Abstract
Homeobox (Hox) transcription factors confer anterior–posterior (AP) axial coordinates to vertebrate embryos. Hox genes are found in clusters that also contain genes for microRNAs (miRNAs). Our analysis of predicted miRNA targets indicates that Hox cluster-embedded miRNAs preferentially target Hox mRNAs. Moreover, the presumed Hox target genes are predominantly situated on the 3′ side of each Hox miRNA locus. These results suggest that Hox miRNAs help repress more anterior programmes, thereby reinforcing posterior prevalence, which is the hierarchical dominance of posterior over anterior Hox gene function that is observed in bilaterians. In this way, miRNA-mediated regulation seems to recapitulate interactions at other levels of gene expression, some more ancestral, within a network under stabilizing selection.
As determinants of regional anatomic identity across Bilateria, homeobox (Hox) gene clusters are under strong evolutionary constraint, with small changes giving rise to profound alterations in body plans1,2. Conservation owing to purifying selection is exemplified by the homeobox motif of the Hox transcription factors, in which 99.7% of non-synonymous mutations are eliminated, in contrast with an average removal estimate of 85% among a random set of human and mouse gene pairs3. Relative to ancestral bilaterian Hox genes, the vertebrate set is also remarkably constrained with respect to cluster organization, gene order, orientation and compactness1. On the other hand, large departures from highly ordered vertebrate-like clusters occur in genomes of clades with widely divergent body plans — as is the case in echinoderms, in which the cluster is scrambled4, or in urochordates, in which it has disintegrated and central genes have been lost5,6. Natural variation in Hox regulatory elements has been used to explain the morphological differences between body segments of related species within arthropods. Among vertebrates, this type of variation (such as gain of a global enhancer to drive expression along a secondary axis) might have enabled the development of structural novelties, including the tetrapod limb7,8.
An iterative code along the AP axis
The four Hox clusters of mammals map to distinct chromosomes, range in size between 100 and 200 kb, and each contain 9 to 11 protein-coding genes dispersed among 13 paralogous groups, all transcribed from one DNA strand. In Hox gene nomenclature, paralogue numbering descends in the direction of transcription, with Hox1 paralogues mapping to the 3′ edge of each cluster (FIG. 1a). The Hox paralogues were derived by tandem duplication of an initial template most closely related to 3′-end coding sequences. A paired set of protoHox1/2 and protoHox3 genes that were present in an early metazoan are thought to have duplicated to generate Hox and paraHox predecessors, each of which subsequently experienced further replication9. The ancestral chordate cluster presumably most resembled the unique ~450 kb cluster of the free-living marine urochordate amphioxus10 minus its most posterior gene Hox14, which is likely to be the product of a urochordate lineage-specific duplication event11. From this ancestral prototype, multiple clusters arose in vertebrates through a series of larger-scale duplications involving the surrounding genome.
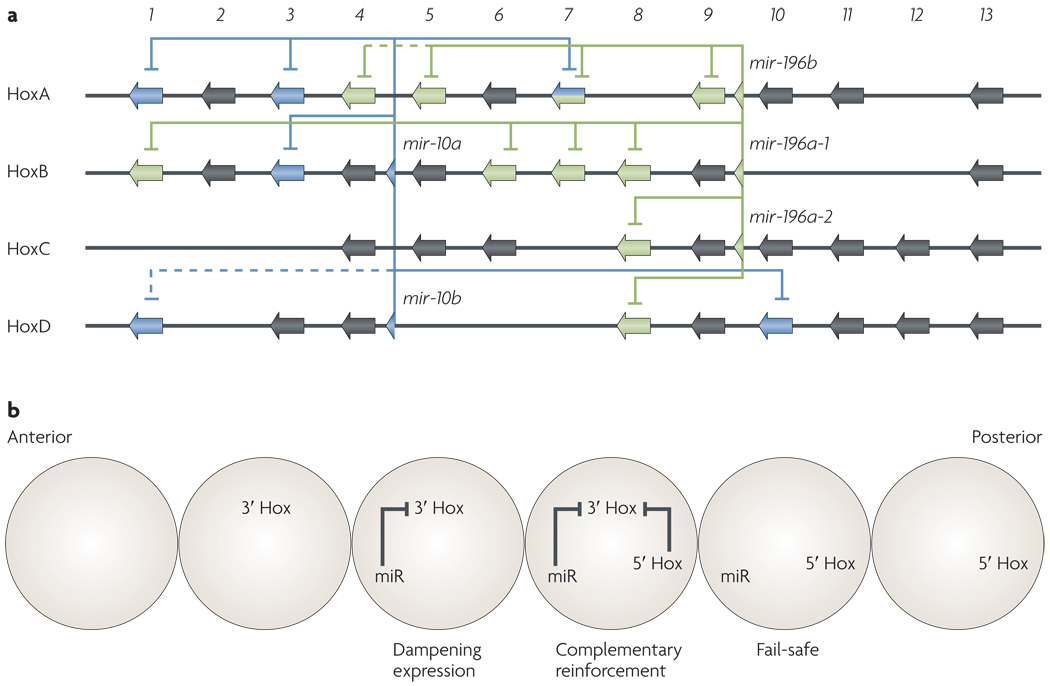
Figure 1. Predicted repression of Hox genes by Hox microRnAs.
a | The mouse Hox clusters. Blue and green lines indicate repression by the microRNAs (miRNAs) miR-10 and miR-196, respectively. All targets are conserved in humans, except Hoxd1 and Hoxa4 (dashed line). b | A model for the role of Hox miRNAs in modulating the Hox code. Hox miRNAs are placed within a scheme of embryonic development along a segmented anterior–posterior axis. The most anterior segment displays the default developmental state that is specified in the absence of Hox expression. This state is modified towards more posterior fates by miRNAs that dampen the activity of Hox genes that are situated 3′ of the miRNA locus. The second most anterior segment is the anterior boundary of expression for the Hox genes that are situated 3′ of the miRNA locus, which specify earlier and more anterior fates. Hox miRNAs dampen the posterior expression of their 3′ Hox targets. In the more posterior domains, they act in parallel with 5′ Hox genes to reinforce the hierarchy of 5′ Hox function. Within the most posterior domains of Hox miRNA expression, the miRNAs provide fail-safe repression of aberrant or low-level and experimentally undetectable transcription. Alternatively, they might linger as stable species following the clearance of 3′ Hox targets. The targets are generally expressed prior to the miRNAs, and thus the miRNA-mediated modulation of expression domains also has a temporal dimension (not shown).
Hox genes are expressed in staggered and overlapping domains in all embryonic germ layers along the anterior–posterior (AP) axis, also called the rostro-caudal axis, with sharp anterior and diffuse posterior boundaries2. The anterior limit of expression is the site with the highest transcript levels and where loss-of-function phenotypes are most overt, hence it is defined as the functional domain2,12. Gene order within a cluster correlates with the coordinates of the functional domains along the AP axis, and with the relative onset of gene expression during vertebrate gastrulation. These conserved properties, whereby genes at the 3′ end of the Hox cluster are expressed earlier and more anteriorly and the more 5′ genes are expressed later and further towards the tail, are referred to as spatial and temporal colinearity2,13,14. The nested expression of Hox genes leads to a modular code that specifies spatial coordinates along the AP axis and determines regional anatomic identities15,16.
An elaborate set of global and local transcriptional regulatory mechanisms seems to be responsible for the spatial and temporal colinearity of Hox expression17,18. Post-transcriptional regulation is also observed19,20, but has received less experimental attention. This includes regulation by microRNAs (miRNAs), which are ~22-nucleotide (nt) non-coding RNAs that guide the post-transcriptional repression of protein-coding genes by pairing to the messages of these genes, usually within their 3′UTRs21. Most important for target recognition is pairing to the 5′ region of the miRNA, particularly to miRNA nucleotides 2–7, which is known as the seed. Conserved targets of a miRNA can be predicted above a background of false-positive predictions by searching for conserved 7-nt matches to the seed region22–25. This approach indicates that over a third of mammalian protein-coding genes have been under selective pressure to maintain pairing to miRNAs23. These targeted messages are repressed through either translational repression, mRNA destabilization or both. Here we consider known and predicted repression of Hox mRNAs by miRNAs and how this miRNA-mediated repression relates to the overall regulation and function of Hox clusters during embryonic development and evolution.
Genomic linkage of Hox miRNAs and their targets
At least 30 of the 39 mammalian Hox 3′UTRs have one or more conserved matches to vertebrate miRNAs, several of which have been supported experimentally. These include Hoxa7, Hoxb8, Hoxc8 and Hoxd8, which have experimental support as conserved targets of miR-196 (ReFS 26,27). In the chicken, Hoxb8 has a seed-matched site but, in most vertebrates, Hoxb8 is atypical as a miRNA target in that it lacks perfect seed-pairing and instead has extensive complementarity to miR-196, making it a substrate for miRNA-directed cleavage. Expression of mir-196 is lower in the forelimb than in the hindlimb, where the miRNA acts as an inhibitor of Hoxb8 and prevents its induction by ectopic retinoic acid28. Hoxc8, Hoxd8 and Hoxa7 have canonical seed matches — the type of sites that mediate translational repression and mRNA destabilization without miRNA-directed cleavage. The 3′UTR fragments containing these sites mediate the repression of reporters in cultured human cervical cancer (Hela) cells26. Similar experiments support the targeting of Hoxd10 mRNA by miR-10 in cultured human non-metastatic breast cancer (sum149) cells29. Altering levels of miR-10 in zebrafish embryos leads to misexpression of Hoxb1a and Hoxb3a, both of which have seed matches within their 3′UTRs30. Moreover, blocking functional miR-10 and miR-196 in chick embryos leads to extensive skeletal defects, including homeotic transformations, consistent with regulation of Hox genes by these two miRNAs (E. McGlinn, S.Y., D.P.B. and C.J.T., unpublished observations). Likewise, experiments in flies support the predicted repression of Drosophila Hox genes by miR-10 and miR-iab miRNAs31–34,48, including a loss-of-function study indicating that the miR-iab miRNAs target Ultrabithorax (Ubx) for repression in more posterior segements31.
Preferential targeting of Hox mRNAs
Both miR-10 and miR-196, the two vertebrate miRNAs experimentally implicated in targeting Hox mRNAs, are expressed from gene families that are themselves encoded by sequences within the Hox clusters26,47 (FIG. 1a), leading to the question of whether these two Hox miRNAs might preferentially target Hox mRNAs. Examination of conserved miRNA sites that matched the 73 highly conserved vertebrate miRNA families23,35 revealed that 15% of sites falling within the Hox 3′UTRs matched the two Hox miRNAs. Moreover, the two Hox miRNAs were ranked first and third highest among the 73 miRNA families when the fraction of conserved sites falling in Hox 3′UTRs was considered (TABLE 1). Thus, although the Hox cluster-encoded miRNAs have many conserved targets other than Hox genes, and although the Hox mRNAs contain target sites to other miRNAs, the two Hox miRNAs seem to preferentially regulate Hox genes.
Table 1.
Targeting of Hox mRNAs by conserved vertebrate microRNAs
| conserved microRnA | conserved 3′UTR sites |
conserved sites in Hox 3′UTRs (number of Hox 3′UTRs targeted) |
Fraction of sites in Hox 3′UTRs |
|---|---|---|---|
| miR-196 | 183 | 14 (6) | 7.7% |
| miR-99/100 | 37 | 1(1) | 2.7% |
| miR-10 | 181 | 4(4) | 2.2% |
| miR-193 | 138 | 2(2) | 1.4% |
| miR-23 | 719 | 9(8) | 1.3% |
| miR-34b | 259 | 3(3) | 1.2% |
| miR-33 | 261 | 3(3) | 1.1% |
| miR-192/215 | 97 | 1(1) | 1.0% |
For the 73 highly conserved microRNA families considered at www.targetscan.org (release 4.0), the eight with the highest percentage of conserved sites in Hox 3′ UTRs are listed.
This preferential targeting is all the more striking because miRNA target predictions are far from perfect and undoubtedly yield many false positives and false negatives. Our analysis, which primarily focused on 7–8-mer seed-matched sites falling in 3′UTRs, would have missed sites with non-canonical pairing or sites located elsewhere in the message. Efficacy and preferential conservation have been reported for sites falling in ORFs, but the efficacy is about one tenth of that observed in 3′UTRs, and the conservation that is due to coding leads to lowered prediction specificity, justifying the exclusion of these sites from consideration23,35. Sites without perfect pairing to the seed region can still function if they have extensive pairing to the 3′ region of the miRNA, which can compensate for a mismatch or bulge in the seed pairing. Indeed, the miR-196 site in the Hoxb8 3′UTR is a well known example of such a 3′-compensatory site, and similar miR-196 sites in Hoxc8 and Hoxd8 have been proposed26. However, experiments examining the effects of site disruption on miRNA-meditated repression have yet to uncover any additional 3′-compensatory sites in vertebrate mRNAs, even though such experiments have supported the function of countless seed-matched sites. A systematic analysis of site conservation indicates that additional 3′-compensatory sites are likely to function in vertebrates, but that they are rare and constitute less than two percent of all selectively maintained sites for vertebrate miRNAs (R. Friedman, K.K. Farh, C.B. Burge and D.P.B., unpublished observations). Of the 30 most probable 3′-compensatory sites, ranked by quality of 3′ pairing and extent of conservation, the only two that fall in Hox 3′UTRs are the miR-196 sites in Hoxb8 (ranked 1) and Hoxc8 (ranked in the top 20). These results are consistent with experiments showing that sites predicted by algorithms that allow for seed mismatches have poor efficacy35. On the basis of these considerations, our analysis included the three miR-196 3′-compensatory sites26 but did not consider other seed-mismatched sites. Pairing to the 3′ region of the miRNA can also supplement seed-matched sites to increase site efficacy but, because consequential 3′-supplementary pairing seems to be rare and imparts only a modest increase to site efficacy35, it was not considered.
Conservation of target sites
Although conservation provides useful information regarding the functional relevance of a regulatory site, not all miRNA-responsive mRNAs have conserved sites. Indeed, non-conserved sites often mediate miRNA-dependent repression in reporter assays, and most messages that are repressed when a miRNA is introduced, as well as most of those that are derepressed when a miRNA is either inhibited or eliminated, have sites that do not meet the conservation criteria typically used for target prediction35–39. If the repression these miRNAs mediate were inconsequential, sites would not be expected to be under purifying selection, and this might be the case for some non-conserved targeting interactions. In some cases, however, the sites might be providing recently evolved but important lineage-specific functions, or they might be a form of ancestral regulation that is under purifying selection but nonetheless lost (or that is not able to be aligned) in one of the reference species. To find potential non-conserved targeting of Hox mRNAs by Hox miRNAs, we independently surveyed the Hox 3′UTRs of five representative vertebrate genomes — human, mouse, chick, zebrafish and pufferfish — for canonical 7–8-mer seed-matched sites (see Box 1 for the methods used). More than a third of the 3′UTRs had at least one and up to six canonical sites for miR-196 or miR-10 (FIG. 1a; TABLE 2). Although conservation, local 3′UTR alignment or synteny were not required for their identification, all but three of the sites were present in both the human and mouse genome. In these three cases the history of the site was assessed by examination of multiple mammalian genome alignments. Hoxa4 and Hoxb13 seem to have gained unique 7-mer seed matches to miR-196 in the rodent and primate lineages, respectively. Hoxd1 has retained an 8-mer site that matches the miR-10 seed region in the mouse, but has lost it in the rat and in the primate lineage. The opossum, which is basal to eutherian mammals, does not have the site, but six other mammals that are basal to both primates and rodents do have a 7-mer site that matches the miR-10 seed region. In a number of cases, including murine Hoxa4 and Hoxa7, which show evidence of alternative polyadenylation, miRNA sites are present in the longer isoform and might contribute to isoform-specific regulation. We conclude that the majority of the targeting by Hox miRNAs existed prior to the emergence of mammals, and has been under high selective pressure to be retained.
Box 1 | Prediction and genomic arrangement of the Hox targets of miR-196 and miR-10
Using the miRNA targeting insights of Lewis et al.23, we predicted targets of miR-10 and miR-196 in the human, mouse, chick, zebrafish and Takifugu Hox clusters without imposing conservation requirements. A target contained at least one canonical 7–8-mer match to miR-196 (8-mer match, ACTACCTA; 7-mer matches, ACTACCT and CTACCTA) or to miR-10 (8-mer match, ACAGGGA; 7-mer matches, ACAGGG and CAGGGA) within its 3′UTR. Also included were the mir-196 3′-compensatory sites identified in the Hoxb8, Hoxc8 and Hoxd8 UTRs26. In mammals, the 3′UTRs were defined by the longest RefSeq annotation, except for the 3′UTR of Hoxb1, which was extended beyond existing annotation owing to the presence of overlapping ESTs and conservation above surrounding intergenic sequence. In the chicken, UTRs were defined using orthology to mammals, as well as EST sequences, and in teleosts UTRs were defined as the 2 kb sequence 3′ to the stop codon.
Table 2.
Genomic distribution of Hox genes targeted by miR-196 and miR-10
| species | Fraction of Hox genes predicted as targets* |
Fraction of 3′ genes targeted‡ |
Fraction of 5′ genes targeted‡ |
P-value§ |
|---|---|---|---|---|
| miR-196 targets in the Hox cluster | ||||
| Pufferfish (Takifugu) | 27% | 12/32 | 1/16 | 0.020 |
| Zebrafish | 31% | 14/32 | 1/17 | 0.005 |
| Chicken | 25% | 8/22 | 0/10 | 0.030 |
| Human | 28% | 10/27 | 1/12 | 0.068 |
| Mouse | 26% | 10/27 | 0/12 | 0.013 |
| miR-10 targets in the Hox cluster | ||||
| Pufferfish (Takifugu) | 29% | 9/15 | 5/33 | 0.003 |
| Zebrafish | 16% | 5/14 | 3/35 | 0.033 |
| Chicken | 9% | 3/10 | 0/22 | 0.024 |
| Human | 13% | 3/12 | 2/27 | 0.159 |
| Mouse | 15% | 4/12 | 2/27 | 0.060 |
Predicted targets contain within their 3′UTR one or more canonical 7–8-mer seed-region match.
Hox genes targeted by the microRNAs (miRNAs) miR-196 and miR-10 were categorized according to their genomic location in the Hox clusters relative to the miRNA locus. In the case of mir-196, Hox1–9 were grouped together as downstream, and Hox10–13 were considered upstream, regardless of which cluster they belonged to. Similarly, Hox1–4 were downstream of mir-10, whereas Hox5–13 were upstream.
P-values for the probability of the observed genomic distributions were obtained by Fisher’s exact test (one-sided).
Expression and function throughout Bilateria
when evaluating the functional significance of the preferential targeting of Hox mRNAs by the Hox miRNAs, it is useful to consider the expression and evolution of these miRNAs. The mir-10 and mir-196 genes are transcribed in the same orientation as the protein-coding genes in each cluster, and are expressed in patterns that approximate the characteristic expression of Hox genes, including anterior limits of expression correlated with their genomic positions within the cluster26,27,30,40,41. Both miRNA families have highest expression in the neural tube and lower expression levels in the trunk mesoderm, with ill defined anterior limits and broad posterior expression through the tail. In mouse embryos, the resolution of miRNA expression is too low to determine exact boundaries. However, the observed expression domains of miRNAs are in agreement with the patterns that are expected on the basis of their locations within the Hox clusters. For example, the predicted anterior limit of mir-196 expression would be slightly posterior to that of Hoxb9 (ref. 27), which is adjacent to mir-196 within the Hox cluster. Hoxb9 expression has an anterior limit in the paraxial mesoderm up to prevertebra 3 in embryonic day 9.5 (E9.5) mice, which is shifted caudally to the thoracic prevertebra 8 by e12.5 (ReF. 15). The anterior limit of miR-10 is caudal to or equivalent to that of transcripts from the adjacent gene Hoxb4 (ReF. 27), which has an expression boundary in the paraxial mesoderm at prevertebra 2 in E10.5 mice20.
Expressed from loci between Hox4 and Hox5 paralogues (FIG. 1a) or within the intron of Hoxd4 (for example, the mouse miR-10b), miR-10 is among the set of core bilaterian miRNAs with orthologues in insects42, nematodes43 and planaria44. RNA-blot studies hint at possible presence of a homologue in the startlet sea anemone, Nematostella vectensis, suggesting that miR-10 sequence might have pre-dated the cnidarian–bilaterian split44,45. The orthologue in nematodes, miR-57, shares the seed region of the chordate miR-10, and thus is expected to recognize essentially the same sites. However, mir-57 is not located among Hox genes, nor does it have canonical seed-pairing to Caenorhabditis elegans Hox mRNAs. The Drosophila mir-10 gene is unusual because it produces mature, apparently functional miRNAs from both arms of the precursor hairpin, which expands the potential for targeting from this locus33,46. Moreover, the fly mir-10 homologue is shifted by one nucleotide at its 5′ end, which is expected to have a profound effect on target recognition as it changes one of two 7-mer sites recognized by this miRNA46. Nonetheless, the fly mir-10 gene resembles its vertebrate counterpart in two aspects: it is found at an orthologous locus within the fly Antennapedia complex47, and both miR-10 miRNAs of flies have the conserved potential to target Hox mRNAs33,46.
The other Hox miRNA gene, mir-196, which is situated between Hox9 and Hox10 paralogues in all but the HoxD cluster (FIG. 1a), seems to have emerged more recently. Absence of mir-196 from non-vertebrate chordates and its presence in the jawless lamprey and in multiple vertebrate clusters indicate an origin in the common ancestor of vertebrates, pre-dating the initial cluster duplication event. Although mir-196 homologues seem to be absent outside of vertebrates, a functional analogue, mir-iab-4, resides at the orthologous location in the fly Bithorax complex26,32. Through transcription from either strand of DNA, the fly locus produces two alternative miRNAs: miR-iab-4s and miR-iab-4as (ReF. 33). Neither of these fly miRNAs has detectable homology to miR-196, but both seem to target nearby Hox mRNAs26,31–34,48.
Asymmetric distribution of target mRNA loci
In our survey of conserved and non-conserved sites in the five representative vertebrate genomes, we found that the predicted target genes were unevenly distributed throughout the clusters. For the purpose of this analysis, the miRNA loci were treated as genomic boundaries and Hox genes were divided into two groups, depending upon which side of this boundary they fell (FIG. 1a). Thus, for the mir-196 locus, Hox1–9 paralogues were interchangeably referred to as 3′ or anterior, and Hox10–13 paralogues as 5′ or posterior. Regardless of conservation of individual sites, a significant majority of target 3′UTRs belonged to genes in paralogous groups located 3′ to the mir-196 loci, thus with more anterior expression boundaries. For example, humans possess ten 3′ targets of miR-196, but only a single target was located in the 5′ side. Within the 3′ set, more than half of the predicted targets were in the immediate vicinity of the miRNA locus, that is, within the central paralogues of Hox5–9. Moreover, the fraction of 3′ Hox genes predicted to be targets of miR-196 was higher than 5′ Hox genes, with a vertebrate average of 38% of 3′ genes and 4% of 5′ genes (TABLE 2). Similarly, in fruitflies the miRNAs of the iab-4 locus have the potential to target 3′UTRs of the downstream and more anterior genes abdominal A (abd-A), Ultrabithorax (Ubx), Antennapedia (Antp) and Sex combs reduced (Scr)32,48, although in flies the number of Hox genes is too low for testing statistical significance. These tendencies in vertebrates and flies implied a recurring logic of Hox gene targeting by miR-iab-4 or miR-196, in which these miRNAs act to repress genes that are expressed in more anterior domains.
In vertebrates, a significant non-random distribution of predicted target genes was also found for the more ancient Hox miRNA family, miR-10, for which Hox1–4 paralogues were defined as 3′ to or anterior to the mir-10 locus, and Hox5–13 paralogues as 5′ or posterior to the mir-10 locus. The genomic position of mir-10 in the clusters (between Hox4 and Hox5) dictated that there were fewer 3′ Hox genes available for targeting. In amphioxus, seed matches to miR-10 were found in Hox1, Hox2 and Hox5 3′UTRs. In vertebrates, although miR-10 seems to target fewer Hox genes than does miR-196, the genomic arrangement of the target loci exhibited the same one-sided skew. As with miR-196, a higher fraction of 3′ genes compared with 5′ genes were predicted as targets of miR-10 (a vertebrate average of 37% of 3′ genes and 8% of 5′ genes; TABLE 2). This trend was not observed, however, in flies, in which miR-10 seems to also target 5′ and more posterior genes such as Abdominal B (Abd-B) and Scr46. This suggests that the altered and expanded targeting in flies, which is due to the shifted miRNA end and enlistment of the miRNA from the other arm of the hairpin, led to a divergence in targeting for miR-10 that is in sharp contrast to the pattern of targeting observed for vertebrate miR-10, vertebrate miR-196 and fly miR-iab-4.
Posterior prevalence
within domains of coexpression, the more posterior vertebrate Hox genes render anterior Hox genes irrelevant in the regions in which their expression patterns overlap, a phenomenon known as posterior prevalence12,16,49. In other words, posterior and 5′ genes are epistatic to anterior and 3′ genes50. The phenomenon was first described as phenotypic suppression on the basis of morphological observations made in fly larvae with mutations at the extra sexcombs (esc) locus12,50. Such mutations inactivate repressors of Polycomb-group proteins and cause general derepression of Hox expression12,50. The resulting segmental pattern in esc mutants reflects the activity of the most posterior-acting Hox gene, Abd-B, such that the head, thoracic and abdominal segments morph into a phenocopy of A8, the most posterior abdominal segment. Mutant esc larvae that also lack Abd-B develop with reiteration of A4 segments, typically specified by abd-A, the second most posterior gene. mutant esc larvae with deletion of all abdominal Hox genes — Ubx, abd–A, and Abd-B — develop with reiterations of thoracic segments normally specified by Scr and Antp. When Scr and Antp are eliminated in addition to the three abdominal genes, esc larvae have cephalic segments throughout. Thus a hierarchy of homeotic gene function has been defined50. Further experiments showed that transcriptional cross-regulation is not the principal driving force of phenotypic suppression. Experimentally derived ubiquitous expression of Hox genes under promoters that are known to be transcriptionally irrepressible leads to transformations only in regions anterior to the functional domain of the gene. For example, the thoracic Antp, when ubiquitously expressed, suppresses Hox genes of the head, resulting in posterior transformation of head segments towards a thoracic identity while not affecting the abdomen — here, the effect of Antp is phenotypically suppressed by Bithorax-complex genes such as Ubx12,51.
Analogous observations have been made in transgenic vertebrate embryos for a number of Hox genes. For instance, the introduction of a Hoxd4 transgene under transcriptional control of the Hoxa1 promoter leads to a rostral shift in the anterior boundary of Hoxd4 expression52 (Hoxd4 is not a predicted target of miR-10 or miR-196). The transgenic embryos exhibit posterior transformations of the occipital bones at the base of the skull towards structures that show characteristics of the segmented vertebral column, in particular of the first two cervical vertebrae. However, the phenotypes are limited to the anterior domain of ectopic expression, even though levels are over-expressed or ectopically expressed elsewhere in the embryo52. The posterior prevalence model explains the general trends of homeotic phenotypes, with loss of function often leading to anterior transformation at rostral boundaries of expression; in the absence of a Hox gene, more anterior acting genes that are typically suppressed are now permitted to function. The model also accounts for the changes seen following gain of function or ectopic expression of a Hox gene, which generally causes posterior transformations in regions anterior to the endogenous domain, where the ectopic expression can suppress the effect of resident Hox genes.
These tendencies generally hold true in flies, but not always in vertebrates, in which deviations from the rule occur, as do defects other than homeotic transformations (for an example see ReF. 53). Fly Hox genes seem to be under the control of more independent regulatory elements and have distinct expression domains, whereas vertebrate Hox genes have more coordinated regulation, redundancy among paralogues and higher overlap in expression. In general, vertebrate systems seem to be more sensitive to quantitative differences in Hox gene expression; in these systems ratios of Hox genes that constitute the Hox code determine fate specification. Nevertheless, as a general rule 5′-located Hox genes modify more anterior programmes.
The hypothetical ancestral condition
The functional hierarchy that is understood as posterior prevalence seems to be a retained property of the ancestral Hox cluster, observed even in the absence of absolute spatial and temporal colinearities, or of segmentation, as is the case in the highly derived nematode Hox cluster54. In its essence, Hox master regulators have an ascending hierarchical ability, which is colinear with gene order, to modify programmes specified by one another. This hierarchy might have been inherent in early neofunctionalization events that followed initial tandem gene duplications, which pre-dated the cnidarian–bilaterian split and the formation of a larger cluster. A hypothetical eubilaterian embryo might have expressed at least a pair of linked Hox genes to polarize its longitudinal axis55 and govern differentiation events during development by reiteration of genetic programmes. such expression would lead to the specification of anterior cellular fates earlier than posterior ones. This trait has persisted among basal insects56 and chordates, in which cells within segments are specified sequentially, with anterior-to-posterior progression. The most anterior hindbrain rhombomere in the zebrafish embryo is specified earliest, a process that does not involve Hox expression, and differentiates to be the default state that posterior rhombomeres revert to following elimination of Hox cofactor function57. During ontogenesis, such a default state might be altered in developmental modules that are formed later or more posteriorly owing to hierarchical molecular activity, thereby creating a range of possible morphological outputs. The ability to overrule and elaborate on a pre-existing programme as a mechanism to evolve a novel unit of development might have been a driving force to increase the copy number of Hox genes with retained hierarchical activity. Thus, posterior prevalence might be invoked as a fundamental property of ancestral Hox genes that helps account for their successful propagation in animal genomes.
Derived reinforcement of established hierarchies
The molecular mechanisms that establish hierarchical activity, although ill explained, are attributed foremost to properties of Hox transcription factors12 and their downstream targets. In at least some cases, posterior-acting Hox proteins are more efficient than anterior ones at exerting downstream function, for instance, by competition for shared promoter binding sites or for cofactors58. In addition, posterior Hox genes might exert dominance by directing expression of different target genes so that a posterior Hox network is dominant in function over an anterior one. within a functional domain, the dominance of a gene is facilitated by higher levels of absolute transcription. Finally, there is evidence for hierarchy by transcriptional cross-regulation, with posterior genes directing the repression of anterior ones12.
The conserved trend in the genomic distribution of the miRNA target genes within the Hox clusters, when considered together with transcriptional expression patterns that are due to spatial and temporal colinearity, indicates that the Hox miRNAs dampen protein output in more posterior regions of vertebrate Hox gene expression domains. Furthermore, Hox miRNAs function as delayed negative post-transcriptional regulators of their 3′ Hox targets. Thus, as suggested recently for individual examples30,48, the Hox miRNAs seem to have a striking general propensity to promote posterior prevalence (FIG. 1b). Regulation of Hox genes by miR-196 is a vertebrate innovation and thus a regulatory addition that appeared after the emergence of functional hierarchy. In vertebrates both Hox miRNAs seem to contribute to and reinforce the functional hierarchy among Hox genes, thus supporting the hypothesis that a multitude of molecular approaches are continuously selected for to retain this ancestral trait.
The retention of mir-10 and mir-196 within Hox clusters might be explained by their proximity to other coding genes and their regulatory elements, leading to a coordinated expression pattern of the miRNAs and their targets. The Hox clusters might possess properties that enable an active mechanism to avoid genomic rearrangements or insertions and deletions, thus maintaining synteny and overall compactness. Moreover, the presence of miRNAs at regular intervals within the clusters and among their targets results in a shared local DNA environment. This environment can produce coordinated reactions with equal response to non-specific perturbations affecting global transcription, thereby preserving constant transcript ratios. This shared response between the miRNAs and their targets might be among the driving forces to retain the miRNAs within the clusters. The conserved genomic landscape with its non-random distribution of seed matches in transcribed sequences might be indicative of a gradient of constraints on 3′UTR sequences, with faster mutation rates of 5′ genes coinciding with co-option into novel expression in derived cells. Hence, the relative depletion of seed matches among 5′ transcripts, and consequent freedom from the constraints of Hox miRNA repression, might have predisposed this group towards acquisition of secondary functions, consistent with the role of Hoxd9–13 expression in the establishment of the proximal–distal axis of the tetrapod limb.
Not all of the more anterior Hox genes have sites matching the Hox miRNAs (FIG. 1a; TABLE 2), further implying that other, possibly more ancestral, regulatory mechanisms predominate in establishing and maintaining posterior prevalence. Moreover, sites matching the Hox miRNAs, although more abundant than for most other specific miRNAs, constitute only 15% of the conserved 7–8-mer sites in Hox 3′UTRs. This ratio suggests that when considering Hox gene regulation by all the miRNAs, reinforcing posterior prevalence is less widespread than the broader, more generic role of miRNAs, which is to adjust protein output for many other purposes, thereby sculpting expression patterns with the complexity and precision that would be difficult to create using transcriptional regulation alone59. Indeed, the Hox miRNAs probably participate in these other functions in addition to their role in posterior prevalence, as they seem to have many non-Hox targets.
Conclusions
The ancestral acquisition of hierarchy among Hox genes has allowed for the specification of cellular identities along a regionalized AP axis by reiteration of a genetic programme with unidirectional modifications, and has been reinforced over time by multiple layers of interactions at many levels of gene expression. The contribution of miRNA-mediated repression to the posterior prevalence model adds a layer of regulatory interactions at the post-transcriptional level, which involves the conscription of only a few nucleotides in a region resistant to mutation. The additive layering of complementary regulation might enable a more stable outcome by limiting dependence on any one regulatory mode. Moreover, enlisting miRNA-mediated gene regulation allows for more complex regulatory output, with the consequent potential for the diversification of animal body plans. As more animals with mutations in the Hox miRNAs and their regulatory sites are generated, the functional consequences of these nodes in the Hox regulatory network will be demonstrated.
Acknowledgements
We thank laboratory members and collegues for helpful discussions. Supported by grants from the National Institutes of Health.
Glossary
- Bilateria
Members of the animal clade that have bilateral symmetry — the property of having two similar sides, with definite upper and lower surfaces, and anterior and posterior ends. Bilaterians include chordates, arthropods, nematodes, annelids and molluscs, among other groups.
- Non-synonymous mutation
A change in nucleotide sequence that alters the encoded amino acid.
- Paralogous
The homology between two genomic segments in the same organism that arose from a duplication event.
- Neofunctionalization
The process whereby a pair of duplicated genes becomes permanently preserved as one copy acquires mutations, conferring a new function.
- Rhombomere
Each of seven neuroepithelial segments found in the embryonic hindbrain that adopt distinct molecular and cellular properties, restrictions in cell mixing, and ordered domains of gene expression.
Footnotes
FURTHER INFORMATION
The Bartel laboratory: http://web.wi.mit.edu/bartel/pub
The Tabin laboratory: http://genetics.med.harvard.edu/~tabin
MicroRnA target predictions: www.targetscan.org
References
- 1. Duboule D. The rise and fall of Hox gene clusters. Development. 2007;134:2549–2560. doi: 10.1242/dev.001065. This paper presents a synthesis of the current understanding of the structure, and the developmental and evolutionary significance, of the organization of the Hox clusters.
- 2.Krumlauf R. Hox genes in vertebrate development. Cell. 1994;78:191–201. doi: 10.1016/0092-8674(94)90290-9. [DOI] [PubMed] [Google Scholar]
- 3.Nei M. The new mutation theory of phenotypic evolution. Proc. Natl Acad. Sci. USA. 2007;104:12235–12242. doi: 10.1073/pnas.0703349104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cameron RA, et al. Unusual gene order and organization of the sea urchin hox cluster. J. Exp. Zool. B Mol. Dev. Evol. 2006;306:45–58. doi: 10.1002/jez.b.21070. [DOI] [PubMed] [Google Scholar]
- 5.Spagnuolo A, et al. Unusual number and genomic organization of Hox genes in the tunicate Ciona intestinalis. Gene. 2003;309:71–79. doi: 10.1016/s0378-1119(03)00488-8. [DOI] [PubMed] [Google Scholar]
- 6.Seo HC, et al. Hox cluster disintegration with persistent anteroposterior order of expression in Oikopleura dioica. Nature. 2004;431:67–71. doi: 10.1038/nature02709. [DOI] [PubMed] [Google Scholar]
- 7.Shubin N, Tabin C, Carroll S. Fossils, genes and the evolution of animal limbs. Nature. 1997;388:639–648. doi: 10.1038/41710. [DOI] [PubMed] [Google Scholar]
- 8.Spitz F, et al. Large scale transgenic and cluster deletion analysis of the HoxD complex separate an ancestral regulatory module from evolutionary innovations. Genes Dev. 2001;15:2209–2214. doi: 10.1101/gad.205701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chourrout D, et al. Minimal ProtoHox cluster inferred from bilaterian and cnidarian Hox complements. Nature. 2006;442:684–687. doi: 10.1038/nature04863. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Fernandez J, Holland PW. Archetypal organization of the amphioxus Hox gene cluster. Nature. 1994;370:563–566. doi: 10.1038/370563a0. [DOI] [PubMed] [Google Scholar]
- 11.Powers TP, Amemiya CT. Evidence for a Hox14 paralog group in vertebrates. Curr. Biol. 2004;14:R183–R184. doi: 10.1016/j.cub.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 12.Morata G. Homeotic genes of Drosophila. Curr. Opin. Genet. Dev. 1993;3:606–614. doi: 10.1016/0959-437x(93)90096-8. [DOI] [PubMed] [Google Scholar]
- 13.Duboule D. The vertebrate limb: a model system to study the Hox/HOM gene network during development and evolution. Bioessays. 1992;14:375–384. doi: 10.1002/bies.950140606. [DOI] [PubMed] [Google Scholar]
- 14.Maconochie M, Nonchev S, Morrison A, Krumlauf R. Paralogous Hox genes: function and regulation. Annu. Rev. Genet. 1996;30:529–556. doi: 10.1146/annurev.genet.30.1.529. [DOI] [PubMed] [Google Scholar]
- 15.Chen F, Capecchi MR. Targeted mutations in hoxa-9 and hoxb-9 reveal synergistic interactions. Dev. Biol. 1997;181:186–196. doi: 10.1006/dbio.1996.8440. [DOI] [PubMed] [Google Scholar]
- 16.Krumlauf R. Mouse Hox genetic functions. Curr. Opin. Genet. Dev. 1993;3:621–625. doi: 10.1016/0959-437x(93)90098-a. [DOI] [PubMed] [Google Scholar]
- 17.Heard E, Bickmore W. The ins and outs of gene regulation and chromosome territory organisation. Curr. Opin. Cell Biol. 2007;19:311–316. doi: 10.1016/j.ceb.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 18.Deschamps J. Ancestral and recently recruited global control of the Hox genes in development. Curr. Opin. Genet. Dev. 2007;17:422–427. doi: 10.1016/j.gde.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 19.Nelson CE, et al. Analysis of Hox gene expression in the chick limb bud. Development. 1996;122:1449–1466. doi: 10.1242/dev.122.5.1449. [DOI] [PubMed] [Google Scholar]
- 20.Brend T, Gilthorpe J, Summerbell D, Rigby PW. Multiple levels of transcriptional and post-transcriptional regulation are required to define the domain of Hoxb4 expression. Development. 2003;130:2717–2728. doi: 10.1242/dev.00471. [DOI] [PubMed] [Google Scholar]
- 21.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 22.Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell. 2003;115:787–798. doi: 10.1016/s0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- 23. Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. References 22 and 23 use comparative sequence analyses to develop seed-based target-prediction methods, uncover key features of mammalian miRNA-target recognition and reveal the widespread scope of targeting in vertebrates.
- 24. Brennecke J, Stark A, Russell RB, Cohen SM. Principles of microRNA-target recognition. PLoS Biol. 2005;3:e85. doi: 10.1371/journal.pbio.0030085. This paper uses comparative sequence analyses and reporter assays to reveal widespread targeting and important features of target recognition in flies.
- 25.Krek A, et al. Combinatorial microRNA target predictions. Nature Genet. 2005;37:495–500. doi: 10.1038/ng1536. [DOI] [PubMed] [Google Scholar]
- 26.Yekta S, Shih IH, Bartel DP. MicroRNA-directed cleavage of HOXB8 mRNA. Science. 2004;304:594–596. doi: 10.1126/science.1097434. [DOI] [PubMed] [Google Scholar]
- 27.Mansfield JH, et al. MicroRNA-responsive ‘sensor’ transgenes uncover Hox-like and other developmentally regulated patterns of vertebrate microRNA expression. Nature Genet. 2004;36:1079–1083. doi: 10.1038/ng1421. [DOI] [PubMed] [Google Scholar]
- 28.Hornstein E, et al. The microRNA miR-196 acts upstream of Hoxb8 and Shh in limb development. Nature. 2005;438:671–674. doi: 10.1038/nature04138. [DOI] [PubMed] [Google Scholar]
- 29.Ma L, Teruya-Feldstein J, Weinberg RA. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature. 2007;449:682–688. doi: 10.1038/nature06174. [DOI] [PubMed] [Google Scholar]
- 30.Woltering JM, Durston AJ. miR-10 represses HoxB1a and HoxB3a in zebrafish. PLoS ONE. 2008;3:e1396. doi: 10.1371/journal.pone.0001396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bender W. MicroRNAs in the Drosophila Bithorax complex. Genes Dev. 2008;22:14–19. doi: 10.1101/gad.1614208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ronshaugen M, Biemar F, Piel J, Levine M, Lai EC. The Drosophila microRNA iab-4 causes a dominant homeotic transformation of halteres to wings. Genes Dev. 2005;19:2947–2952. doi: 10.1101/gad.1372505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruby JG, et al. Evolution, biogenesis, expression, and target predictions of a substantially expanded set of Drosophila microRNAs. Genome Res. 2007;17:1850–1864. doi: 10.1101/gr.6597907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tyler DM, et al. Functionally distinct regulatory RNAs generated by bidirectional transcription and processing of microRNA loci. Genes Dev. 2008;22:26–36. doi: 10.1101/gad.1615208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grimson A, et al. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol. Cell. 2007;27:91–105. doi: 10.1016/j.molcel.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krutzfeldt J, et al. Silencing of microRNAs in vivo with ‘antagomirs’. Nature. 2005;438:685–689. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- 37.Farh KK, et al. The widespread impact of mammalian microRNAs on mRNA repression and evolution. Science. 2005;310:1817–1821. doi: 10.1126/science.1121158. [DOI] [PubMed] [Google Scholar]
- 38.Lim LP, et al. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433:769–773. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- 39.Giraldez AJ, et al. Zebrafish miR-430 promotes deadenylation and clearance of maternal mRNAs. Science. 2006;312:75–79. doi: 10.1126/science.1122689. [DOI] [PubMed] [Google Scholar]
- 40.Wienholds E, et al. MicroRNA expression in zebrafish embryonic development. Science. 2005;309:310–311. doi: 10.1126/science.1114519. [DOI] [PubMed] [Google Scholar]
- 41.Kloosterman WP, Wienholds E, de Bruijn E, Kauppinen S, Plasterk RH. In situ detection of miRNAs in animal embryos using LNA-modified oligonucleotide probes. Nature Methods. 2006;3:27–29. doi: 10.1038/nmeth843. [DOI] [PubMed] [Google Scholar]
- 42.Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T. Identification of novel genes coding for small expressed RNAs. Science. 2001;294:853–858. doi: 10.1126/science.1064921. [DOI] [PubMed] [Google Scholar]
- 43.Lau NC, Lim LP, Weinstein EG, Bartel DP. An abundant class of tiny RNAs with probable regulatory roles in Caenorhabditis elegans. Science. 2001;294:858–862. doi: 10.1126/science.1065062. [DOI] [PubMed] [Google Scholar]
- 44.Palakodeti D, Smielewska M, Graveley BR. MicroRNAs from the planarian Schmidtea mediterranea, a model system for stem cell biology. RNA. 2006;12:1640–1649. doi: 10.1261/rna.117206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sempere LF, Cole CN, McPeek MA, Peterson KJ. The phylogenetic distribution of metazoan microRNAs: insights into evolutionary complexity and constraint. J. Exp. Zool. B Mol. Dev. Evol. 2006;306:575–588. doi: 10.1002/jez.b.21118. [DOI] [PubMed] [Google Scholar]
- 46.Stark A, et al. Systematic discovery and characterization of fly microRNAs using 12 Drosophila genomes. Genome Res. 2007;17:1865–1879. doi: 10.1101/gr.6593807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lagos-Quintana M, Rauhut R, Meyer J, Borkhardt A, Tuschl T. New microRNAs from mouse and human. RNA. 2003;9:175–179. doi: 10.1261/rna.2146903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stark A, et al. A single Hox locus in Drosophila produces functional microRNAs from opposite DNA strands. Genes Dev. 2008;22:8–13. doi: 10.1101/gad.1613108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Duboule D, Morata G. Colinearity and functional hierarchy among genes of the homeotic complexes. Trends Genet. 1994;10:358–364. doi: 10.1016/0168-9525(94)90132-5. This paper develops the concept of posterior prevalence between genes of the Hox clusters.
- 50. Struhl G. Role of the esc+ gene product in ensuring the selective expression of segment-specific homeotic genes in Drosophila. J. Embryol. Exp. Morphol. 1983;76:297–331. This paper is the original source for the concept of hierarchical activity among Hox genes, which was deduced from classical genetics experiments in flies.
- 51.Gibson G, Gehring W. Head and thoracic transformations caused by ectopic expression of Antennapedia during Drosophila development. Development. 1988;102:657–675. [Google Scholar]
- 52.Lufkin T, et al. Homeotic transformation of the occipital bones of the skull by ectopic expression of a homeobox gene. Nature. 1992;359:835–841. doi: 10.1038/359835a0. [DOI] [PubMed] [Google Scholar]
- 53.Pollock RA, Sreenath T, Ngo L, Bieberich CJ. Gain of function mutations for paralogous Hox genes: implications for the evolution of Hox gene function. Proc. Natl Acad. Sci. USA. 1995;92:4492–4496. doi: 10.1073/pnas.92.10.4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Burglin TR, Ruvkun G. The Caenorhabditis elegans homeobox gene cluster. Curr. Opin. Genet. Dev. 1993;3:615–620. doi: 10.1016/0959-437x(93)90097-9. [DOI] [PubMed] [Google Scholar]
- 55.Martindale MQ. The evolution of metazoan axial properties. Nature Rev. Genet. 2005;6:917–927. doi: 10.1038/nrg1725. [DOI] [PubMed] [Google Scholar]
- 56.Davis GK, Patel NH. The origin and evolution of segmentation. Trends Cell Biol. 1999;9:M68–M72. [PubMed] [Google Scholar]
- 57.Waskiewicz AJ, Rikhof HA, Moens CB. Eliminating zebrafish pbx proteins reveals a hindbrain ground state. Dev. Cell. 2002;3:723–733. doi: 10.1016/s1534-5807(02)00319-2. [DOI] [PubMed] [Google Scholar]
- 58.Williams ME, Lehoczky JA, Innis JW. A group 13 homeodomain is neither necessary nor sufficient for posterior prevalence in the mouse limb. Dev. Biol. 2006;297:493–507. doi: 10.1016/j.ydbio.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 59.Bartel DP, Chen CZ. Micromanagers of gene expression: the potentially widespread influence of metazoan microRNAs. Nature Rev. Genet. 2004;5:396–400. doi: 10.1038/nrg1328. [DOI] [PubMed] [Google Scholar]