States and Japan. Medicine (Baltimore) 2004;83:193–207. doi: 10.1097/01.md.0000126763.68170.46. [DOI] [PubMed] [Google Scholar]
- 13.Mukhina GL, Buckley JT, Barber JP, Jones RJ, Brodsky RA. Multilineage glycosylphosphatidylinositol anchor deficient hematopoiesis in untreated aplastic anemia. Br J Haematol. 2001;115:476–482. doi: 10.1046/j.1365-2141.2001.03127.x. [DOI] [PubMed] [Google Scholar]
- 14.Dunn DE, Tanawattanacharoen P, Boccuni P, et al. Paroxysmal nocturnal hemoglobinuria cells in patients with bone marrow failure syndromes. Ann Intern Med. 1999;131:401–408. doi: 10.7326/0003-4819-131-6-199909210-00002. [DOI] [PubMed] [Google Scholar]
- 15.Wang H, Chuhjo T, Yamazaki H, et al. Relative increase of granulocytes with a paroxysmal nocturnal haemoglobinuria phenotype in aplastic anaemia patients: the high prevalence at diagnosis. Eur J Haematol. 2001;66:200–205. doi: 10.1034/j.1600-0609.2001.00376.x. [DOI] [PubMed] [Google Scholar]
- 16.Maciejewski JP, Selleri C, Sato T, Anderson S, Young NS. Increased expression of Fas antigen on bone marrow CD34+ cells of patients with aplastic anaemia. Br J Haematol. 1995;91:245–252. doi: 10.1111/j.1365-2141.1995.tb05277.x. [DOI] [PubMed] [Google Scholar]
- 17.Young NS, Calado RT, Scheinberg P. Current concepts in the pathophysiology and treatment of aplastic anemia. Blood. 2006;108:2509–2519. doi: 10.1182/blood-2006-03-010777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagarajan S, Brodsky RA, Young NS, Medof ME. Genetic defects underlying paroxysmal nocturnal hemoglobinuria that arises out of aplastic anemia. Blood. 1995;86:4656–4661. [PubMed] [Google Scholar]
- 19.Ham T. Chronic hemolytic anemia with paroxysmal nocturnal hemoglobinuria: a study of the mechanism of hemolysisin relation to acid-base equilibrium. N Engl J Med. 1937;217:915–917. [Google Scholar]
- 20.Hartmann RC, Jenkins DE. The “sugar-water” test for paroxysmal nocturnal hemoglobinuria. N Engl J Med. 1966;275:155–157. doi: 10.1056/NEJM196607212750308. [DOI] [PubMed] [Google Scholar]
- 21.Rosse WF, Dacie JV. Immune lysis of normal human and paroxysmal nocturnal hemoglobinuria (PNH) red blood cells: I. The sensitivity of PNH red cells to lysis by complement and specific antibody. J Clin Invest. 1966;45:736–748. doi: 10.1172/JCI105388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bessler M, Fehr J. Fc III receptors (FcRIII) on granulocytes: a specific and sensitive diagnostic test for paroxysmal nocturnal hemoglobinuria (PNH). Eur J Haematol. 1991;47:179–184. doi: 10.1111/j.1600-0609.1991.tb01552.x. [DOI] [PubMed] [Google Scholar]
- 23.Hall SE, Rosse WF. The use of monoclonal antibodies and flow cytometry in the diagnosis of paroxysmal nocturnal hemoglobinuria. Blood. 1996;87:5332–5340. [PubMed] [Google Scholar]
- 24.Telen MJ, Green AM. The Inab phenotype: characterization of the membrane protein and complement regulatory defect. Blood. 1989;74:437–441. [PubMed] [Google Scholar]
- 25.Yamashina M, Ueda E, Kinoshita T, et al. Inherited complete deficiency of 20-kilodalton homologous restriction factor (CD59) as a cause of paroxysmal nocturnal hemoglobinuria. N Engl J Med. 1990;323:1184–1189. doi: 10.1056/NEJM199010253231707. [DOI] [PubMed] [Google Scholar]
- 26.Brodsky RA, Mukhina GL, Li S, et al. Improved detection and characterization of paroxysmal nocturnal hemoglobinuria using fluorescent aerolysin. Am J Clin Pathol. 2000;114:459–466. doi: 10.1093/ajcp/114.3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parker MW, van der Goot FG, Buckley JT. Aerolysin: the ins and outs of a model channel-forming toxin. Mol Microbiol. 1996;19:205–212. doi: 10.1046/j.1365-2958.1996.355887.x. [DOI] [PubMed] [Google Scholar]
- 28.Nelson KL, Raja SM, Buckley JT. Theglycosylphosphatidylinositol-anchored surface glycoprotein Thy-1 is a receptor for the channel-forming toxin aerolysin. J Biol Chem. 1997;272:12170–12174. doi: 10.1074/jbc.272.18.12170. [DOI] [PubMed] [Google Scholar]
- 29.Brodsky RA, Mukhina GL, Nelson KL, et al. Resistance of paroxysmal nocturnal hemoglobinuria cells to the glycosylphosphatidylinositol-binding toxin aerolysin. Blood. 1999;93:1749–1756. [PubMed] [Google Scholar]
- 30.Hu R, Mukhina GL, Piantadosi S, et al. PIG-A mutations in normal hematopoiesis. Blood. 2005;105:3848–3854. doi: 10.1182/blood-2004-04-1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Araten DJ, Nafa K, Pakdeesuwan K, Luzzatto L. Clonal populations of hematopoietic cells with paroxysmal nocturnal hemoglobinuria genotype and phenotype are present in normal individuals. Proc Natl Acad Sci U S A. 1999;96:5209–5214. doi: 10.1073/pnas.96.9.5209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ware RE, Pickens CV, DeCastro CM, Howard TA. Circulating PIG-A mutant T lymphocytes in healthy adults and patients with bone marrow failure syndromes. Exp Hematol. 2001;29:1403–1409. doi: 10.1016/s0301-472x(01)00746-9. [DOI] [PubMed] [Google Scholar]
- 33.Traulsen A, Pacheco JM, Dingli D. On the origin of multiple mutant clones in paroxysmal nocturnal hemoglobinuria. Stem Cells. 2007;25:3081–3084. doi: 10.1634/stemcells.2007-0427. [DOI] [PubMed] [Google Scholar]
- 34.Wang H, Chuhjo T, Yasue S, Omine M, Nakao S. Clinical significance of a minor population of paroxysmal nocturnal hemoglobinuria-type cells in bone marrow failure syndrome. Blood. 2002;100:3897–3902. doi: 10.1182/blood-2002-03-0799. [DOI] [PubMed] [Google Scholar]
- 35.Matsui WH, Brodsky RA, Smith BD, Borowitz MJ, Jones RJ. Quantitative analysis of bone marrow CD34 cells in aplastic anemia and hypoplastic myelodysplastic syndromes. Leukemia. 2006;20:458–462. doi: 10.1038/sj.leu.2404119. [DOI] [PubMed] [Google Scholar]
- 36.Rother RP, Rollins SA, Mojcik CF, Brodsky RA, Bell L. Discovery and development of the complement inhibitor eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria. Nat Biotechnol. 2007;25:1256–1264. doi: 10.1038/nbt1344. [DOI] [PubMed] [Google Scholar]
- 37.Brodsky R. Paroxysmal nocturnal hemoglobinuria. In: Hoffman R, Benz EJ Jr, Shattil SJ, et al., editors. Hematology: Basic Principles and Practice. Philadelphia, PA: Churchill Livingstone; 2009. pp. 385–394. [Google Scholar]
- 38.Hillmen P, Young NS, Schubert J, et al. The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2006;355:1233–1243. doi: 10.1056/NEJMoa061648. [DOI] [PubMed] [Google Scholar]
- 39.Brodsky RA, Young NS, Antonioli E, et al. Multicenter phase 3 study of the complement inhibitor eculizumab for the treatment of patients with paroxysmal nocturnal hemoglobinuria. Blood. 2008;111:1840–1847. doi: 10.1182/blood-2007-06-094136. [DOI] [PubMed] [Google Scholar]
- 40.Hillmen P, Muus P, Duhrsen U, et al. Effect of the complement inhibitor eculizumab on thromboembolism in patients with paroxysmal nocturnal hemoglobinuria. Blood. 2007;110:4123–4128. doi: 10.1182/blood-2007-06-095646. [DOI] [PubMed] [Google Scholar]
- 41.Brodsky RA, Jones RJ. Aplastic anaemia. Lancet. 2005;365:1647–1656. doi: 10.1016/S0140-6736(05)66515-4. [DOI] [PubMed] [Google Scholar]
- 42.Young NS. Acquired aplastic anemia. Ann Intern Med. 2002;136:534–546. doi: 10.7326/0003-4819-136-7-200204020-00011. [DOI] [PubMed] [Google Scholar]
- 43.Risitano AM, Notaro R, Marando L, et al. Complement fraction 3 binding on erythrocytes as additional mechanism of disease in paroxysmal nocturnal hemoglobinuria patients treated by eculizumab. Blood. 2009;113:4094–4100. doi: 10.1182/blood-2008-11-189944. [DOI] [PubMed] [Google Scholar]
- 44.Schubert J, Hillmen P, Roth A, et al. Eculizumab, a terminal complement inhibitor, improves anaemia in patients with paroxysmal nocturnal haemoglobinuria. Br J Haematol. 2008 May 22; doi: 10.1111/j.1365-2141.2008.07183.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saso R, Marsh J, Cevreska L, et al. Bone marrow transplants for paroxysmal nocturnal haemoglobinuria. Br J Haematol. 1999;104:392–396. doi: 10.1046/j.1365-2141.1999.01195.x. [DOI] [PubMed] [Google Scholar]
- 46.Perfault de Latour R, Schrezenmeier H, Mary JY, et al. Stem cell transplantation for paroxysmal nocturnal hemoglobinuria: an ongoing joint study of the AAWP EBMT group and the French Society of Hematology [abstract]. Blood. 2008;112 Abstract 1181. [Google Scholar]
- 47.Antin JH, Ginsburg D, Smith BR, et al. Bone marrow transplantation for paroxysmal nocturnal hemoglobinuria: eradication of the PNH clone and documentation of complete lymphohematopoietic engraftment. Blood. 1985;66:1247–1250. [PubMed] [Google Scholar]
- 48.Cho SG, Lim J, Kim Y, et al. Conditioning with high-dose cyclophosphamide may not be sufficient to provide a long-term remission of paroxysmal nocturnal hemoglobinuria following syngeneic peripheral blood stem cell transplantation. Bone Marrow Transplant. 2001;28:987–988. doi: 10.1038/sj.bmt.1703259. [DOI] [PubMed] [Google Scholar]
- 49.Suenaga K, Kanda Y, Niiya H, et al. Successful application of nonmyeloablative transplantation for paroxysmal nocturnal hemoglobinuria. Exp Hematol. 2001;29:639–642. doi: 10.1016/s0301-472x(01)00632-4. [DOI] [PubMed] [Google Scholar]
- 50.Brodsky RA, Luznik L, Bolanos-Meade J, et al. Reduced intensity HLA-haploidentical BMT with post transplantation cyclophosphamide in nonmalignant hematologic diseases. Bone Marrow Transplant. 2008;42:523–527. doi: 10.1038/bmt.2008.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kuo GP, Brodsky RA, Kim HS. Catheter-directed thrombolysis and thrombectomy for the Budd-Chiari syndrome in paroxysmal nocturnal hemoglobinuria in three patients. J Vasc Interv Radiol. 2006;17:383–387. doi: 10.1097/01.RVI.0000196338.87954.CE. [DOI] [PubMed] [Google Scholar]
- 52.Hall C, Richards S, Hillmen P. Primary prophylaxis with warfarin prevents thrombosis in paroxysmal nocturnal hemoglobinuria (PNH). Blood. 2003;102:3587–3591. doi: 10.1182/blood-2003-01-0009. [DOI] [PubMed] [Google Scholar]
- 53.Ray JG, Burows RF, Ginsberg JS, Burrows EA. Paroxysmal nocturnal hemoglobinuria and the risk of venous thrombosis: review and recommendations for management of the pregnant and nonpregnant patient. Haemostasis. 2000;30:103–117. doi: 10.1159/000022532. [DOI] [PubMed] [Google Scholar]
- 54.Danilov AV, Smith H, Craigo S, et al. Paroxysmal nocturnal hemoglobinuria (PNH) and pregnancy in the era of eculizumab. Leuk Res. 2009;33:e4–e5. doi: 10.1016/j.leukres.2008.09.008. [DOI] [PubMed] [Google Scholar]