Abstract
CD8+ T cells are major players in the immune response against HIV. However, recent failures in the development of T cell–based vaccines against HIV-1 have emphasized the need to reassess our basic knowledge of T cell–mediated efficacy. CD8+ T cells from HIV-1–infected patients with slow disease progression exhibit potent polyfunctionality and HIV-suppressive activity, yet the factors that unify these properties are incompletely understood. We performed a detailed study of the interplay between T-cell functional attributes using a bank of HIV-specific CD8+ T-cell clones isolated in vitro; this approach enabled us to overcome inherent difficulties related to the in vivo heterogeneity of T-cell populations and address the underlying determinants that synthesize the qualities required for antiviral efficacy. Conclusions were supported by ex vivo analysis of HIV-specific CD8+ T cells from infected donors. We report that attributes of CD8+ T-cell efficacy against HIV are linked at the level of antigen sensitivity. Highly sensitive CD8+ T cells display polyfunctional profiles and potent HIV-suppressive activity. These data provide new insights into the mechanisms underlying CD8+ T-cell efficacy against HIV, and indicate that vaccine strategies should focus on the induction of HIV-specific T cells with high levels of antigen sensitivity to elicit potent antiviral efficacy.
Introduction
CD8+ T cells are essential for effective immunity against HIV-1, and the induction of such responses using vaccines has become a major objective in the strategy to halt the pandemic.1 However, the recent outcome of the Merck STEP study, the most ambitious trial of an anti-HIV T cell–based vaccine conducted to date, has been a major disappointment.2 Despite its immunogenicity, the vaccine failed both to prevent infection of vaccinated volunteers at high risk of acquiring HIV and to reduce viral load set points in infected vaccinees. This failure has roused the scientific community to step back and reconsider its basic knowledge of T cell–mediated efficacy.3,4 Indeed, consensual opinion is that our general understanding of T-cell efficacy in HIV-1 infection is actually still limited, which represents a clear obstacle to the design of successful vaccines.
Over recent years, qualitative attributes of CD8+ T cells have increasingly become the focus of attempts to identify reliable correlates of immune protection against HIV. Among these, polyfunctionality5 and HIV-suppressive activity6 have been associated with spontaneous control of HIV infection and slower disease progression rates in infected patients. Of note, polyfunctionality is currently seen as the best correlate of T-cell efficacy measurable directly ex vivo.7 Polyfunctional CD8+ T cells are those that exhibit multiple effector functions (ie, degranulation and production of antiviral factors) simultaneously upon antigen encounter; this can be assessed after stimulation with cognate peptides by multiparametric flow cytometry (eg, mobilization of CD107 and intracellular production of interferon [IFN]–γ, tumor necrosis factor [TNF]–α, interleukin-2 [IL-2], and macrophage-inflammatory protein [MIP]–1β).5 HIV-suppressive activity reflects the capacity of HIV-specific CD8+ T cells to eliminate HIV-infected targets via classical class I major histocompatibility complex (MHC)-restricted cytotoxic lysis.6,8 It can be assessed using primary CD4+ T cells infected in vitro with HIV in the presence of HIV-specific CD8+ T cells. However, although consistent qualitative properties generally identify HIV-specific CD8+ T-cell populations that are associated with lower HIV replication rates, the issue of cause and effect remains difficult to determine in ex vivo studies. Furthermore, the factors that link these functional outcomes are not well defined.
We recently reported that, in addition to polyfunctional profiles, HIV-specific CD8+ T cells that are associated with superior control of HIV-1 replication display high levels of antigen sensitivity.9 Based on these observations and a review of the literature, we proposed that antigen sensitivity may be an important facet of T-cell efficacy in HIV infection.10 However, contrasting data have been reported. For instance, it was recently suggested that HIV-specific CD8+ T cells with polyfunctional profiles are actually those that display low, rather than high, levels of antigen sensitivity.11 Moreover, CD8+ T-cell polyfunctionality has been shown to depend inversely on HIV load;12,13 such observations suggest that polyfunctionality is a consequence of low antigen levels rather than the cause of improved viral containment.
Virus-specific CD8+ T cells differ with respect to many parameters, including (1) intrinsic factors, such as human histocompatibility leukocyte antigen (HLA) restriction, target antigen, and T-cell receptor (TCR); and (2) ontogenetic factors, such as replicative history, differentiation status, and activation profile. Each of these parameters is known to affect T-cell properties directly and may influence the control of HIV-1 replication. This heterogeneity in the nature and environment of individual CD8+ T cells is a substantial obstacle to the deconvolution of functional complexity in relation to the control of HIV-1 replication; it may also explain the contrasting findings in the literature, and hence, the current confusion regarding the determinants of CD8+ T-cell efficacy. To overcome this issue and investigate the relationship between different functional attributes associated with T-cell efficacy in HIV-1 infection, we established a controlled system using HIV-specific CD8+ T-cell clones isolated in vitro; importantly, all of these clones were specific for the same HIV-derived epitope (p24 Gag KK10; residues 263-272) and restricted by the same HLA class I molecule (HLA B*2705). This approach allowed direct comparison of the functional properties of individual CD8+ T-cell clonotypes, which represent the fundamental units of T-cell immunity, in the setting of a fixed antigen target and comparable activation status. The principal finding of these studies, supported by ex vivo observations, was that CD8+ T-cell polyfunctionality and HIV-suppressive activity are directly linked to the level of antigen sensitivity.
Methods
Patients
Samples were obtained from HIV-1–infected patients enrolled in the French Agence Nationale de la Recherche sur le SIDA (ANRS) ALT14 and IMMUNOCO15 Cohorts. Antiretroviral therapy-naive patients during the chronic phase of infection, with CD4 counts greater than 400 cells/mm3, no clinical symptoms, and plasma viral load ranging from 200 to 350 000 copies of HIV-1 RNA/mL, were selected for the study. The study was approved by the relevant local institutional (at the Hospital Pitié Salpétrière) review board and ethics committee, and informed consent was obtained in accordance with the Declaration of Helsinki. Peripheral blood mononuclear cells (PBMCs) were separated from citrate anticoagulated blood and cryopreserved for subsequent studies. HLA genotyping was carried out by amplification refractory mutation system–polymerase chain reaction (PCR) using sequence-specific primers, as previously described.16 HLA-genotyped patients were screened for HIV-specific CD8+ T-cell responses with IFN-γ enzyme-linked immunospot (ELISPOT) assays using a panel of 49 known HLA class I–restricted viral epitope peptides to identify the immunodominant response in each donor.
Isolation and expansion of HIV-specific CD8+ and CD4+ T-cell clones
HIV-specific CD8+ T-cell clones were isolated from PBMC samples obtained from 3 HLA B*2705+ patients infected with HIV-1. Single KK10/HLA B*2705 tetramer+ CD8+ T cells were sorted using a FACSAria flow cytometer in a biosafety containment level III laboratory and expanded in microtiter plates by periodic stimulation in the presence of mixed irradiated allogeneic PBMC, phytohemagglutinin (PHA; 1 μg/mL), and recombinant human (rh)IL-2 (150 IU/mL). HIV-specific CD4+ T-cell clones were generated by in vitro priming using HIV-infected autologous monocyte-derived dendritic cells and subsequently expanded, as previously described.17 Clones demonstrated specificity for an epitope in HIV-1 p24 Gag (residues 271-290). HLA-matched Epstein-Barr virus (EBV)–transformed B-cell lines used to present antigen in cytolytic and polyfunctional assays were cultured in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS), antibiotics, and l-glutamine. Peptide-specific clones were screened using IFN-γ enzyme-linked immunosorbent assay (ELISPOT) and tetramer staining.
Monoclonal antibodies, amine reactive dyes, and peptide/HLA class I tetramers
Tetrameric antigen complexes for KK10/HLA-B*2705 and NV9/HLA-A*0201 NL9 were generated, as described previously.18,19 Directly conjugated monoclonal antibodies (mAbs) were obtained from the following vendors: (1) αCD3-Cy5.5 peridinin chlorophyll protein, αCD8-allophycocyanin (APC), αCD4-APCCy7, αCD107a-Cy5 phycoerythrin (PE), αCD40L-PE, αIL-2-APC, αIFNγ-Alexa700, and αTNFα-PECy7 (BD Biosciences, San Diego, CA); (2) αCD8-Alexa405, αperforin-PE, and αgranzyme B–Texas Red PE (Caltag Laboratories, Burlingame, CA); (3) αMIP-1β-fluorescein isothiocyanate (FITC; R&D Systems, Minneapolis, MN); and (4) αCD4–Texas Red PE, αCD8-Cy5PE, and αKC57/p24-FITC (Beckman Coulter, Miami, FL). The amine-reactive viability dye ViViD (Molecular Probes, Eugene, OR) was used to eliminate dead cells from the analysis, as described previously.20 Staining with all reagents was conducted according to standard procedures.21,22
Clonotype analysis
Clonality of expanded clones was confirmed by molecular analysis of TCR gene expression, conducted using a template-switch–anchored reverse transcription-PCR, as described previously.23,24 Twenty TCRB sequences tested were generated for each clone; clonality was validated when a single productive TCRB sequence was repeatedly obtained for a given clone. The ImMunoGeneTics nomenclature system is used throughout.19,23
Measure of antigen sensitivity
Antigen sensitivity of CD8+ T cells refers to activation threshold in response to defined concentrations of exogenous peptide. It was assessed by performing peptide dilutions and determining the peptide concentration required to induce half-maximum response in functional assays. For CD8+ T-cell clones, antigen sensitivity was measured in chromium release assays. Briefly, target cells were labeled with 51Cr (70 μCi for 106 cells) for 1 hour at 37°C, washed 3 times, and then incubated in the presence of cognate peptide at the concentrations indicated for 15 minutes at room temperature before the addition of T-cell clones at an effector to target (E:T) ratio of 10:1. After incubation for 4 hours at 37°C, chromium release was measured in harvested supernatants. Activity was determined as the percentage lysis: 100 × (experimental − spontaneous release)/(total − spontaneous release). For CD8+ T-cell populations within PBMCs, antigen sensitivity was measured in ex vivo IFN-γ ELISPOT assays. Briefly, thawed PBMCs were plated in the presence of cognate peptide at 105 cells/well in 96-well polyvinylidene plates (Millipore, Molsheim, France) precoated with capture anti–human IFN-γ mAb (Diaclone, Besançon, France) at 4°C overnight. Plates were incubated overnight at 37°C in 5% CO2 and developed according to the manufacturer's recommendations. Spots were counted using an automated ELISPOT reader (Zeiss, Le Pecq, France).
Polyfunctional analysis
For polyfunctional profiling, PBMC or T-cell clones together with antigen-presenting cells as appropriate were incubated in the presence of specific peptide at the indicated concentrations and pretitered αCD107a mAb for 1 hour at 37°C/5% CO2, followed by an additional 5 hours in the presence of monensin (2.5 μg/mL; Sigma-Aldrich, St Louis, MO) and brefeldin A (5 μg/mL; Sigma-Aldrich); negative controls were processed in parallel for all experiments in the absence of peptide. The BD Cytofix/Cytoperm kit was used to permeabilize cells, according to the manufacturer's instructions, before staining for intracellular markers. Analysis was performed using DIVA and FlowJo version 8.2 (TreeStar, Ashland, OR) software after acquisition of stained cells on a standard LSRII flow cytometer (BD Biosciences). Multifunctional data were analyzed with PESTLE version 1.3.2 and SPICE version 3.1 (provided by Mario Roederer, ImmunoTechnology Section, Vaccine Research Center/National Institute of Allergy and Infectious Diseases/National Institutes of Health, Bethesda, MD).
Assessment of HIV-suppressive activity
Primary HLA B*2705+ CD4+ T cells were purified (> 99%) from freshly isolated PBMC by positive selection with antibody-coated magnetic beads (Miltenyi Biotec, Paris, France). CD4+ cells were stimulated for 3 days with PHA at 2 μg/mL in the presence of IL-2 (Chiron, Suresnes, France) at 100 U/mL; the culture medium was RPMI 1640 supplemented with 10% FCS and 100 U/mL penicillin/streptomycin. The results shown were obtained by performing infections in vitro with HIV-1 NL4.3 (X4) at the indicated multiplicity of infection (MOI). CD4+ T cells (105) were infected in 96-well plates with a spinoculation protocol. For coculture, 105 CD4+ T cells were mixed with CD8+ T cells (at different CD8/CD4 ratios) at the moment of infection. After infection, the cells were washed and cultured for 3 days. Cells were then harvested and stained with CD4 and CD8 mAb, permeabilized (Cytofix/Cytoperm; BD Biosciences), and stained with anti-p24 mAb to monitor HIV-1 infection.
Statistics
Group medians and distributions were compared using the Mann-Whitney U test or the Wilcoxon signed rank test. Associations between variables were determined by the nonparametric Spearman rank correlation test. P values less than .05 were considered significant.
Results
Generation of CD8+ T-cell clones with different antigen sensitivities
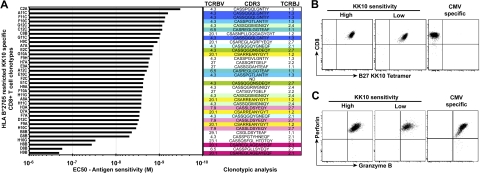
To assess the impact of antigen sensitivity on the attributes of CD8+ T-cell efficacy, we generated a bank of HIV-specific CD8+ T-cell clones specific for the immunodominant HLA B*2705-restricted p24 Gag KK10 epitope (residues 263-272); this restriction element and targeted antigenic protein are both associated with improved control of HIV-1 replication.25,26 These HIV-specific CD8+ T-cell clones were isolated from the peripheral blood of 3 HIV-infected donors using KK10/HLA B*2705 tetramers combined with flow cytometric sorting and then expanded in vitro according to standard protocols; clonality was confirmed by TCR sequencing (Figure 1A). A total of 36 from 150 clones was selected for further investigation based on robust expansion upon repeated restimulation; this necessary selection criterion avoids bias that might be introduced by replicative senescence or exhaustion. The antigen sensitivity of these clones was determined as the 50% effective peptide concentration (EC50) required to elicit half-maximal lytic activity in chromium release assays using cognate peptide titration and a common antigen-presenting HLA B*2705+ target cell line. As shown in Figure 1A, the CD8+ T-cell clones displayed a range of antigen sensitivities with a difference of more than 2 orders of magnitude with respect to the EC50 value (2.1 × 10−7 M to 3.3 × 10−10 M). Regardless of antigen sensitivity, all clones stained comparably with KK10/HLA B*2705 tetramers (Figure 1B) and were fully armed cytotoxic cells (Figure 1C): there was no relationship between the antigen sensitivity of the clones and the percentage (or median fluorescence intensity [MFI]) of tetramer staining, or of perforin and granzyme B expression.
Figure 1.
Identification of HIV-specific CD8+ T-cell clones with distinct antigen sensitivities. (A) Half-maximal effective concentrations (EC50) of HLA B*2705-restricted KK10-specific CD8+ T-cell clones were determined in standard chromium release assays using peptide titrations and a common antigen-presenting HLA B*2705+ B-cell line. The tested CD8+ T-cell clones are classified according to increasing antigen sensitivity from bottom to top. TCRBV usage, CDR3 amino acid sequence, and TCRBJ usage are shown for each clone (ND, not done). Same colors indicate identical clonotypes. The clone reference is indicated on the y-axis (the code last letter corresponds to the patient from which the clone was obtained: A and F for patient 1, B and G for patient 2, and C and H for patient 3). (B) Representative flow cytometric data showing combined αCD8 mAb and KK10/HLA B*2705 tetramer staining of KK10-specific CD8+ T-cell clones with higher or lower levels of antigen sensitivity; staining of a HLA A*0201-restricted CMV pp65-specific clone is shown for comparison. (C) Representative intracellular stainings for perforin and granzyme B in the same CD8+ T-cell clones.
Link between antigen sensitivity and CD8+ T-cell polyfunctionality
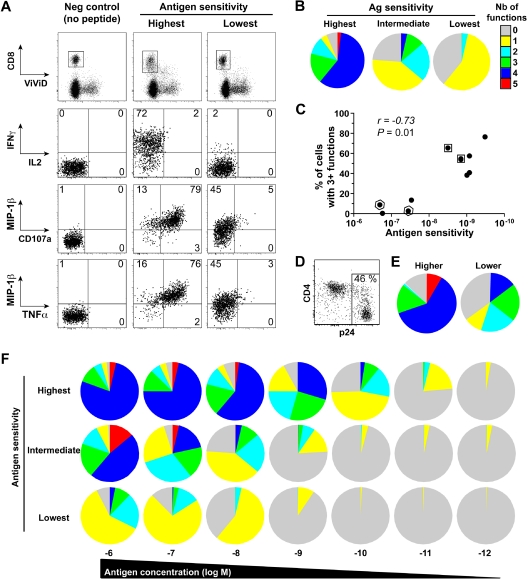
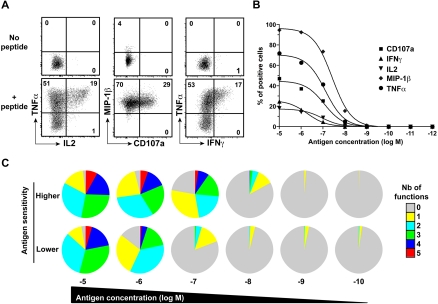
The polyfunctional profile of representative clones that displayed different levels of antigen sensitivity was then assessed by multiparametric flow cytometry (Figure 2A). For stimulation, target cells were incubated with 10−8 M peptide concentration, thought to be close to the amount of peptide that real infection generates on cell surface.27 Importantly, these assays were performed on equivalently “resting” clones 2 weeks postrestimulation to circumvent any potential bias related to activation status. Among the clones selected for these assays (n = 10), we included clones with identical TCRB sequence for internal control (see framed dots in Figure 2C). A caveat is that we only performed TCRB sequencing, and it is possible that clones with the same TCRB chain might have different TCRA chain and therefore not be identical clonotypes. The antigen sensitivity of all these clones was superior for the wild-type KK10 peptide, compared with a L268M variant KK10 peptide, which corresponds to an escape mutation commonly observed in HIV-infected patients (data not shown). Notably, whereas all clones elicited at least one function upon antigen encounter, highly sensitive clones displayed more polyfunctional profiles compared with clones with lower levels of antigen sensitivity (representative examples are shown in Figure 2B). Indeed, there was a direct link between CD8+ T-cell antigen sensitivity and polyfunctionality, as shown by a significant positive correlation between these 2 parameters (Figure 2C). Likewise, in the presence of primary CD4+ T cells infected with HIV (Figure 2D; ie, endogenous presentation of the antigen), CD8+ T-cell clones with higher antigen sensitivity showed a stronger polyfunctional profile (Figure 2E).
Figure 2.
Functional characterization of HIV-specific CD8+ T-cell clones with distinct antigen sensitivities. (A) Representative data showing the simultaneous and independent measurement of 5 separate functions in KK10-specific CD8+ T-cell clones using 8-color flow cytometry. Cells were stimulated for 6 hours in the presence of EBV-transformed HLA B*2705+ B cells and 10−8 M peptide before intracellular staining. Function plots are gated on CD8+ViViD– cells; percentages of cells in the different quadrants, gated with respect to the corresponding negative controls (displayed for reference in the left panels for the clone with low antigen sensitivity), are shown. (B) The pie charts depict the background-adjusted polyfunctional profile of 3 representative KK10-specific CD8+ T-cell clones with different antigen sensitivities (highest, C2A; intermediate, H10G; lowest, D8B). For simplicity, responses are grouped according to the number of functions (from CD107a, IFN-γ, TNF-α, IL-2, and MIP-1β) elicited in response to antigen encounter; individual segments represent the proportions of cells within each total clonal population that exhibited the number of functions indicated. (C) Polyfunctionality, defined as the percentage of cells displaying 3 or more functions simultaneously, is plotted as a function of antigen sensitivity. Each dot represents a distinct clone; dots framed by a square or hexagon indicate clones with identical TCRB sequences. The correlation was determined using Spearman rank test. (D) Representative staining for p24 and CD4 from primary CD4+ T cells 3 days postinfection with the replicative HIV-1 strain NL4.3 pseudotyped with vesicular stomatitis virus. (E) Polyfunctional profile of 2 CD8+ T-cell clones with different antigen sensitivity for KK10 (higher, C2A; lower, H8B) after 6-hour incubation with HLA-B27 primary CD4+ T cells infected with HIV-1 (1:10 CD8 to CD4 ratio). (F) Polyfunctional profiling of 3 representative KK10-specific CD8+ T-cell clones (highest, C2A; intermediate, H10G; lowest, D8B) along a peptide concentration gradient.
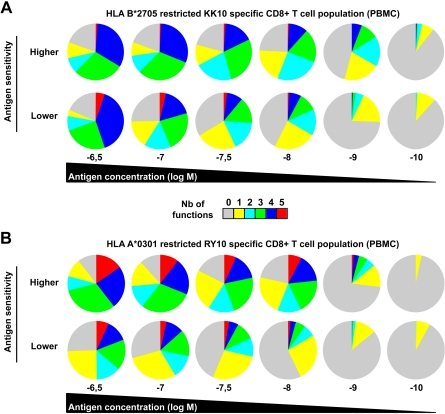
Functional outcomes upon antigen engagement depend on the strength of the stimulus,28 which can be directly affected by the level of antigen sensitivity. In a related manner, several studies have also shown that the functional profile of T cells can be dictated by antigen concentration.29–32 To address these issues in our study, we determined the polyfunctional profiles of representative KK10-specific CD8+ T-cell clones in peptide titration assays. Progressive increases in antigen concentration stimulated more polyfunctional outcomes (Figure 2F). Although apparently paucifunctional during initial screening (Figure 2B), CD8+ T-cell clones with lower levels of antigen sensitivity displayed enhanced polyfunctional profiles at supraoptimal peptide concentrations. Nonetheless, at any given antigen density, greater proportions of the population were polyfunctional in highly sensitive CD8+ T-cell clones compared with their lower sensitivity counterparts. Similar observations were obtained using CD8+ T-cell clones specific for the HLA A*0201-restricted cytomegalovirus (CMV) pp65 NV9 epitope (residues 495-503) with distinct antigen sensitivity (data not shown), extending the relationship between polyfunctionality and antigen sensitivity to another specificity. The relevance of these observations was confirmed in assays conducted directly ex vivo. We assessed the functional profile of CD8+ T-cell populations specific for 2 distinct HIV antigens in peptide titration assays. Figure 3 shows CD8+ T-cell populations (from distinct donors) specific for the HLA B*2705-restricted KK10 epitope (Figure 3A) or the HLA A*0301-restricted HIV-1 p24 Gag epitope RY10 (residues 20-29; Figure 3B) that exhibited distinct antigen sensitivities. Ex vivo observation mirrored those obtained with the CD8+ T-cell clones; thus, regardless of specificity or experimental conditions, polyfunctionality was determined by antigen sensitivity and peptide concentration.
Figure 3.
Ex vivo polyfunctional profiles of HIV-specific CD8+ T-cell populations. Polyfunctional profiling of representative HLA B*2705-restricted KK10 (A)– or HLA A*0301-restricted RY10 (B)–specific CD8+ T-cell populations (from 2 patients of 5 in each case) with different antigen sensitivities along a peptide concentration gradient. Antigen sensitivity of these populations was determined in standard intracellular cytokine-staining assays for IFN-γ secretion conducted directly ex vivo.
Sequential induction of functions with signal intensity
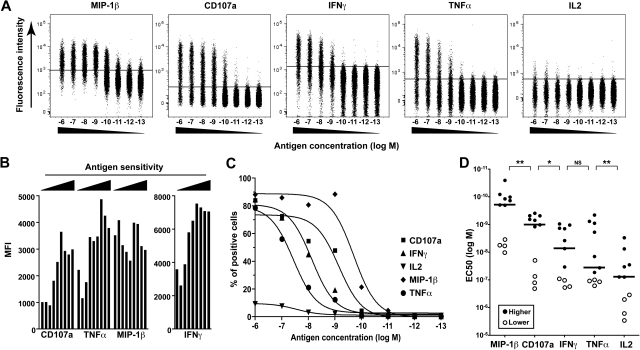
To gain mechanistic insights into the relationship between polyfunctionality and antigen sensitivity, we analyzed the induction of individual functions independently (Figure 4A). In addition to being more polyfunctional, high-sensitivity clones also produced more IFN-γ and/or TNF-α and mobilized more CD107a on a per-cell basis compared with clones with lower levels of antigen sensitivity; this quantification of individual functions was assessed by MFI measurements gated solely on responsive cells stimulated at the highest peptide concentration (Figure 4B). Thus, the amount of produced cytokines is directly associated with the degree of activation at the single-cell level. These observations are consistent with recent reports that showed an association between the magnitude of individual functions at the single-cell level and the degree of polyfunctionality in polyclonal antigen-specific T-cell populations ex vivo.33,34 Only MIP-1β failed to show such hierarchical induction, thereby suggesting that this function is rapidly elicited at maximal output; low IL-2 production levels precluded such analyses for this function. Further examination of individual functions revealed a sequential induction of different functions according to stimulus strength (Figure 4C). Thus, although interclonal variations could be observed (mostly with respect to the differential induction of IFN-γ and TNF-α), the individual functions appeared in a predictable linear order along the gradient of antigen concentration as follows: MIP-1β > CD107a > IFN-γ ≥ TNF-α > IL-2 (P = .009; Kruskal-Wallis test; Figure 4D). This sequential induction of functions with increasing TCR signal intensity provides an explanation for the relationship between antigen sensitivity and functional attributes. In sum, these data demonstrate the existence of a functional hierarchy in HIV-specific CD8+ T-cell clones that is determined by their antigen sensitivity.
Figure 4.
Sequential induction of functions with increasing antigen concentration. (A) Representative example of the assessment of individual functions for a KK10-specific CD8+ T-cell clone (G11C) upon activation with a gradient of peptide concentration. Cells are gated on CD8+ViViD−. (B) The MFI values for CD107a, IFN-γ, TNF-α, and MIP-1β are plotted for distinct KK10-specific CD8+ T-cell clones ordered according to their antigen sensitivity. Cells were stimulated with 10−6 M cognate peptide in the presence of antigen-presenting EBV-transformed HLA B*2705+ B cells; MFI values were determined after gating solely on cells that were positive for the relevant function, excluding responses less than 20% of the total clonal population in each case. (C) Representative peptide titration assays for the induction of individual functions in a KK10-specific CD8+ T-cell clone (G11C). (D) EC50 values for each individual function obtained with different KK10-specific CD8+ T-cell clones are plotted to highlight the sequential induction of separate functions with antigen concentration. Horizontal bars indicate median values; ○ represent clones with lower levels of antigen sensitivity. *P < .05, **P < .01 calculated using the Wilcoxon signed rank test.
Hierarchical functionality in CD4+ T cells
To extend the applicability of these findings, we investigated also the functional responsiveness of cells from the CD4+ lineage. For this purpose, 2 CD4+ T-cell clones specific for an epitope in HIV-1 p24 Gag (residues 271-290) were isolated from a single donor and expanded in vitro; these clones displayed distinct levels of antigen sensitivity, as measured by expression of CD40L after stimulation with a range of peptide concentrations (lower, EC50 = 5.5 × 10−7 M; higher, EC50 = 5.3 × 10−8 M), and used different HLA restriction elements (HLA-DR*01 or HLA-DR*04). Functional profiling was conducted using an immortalized B-cell line derived from the original donor, thus expressing both HLA molecules of interest, as a common target for antigen presentation (Figure 5A). The functional hierarchy was different from that observed for CD8+ T cells; however, there was clear sequential induction of individual functions (Figure 5B). Furthermore, CD4+ T-cell polyfunctionality increased with the stimulus gradient and was more pronounced for the clone with higher sensitivity for antigen (Figure 5C). Thus, antigen sensitivity dictates T-cell functional response profiles regardless of specificity and lineage.
Figure 5.
Polyfunctional profiling of HIV-specific CD4+ T-cell clones. (A) Representative data showing the simultaneous and independent measurement of 5 separate functions in a CD4+ T-cell clone specific for HIV-1 Gag. Cells were stimulated for 6 hours in the presence of autologous EBV-transformed B cells and cognate peptide before intracellular staining; αCD107 mAb was added to the assays immediately before stimulation. Function plots are gated on CD3+CD4+ViViD− cells; percentages of cells in the different quadrants, gated with respect to the corresponding negative controls, are shown. (B) Representative peptide titration assays for the induction of individual functions in a CD4+ T-cell clone specific for HIV-1 Gag. (C) Polyfunctional profiles of HIV-1 Gag-specific CD4+ T-cell clones with lower or higher levels of antigen sensitivity along a peptide concentration gradient. Antigen sensitivity was determined in CD40L up-regulation assays with immortalized autologous B cells as targets. For simplicity, responses are grouped according to the number of functions (from CD107a, IFN-γ, TNF-α, IL-2, and MIP-1β) elicited in response to antigen encounter; individual segments of the pie charts represent the proportions of cells within each total population that exhibited the number of functions indicated.
Potent suppression of HIV replication by highly sensitive CD8+ T cells
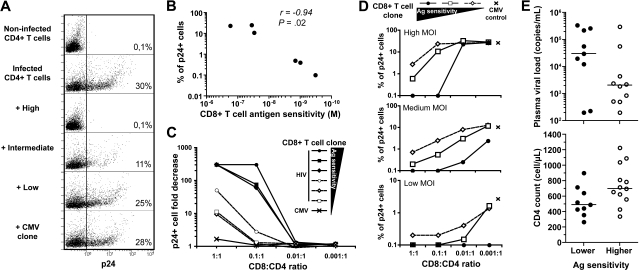
Recently, it has been shown that the in vivo control of HIV-1 replication is associated with the capacity of CD8+ T cells to suppress p24 production in primary CD4+ T-cell cultures infected with HIV-1, by eliminating target cells via perforin/granzyme B-mediated cytolytic pathways.6,8 The measurement of HIV suppression has emerged as one of the most relevant approaches for assessing CD8+ T-cell efficacy against HIV.4 We therefore tested the capacity of our KK10-specific CD8+ T-cell clones to suppress HIV replication under these conditions, using infected HLA B*2705+ primary CD4+ T cells. Notably, the presence of highly sensitive CD8+ T-cell clones prevented the production of p24 in the infected CD4+ T-cell cultures to levels approaching those of the negative controls, that is, in the complete absence of virus; thus, these CD8+ T cells had almost totally suppressed HIV replication in this assay (Figure 6A). However, KK10-specific clones with lower levels of antigen sensitivity were not as effective. In fact, the presence of clones with the lowest antigen sensitivity had virtually no effect on the dynamics of HIV infection and replication (Figure 6A). Importantly, we found an inverse correlation between the proportion of infected cells expressing intracellular p24 and the antigen sensitivity of the CD8+ T-cell clones present in the cultures under equivalent conditions (Figure 6B).
Figure 6.
Suppression of HIV-1 infection in vitro by HIV-specific CD8+ T-cell clones. (A) Primary CD4+ T cells isolated from a HLA B*2705+ healthy donor and blasted with PHA were infected with the replicative HIV-1 strain NL4.3 (MOI = 10−1.8) in the presence or absence of KK10-specific CD8+ T-cell clones with distinct levels of antigen sensitivity (highest, C2A; intermediate, H10G; lowest, D8B) or a control NV9-specific CD8+ T-cell clone; E:T ratio 1:10. After 3 days, HIV-1 infection levels were measured using intracellular p24 staining. Numbers show the percentages of p24+ cells in the cultures. (B) Inverse correlation between CD8+ T-cell antigen sensitivity and HIV-1 infection in vitro (% of p24+ cells) determined using Spearman rank test (each dot represents 1 clone). (C) Assessment of suppressive activity (fold decrease of p24+ cells compared with infected CD4+ T-cell controls in the absence of CD8+ T cells) for CD8+ T-cell clones at different E:T ratios. (D) Measurement of p24+ cells at decreasing MOI (10−1.8, 10−2.2, 10−3.3) and different E:T ratios for CD8+ T-cell clones with highest (●), intermediate (□), or lowest (◇) levels of antigen sensitivity. CD8neg cells were gated for the analyses. (E) Plasma viral loads and CD4 counts in 21 untreated HIV-infected donors grouped according to the antigen sensitivity (lower ● or higher ○) of their individual immunodominant CD8+ T-cell response, determined in peptide titration IFN-γ ELISPOT assays conducted ex vivo. Immunodominant responses (including HLA-A2 Nef PL10, A3 Gag RY10, A3 Nef QK10, A24 RW8, A26 Gag EL9, B7 Gag GL9, B7 Nef TL10, B8 Gag GI9, B8 Nef FL8, B8 Gag DL10, B27 Gag KK10, and B51 Env RL9) were screened using a panel of 49 optimized cytotoxic T lymphocyte epitopes.
To further explore the mechanistic basis of HIV-suppressive activity, we tested in these assays the influences of the E:T ratio as well as viral input. In the presence of disproportionate numbers of T-cell clones with lower levels of antigen sensitivity, apparent levels of HIV suppression could nonetheless be observed (Figure 6C). Finally, at lower MOI, suppression of HIV replication could be achieved at lower E:T ratios (Figure 6D). This shows that, although the frequency of effector cells and the level of virus challenge are important parameters and can influence the efficiency of HIV suppression, under all conditions, highly sensitive clones exhibit greater potency in their ability to suppress HIV infection and replication, most likely through more rapid recognition and elimination of target cells, as shown in mouse models.35 Overall, this provides an explanation for our findings of an inverse correlation between the antigen sensitivity of immunodominant HIV-specific CD8+ T-cell populations measured ex vivo and the cell-associated viral load in patients.9 Of note, HIV-infected patients harboring immunodominant HIV-specific CD8+ T-cell responses with higher antigen sensitivity presented clear trends toward lower plasma viral loads and higher CD4 counts (Figure 6E).
Discussion
Effective control of HIV by CD8+ T cells is thought to rely on qualitative aspects of the virus-specific response. Indeed, the deployment of potent effector functions, such as polyfunction and suppression of viral replication, has been identified as hallmarks of CD8+ T-cell efficacy in HIV-1 infection. The principal finding of the current study is that these functional attributes of CD8+ T-cell populations are governed by the level of antigen sensitivity. It is established that the functional profile of T cells in response to antigen-induced activation can be influenced by several factors, among which the intensity of the TCR-mediated signal is crucial. Consistent with previous reports,28–32 we found that polyfunctionality is a composite of individual functions triggered in a hierarchical manner along a gradient of antigen concentration. Because highly sensitive cells receive a systematically stronger signal for a given antigenic stimulus, they display a more polyfunctional profile compared with their lower sensitivity counterparts. This principle applied generally to CD8+ T cells specific for different viral epitopes and to CD4+ T cells. Moreover, CD8+ T cells with high levels of antigen sensitivity can recognize low densities of cognate antigen on the target cell surface, thereby resulting in effective target cell clearance;36,37 this corresponds to the superior capacity of these cells to suppress HIV replication (Figure 6). Thus, overall, the qualitative attributes associated with T-cell efficacy are interlinked and governed by antigen sensitivity.
Previous studies that used isolated HIV-specific CD8+ T-cell clones to assess their functional attributes reached diverging conclusions as to the importance of antigen sensitivity.38,39 These contrasting results may be related to the heterogeneity (in terms of target epitope and HLA restriction)38 or the number (n = 2)39 of clones used in these studies, which may hinder definite insights into the relevance of a specific parameter for CD8+ T-cell efficacy. This reinforces our strategy to generate a large number of clones, with a unique specificity, to perform adequate comparative analysis and explore the mechanistic basis of CD8+ T-cell efficacy, supported by ex vivo data. It is noteworthy that, despite being restricted by HLA B*2705 and cognate for an epitope derived from the Gag protein (parameters associated with better control of HIV replication), the KK10-specific CD8+ T-cell clones with low antigen sensitivity in our study exhibited paucifunctionality and poor HIV-suppressive capacity in vitro. These findings serve to emphasize further that the control of HIV replication is not solely a function of HLA restriction or epitope target.
The findings presented in this study are directly relevant to the development and assessment of effective T-cell–based vaccines. Currently, the majority of studies use functional assays, such as IFN-γ ELISPOT or intracellular cytokine staining for polyfunctional immunoprofiling, performed in the presence of saturating peptide concentrations. However, it is clear from the present dataset that the functional profiles of antigen-specific T-cell populations approximate when assessed under supraoptimal stimulation conditions; thus, whereas such assays can provide information on total antigen reactivity, they are limited in terms of dissecting the finer qualitative attributes of CD8+ T-cell populations. These considerations may argue for the routine assessment of antigen sensitivity as an independent parameter in our attempts to define the correlates of protective immunity and inform vaccine design.
Antigen sensitivity is known to depend on multiple factors. These include, of course, the affinity of the expressed TCR for antigen, but also additional factors not directly related to this primary interaction, such as molecular density and topography on the T-cell surface, coreceptor-mediated effects, membrane flexibility, and differential triggering thresholds.40–42 Recent work suggests that the sensitivity of T cells to signals from the TCR may also be controlled at the level of miRNA expression. For instance, it was shown that during thymopoiesis, miR-181 changes the outcome of signaling triggered by TCR engagement by peptide-MHC complexes in double-positive (CD4+CD8+) thymocytes through the modulation of negative regulators of TCR signaling.43 Furthermore, several factors primarily related to cellular ontogeny and environment can influence the functional profile of T cells and their antigen sensitivity. For instance, T-cell activation status exerts an effect, because antigenic stimulation can result in transient hyporesponsiveness.44–47 This may be due to activation-induced regulatory pathways, such as the expression of inhibitory receptors like programmed death 1,48 TCR-CD8 dissociation,49 or factors related to TCR down-regulation,50,51 and may explain why T cells from HIV-infected patients with high viral loads appear less functional in ex vivo assays.12,13,52
Although complex, this nonhierarchical combination of in vivo factors needs to be integrated into our comprehension of CD8+ T-cell–mediated control of HIV infection; the present work in this light simply highlights the importance of efforts that aim to dissect the respective contributions of these parameters to T-cell efficacy. We are currently engaged in biophysical and structural analyses of specific TCR peptide-MHC class I complex interactions, as well as genomics analysis (macroRNA and microRNA) of higher versus lower antigen sensitivity T cells, to deconvolute the major determinants of antigen sensitivity with regard to CD8+ T-cell efficacy. The induction of T cells with high levels of antigen sensitivity could emerge as a major focus of vaccine design, and more knowledge is needed with respect to the basis of antigen sensitivity. We recently reviewed possible approaches that may contribute to this goal, emphasizing the importance of antigen levels for T-cell priming and boosting, as well as the potentially crucial role of costimulatory pathways.10 In this context, a recent study has shown that the use of Toll-like receptor ligands as adjuvants in vaccination formulations can result in increasing TCR selection thresholds, thereby leading to the preferential expansion of clonotypes with high levels of antigen sensitivity.53
However, whereas highly sensitive T cells may be the most efficacious in terms of halting HIV replication and spread, 2 important issues need to be considered in the vaccine setting. First, the extreme mutability and rapid evolution of HIV in vivo in response to selection pressure mediated by highly sensitive CD8+ T cells54,55 dictate that the choice of immunogen and the breadth of response, both in terms of the number of targeted epitopes and the clonotypic composition of each antigen-specific population, are primary considerations.56 Interestingly, transduction in CD8+ T cells of a TCR with supraphysiologic affinity for an HIV epitope has been recently reported to enable recognition of variants.27 Nonetheless, induction of T-cell responses targeting simultaneously several conserved epitopes with high sensitivities is likely to be crucial to exert pressure on points where the virus has difficulties to mutate. Second, clones with higher levels of antigen sensitivity expand more readily upon cognate stimulation (data not shown). In the setting of viral persistence and continued antigenic challenge, this stronger expansion and turnover can result in a limited replicative lifespan,57 and eventually the irreversible exhaustion of effective T-cell clonotypes.9,58 Induction of durable effective T-cell immunity is therefore conditional on T-cell proliferative and renewal capacities. Thus, defining the optimal equilibrium for the induction and maintenance of highly sensitive T cells from the available repertoire may prove to be one of the keys to the development of a successful T-cell–based vaccine against HIV infection.
Supplementary Material
Acknowledgments
We are very grateful to the staff and patients who participated in this study and to the ANRS Cohort ALT group. Martha Nason provided invaluable help with statistical analysis. We thank Catherine Blanc for her skills in infected live cell sorting in the Pitié-Salpêtrière Flow Cytometry Platform. We are also indebted to Nathalie Rufer for the provision of rhIL-2. For a complete list of the ANRS Cohort ALT group participants, see the supplemental appendix (available on the Blood website; see the Supplemental Materials link at the top of the online article).
This work was supported by the Inserm AVENIR grant, the French ANRS, Sidaction, the National Institutes of Health, and the Medical Research Council (MRC) of the United Kingdom. D.A.P. is a MRC Senior Clinical Fellow. J.R.A. is supported by a fellowship from the Fundação para a Ciência e Tecnologia. S.Y.S. is supported by Korea Science and Engineering Foundation and the Institut Pasteur Korea.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: J.R.A. performed research and analyzed data; D.S. contributed vital new reagents and performed research; D.A.P. contributed vital new reagents and wrote the paper; L.P. performed research and analyzed data; S.Y.S. performed research; A.M. contributed vital new reagents; M.L. contributed vital new reagents; G.P. designed the research and contributed vital new reagents; D.C.D. designed the research and contributed vital new reagents; B.A. designed the research and contributed vital new reagents; A.S.-C. designed the research and analyzed data; and V.A. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Victor Appay, Cellular Immunology Laboratory, Inserm Unité 945, Hôpital Pitié-Salpêtrière, Paris, France; e-mail: victor.appay@upmc.fr.
References
- 1.McMichael A, Hanke T. The quest for an AIDS vaccine: is the CD8+ T-cell approach feasible? Nat Rev Immunol. 2002;2:283–291. doi: 10.1038/nri779. [DOI] [PubMed] [Google Scholar]
- 2.Steinbrook R. One step forward, two steps back—will there ever be an AIDS vaccine? N Engl J Med. 2007;357:2653–2655. doi: 10.1056/NEJMp0708117. [DOI] [PubMed] [Google Scholar]
- 3.Sekaly RP. The failed HIV Merck vaccine study: a step back or a launching point for future vaccine development? J Exp Med. 2008;205:7–12. doi: 10.1084/jem.20072681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watkins DI, Burton DR, Kallas EG, Moore JP, Koff WC. Nonhuman primate models and the failure of the Merck HIV-1 vaccine in humans. Nat Med. 2008;14:617–621. doi: 10.1038/nm.f.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Betts MR, Nason MC, West SM, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107:4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saez-Cirion A, Lacabaratz C, Lambotte O, et al. HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar cytotoxic T lymphocyte activation phenotype. Proc Natl Acad Sci U S A. 2007;104:6776–6781. doi: 10.1073/pnas.0611244104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol. 2008;8:247–258. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- 8.Migueles SA, Osborne CM, Royce C, et al. Lytic granule loading of CD8+ T cells is required for HIV-infected cell elimination associated with immune control. Immunity. 2008;29:1009–1021. doi: 10.1016/j.immuni.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Almeida JR, Price DA, Papagno L, et al. Superior control of HIV-1 replication by CD8+ T cells is reflected by their avidity, polyfunctionality, and clonal turnover. J Exp Med. 2007;204:2473–2485. doi: 10.1084/jem.20070784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Appay V, Douek DC, Price DA. CD8+ T cell efficacy in vaccination and disease. Nat Med. 2008;14:623–628. doi: 10.1038/nm.f.1774. [DOI] [PubMed] [Google Scholar]
- 11.Harari A, Cellerai C, Enders FB, et al. Skewed association of polyfunctional antigen-specific CD8 T cell populations with HLA-B genotype. Proc Natl Acad Sci U S A. 2007;104:16233–16238. doi: 10.1073/pnas.0707570104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Streeck H, Brumme ZL, Anastario M, et al. Antigen load and viral sequence diversification determine the functional profile of HIV-1-specific CD8+ T cells. PLoS Med. 2008;5:e100. doi: 10.1371/journal.pmed.0050100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rehr M, Cahenzli J, Haas A, et al. Emergence of polyfunctional CD8+ T cells after prolonged suppression of human immunodeficiency virus replication by antiretroviral therapy. J Virol. 2008;82:3391–3404. doi: 10.1128/JVI.02383-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magierowska M, Theodorou I, Debre P, et al. Combined genotypes of CCR5, CCR2, SDF1, and HLA genes can predict the long-term nonprogressor status in human immunodeficiency virus-1-infected individuals. Blood. 1999;93:936–941. [PubMed] [Google Scholar]
- 15.Chouquet C, Autran B, Gomard E, et al. Correlation between breadth of memory HIV-specific cytotoxic T cells, viral load and disease progression in HIV infection. AIDS. 2002;16:2399–2407. doi: 10.1097/00002030-200212060-00004. [DOI] [PubMed] [Google Scholar]
- 16.Bunce M, O'Neill CM, Barnardo MC, et al. Phototyping: comprehensive DNA typing for HLA-A, B, C, DRB1, DRB3, DRB4, DRB5 & DQB1 by PCR with 144 primer mixes utilizing sequence-specific primers (PCR-SSP). Tissue Antigens. 1995;46:355–367. doi: 10.1111/j.1399-0039.1995.tb03127.x. [DOI] [PubMed] [Google Scholar]
- 17.Moris A, Pajot A, Blanchet F, Guivel-Benhassine F, Salcedo M, Schwartz O. Dendritic cells and HIV-specific CD4+ T cells: HIV antigen presentation, T-cell activation, and viral transfer. Blood. 2006;108:1643–1651. doi: 10.1182/blood-2006-02-006361. [DOI] [PubMed] [Google Scholar]
- 18.Altman JD, Moss PAH, Goulder PJR, et al. Phenotypic analysis of antigen-specific T lymphocytes. [Published erratum appears in Science. 1998;280:1821.] Science. 1996;274:94–96. doi: 10.1126/science.274.5284.94. [DOI] [PubMed] [Google Scholar]
- 19.Price DA, Brenchley JM, Ruff LE, et al. Avidity for antigen shapes clonal dominance in CD8+ T cell populations specific for persistent DNA viruses. J Exp Med. 2005;202:1349–1361. doi: 10.1084/jem.20051357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perfetto SP, Chattopadhyay PK, Lamoreaux L, et al. Amine reactive dyes: an effective tool to discriminate live and dead cells in polychromatic flow cytometry. J Immunol Methods. 2006;313:199–208. doi: 10.1016/j.jim.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 21.Whelan JA, Dunbar PR, Price DA, et al. Specificity of CTL interactions with peptide-MHC class I tetrameric complexes is temperature dependent. J Immunol. 1999;163:4342–4348. [PubMed] [Google Scholar]
- 22.Papagno L, Almeida JR, Nemes E, Autran B, Appay V. Cell permeabilization for the assessment of T lymphocyte polyfunctional capacity. J Immunol Methods. 2007;328:182–188. doi: 10.1016/j.jim.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Douek DC, Betts MR, Brenchley JM, et al. A novel approach to the analysis of specificity, clonality, and frequency of HIV-specific T cell responses reveals a potential mechanism for control of viral escape. J Immunol. 2002;168:3099–3104. doi: 10.4049/jimmunol.168.6.3099. [DOI] [PubMed] [Google Scholar]
- 24.Price DA, West SM, Betts MR, et al. T cell receptor recognition motifs govern immune escape patterns in acute SIV infection. Immunity. 2004;21:793–803. doi: 10.1016/j.immuni.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 25.Kaslow RA, Carrington M, Apple R, et al. Influence of combinations of human major histocompatibility complex genes on the course of HIV-1 infection. Nat Med. 1996;2:405–411. doi: 10.1038/nm0496-405. [DOI] [PubMed] [Google Scholar]
- 26.Kiepiela P, Ngumbela K, Thobakgale C, et al. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat Med. 2007;13:46–53. doi: 10.1038/nm1520. [DOI] [PubMed] [Google Scholar]
- 27.Varela-Rohena A, Molloy PE, Dunn SM, et al. Control of HIV-1 immune escape by CD8 T cells expressing enhanced T-cell receptor. Nat Med. 2008;14:1390–1395. doi: 10.1038/nm.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Viola A, Lanzavecchia A. T cell activation determined by T cell receptor number and tunable thresholds. Science. 1996;273:104–106. doi: 10.1126/science.273.5271.104. [DOI] [PubMed] [Google Scholar]
- 29.Valitutti S, Muller S, Dessing M, Lanzavecchia A. Different responses are elicited in cytotoxic T lymphocytes by different levels of T cell receptor occupancy. J Exp Med. 1996;183:1917–1921. doi: 10.1084/jem.183.4.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Price DA, Sewell AK, Dong T, et al. Antigen-specific release of β-chemokines by anti-HIV-1 cytotoxic T lymphocytes. Curr Biol. 1998;8:355–358. doi: 10.1016/s0960-9822(98)70138-1. [DOI] [PubMed] [Google Scholar]
- 31.Betts MR, Price DA, Brenchley JM, et al. The functional profile of primary human antiviral CD8+ T cell effector activity is dictated by cognate peptide concentration. J Immunol. 2004;172:6407–6417. doi: 10.4049/jimmunol.172.10.6407. [DOI] [PubMed] [Google Scholar]
- 32.La Gruta NL, Turner SJ, Doherty PC. Hierarchies in cytokine expression profiles for acute and resolving influenza virus-specific CD8+ T cell responses: correlation of cytokine profile and TCR avidity. J Immunol. 2004;172:5553–5560. doi: 10.4049/jimmunol.172.9.5553. [DOI] [PubMed] [Google Scholar]
- 33.Precopio ML, Betts MR, Parrino J, et al. Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8+ T cell responses. J Exp Med. 2007;204:1405–1416. doi: 10.1084/jem.20062363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Darrah PA, Patel DT, De Luca PM, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13:843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 35.Derby M, Alexander-Miller M, Tse R, Berzofsky J. High-avidity CTL exploit two complementary mechanisms to provide better protection against viral infection than low-avidity CTL. J Immunol. 2001;166:1690–1697. doi: 10.4049/jimmunol.166.3.1690. [DOI] [PubMed] [Google Scholar]
- 36.Alexander-Miller MA, Leggatt GR, Berzofsky JA. Selective expansion of high- or low-avidity cytotoxic T lymphocytes and efficacy for adoptive immunotherapy. Proc Natl Acad Sci U S A. 1996;93:4102–4107. doi: 10.1073/pnas.93.9.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Messaoudi I, Guevara Patino JA, Dyall R, LeMaoult J, Nikolich-Zugich J. Direct link between MHC polymorphism, T cell avidity, and diversity in immune defense. Science. 2002;298:1797–1800. doi: 10.1126/science.1076064. [DOI] [PubMed] [Google Scholar]
- 38.Yang OO, Sarkis PT, Trocha A, Kalams SA, Johnson RP, Walker BD. Impacts of avidity and specificity on the antiviral efficiency of HIV-1-specific CTL. J Immunol. 2003;171:3718–3724. doi: 10.4049/jimmunol.171.7.3718. [DOI] [PubMed] [Google Scholar]
- 39.Ueno T, Tomiyama H, Fujiwara M, Oka S, Takiguchi M. Functionally impaired HIV-specific CD8 T cells show high affinity TCR-ligand interactions. J Immunol. 2004;173:5451–5457. doi: 10.4049/jimmunol.173.9.5451. [DOI] [PubMed] [Google Scholar]
- 40.Cawthon AG, Lu H, Alexander-Miller MA. Peptide requirement for CTL activation reflects the sensitivity to CD3 engagement: correlation with CD8αβ versus CD8αα expression. J Immunol. 2001;167:2577–2584. doi: 10.4049/jimmunol.167.5.2577. [DOI] [PubMed] [Google Scholar]
- 41.Schamel WW, Arechaga I, Risueno RM, et al. Coexistence of multivalent and monovalent TCRs explains high sensitivity and wide range of response. J Exp Med. 2005;202:493–503. doi: 10.1084/jem.20042155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park JH, Adoro S, Lucas PJ, et al. ‘Coreceptor tuning’: cytokine signals transcriptionally tailor CD8 coreceptor expression to the self-specificity of the TCR. Nat Immunol. 2007;8:1049–1059. doi: 10.1038/ni1512. [DOI] [PubMed] [Google Scholar]
- 43.Li QJ, Chau J, Ebert PJ, et al. miR-181a is an intrinsic modulator of T cell sensitivity and selection. Cell. 2007;129:147–161. doi: 10.1016/j.cell.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 44.De Mattia F, Chomez S, Van Laethem F, et al. Antigen-experienced T cells undergo a transient phase of unresponsiveness following optimal stimulation. J Immunol. 1999;163:5929–5936. [PubMed] [Google Scholar]
- 45.Demotte N, Colau D, Ottaviani S, et al. A reversible functional defect of CD8+ T lymphocytes involving loss of tetramer labeling. Eur J Immunol. 2002;32:1688–1697. doi: 10.1002/1521-4141(200206)32:6<1688::AID-IMMU1688>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 46.Drake DR, Ream RM, Lawrence CW, Braciale TJ. Transient loss of MHC class I tetramer binding after CD8+ T cell activation reflects altered T cell effector function. J Immunol. 2005;175:1507–1515. doi: 10.4049/jimmunol.175.3.1507. [DOI] [PubMed] [Google Scholar]
- 47.Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 48.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Demotte N, Stroobant V, Courtoy PJ, et al. Restoring the association of the T cell receptor with CD8 reverses anergy in human tumor-infiltrating lymphocytes. Immunity. 2008;28:414–424. doi: 10.1016/j.immuni.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 50.Niedergang F, Dautry-Varsat A, Alcover A. Peptide antigen or superantigen-induced down-regulation of TCRs involves both stimulated and unstimulated receptors. J Immunol. 1997;159:1703–1710. [PubMed] [Google Scholar]
- 51.San Jose E, Borroto A, Niedergang F, Alcover A, Alarcon B. Triggering the TCR complex causes the down-regulation of nonengaged receptors by a signal transduction-dependent mechanism. Immunity. 2000;12:161–170. doi: 10.1016/s1074-7613(00)80169-7. [DOI] [PubMed] [Google Scholar]
- 52.Oxenius A, Sewell AK, Dawson SJ, et al. Functional discrepancies in HIV-specific CD8+ T-lymphocyte populations are related to plasma virus load. J Clin Immunol. 2002;22:363–374. doi: 10.1023/a:1020656300027. [DOI] [PubMed] [Google Scholar]
- 53.Malherbe L, Mark L, Fazilleau N, McHeyzer-Williams LJ, McHeyzer-Williams MG. Vaccine adjuvants alter TCR-based selection thresholds. Immunity. 2008;28:698–709. doi: 10.1016/j.immuni.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O'Connor DH, Allen TM, Vogel TU, et al. Acute phase cytotoxic T lymphocyte escape is a hallmark of simian immunodeficiency virus infection. Nat Med. 2002;8:493–499. doi: 10.1038/nm0502-493. [DOI] [PubMed] [Google Scholar]
- 55.Betts MR, Exley B, Price DA, et al. Characterization of functional and phenotypic changes in anti-Gag vaccine-induced T cell responses and their role in protection after HIV-1 infection. Proc Natl Acad Sci U S A. 2005;102:4512–4517. doi: 10.1073/pnas.0408773102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Davenport MP, Price DA, McMichael AJ. The T cell repertoire in infection and vaccination: implications for control of persistent viruses. Curr Opin Immunol. 2007;19:294–300. doi: 10.1016/j.coi.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 57.Lichterfeld M, Mou D, Cung TD, et al. Telomerase activity of HIV-1-specific CD8+ T cells: constitutive up-regulation in controllers and selective increase by blockade of PD ligand 1 in progressors. Blood. 2008;112:3679–3687. doi: 10.1182/blood-2008-01-135442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lichterfeld M, Yu XG, Mui SK, et al. Selective depletion of high-avidity human immunodeficiency virus type 1 (HIV-1)-specific CD8+ T cells after early HIV-1 infection. J Virol. 2007;81:4199–4214. doi: 10.1128/JVI.01388-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.