Abstract
Background
Human hypomorphic NEMO mutations cause diverse clinical immunologic phenotypes, but understanding their scope and mechanistic links immune function and genotype is incomplete.
Objective
We created and analyzed a database of hypomorphic NEMO mutations to determine the spectrum of phenotypes and their associated genotypes and sought to establish a standardized NEMO reconstitution system to obtain mechanistic insights.
Methods
Phenotypes of 72 individuals with NEMO mutations were compiled. NEMO L153R and C417R were investigated further in a reconstitution system. TNF-α or Toll-like receptor 5 signals were evaluated for NF-κB activation, programmed cell death, and A20 gene expression.
Results
32 different mutations were identified; 53% affect the zinc finger domain. 81% were associated with Ectodermal dysplasia, 76% with serious pyogenic infection, 39% with mycobacterial infection, 19% with serious viral infection, 21% with inflammatory diseases. 36% died at a mean age of 6.4 years. CD40, IL-1, TNF-α, TLR, and TCR signals were impaired in 15/16 (94%), 6/7 (86%), 9/11 (77%), 9/14 (64%), and 7/18 (39%), respectively. Hypomorphism-reconstituted NEMO-deficient cells demonstrated partial restoration of NEMO functions. Although both L153R and C417R impaired TLR and TNF-α induced NF-κB activation, L153R also increased TNF-α-induced programmed cell death with decreased A20 expression.
Conclusion
Distinct NEMO hypomorphs define specific disease and genetic characteristics. A reconstitution system can identify attributes of hypomorphisms independent of an individual’s genetic background. Apoptosis susceptibility in L153R reconstituted cells defines a specific phenotype of this mutation that likely contributes to the excessive inflammation with which it is clinically associated.
Keywords: NEMO, immunodeficiency, genetic database, Jurkat reconstitution, NF-κB activation, A20
INTRODUCTION
NEMO is a 419 amino acid regulatory protein encoded by 10 exons X-chromosome1. NEMO participates in the IκB kinase (IKK) complex contains IKKα and IKKβ kinases2. The IKK complex enables nuclear translocation of NF-κB dimers by phosphorylating the inhibitor of NF-κB, This targets IκB for proteosomal degradation releasing its hold on NF-κB in the cytoplasm.
Amorphic NEMO mutations are lethal to males, but hypomorphic mutations can result in ectodermal dysplasia and immunodeficiency. This disease was defined by familial susceptibility to mycobacterial infection, infection with pyogenic bacteria, and abnormal immunoglobulin production in the setting of variable T and B cell defects.3,4,5
The ectodermal dysplasia results from an inability of the Ectodysplasin A Receptor (a TNF receptor family member) to induce NF-κB activation following ligation4. A variety of immunoreceptor functions that depend on NEMO-induced NF-κB activation are similarly defective in patients with NEMO hypomophisms. The clinical and immunological phenotypes attributed to NEMO hypomorphs has expanded substantially in recent years. Thus, we have compiled these in to a database to further define the clinical syndrome. We have also utilized reconstitution system to exploit mechanistic insights derived from the naturally occurring mutations and to test the hypothesis that they are independent of genetic background.
METHODS
Database
72 individuals with hypomorphic NEMO mutations were identified using Medline, our own patient evaluations, and conference abstracts. Brothers of index cases having characteristic disease features were assumed to carry the same mutation. Detailed definitions of specific clinical and immunologic categories are provided in supplemental methods. Patients evaluated through our center were done so in accordance with our Institutional Review Board for the protection of human subjects.
Constructs and cell lines
3T8 is a previously described6 Jurkat cell line expressing an NF-κB reporter construct containing the rat Thy-1 gene, and is designated parental NEMO (pNEMO). pNEMO was previously mutagenized to generate a NEMO deficient line, 83216, herein designated NEMO(−), genomic sequencing of which revealed a hemizygous point mutation 1000G>T leading to a predicted Glu334X (Figure S1). Direct evaluation of NEMO protein in cells using polyclonal and monoclonal antibodies raised against full-length and the leucine zipper of NEMO, respectively, however, demonstrated negligible specific protein (Figure S2). NEMO cDNA was cloned to generate the following cell lines: wild-type reconstituted NEMO(−) (rNEMO); L153R reconstituted NEMO(−) (L153R); C417R reconstituted NEMO(−) (C417R); empty vector reconstituted NEMO(−) (GFP-NEMO(−); and empty vector transduced pNEMO (GFP-pNEMO) (details in supplemental methods).
Western blot
Western blotting was performed as previously described8 (details in supplemental methods).
Statistics
Student’s t-test was performed to evaluate mean data where indicated.
NF-κB reporter and Apoptosis assays
1×106 cells were treated with 10ng/mL recombinant human TNF-α (R&D Systems) or 50ng/mL recombinant Salmonella Flagellin (Invivogen) after which cells were collected, washed and incubated with phycoerythrin-conjugated anti-rat Thy-1 antibody (BD). Apoptosis was concurrently assayed by resuspension in binding buffer (10uM HEPES, 140mM NaCl and 2.5 mM CaCl2) containing AnnexinV-Cy5 and 7-AAD (BD).
Intracellular NEMO FACS
Cells were fixed and permeabilized in cytofix-cytoperm solution (BD), washed and incubated with mouse anti-NEMO mAb (clone 54, BD), or isotype-matched IgG control (clone MOPC-21, BD) for 1h. After washing, cells were incubated for 1 hr with Alexaflour 647-conjugated anti-Mouse IgG (Invitrogen), and analyzed by FACS.
mRNA isolation and analysis
RNA was extracted from cells, cDNA generated and A20 or actin targets amplified as described8.
RESULTS
IKBKG hypomorphism and spectrum of disease
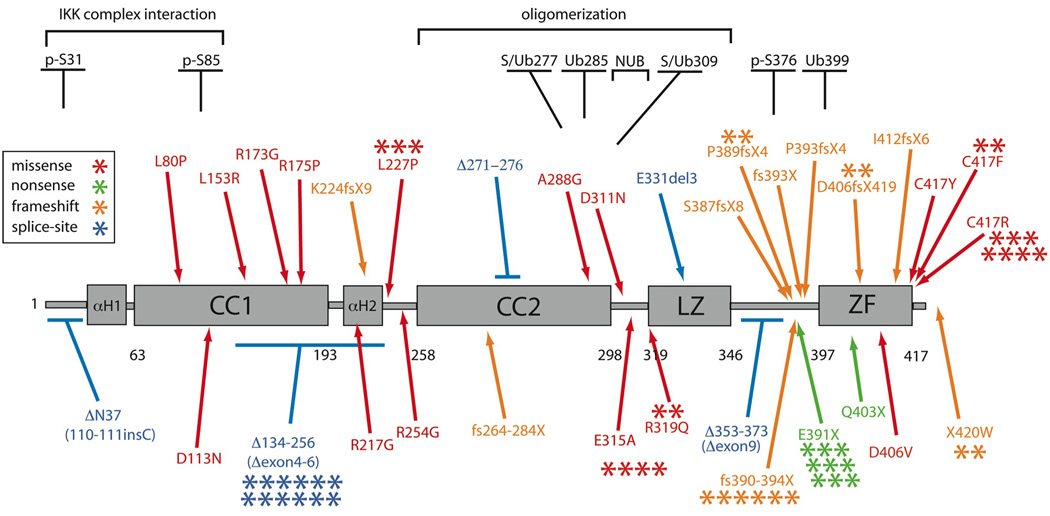
As IKBKG hypomorphisms cause a variety of phenotypes, a database was compiled to gage diversity and potentially identify genotype/phenotype correlations. 72 individuals were included (Figure 1). Missense mutations account for 40%, splice-site 21%, frameshift 25%, and nonsense 14%. 11 mutations were shared by 51 patients; the other 21 mutations were unique. 53% of mutations specifically affected the Zinc Finger domain, either due to missense, nonsense or frameshift. 3% were within the region aa50–120 important for interacting with the other members of the IKK complex9 and 15% were in the region responsible for allowing NEMO oligomerization10. 7% of mutations affected the NEMO ubiquitin binding domain (NUB), important for binding K63-linked polyubiquitin11. Two patients were female12,13 but had defective X chromosome lyonization and characteristics of the disease.
Figure 1. Hypomorphic NEMO mutations.
Each asterisk represents an individual patient, and mutation types are color-coded. Structural predictions indicate an extended alpha helix structure with 2 coiled coils, a leucine zipper and zinc finger motifs. The minimal oligomerization domain, serine phosphorylation (p-S), ubiquitination (U), sumoylation (S), ubiquitin binding (NUB), and IKK binding/NEMO dimerization regions are shown.
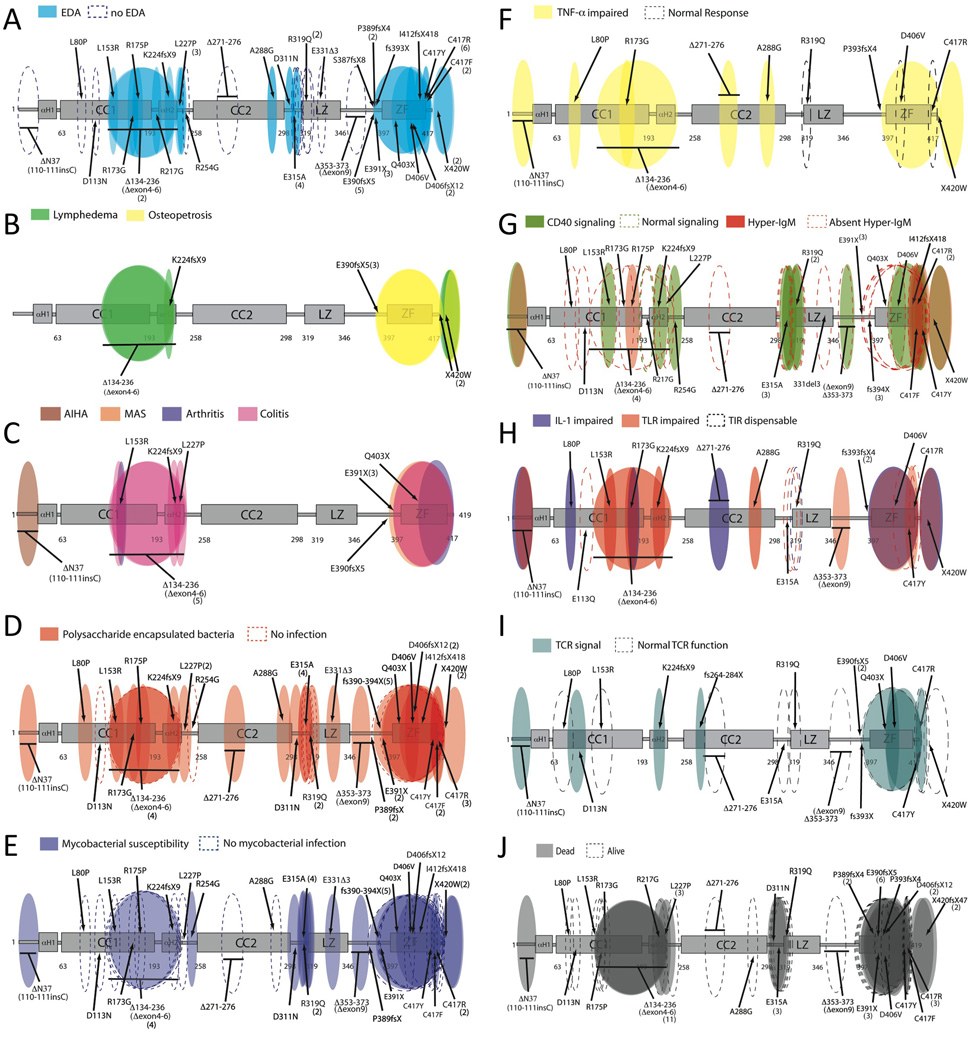
Patient clinical and immunologic characteristics were compiled according to clinical phenotype, infectious susceptibility, and immune capacity (see supplementary methods for definitions). 50 categories were defined and considered for each patient (Table S1–S4). For any category where insufficient details were available, patients were excluded from calculations. 77% (40/52) of patients were diagnosed with EDA or met our definition. 4% (2/52) of patients had dental abnormalities alone and were not included as having EDA. Three discrete regions of NEMO contained alterations not resulting in an ectodermal phenotype (Fig 2A). Osteopetrosis has been described in 7.5% (5/65) of patients (Fig 2B). In one, bone demonstrated no osteoclasts14, but in others varying severities of pathology were identified.15,16 10% (6/65) of patients had vascular anomalies affecting lymphatic or venous systems4,15,17,18,19,20 (Fig 2B), ranging from transient lower limb edema17 to persistent defects with abnormal lymphoscintigrams15 or multiple lymphangiomas.16
Figure 2. NEMO Phenotype maps.
The following phenotypes are shown: Ectodermal dysplasia (A), lymphedema/osteopetrosis (B), Inflammatory disease (C), Pyogenic infection (D), Mycobacterial infection (E), TNF-α response (F), Hyper-IgM phenotype/CD40 (G), IL-1/TLR response (H), TCR response (I), Mortality (J). Each oval represents the reported presence (shaded) or absence (dashed) of the indicated phenotype, and is intended to reflect the protein region affected.
Inflammatory conditions or auto-immunity affected 25% (15/61) of patients (Fig 2C). The most frequent was inflammatory colitis21, and occurred in 21% (13/61). 46% (6/13) of these individuals had intractable diarrhea, and 30% (4/13) were diagnosed with failure to thrive. Autoantibody-associated disease was described in 1 patient with autoimmune hemolytic anemia22. Chronic arthritis affected 3% (2/66)23. Hemophagoctyic syndrome following Klebsiella pneumoniae infection was identified in one patient14. 14% (9/66) of individuals were small for gestational age, but most were from a single kindred18. Pre-eclampsia complicated 3% (2/66) of deliveries.20,24
The most common infections included pneumonia (31%-19/61) leading to bronchiectasis in 9%, bacteremia or sepsis (33%-20/61), skin and deep tissue abscess formation (30%-18/61), intestinal infection (23%-14/61), encephalitis or meningitis (20%-12/61), sinusitis (11%-7/61), and osteomyelitis (11%-6/61) – usually with atypical mycobacteria (Fig 2D, TableS2). Pyogenic bacterial infection was identified in 87% (45/52) of patients in whom an organism of any kind was identified. Pathogens identified in greater than 10% included Streptococcus pneumoniae, Haemophilus influenza, and Staphylococcus aureus Mycobacterial infection, most commonly due to mycobacterium avium intracellulare affected 44% (23/52) (Fig 2E), and included cellulitis, osteomyelitis, lymphadenitis, pneumonia, and disseminated forms. Serious viral infection occurred in 21% (11/52) and included herpes simplex virus encephalitis22, severe adenoviral gastroenteritis16, and cytomegalovirus sepsis23. Fungal and opportunistic infections occurred in 10% (6/52) of patients; Pneumocystis and oral candidiasis were predominant.
IVIG replacement therapy was documented in 29/58 (50 %) individuals who survived beyond 6 months. Antibiotic prophylaxis to prevent Pneumocystis and/or mycobacteria was provided to 11/58 patients (19%). Additional interventions documented included cytokine therapy to augment immune function27, IFN-γ as an anti-mycobacterial25,28, and hematopoietic stem-cell transplantation15.
Immunologic Functions in patients with IKBKG hypomorphisms
Given the range of immunoreceptors that utilize NEMO it is possible that specific infectious susceptibilities are defined by the impact of individual mutations on immune signaling. Evaluation of TNFαR, CD40, TLR, IL-1R, and T cell receptor signaling, as well as antigen presenting cell costimulation, antibody repertoire generation, B and T cell development and memory, NK cell function, and monocyte activation have all been recorded. All mutations tested demonstrated some impairment in NF-κB signaling as defined in the supplemental methods. Defects in the TNFR superfamily functions were common with 82% (9/11) impairing TNF-α-induced NF-κB activation, but R319Q25, D406V5 and C417R5 mutations did not (Fig 2F, Table S3). CD40 signaling impairment was found in 94% (15/16), however only 40% (6/15) of these had an immunoglobulin class switch defect (Fig 2G, Table S3). Hypogammaglobulinemia occurred in 24/41 (59%), but correlated with impaired CD40 signaling only in Zinc Finger mutations. Defects in specific antibody production occurred in 64% (18/28), and deficits in specific antibodies against Streptococcus pneumoniae were identified in 72% (13/16) of patients tested. Of patients with specific antibody defects, only 15% (6/40) had low IgG with normal or elevated IgM. Defects in other immune responses and pathways were also common. 86% (6/7) patients had abnormal IL-1 signaling, and 64% (9/14) had abnormal TLR signaling (Fig 2H). 39% (7/18) of individuals in which innate signaling pathways were tested had no detectible abnormality in at least one test (TNFR, IL-1, TLR4 or other TLR). Lymphocyte quantitation and proliferative function were frequently normal. 65% (11/17) and 73% (8/11) had normal or elevated CD4 and CD8 counts, respectively. Mitogen-induced, and antigen induced proliferation was normal in 91% (20/22) and 76% (11/14), respectively (Fig 2I, Table S3). DTH testing, however, was normal in only 3/7 43%. Patients with C417R mutation had impaired DC IL-12 secretion and failure to upregulate costimulatory molecules26. NK cell cytotoxicity was globally deficient .14,23,27,28
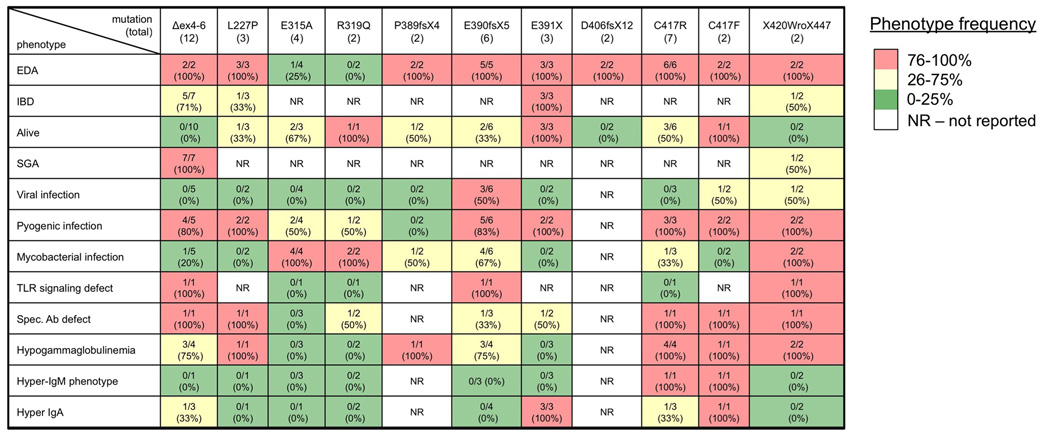
To evaluate consistency of expression of the most common phenotypes, the 11 shared mutations were analyzed (Figure 3). The frequency of EDA was 100% in 9 of these mutations, and 0–25% in the E315A and R319Q mutations. Inflammatory colitis occurred in 100% of the E391X individuals, but in only 25–75% in the three other mutions in which it was reported, although it appeared in 71% (5/7) of Δexon 4–6 mutations. This latter mutation was highly correlated with mortality, 10/10 (100%) and SGA, 7/7 (100%). Susceptibility to mycobacterial infection was generally absent in individuals with mutations in the first coiled-coil and alpha-helix. Hypogammaglobulinemia affected patients with Δex4–6, L227P and C417R substitutions, and ZF truncations (with the exception of E391X). The hyper-IgM phenotype was particular to individuals with C417 mutations
Figure 3. Phenotype frequency of shared mutations.
Each column represents a mutation which occurred in more than one individual. Frequency is depicted by quartile and is color coded: High (red), intermediate (yellow), and low (green) phenotype presence.
Hypomorphic NEMO complementation
We next wanted to determine the effect of particular NEMO mutations on NF-κB-dependent signaling pathways. If we could establish that individual hypomorphisms possessed differential properties in the context of a standardized genetic background it would support a mechanism of genotype-phenotype correlations, thereby substantiating our central hypothesis. Thus a NEMO(−) deficient Jurkat T cell line stably expressing an NF-κB reporting construct was used. Wild-type or patient derived hypomorphic sequences were cloned into a retroviral vector preceding internal ribosomal entry site (IRES) and green fluorescent protein (GFP) sequences. The L153R and C417R mutations were selected because of their similarities and differences. Both result in the originally described syndrome of EDA and immunodeficiency and both are caused by missense mutations introducing an arginine. Differences included: 1) presence of inflammatory colitis (L153R only); impaired LPS response (L153R only); and 3) Hyper-IgM phenotype (C417R only). Recombinant retroviruses encoding wild-type NEMO sequences, L153R, or C417R mutations were therefore generated and used to infect NEMO(−) cells. Non-clonal populations which had stably incorporated the construct were selected by GFP FACS and maintained as stable cultures. These were refined to express equal and physiological levels of NEMO as determined by Western blot (Fig. 4A). The level of reconstituted NEMO in individual cells was also comparable to that in parental Jurkat cells (pNEMO) as demonstrated by intercellular NEMO FACS (Fig 4B). This correlated with GFP fluorescence in individual reconstituted cells (Fig 4C), further demonstrating equivalent expression.
Figure 4. Expression levels of reconstituted NEMO are equivalent by anti-NEMO Western blot, intracellular FACS, and GFP FACS.
A, Cells from reconstituted lines were lysed and probed with anti-NEMO monoclonal antibody specific for the C-terminus. Actin blotting demonstrates equal loading. B, FACS to determine GFP expression was performed on NEMO reconstituted cells lines, C, which was evaluated by intracellular staining (n=2).
Activation of the NF-κB pathway measured by flow cytometry
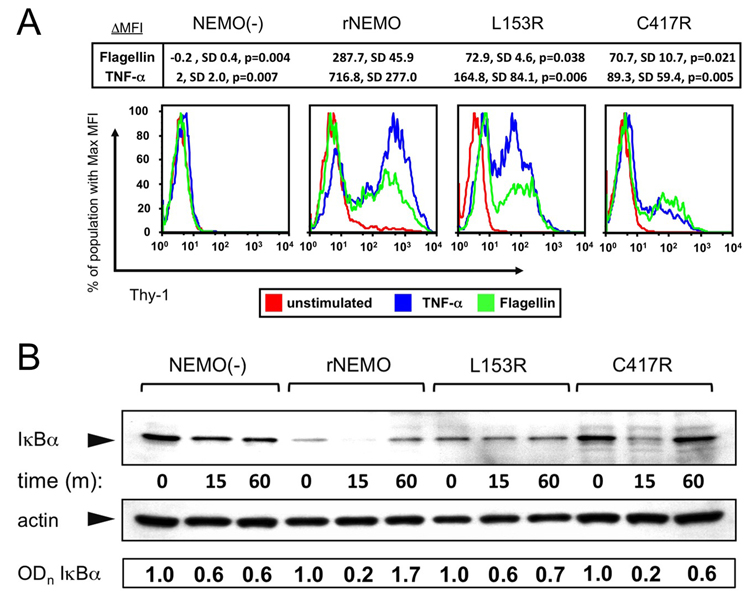
To investigate the effects of NEMO hypomorphism on innate immune signaling in T cells, Jurkat cells were cultured for 8h in the presence of Flagellin or TNF-α. Surface levels of rat Thy1 expressed by the NF-κB reporter contruct were determined by FACS. Jurkat T cells express TLR5 and exposure to the TLR5-ligand Flagellin leads to activation of NF-κB29,30. Rat Thy-1 expression was not upregulated in NEMO(−) cells after TNF-α or Flagellin stimulation but was in wild-type reconstituted NEMO cells (rNEMO) (Fig 5A). Rat Thy-1 upregulation in rNEMO cells was comparable to that in pNEMO cells (not shown). In contrast, NEMO(−) cells reconstituted with L153R and C417R constructs had reduced NF-κB activation in response to either TNF-α or Flagellin. Mean fluorescence intensity of induced rat Thy1 in repeated experiments was significantly decreased by ~75–90%, respectively, compared to control (Fig 5A).
Figure 5. Decreased NF-κB reporter expression after stimulation with TNF-α and Flagellin in reconstituted NEMO(−) cells and impaired IκB degradation in the L153R but not C417R cell line.
A, Cells were stained with rat-Thy-1PE and analyzed by FACS – decreased levels of expression indicate decreased NF-κB activation in response to TNF-α and Flagellin in L153R and C417R . Replicates of experiments indicate significant differences compared to rNEMO; means, SD and p values are shown. B, Western blot of IκB levels from the various cell lines following TNF-α activation. Densitometry measurements of IκBα/actin normalized to time=0 for each cell line are indicated below individual bands.
IκB degradation
To dissect the mechanisms by which each NEMO hypomorphism affects NF-κB activation, and delineate signaling pathways relative to the IKK complex, we initially measured IκBα degradation at different times after TNF-α stimulation. TNF-α failed to induce rapid degradation of IκB in NEMO(−) or L153R-NEMO cells (Fig 5B). In C417R-NEMO cells, however, there was initial IκB degradation, and restoration of IκB levels at 60min. Quantitative analysis of IκBα levels relative to actin confirmed these patterns (Fig 5B) and thus defines differences between hypomorphism expressing and NEMO(−) cells.
NF-κB directed anti-apoptotic function in reconstituted cells
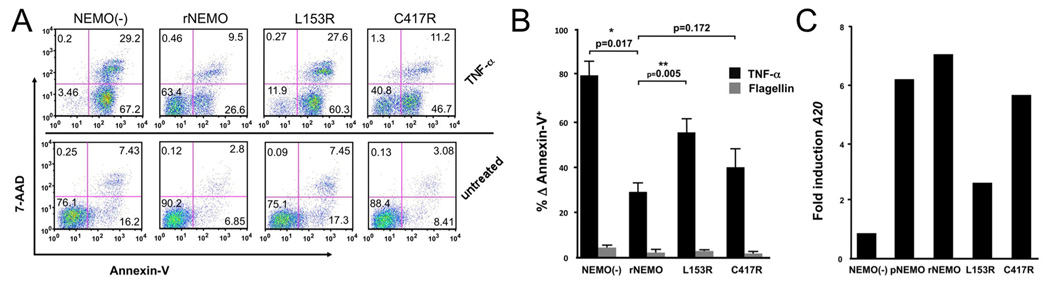
To determine the effects of NEMO hypomorphism on TNF-α-induced programmed cell death in T cells, the individual cell lines were cultured in the presence of TNF-α for 8h. Cell surface binding of Annexin-V, which occurs during the early and late phases of apoptosis, and 7-amino actinomycin D (7-AAD) uptake, which occurs only in dead cells was determined by FACS. Following TNF-α activation of NEMO(−) cells, almost all (96%) cells bind Annexin-V, of which 29% were in later phases of cell death as determined by 7-AAD retention (Fig 6A). In rNEMO cells there was reduced Annexin-V binding (30%), and only 9.5% retained 7-AAD; similar to pNEMO cells (not shown). L153R-NEMO cells bound Annexin-V substantially (88%) after TNF-α stimulation and retained 7-AAD similarly to NEMO(−) cells (28%). In contrast, C417R-NEMO cells demonstrated intermediate Annexin-V binding (55%) and 7-AAD retention (11%), more closely resembling rNEMO cells. These differences were confirmed in independently repeated experiments and Annexin-V binding in NEMO(−) and L153R was significantly higher than in rNEMO cells (Fig 6B). rNEMO and C417R-NEMO were not statistically different (P=0.17). As expected, Flagellin did not induce programmed cell death (Fig 6B).
Figure 6. Apoptosis in TNF-α stimulated cells and A20 expression.
A, Cell lines were activated with TNF-a and apoptosis was measured by Annexin-V and 7-AAD. B, Replicates and statistical evaluation of repeated apoptosis assays. C, A20 transcripts were quantified using real-time PCR and fold induction of A20 expression is reduced in L153R reconstituted NEMO(−) cells, the result is representative of two independently conducted experiments.
TNF-α-induced A20 gene expression
To evaluate whether differences in programmed cell death observed in L153R-NEMO correlated with aberrant TNF-α-induced survival-gene expression, quantitative Real Time PCR was performed. The anti-apoptotic A20 gene constitutively expressed in Jurkat cells is strongly induced by TNF-α and requires NEMO function6. In rNEMO and pNEMO cells A20 expression was induced ~7 fold after TNF-α stimulation (Fig 6C). In contrast, induced A20 expression in L153R-NEMO cells was >50% reduced compared to rNEMO cells. In C417R-NEMO cells, however, TNF-α induced A20 expression at levels similar to that in rNEMO cells. Thus, hypomorphic NEMO mutations demonstrated differential ability to protect T cells from TNF-α-induced programmed cell death, which may be at least in part due to impaired expression of A20. This may help explain differences in clinical phenotype in patients with these mutations, as L153R, but not C417R has been associated with an auto-inflammatory phenotype.
DISCUSSION
The previous conception of human disease due to hypomorphic NEMO mutation is one, which affects males, is associated with EDA in all but very rare cases, and is characterized by bacterial infection with poor production of specific antibody. We assembled a database of known mutations to discern phenotypic diversity of mutations and discover potential genotype/phenotype correlations. Although many of the previous characteristics of disease are apparent in the 72 patients considered here, the spectrum of disease due to NEMO hypomorphism is different than what has been based on earlier series.3,4,21
Although originally described as EDA-ID, only 77% of individuals with NEMO mutation and immunodeficiency in our database had EDA. As essentially all had immunodeficiency, a more appropriate name for this syndrome might be NEMO mutation with immunodeficiency, or NEMO-ID. Individuals demonstrated susceptibilities to pyogenic bacteria, atypical mycobacteria, viruses, and Pneumocystis, with cases affected by the latter two increasing in recent years. Autoinflammatory disease occurred frequently, most commonly affecting the gut.21,23 Signaling defects were varied and increasing numbers of mutations that permit partial TNF-α and TLR signaling have been identified (Fig F, H). Early mortality has been increasingly described as the mean age at death in patients reported over the last 3 years was 2.3 as compared to 6.4 for all patients in the database.
A finding consistent with previous understanding of disease was the high proportion of patients affected by pyogenic infection. Similarly, CD40 signaling impaired in most mutations tested. The classical hyper-IgM phenotype, however, affected a minority of patients, most specifically ΔN3722, R175P31, C417 alterations3,5,23 or with X420 frameshift mutation4. Also, as expected approximately two-thirds of individuals had defective specific antibody production, with a suggested selective inability to generate pneumococcus-specific antibodies. Hypogammaglobulinemia was still present in the majority (~60%) and osteopetrosis and lymphedema in the minority (~7.5%).
As this was a retrospective investigation of anecdotal reports and case series, both an ascertainment and reporting bias exist due to over-representation of severe and extraordinary cases. Generalizations about disease in the native population, therefore, should be made with caution. Recently reported cases have appeared to be more severe, but this is likely skewed because of one large kindred18. Longitudinal evaluation of patients in prospective studies would address these issues.
Interestingly, some phenotypes were characteristic of particular NEMO domains, while others were private to few mutations. EDA was attributed to 3 distinct NEMO regions, largely sparing mutations of the leucine zipper, and C-terminal portions of the first and second coiled-coil domain (Fig 2A). The hyper-Igm phenotype occurred with mutations affecting the Zinc Finger domain (Fig 2G) The region immediately preceeding the leucine zipper is required for signaling by CD40 and TNF-α, but not IL-1/TLR or TCR. Certain mutations, such as Zinc Finger truncations and Δexon4–6 splice mutations appear to globally affect function (Fig 2, all and Tables S1–S4). Importantly, evaluation of grouped-mutations fail to define uniform characteristics and thus the disease is quite variable.
In order to consider genotypic association independently of a patient’s genetic background, we established a reconstitution system and studied 2 patient-derived hypomorphisms. These mutations were selected not based upon the frequency with which they occurred, but rather due to important similarities and differences, which made them suitable candidates to demonstrate the proof-of-principal that functional differences between mutations could be attributed to a specific hypomorphism. Wild-type NEMO reconstitution restored physiologic function, but hypomorphisms did not. The C417R mutation permitted IκB degradation following TNF-α stimulation, in agreement with results obtained in patient-derived cells5, and was accompanied by A20 transcription and protection of cells from TNF-induced apoptosis (Fig 5, Fig 6). Mutation of C417 is known to affect NEMO folding32. This may prevent physical interaction between NEMO and proteins required for full signal transduction, but still permit some kinase activity of the complex. In contrast, L153R resulted in full impairment of IKK activity, with no IκB degradation following TNF stimulation, and increased apoptosis after T cell exposure to TNF and failure to induce A20 expression. This may address mechanisms for NEMO deficiency and inflammation, as the patient with an L153R hypomorphism had severe intestinal inflammation23. Complete NEMO deficiency in epithelial cells in mice causes inflammatory colitis and apoptosis, likely due to impaired barrier to intestinal flora.33,34 An additional role in promoting inflammation after exposure of T cells to innate immune signals, however, may also contribute to clinical phenotype. It may further explain why not all individuals with NEMO-ID have intestinal inflammation.
Our analysis defines disease due to hypomorphic NEMO mutations as diverse and complex, but there is suggestion of associations of particular phenotypes with NEMO genotypes, which raise important biological specificities of altered regions of NEMO. The use of reconstitution systems will help further important biological insights that can be derived from the disease. Clinically the list of phenotypes attributed to mutations is expanding and warrants careful consideration of patients with undiagnosed immunodeficiency.
Key messages
A database of human NEMO mutations reveals a broad associated spectrum of infectious susceptibilities and immune dysfunction.
The database and in vitro reconstitution of mutations illustrates important genotypic associations.
Supplementary Material
Table 1.
Clinical and immune function of individuals with hypomorphic NEMO mutation.
| Functional or clinical category | Observed deficiency |
Percent affected |
|---|---|---|
| B cell costimulation/CD40 signaling1 | 15/16* | 94% |
| Infectious susceptibility2 | 60/61 | 98% |
| bacterial infection | 45/52 | 86% |
| myobacterial infection | 23/52 | 44% |
| PCP | 4/52 | 8% |
| DNA viral infection | 11/52 | 21% |
| meningitis | 12/61 | 21% |
| pneumonia | 19/61 | 31% |
| sepsis/bacteremia | 20/61 | 33% |
| NK function3 | 10/10* | 100% |
| TNF Response4 | 9/11* | 82% |
| Ectodermal dysplasia (ED)5 | 40/52 | 77% |
| hyper IgM6 | 6/40 | 15% |
| hypogammaglobulinemia | 24/41 | 59% |
| hyper IgA | 13/35 | 37% |
| hyper IgD | 2/5 | 40% |
| specific antibody deficiency | 18/28 | 64% |
| specific Pneumococcal antibody | 13/16 | 81% |
| IL-1 Response7 | 6/7* | 86 % |
| TLR Response | 9/14* | 64% |
| Auto-immune/Inflammatory disease8 | 14/66 | 23% |
| Lymphedema | 5/65 | 8% |
| Osteopetrosis | 5/65 | 8% |
| Small for gestational age9 | 9/65 | 14% |
| Dead | 24/66 | 36% |
Impaired CD40 signaling by EMSA, reporter assay or ELISA or documented inability to undergo T-cell dependent immunoglobulin class switching or memory B cell development
Persistent or recurrent infection or life threatening infection.
Cytotoxicity by standard chromium release assay.
Impaired signaling by EMSA, reporter assay, IκB degradation or ELISA
Absence of eccrine sweat glands, hair and sparse or absent conical teeth.
Hyper IgM phenotype (elevated or normal IgM, low IgG and specific antibody production defect or class switch recombination defect)
Impaired IL-1β or TLR signaling by EMSA, reporter assay, CD62L shedding defect or ELISA
The presence of one or more of the following: sustained elevated serum inflammatory markers, chronic arthritis, autoimmune hemolytic anemia, macrophage activation syndrome, non-infecctious colitis.
Lower than two standard deviations below the average weight for gestational age.
These assays were performed on a subset of individuals.
Acknowledgments
This work was supported by NIH AI079731 (to J.S.O.), US Immunodeficiency Network Grant NIH N01 AI-22070 (to J.S.O. and M.J.M), NIH HL080612 (to M.J.M), the Pennsylvania Department of Health (to J.S.O.) (The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations, or conclusions from this study.) , a career development award from the American Academy of Allergy, Asthma and Immunology (to J.S.O.), NIH 5T32CA009140-33 and the Penn Center for Clinical Immunology Jackson-Wade Fellowship (to E.P.H).
Abbreviations
- TNF-α
Tumor Necrosis Factor-alpha
- NEMO
NF-κB essential modulator
- IL-1
Interleukin-1
- PBMCs
peripheral blood mononuclear cells
- FACS
fluorescence-activated cell sorting
- GFP
Green Fluorescent Protein
- DTH
Delayed Type Hypersensitivity
- TCR
T-Cell Receptor
- EDA
Ectodermal Dysplasia and Anhidrosis
Footnotes
No financial relationships with biotechnology and/or pharmaceutical manufacturers exist
REFERENCES
- 1.Aradhya S, Bardaro T, Galgoczy P, Yamagata T, Esposito T, Patlan H, et al. Multiple pathogenic and benign genomic rearrangements occur at a 35 kb duplication involving the NEMO and LAGE2 genes. Hum Mol Genet. 2001;10:2557–2567. doi: 10.1093/hmg/10.22.2557. [DOI] [PubMed] [Google Scholar]
- 2.Gilmore TD. Introduction to NF-kappaB: players, pathways, perspectives. Oncogene. 2006;25:6680–6684. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- 3.Zonana J, Elder ME, Schneider LC, Orlow SJ, Moss C, Golabi M, et al. A novel X-linked disorder of immune deficiency and hypohidrotic ectodermal dysplasia is allelic to incontinentia pigmenti and due to mutations in IKK-gamma (NEMO) Am J Hum Genet. 2000;67:1555–1562. doi: 10.1086/316914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doffinger R, Smahi A, Bessia C, Geissmann F, Feinberg J, Durandy A, et al. X-linked anhidrotic ectodermal dysplasia with immunodeficiency is caused by impaired NF-kappaB signaling. Nat Genet. 2001;27:277–285. doi: 10.1038/85837. [DOI] [PubMed] [Google Scholar]
- 5.Jain A, Ma CA, Liu S, Brown M, Cohen J, Strober W. Specific missense mutations in NEMO result in hyper-IgM syndrome with hypohydrotic ectodermal dysplasia. Nat Immunol. 2001;2:223–228. doi: 10.1038/85277. [DOI] [PubMed] [Google Scholar]
- 6.He KL, Ting AT. A20 inhibits tumor necrosis factor (TNF) α-induced apoptosis by disrupting recruitment of TRADD and RIP to the TNF receptor 1 complex in Jurkat T cells. Mol Cell Biol. 2002;22:6034–6045. doi: 10.1128/MCB.22.17.6034-6045.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swift S, Lorens J, Achacoso P, Nolan GP. Rapid production of retroviruses for efficient gene delivery to mammalian cells using 293T cell-based systems. Curr Protoc Immunol. 2001;(Chapter 10) doi: 10.1002/0471142735.im1017cs31. Unit 10 7C. [DOI] [PubMed] [Google Scholar]
- 8.Pandey R, Destephan CM, Madge LA, May MJ, Orange JS. NKp30 Ligation Induces Rapid Activation of the Canonical NF-κB Pathway in NK Cells. J Immunol. 2007;179:7385–7396. doi: 10.4049/jimmunol.179.11.7385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marienfeld RB, Palkowitsch L, Ghosh S. Dimerization of the I kappa B kinase-binding domain of NEMO is required for tumor necrosis factor alpha-induced NF-kappa B activity. Mol Cell Biol. 2006;26:9209–9219. doi: 10.1128/MCB.00478-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agou F, Traincard F, Vinolo E, Courtois G, Yamaoka S, Israel A, et al. The trimerization domain of NEMO is composed of the interacting C-terminal CC2 and LZ coiled-coil subdomains. J Biol Chem. 2004;279:27861–27869. doi: 10.1074/jbc.M314278200. [DOI] [PubMed] [Google Scholar]
- 11.Wu CJ, Conze DB, Li T, Srinivasula SM, Ashwell JD. Sensing of Lys 63-linked polyubiquitination by NEMO is a key event in NF-κB activation [corrected] Nat Cell Biol. 2006;8:398–406. doi: 10.1038/ncb1384. [DOI] [PubMed] [Google Scholar]
- 12.Martinez-Pomar N, Munoz-Saa I, Heine-Suner D, Martin A, Smahi A, Matamoros N. A new mutation in exon 7 of NEMO gene: late skewed X-chromosome inactivation in an incontinentia pigmenti female patient with immunodeficiency. Hum Genet. 2005;118:458–465. doi: 10.1007/s00439-005-0068-y. [DOI] [PubMed] [Google Scholar]
- 13.Kosaki K, Shimasaki N, Fukushima H, Hara M, Ogata T, Matsuo N. Female patient showing hypohidrotic ectodermal dysplasia and immunodeficiency (HED-ID) Am J Hum Genet. 2001;69:664–666. doi: 10.1086/323003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmid JM, Junge SA, Hossle JP, Schneider EM, Roosnek E, Seger RA, et al. Transient hemophagocytosis with deficient cellular cytotoxicity, monoclonal immunoglobulin M gammopathy, increased T-cell numbers, and hypomorphic NEMO mutation. Pediatrics. 2006;117:e1049–e1056. doi: 10.1542/peds.2005-2062. [DOI] [PubMed] [Google Scholar]
- 15.Dupuis-Girod S, Corradini N, Hadj-Rabia S, Fournet JC, Faivre L, Le Deist F, et al. Osteopetrosis, lymphedema, anhidrotic ectodermal dysplasia, and immunodeficiency in a boy and incontinentia pigmenti in his mother. Pediatrics. 2002;109:e97. doi: 10.1542/peds.109.6.e97. [DOI] [PubMed] [Google Scholar]
- 16.Mansour S, Woffendin H, Mitton S, Jeffery I, Jakins T, Kenwrick S, et al. Incontinentia pigmenti in a surviving male is accompanied by hypohidrotic ectodermal dysplasia and recurrent infection. Am J Med Genet. 2001;99:172–177. doi: 10.1002/1096-8628(2001)9999:9999<::aid-ajmg1155>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 17.Nishikomori R, Akutagawa H, Maruyama K, Nakata-Hizume M, Ohmori K, Mizuno K, et al. X-linked ectodermal dysplasia and immunodeficiency caused by reversion mosaicism of NEMO reveals a critical role for NEMO in human T-cell development and/or survival. Blood. 2004;103:4565–4572. doi: 10.1182/blood-2003-10-3655. [DOI] [PubMed] [Google Scholar]
- 18.Orstavik KH, Kristiansen M, Knudsen GP, Storhaug K, Vege A, Eiklid K, et al. Novel splicing mutation in the NEMO (IKK-γ) gene with severe immunodeficiency and heterogeneity of X-chromosome inactivation. Am J Med Genet A. 2006;140:31–39. doi: 10.1002/ajmg.a.31026. [DOI] [PubMed] [Google Scholar]
- 19.Smahi A, Courtois G, Vabres P, Yamaoka S, Heuertz S, Munnich A, et al. Genomic rearrangement in NEMO impairs NF-kappaB activation and is a cause of incontinentia pigmenti. The International Incontinentia Pigmenti (IP) Consortium. Nature. 2000;405:466–472. doi: 10.1038/35013114. [DOI] [PubMed] [Google Scholar]
- 20.Risma K, Deering R, Monaco-Shawver L, Heltzer M, Burnham J, Niemela J, et al. Ectodermal Dysplasia with Immunodeficiency and lymphedema, but not osteopetrosis, is associated with a unique NF-κB essential Modulator (NEMO) mutation. Clin Immunol. 2005;116:300–301. [Google Scholar]
- 21.Orange JS, Levy O, Geha RS. Human disease resulting from gene mutations that interfere with appropriate nuclear factor-kappaB activation. Immunol Rev. 2005;203:21–37. doi: 10.1111/j.0105-2896.2005.00221.x. [DOI] [PubMed] [Google Scholar]
- 22.Niehues T, Reichenbach J, Neubert J, Gudowius S, Puel A, Horneff G, et al. Nuclear factor kappaB essential modulator-deficient child with immunodeficiency yet without anhidrotic ectodermal dysplasia. J Allergy Clin Immunol. 2004;114:1456–1462. doi: 10.1016/j.jaci.2004.08.047. [DOI] [PubMed] [Google Scholar]
- 23.Orange JS, Jain A, Ballas ZK, Schneider LC, Geha RS, Bonilla FA. The presentation and natural history of immunodeficiency caused by nuclear factor κB essential modulator mutation. J Allergy Clin Immunol. 2004;113:725–733. doi: 10.1016/j.jaci.2004.01.762. [DOI] [PubMed] [Google Scholar]
- 24.Salt M BH, Jain A, MD2, Pandey R, PhD3, Hanson EP, MD3, Niemela2 JE, Deering3 RP, Quinones R, MD4, Orange JS, MD/PhD3,5, Gelfand EW., MD1,5 IKBKG (NEMO) Mutation can be Associated With Opportunistic Infection Without Impairing TLR Function. JACI. 2007 doi: 10.1016/j.jaci.2007.11.014. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Filipe-Santos O, Bustamante J, Haverkamp MH, Vinolo E, Ku CL, Puel A, et al. X-linked susceptibility to mycobacteria is caused by mutations in NEMO impairing CD40-dependent IL-12 production. J Exp Med. 2006;203:1745–1759. doi: 10.1084/jem.20060085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Temmerman ST, Ma CA, Borges L, Kubin M, Liu S, Derry JM, et al. Impaired dendritic-cell function in ectodermal dysplasia with immune deficiency is linked to defective NEMO ubiquitination. Blood. 2006;108:2324–2331. doi: 10.1182/blood-2006-04-017210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orange JS, Brodeur SR, Jain A, Bonilla FA, Schneider LC, Kretschmer R, et al. Deficient natural killer cell cytotoxicity in patients with IKK-gamma/NEMO mutations. J Clin Invest. 2002;109:1501–1509. doi: 10.1172/JCI14858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orange JS, Levy O, Brodeur SR, Krzewski K, Roy RM, Niemela JE, et al. Human nuclear factor kappa B essential modulator mutation can result in immunodeficiency without ectodermal dysplasia. J Allergy Clin Immunol. 2004;114:650–656. doi: 10.1016/j.jaci.2004.06.052. [DOI] [PubMed] [Google Scholar]
- 29.Kabelitz D, Medzhitov R. Innate immunity--cross-talk with adaptive immunity through pattern recognition receptors and cytokines. Curr Opin Immunol. 2007;19:1–3. doi: 10.1016/j.coi.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 30.Kabelitz D. Expression and function of Toll-like receptors in T lymphocytes. Curr Opin Immunol. 2007;19:39–45. doi: 10.1016/j.coi.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 31.Abinun M, Spickett G, Appleton AL, Flood T, Cant AJ. Anhidrotic ectodermal dysplasia associated with specific antibody deficiency. Eur J Pediatr. 1996;155:146–147. doi: 10.1007/BF02075774. [DOI] [PubMed] [Google Scholar]
- 32.Cordier F, Vinolo E, Veron M, Delepierre M, Agou F. Solution structure of NEMO zinc finger and impact of an anhidrotic ectodermal dysplasia with immunodeficiency-related point mutation. J Mol Biol. 2008;377:1419–1432. doi: 10.1016/j.jmb.2008.01.048. [DOI] [PubMed] [Google Scholar]
- 33.Nenci A, Becker C, Wullaert A, Gareus R, van Loo G, Danese S, et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 2007;446:557–561. doi: 10.1038/nature05698. [DOI] [PubMed] [Google Scholar]
- 34.Zaph C, Troy AE, Taylor BC, Berman-Booty LD, Guild KJ, Du Y, et al. Epithelial-cell-intrinsic IKK-beta expression regulates intestinal immune homeostasis. Nature. 2007;446:552–556. doi: 10.1038/nature05590. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.