Abstract
Since TSH receptor (TSHR) expression increases during adipogenesis and signals via cAMP/phospho-cAMP-response element binding protein (CREB), reported to be necessary and sufficient for adipogenesis, we hypothesised that TSHR activation would induce preadipocyte differentiation. Retroviral vectors introduced constitutively active TSHR (TSHR*) into 3T3L1 preadipocytes; despite increased cAMP (RIA) and phospho-CREB (western blot) there was no spontaneous adipogenesis (assessed morphologically, using oil red O and QPCR measurement of adipogenesis markers). We speculated that Gβγ signalling may be inhibitory but failed to induce adipogenesis using activated Gsα (gsp*). Inhibition of phosphodiesterases did not promote adipogenesis in TSHR* or gsp* populations. Furthermore, differentiation induced by adipogenic medium with pioglitazone was reduced in TSHR* and abolished in gsp* expressing 3T3L1 cells. TSHR* and gsp* did not inactivate PPARγ (PPARG as listed in the HUGO database) by phosphorylation but expression of PPARγ1 was reduced and PPARγ2 undetectable in gsp*. FOXO1 phosphorylation (required to inactivate this repressor of adipogenesis) was lowest in gsp* despite the activation of AKT by phosphorylation. PROF is a mediator that facilitates FOXO1 phosphorylation by phospho-Akt. Its transcript levels remained constantly low in the gsp* population. In most measurements, the TSHR* cells were between the gsp* and control 3T3L1 preadipocytes. The enhanced down-regulation of PREF1 (adipogenesis inhibitor) permits retention of some adipogenic potential in the TSHR* population. We conclude that Gsα signalling impedes FOXO1 phosphorylation and thus inhibits PPARγ transcription and the alternative promoter usage required to generate PPARγ2, the fat-specific transcription factor necessary for adipogenesis.
Introduction
Adipose tissue expands via two mechanisms, hypertrophy of individual adipocytes and hyperplasia due to the proliferation and differentiation of preadipocyte precursors (Drolet et al. 2008). In vitro protocols to induce preadipocyte differentiation (adipogenesis) use agents to increase cAMP. Furthermore, studies in the 3T3L1 murine preadipocytes, the cell line that has been central in unravelling the complex mechanisms driving adipogenesis, indicated that treatment with forskolin or isobutylmethylxanthine (IBMX) alone suffices to trigger the process (Boone et al. 1999) and it has been shown subsequently that cAMP-response element binding protein (CREB) activation is both necessary and sufficient to induce adipogenesis (Reusch et al. 2000).
Many G protein-coupled receptors, including the thyrotropin receptor (TSHR), which is the main regulator of thyroid function and growth, signal predominantly via the cAMP/protein kinase A (PKA) pathway, ultimately leading to phosphorylation of CREB (Vassart & Dumont 1992). TSHR expression is also increased during adipogenesis (Haraguchi et al. 1996). Does this suggest a role for TSHR activation in adipocyte biology? The question is clinically relevant since thyroid dysfunction is common, affecting up to 2% of the population, and the majority of these individuals will have overactivation of the TSHR either by supraphysiological concentrations of TSH in hypothyroidism or from thyroid stimulating antibodies in Graves' hyperthyroidism (Vanderpump et al. 1995).
On the basis of the known signalling pathways following from TSHR activation, we hypothesise that it should trigger adipogenesis spontaneously or at least enhance that induced by known differentiating agents such as synthetic PPARγ (PPARG as listed in HUGO database) agonists. We have used an in vitro model in which naturally occurring constitutively active mutant forms of TSHR, found in e.g. toxic nodules (Paschke & Ludgate 1997), are introduced using retroviral vectors (Fuhrer et al. 2003). Our previous studies used the model to investigate the effect of TSHR activation on human orbital primary preadipocytes (of relevance to the eye disease occurring in Graves' patients). We demonstrated spontaneous induction of early, but inhibition of the later stages of adipogenesis, even in the presence of PPARγ agonists (Zhang et al. 2006). Orbital preadipocytes are a particular fat depot, being derived from the neural crest, and this may explain the differences between our results following activation of the cAMP pathway and those reported using a murine preadipocyte cell line. In the current study, we have applied the model (activating mutant TSHR–L629F) to 3T3L1. To our surprise, despite modestly increasing intracellular cAMP and elevating phospho-CREB levels, TSHR activation did not induce adipogenesis and this was not changed by adding a phosphodiesterase inhibitor. Furthermore, adipogenesis induced by PPARγ agonists was significantly reduced in the mutant TSHR expressing cells compared with the non-modified population. Since TSHR activation liberates two functional moieties of the Gs protein, α (which acts via PKA) and βγ (signals via PI3K), we suggested that the inhibitory effects were due to the latter. Thus, we have introduced constitutively active Gsα (gsp*), Q227L (Ludgate et al. 1999), which yields only the α subunit. Again there was no spontaneous adipogenesis and in vitro induced differentiation was completely abolished. Further investigations revealed a reduction in PPARγ1 and the complete absence of PPARγ2 proteins, the fat-specific isoform, in gsp* expressing 3T3L1.
Materials and Methods
Reagent source; cell culture and adipogenesis protocols
All chemicals were obtained from Sigma–Aldrich and tissue culture media and serum from BioWhittaker-Lonza (Verviers, Belgium) unless otherwise stated. The 3T3L1 cell line was purchased from the ATCC (Atlanta, GA, USA).
3T3L1 murine preadipocytes were routinely cultured in DMEM/F12 10% FCS (complete medium, CM). Adipogenesis was induced in confluent cells by replacing with differentiation medium (DM) containing 5% FCS, biotin (33 μM), panthothenate (17 μM), tri-iodothyronine (1 nM), dexa-methasone (100 nM), thiazolidinedione (1 μM) and insulin (500 nM), for 10–12 days, as previously described (Zhang et al. 2006).
Activating mutant human TSHR, L629F (TSHR*) and rat Gsα, Q227L (gsp*), were introduced using retroviral vectors, previously produced in our laboratory (Ivan et al. 1997, Fuhrer et al. 2003). All experiments were performed on the mixed pools of cells that resulted from geneticin selection. Furthermore, at least two independent mixed pools were generated for each cell population, with results being comparable. Initial characterisation of the G418 resistant pools of 3T3L1 employed RT-PCR and direct sequencing to demonstrate the expression of human TSHR and rat Gsα in the appropriate populations as previously described (Ivan et al. 1997, Fuhrer et al. 2003). In many of our experiments we also included 3T3L1 populations transduced with the wild-type (WT) and a second mutant form of TSHR, M453T. Results obtained (basal levels of cAMP/pCREB, transcript measures for PPARγ and GPDH, etc.) indicated that there was no significant difference in the behaviour of WT compared with non-modified or M453T compared with L629F. In the interests of brevity, the results are reported for the non-modified, L629F and gsp* expressing populations.
Effect on cAMP levels
The non-modified 3T3L1 and populations expressing gsp* or TSHR* were plated at 5×104/well in 12-well plates. The following day they were incubated for 4 h in medium containing 10−4 M IBMX with no further addition (basal conditions), or plus 10 mU/ml bovine TSH. Forskolin (in addition to IBMX) at 10−5 M was included as a positive control in all populations. cAMP was extracted in 0·1 M HCl and measured using an in-house RIA capable of detecting femtogram quantities of the second messenger, as previously described (Fuhrer et al. 2003). Coulter counting of adjacent wells provided an accurate cell number for results to be expressed as picomoles cAMP/104 cells.
Western blotting
The three different 3T3L1 populations were propagated in duplicate in 6-well plates in CM or DM. Proteins were extracted, at various time points, in Laemmli buffer containing 1 mM sodium orthovanadate and 1 mM phenylmethylsulphonyl fluoride. To prepare nuclear and cytosolic fractions, cells were harvested in ice-cold PBS, centrifuged and resuspended in HEPES buffer containing protease inhibitors. The supernatant produced by centrifugation at 12 000 g provided the cytosolic and high-salt extraction of the pellet, the nuclear fractions respectively.
Samples (containing 20 μg protein) were separated by 10% SDS-PAGE and then the gel electroblotted onto PVDF membrane as previously described (Al-Khafaji et al. 2005). To investigate various signalling pathways, the blots were probed with the following rabbit antibodies (all from Cell Signalling Technology unless otherwise stated): anti phospho-CREB (Ser 133, 1:2000 overnight; 4 °C); anti total-CREB (1:1000, room temperature, 1 h; Santa CruzBiotechnology, Santa Cruz, CA, USA); anti phospho-Akt (Thr 308, 1:1000, 4 °C, overnight); anti total-Akt (1:1000, room temperature, 1 h, Santa Cruz); and anti phospho-SYK (Tyr 352, 1:5000, overnight, 4 °C, BD Biosciences, San Jose, CA, USA). To investigate transcription factors, rabbit antibodies to phospho-PPARγ (Ser 82, 1:1000 overnight, 4 °C, Upstate), anti total-PPARγ (1:1000, overnight, 4 °C), anti total-FOXO1 (1:1000, overnight, 4 °C) and anti phospho-FOXO1 (Ser 256, 1:1000, overnight, 4 °C) were employed. In all cases proteins were detected using either an anti-mouse IgG-HRP conjugate or an anti-rabbit IgG-HRP conjugate (1:5000, room temperature for 1 h, GE Healthcare, Amersham, UK) and visualised by enhanced chemiluminescence (ECL Plus, GE Healthcare).
Films were analysed using the Alpha Imager 1200 digital imaging system (Alpha Innotech Corp., San Leandro, CA, USA). The blots were initially probed with the phospho-specific antibodies; they were then stripped and reprobed with antibodies that recognise total proteins.
Effect on spontaneous and PPARγ induced adipogenesis
The various cell populations (in 12-well plates) were examined in CM and DM. Microscopic examination provided a means of determining whether morphological changes, for e.g. rounding-up of cells and/or acquisition of lipid-filled droplets (oil red O staining), had occurred.
Effect on mitotic clonal expansion
To investigate whether the expression of the various mutants had any effect on the mitotic clonal expansion phase, reported by some authors to be required for differentiation in 3T3L1 preadipocytes (Tang et al. 2003), the cells were counted 1, 2, 3 and 10 days after addition of DM, using a Coulter particle counter; results are expressed (mean±s.e.m.) fold increase in cell number.
QRT-PCR measurement of transcript copy number
The various cell populations were plated in 6-well plates in CM or DM. Ten days later, RNA was extracted, reverse transcribed and transcript copy numbers for PPARγ, GPDH, PREF1, ID2, PROF, and acidic ribosomal phosphoprotein (ARP) were measured using Sybr green and a Stratagene MX3000 light cycler. Primers used are listed in Table 1.
Table 1.
Primer sequences (and amplicon sizes) employed for QPCR measurements
| Forward primer | Reverse primer | |
|---|---|---|
| Gene | ||
| PPARγ 220 bp | TTTTCAAGGGTGCCAGTTTC | AATCCTTGGCCCTCTGAGAT |
| GPDH 124 bp | ATGCTCGCCACAGAATCCACAC | AACCGGCAGCCCTTGACTTG |
| PREF1 148 bp | CGTGATCAATGGTTCTCCCT | AGGGGTACAGCTGTTGGTTG |
| ARP 72 bp | GAGGAATCAGATGAGGATATGGGA | AAGCAGGCTGACTTGGTTGC |
| PROF 72 bp | GATCACTCTGTCATCATGTGG | CTTACTGTCCCACATTTGCTTG |
| ID2 141 bp | GGACCACAGCTTGGGCAT | CGTTCATGTTGTAGAGCAGACTCAT |
Standard curves (the PCR amplicon subcloned into pGEM-T at 106 to 102 copies) were included for each gene and results are expressed relative to the housekeeping gene ARP. This gene was selected on the basis of its high expression level (Ct value 16±0·29 in non-modified 3T3L1 in CM), which was not modified in the L629F and gsp* populations (16±0·31 and 16±0·32 respectively in CM) or during adipocyte differentiation (16±0·28 in non-modified cells on day 9 in DM; the Ct values are the mean±s.e.m. obtained from four separate experiments).
Statistical analysis
Means were compared using Student's t-test for parametric data and non-parametric data were analysed using the Wilcoxon signed ranks test.
Results
TSHR* and gsp* increase unstimulated cAMP levels
In non-modified 3T3L1, the unstimulated level of cAMP was 397±18 pmol/104 cells (results are the mean±s.e.m. of three experiments all performed in at least triplicate). Unstimulated cAMP levels were significantly increased in cells expressing TSHR* or gsp* reaching 129±3·9% (P<0·02) and 140±5·9% (P<0·01) respectively of the non-modified population. TSH (1 mU/ml) elicited a cAMP response in cells expressing mutant TSHR (650±218% of unstimulated) but not in the non-modified or gsp* 3T3L1 cells, in keeping with the very low level of endogenous TSHR in these cells prior to differentiation (Haraguchi et al. 1996). There was no significant difference in the response to forskolin, which induced a robust >20-fold increase in cAMP in all the three populations.
Phosphorylated CREB levels are increased in TSHR* and gsp* expressing cells and are modulated during adipogenesis
Western blots to investigate activation of CREB by phosphorylation are usually performed following acute stimulation of the cells followed by analysis in the minutes after exposure to the stimulant. By contrast, we have examined the basal levels of phospho-CREB in the three populations of 3T3L1 to assess the effects of chronic activation via the various mutants. We have used a value of 1 for the phosphorylated:total CREB ratio of the non-modified preadipocytes. Based on at least seven experiments using two independently generated populations of TSHR* or gsp* expressing cells, there was a modest, but significant increase in the ratio in L629F (1·5±0·58 P<0·02) and gsp* (1·19±0·22 P<0·02), reflecting chronic activation of this pathway. A representative western blot is shown in Fig. 1.
Figure 1.
Representative (one out of seven performed) western blot of the three 3T3L1 populations in complete medium (basal conditions) showing phosphorylated CREB (upper panel) and total CREB (lower panel) both having an apparent molecular weight of 43 kDa.
To investigate whether CREB phosphorylation is affected by the adipogenic process, we have measured the phosphorylated:total CREB ratios in samples obtained from the different 3T3L1 populations at various time points following addition of DM. In the non-modified cells there is a slight reduction in the ratio in mid-differentiation, but a significant increase in the later stages (P<0·05), to achieve levels 150–300% of those on day 0 (prior to addition of DM). Apart from the higher basal levels of phospho-CREB, a similar pattern of expression was apparent in the TSHR* and gsp* expressing cells through adipogenesis (data not shown).
The increased phospho-CREB levels do not produce spontaneous adipogenesis
In CM, even when the cells had been confluent for up to 10 days, there were no morphological changes consistent with adipogenesis in cells expressing gsp* or TSHR* when compared with the control 3T3L1. Occasional cells containing small lipid vacuoles were observed in all populations.
Furthermore, we did not see a consistent change in transcript levels of PREF1, PPARγ or GPDH in cells expressing TSHR* or gsp* when compared with the non-modified population (data not shown).
Since chronic stimulation of the cAMP pathway might induce up-regulation of e.g. phosphodiesterases, we included a phosphodiesterase inhibitor (IBMX) in the culture medium. Cells were allowed to reach confluence in CM and 0·5 mM IBMX was added for various time periods, but there was still no morphological evidence for adipogenesis and the transcript levels for markers of differentiation were not significantly changed (data not shown).
TSHR* and gsp* inhibit induced adipogenesis by affecting PPARγ isoforms
PPARγ agonist induced differentiation of the non-modified cells produced the expected change in morphology and similar signs of adipogenesis were also present in the L629F expressing cells, but at a reduced level compared with the control; by contrast, the gsp* population were completely devoid of differentiating cells, as illustrated in Fig. 2, by the absence of oil red O stained cells.
Figure 2.
Oil red O staining in 3T3L1 cells following nine days in differentiation medium containing pioglitazone. A, non-modified; B, L629F; and C, gsp* expressing populations. Magnification ×200.
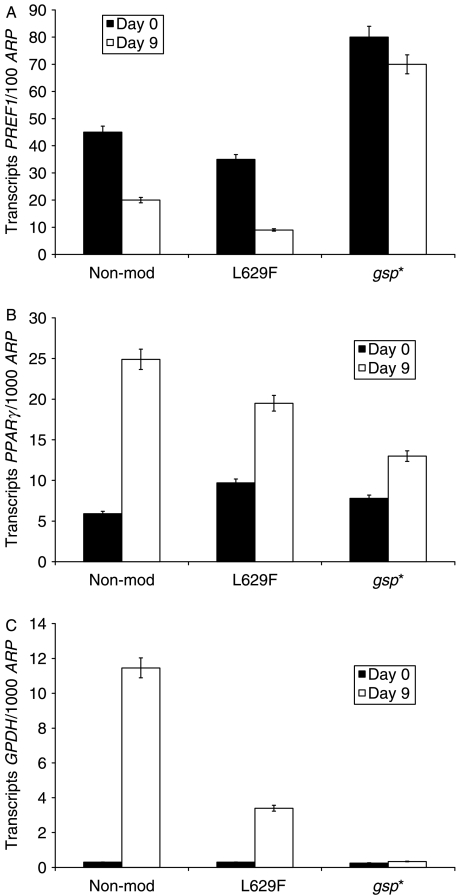
We compared transcripts for markers of adipogenesis in non-modified, L629F and gsp* expressing cells; statistical analyses of the results reported as fold changes are shown in Table 2. Figure 3 is a representative experiment illustrating that PPARγ agonist induced differentiation of non-modified 3T3L1 resulted in sustained and significant increases in PPARγ and GPDH (P<0·05 and 0·02 respectively). The L629F and gsp* populations displayed an attenuated increase in PPARγ (although not significantly different from non-modified) and in GPDH (both significantly less than non-modified) being more severe in the gsp* expressing cells in keeping with their morphological appearance. Furthermore, expression of PREF1, an EGF-like transmembrane protein that inhibits adipogenesis (Smas & Sul 1993), is significantly down-regulated (P<0·04), by the differentiation protocol, to 54±0·12% of the day 0 value, in the non-modified cells. The behaviour of PREF1 in the other populations diverges. PREF1 expression in L629F cells after exposure to DM was reduced to 30±0·06% of day 0 levels (P<0·005), and this differentiation induced PREF1 down-regulation is significantly greater than in the non-modified and gsp* populations (P<0·04 and 0·02 respectively). By contrast, the reduction to 89±0·07% of pre-treatment values in the gsp* population is not significantly different from day 0 PREF1 transcripts in CM in these cells.
Table 2.
Fold changes in transcript levels of adipogenesis markers in the three populations of 3T3L1 cells following exposure to differentiation medium. The three populations were plated in 12-well plates; once confluent, the cells were cultured for 9 days in differentiation medium containing pioglitazone. mRNA was extracted on days 0 and 9 and QPCR measurement of the adipogenesis markers was performed. Results (mean±s.e.m. of the three experiments were all performed in at least duplicate) are the fold changes in transcripts for each gene (relative to the ARP housekeeper) comparing day 9 and day 0
| Non-modified cells | L629F population | gsp* population | |
|---|---|---|---|
| Marker | |||
| PPARγ | 6·5±2·5 | 2·7±0·7 | 2·2±0·5 |
| GPDH | 159±43 | 19·5±8* | 2·2±0·43† |
| PREF1 | 0·54±0·12 | 0·3±0·06‡ | 0·89±0·07§ |
*P<0·03 compared with non-modified; †P<0·02 compared with non-modified; ‡P<0·04 compared with non-modified; §P<0·02 compared with L629F.
Figure 3.
QPCR measurement of adipogenesis markers – A, PREF1; B, PPARγ; and C, GPDH on day 0 (cells in complete medium) and day 9 (cells in differentiation medium containing pioglitazone) in non-modified, L629F and gsp* 3T3L1 cells. Results are the means±s.e.m. of triplicates, expressed as absolute transcript copy numbers (transcripts) of the gene of interest per 1000 copies (100 for PREF-1) of acidic ribosomal phosphoprotein (ARP). Representative experiment, one out of three performed.
Coulter counting of the cells in the first 3 days after addition of DM (n=3) revealed that the non-modified 3T3L1 had a 2·4±0·9-fold increase in cell number on day 3 compared with day 0, indicating mitotic clonal expansion (MCE). Both L629F and gsp* populations displayed significantly increased proliferation compared with the non-modified, 4·9±0·5 (P<0·005) and 3·3±0·7 (P<0·01) fold respectively, suggesting no influence of either mutation on MCE. In the following seven days, there was no further increase in either the non-modified or L629F cell number, but the gsp* population continued to proliferate, in keeping with the absence of differentiation.
The transcriptional activity of PPARγ is reduced when it is phosphorylated (Hu et al. 1996). We were unable to detect any phosphorylated PPARγ either in total cell lysates or nuclear extracts. However, as shown in Fig. 4, the expression of PPARγ1 is greatly reduced (at all time points) in L629F and gsp* and PPARγ2 is completely absent from the latter, in contrast to the non-modified 3T3L1.
Figure 4.
Western blot analysis of PPARγ protein expression on day 0 (cells in complete medium) and at various time points following addition of differentiation medium containing pioglitazone in non-modified, L629F and gsp* 3T3L1 cells. Representative experiment, one out of three performed.
Furthermore, the expression of PPARγ1 and PPARγ2 proteins in non-modified cells is increased in the first 24 h following induction. PPARγ1 expression continues to increase throughout adipogenesis, but PPARγ2 expression is at the limit of detection during the MCE stage, but then resumes in the terminal stages of differentiation.
Reduced FOXO1 phosphorylation may explain the lack of PPARγ2
The transcription factor FOXO1 represses the promoters for PPARγ1 and PPARγ2 (Armoni et al. 2006); it is inactivated by phosphorylation when it translocates from the nucleus to the cytoplasm (Nakae et al. 2003). As can be seen in Fig. 5, in the non-modified cells total FOXO1 protein expression increases in the first 24 h after addition of DM in the nucleus, where it is highly phosphorylated. It is essentially absent from the cytoplasm during the MCE phase. In the gsp* population, total FOXO1 protein expression is higher in basal conditions, its expression is increased in DM and we observe its translocation from the cytoplasm to the nucleus between days 1 and 3. The main difference is the considerably decreased level of phosphorylation from day 3 onwards compared with the non-modified 3T3L1. The TSHR* cells' behaviour is midway between that of the other two populations.
Figure 5.
Western blot analysis of FOXO1 protein expression on day 0 (cells in complete medium) and at various time points following addition of differentiation medium containing pioglitazone in non-modified, L629F and gsp* 3T3L1 cells. A, Nuclear extracts probed with anti-phospho-FOXO1 (upper panel) and anti-total FOXO1 (lower panel). B, cytosolic extracts (panels as in A). Representative experiment, one out of three performed.
What accounts for the behaviour of FOXO1?
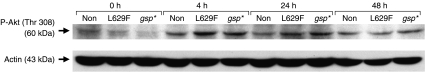
Phosphorylation-dependent inactivation of FOXO1 depends chiefly on the PI3K and phospho-Akt pathway (Sakaue et al. 1998). We investigated and observed an early increase in the proportion of phospho-Akt in all populations of 3T3L1 cells after addition of DM (Fig. 6), indicating the need for an alternative explanation for the reduced FOXO1 phosphorylation in gsp*.
Figure 6.
(A) Western blot analysis of phospho-Akt (upper panel) and actin (lower panel) in nuclear extracts on day 0 (cells in complete medium) and at various time points following addition of differentiation medium containing pioglitazone in non-modified, L629F and gsp* 3T3L1 cells.
Two novel modulators of adipogenesis have recently been described. PROF is a mediator between AKT and FOXO1 whose expression is transiently up-regulated during adipogenesis (Fritzius & Moelling 2008). ID2 is a small molecule, whose transcription also increases during adipogenesis and interacts with as yet to be identified factors to stimulate PPARγ expression (Park et al. 2008). We investigated their expression and found a transient increase in ID2 TCN in all three populations in the first 4–8 h following addition of DM, although transcript levels of ID2 were lowest in gsp* cells at all time points (data not shown). By contrast, as shown in Fig. 7, PROF transcription was increased in the non-modified and, to a lesser extent, in the L629F populations, but remained at a constant low level in the gsp* cells. Thus, despite the abundance of phospho-Akt in the gsp* population, the paucity of PROF prevents inactivation of FOXO1 by phosphorylation, PPARγ2 is not expressed and adipogenesis does not occur.
Figure 7.
QPCR measurement of ProF expression on day 0 (cells in complete medium) and at various time points following the addition of differentiation medium containing pioglitazone in non-modified, L629F and gsp* 3T3L1 cells. Results are the means±s.d. of triplicates, expressed as absolute transcript copy numbers (transcripts) per 1000 copies of acidic ribosomal phosphoprotein (ARP). Representative experiment one out of two performed.
Discussion
Our initial aim was to investigate the effects of TSHR activation on 3T3L1 preadipocytes, following our previous studies demonstrating increased adipogenesis in ex vivo fat samples from Graves' patients, in whom thyroid stimulating antibodies (as opposed to gain-of-function mutation) produce chronic activation of the TSHR (Starkey et al. 2003). From reports of a pro-adipogenic effect of agents capable of elevating cAMP, we hypothesised that spontaneous adipogenesis would arise in preadipocytes expressing naturally occurring (e.g. in toxic thyroid adenoma) gain-of-function TSHR mutations (which like thyroid stimulating antibodies, activate adenylate cyclase/PKA), but this was not the case. It should be noted that the increase in cAMP in preadipocytes using retroviral vectors to express the mutant receptor is considerably lower than that obtained following overexpression by transfection of COS cells. The model more closely resembles the in vivo situation, in which modest elevation of cAMP is sufficient to generate a thyroid toxic nodule, and concurs with our previous findings in thyroid cells (Ludgate et al. 1999, Fuhrer et al. 2003). The constitutively active mutant TSHR responded further when stimulated with TSH, in common with other such mutants, although its TSH responsiveness was less than the WT TSHR (data not shown) as reported by others http://innere.uniklinikum-leipzig.de/tsh/frame.html.
We have then used a gain-of-function mutation in a second component of the PKA pathway, Gsα subunit, to achieve activation of CREB. Neither the receptor, nor its downstream G protein, induced spontaneous adipogenesis (even in the presence of IBMX) as would be expected from previous reports indicating that CREB activation is ‘necessary and sufficient for adipogenesis in 3T3L1’ (Reusch et al. 2000).
Experiments were then conducted to investigate the effects of TSHR* and gsp* on in vitro induced adipogenesis. The reduced differentiation in 3T3L1 expressing TSHR* mirror the results we obtained in human primary orbital preadipocytes (Zhang et al. 2006). Our demonstration of an inhibitory role for Gsα in adipogenesis confirms and extends the findings of Wang et al. (1992) who demonstrated enhanced differentiation in 3T3L1 cells treated with oligomers antisense to the protein. Subsequently, they (Wang & Malbon 1999) reported that Gsα repression of adipogenesis is mediated by the tyrosine kinase Syk, but how this may impact adipogenesis is not clear and we are not aware of further studies to address this question. Our investigations of the pathway confirmed their findings, i.e. an early and transient increase in phospho-SYK in the first day following induced adipogenesis, but only in the non-modified population. The L629F and gsp* expressing cells showed a decrease in phospho-SYK levels (data not shown).
Our experiments illustrate that the failure to differentiate in gsp* is due to reduced expression of PPARγ1 and interference in the alternate promoter usage required to generate PPARγ2, the isoform specific to adipose tissue (Tontonoz et al. 1995).
There is some controversy concerning how PPARγ2 expression changes following addition of DM. Some authors report that it is induced, others that it is up-regulated. In our differentiation protocol; PPARγ2 protein expression increases in the first 24 h, but is then repressed until day 5 post-induction. Its reappearance seems to coincide with the start of the terminal differentiation phase and was a consistent finding in all experiments, but contrasts with the results obtained by others reporting that PPARγ2 expression steadily increases through all stages of differentiation (Saladin et al. 1999).
FOXO1 represses transcription from both the PPARγ1 and PPARγ2 promoters (Armoni et al. 2006) and so we investigated its participation in our different 3T3L1 populations. We observed a rapid increase in FOXO1 protein expression and its subsequent translocation to the nucleus during the first 3 days post-induction in non-modified 3T3L1. At later time points, the nuclear FOXO1 sustained a high degree of phosphorylation. The antibody we have used detects phosphorylation of FOXO1 at serine 256, located in the forkhead domain and indicative of inactivation of the transcription factor; there are two additional phosphorylation sites. Nakae et al. (2003) transduced cells with a tagged FOXO1 and were able to demonstrate a cytoplasmic location for phosphorylated FOXO1. In our experiments investigating endogenous FOXO1, it is predominantly cytoplasmic, then nuclear and then present in both fractions, in agreement with this work. Any phosphorylated endogenous FOXO1 in our nuclear extracts is probably partially phosphorylated (on serine 256, the preferred target for the kinase) and thus inactive. Our experiment also revealed that phosphorylation of FOXO1 is impeded in the gsp* population from day 3 post-induction onwards and so provides some explanation for their lack of adipogenesis.
At no time point is signalling via phospho-Akt compromised and yet FOXO1 phosphorylation had remained low in the gsp* cells. Investigation of two recently described modulators of adipogenesis, ID2 and PROF suggested they may have a role in explaining this anomaly. PROF, also known as WDFY2, is a member of the WD-repeat propeller-FYVE protein family, which is a binding partner for phospho-Akt and facilitates FOXO1 phosphorylation (Fritzius & Moelling 2008). In silico analysis of its proximal promoter reveals the presence of a potentially functional half-site cAMP response element; however, it lacks a ‘tata’ box, generally thought to be required for robust transcriptional induction by cAMP (Zhang et al. 2005). Our investigations demonstrate that its expression level, although higher in basal conditions, remained fairly constant at all time points in the gsp* cells following addition of DM, in contrast to the marked increase in the non-modified 3T3L1.
We also considered the possibility that the excess Gsα in the gsp* expressing cells might sequester free Gβγ subunits generated during adipogenesis and thus impede their downstream effects. However, the mutation in α renders it permanently in the GTP-bound form and thus less likely to bind βγ (Ford et al. 1998).
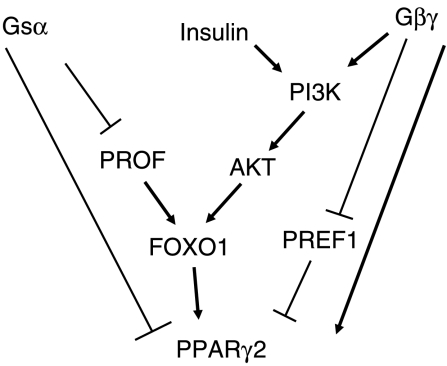
In the majority of our experiments, the behaviour of the TSHR* population is mid-way between that of the gsp* expressing and parent cell lines, indicating that the inhibitory effects of Gsα are abrogated, presumably by the β/γ subunits, which in the thyroid have been reported to signal by PI3K (Zaballos et al. 2008). PREF1 inhibits adipogenesis by preventing induction of PPARγ2 (Kim et al. 2007). The significantly enhanced down-regulation of PREF1 in the TSHR* expressing cells may contribute to the rescue mechanism. The drawing depicted in Fig. 8 summarises the mechanism we propose to explain the difference in adipogenic potential of the TSHR* and gsp* expressing 3T3L1 populations.
Figure 8.
A drawing summarising the effects of signalling via Gsα and Gβγ subunits in regulating adipogenesis via expression of PPARγ2.
In conclusion, our results identify an important role for Gsα signalling in inhibiting the alternative promoter usage necessary to produce PPARγ2 transcripts.
Declaration of interest
There is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
Supported by The Wellcome Trust (grant number WT076003MA).
Acknowledgements
We would like to thank Prof. Maurice Scanlon for his continued support.
References
- Al-Khafaji F, Wiltshire M, Fuhrer D, Mazziotti G, Lewis MD, Smith PJ, Ludgate M. Biological activity of activating TSHR mutants: modulation by iodide. Journal of Molecular Endocrinology. 2005;34:209–220. doi: 10.1677/jme.1.01590. [DOI] [PubMed] [Google Scholar]
- Armoni M, Harel C, Karni S, Chen H, Bar-Yoseph F, Ver MR, Quon MJ, Karnieli E. FOXO1 represses peroxisome proliferator activator receptor gamma 1 and gamma 2 gene promoters in primary adipocytes – a novel paradigm to increase insulin sensitivity. Journal of Biological Chemistry. 2006;28:19881–19891. doi: 10.1074/jbc.M600320200. [DOI] [PubMed] [Google Scholar]
- Boone C, Gregoire F, Remacle C. Various stimulators of the cyclic AMP pathway fail to promote adipose conversion of porcine preadipocytes in primary culture. Differentiation. 1999;64:255–262. doi: 10.1046/j.1432-0436.1999.6450255.x. [DOI] [PubMed] [Google Scholar]
- Drolet R, Richard C, Sniderman AD, Mailloux J, Fortier M, Huot C, Rheaume C, Tchernov A. Hypertrophy and hyperplasia of abdominal adipose tissues in women. International Journal of Obesity. 2008;32:283–291. doi: 10.1038/sj.ijo.0803708. [DOI] [PubMed] [Google Scholar]
- Ford CE, Skiba NP, Bae H, Daaka Y, Reuveny E, Shekter LR, Rosal R, Weng G, Yang C-S, Iyengar R, et al. Molecular basis for interactions of G protein βγ subunits with effectors. Science. 1998;280:1271–1274. doi: 10.1126/science.280.5367.1271. [DOI] [PubMed] [Google Scholar]
- Fritzius T, Moelling K. Akt- and Foxo1-interacting WD-repeat-FYVE protein promotes adipogenesis. EMBO Journal. 2008;27:1399–1410. doi: 10.1038/emboj.2008.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrer D, Lewis MD, Al-Khafaji F, Starkey K, Paschke R, Wynford-Thomas D, Eggo M, Ludgate M. Biological activity of activating TSHR mutants depends on the cellular context. Endocrinology. 2003;144:4018–4030. doi: 10.1210/en.2003-0438. [DOI] [PubMed] [Google Scholar]
- Haraguchi K, Shimura H, Lin L, Saito T, Endo T, Onaya T. Functional expression of thyrotropin receptor in differentiated 3T3-L1 cells: a possible model cell line of extrathyroidal expression of thyrotropin receptor. Biochemical and Biophysical Research Communications. 1996;223:193–198. doi: 10.1006/bbrc.1996.0868. [DOI] [PubMed] [Google Scholar]
- Hu E, Kim JB, Sarraf P, Spiegelman BM. Inhibition of adipogenesis through MAP kinase-mediated phosphorylation of PPAR. Science. 1996;274:2100–2103. doi: 10.1126/science.274.5295.2100. [DOI] [PubMed] [Google Scholar]
- Ivan M, Ludgate M, Gire V, Bond J, Wynford-Thomas D. An amphotropic retroviral vector expressing a mutant Gsp oncogene: effects on human thyroid cells in vitro. Journal of Clinical Endocrinology and Metabolism. 1997;82:2702–2709. doi: 10.1210/jcem.82.8.4122. [DOI] [PubMed] [Google Scholar]
- Kim KA, Kim JH, Wang Y, Sul HS. Pref-1 (preadipocyte factor 1) activates the MEK/extracellular signal-regulated kinase pathway to inhibit adipocyte differentiation. Molecular and Cellular Biology. 2007;27:2294–2308. doi: 10.1128/MCB.02207-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludgate M, Gire V, Crisp M, Ajjan R, Weetman A, Ivan M, Wynford-Thomas D. Contrasting effects of Gsp and activating mutations of the thyrotropin receptor on the function and proliferation of thyroid follicular cells. Oncogene. 1999;18:4798–4807. doi: 10.1038/sj.onc.1202864. [DOI] [PubMed] [Google Scholar]
- Nakae J, Kitamura T, Kitamura Y, Biggs WH, III, Arden KC, Accili D. The forkhead transcription factor Foxo1 regulates adipocyte differentiation. Developmental Cell. 2003;4:119–129. doi: 10.1016/s1534-5807(02)00401-x. [DOI] [PubMed] [Google Scholar]
- Park KW, Waki H, Villanueva CJ, Monticelli LA, Hong C, Kang S, MacDougald OA, Goldrath AW, Tontonoz P. Inhibitor of DNA binding 2 is a small molecule-inducible modulator of peroxisome proliferator-activated receptor-γ expression and adipocyte differentiation. Molecular Endocrinology. 2008;22:2038–2048. doi: 10.1210/me.2007-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschke R, Ludgate M. The thyrotropin receptor and thyroid disease. New England Journal of Medicine. 1997;337:1675–1681. doi: 10.1056/NEJM199712043372307. [DOI] [PubMed] [Google Scholar]
- Reusch JEB, Colton LA, Klemm DJ. CREB activation induces adipogenesis in 3T3-L1 cells. Molecular and Cellular Biology. 2000;20:1008–1020. doi: 10.1128/mcb.20.3.1008-1020.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaue H, Ogawa W, Matsumoto M, Kuroda S, Takata M, Sugimoto T, Spiegelman BM, Kasuga M. Posttranscriptional control of adipocyte differentiation through activation of phosphoinositide 3-kinase. Journal of Biological Chemistry. 1998;273:28945–28952. doi: 10.1074/jbc.273.44.28945. [DOI] [PubMed] [Google Scholar]
- Saladin R, Fajas L, Dana S, Halvorsen YD, Auwerx J, Briggs M. Differential regulation of peroxisome proliferator activator receptor gamma 1 (PPAR gamma 1) and PPAR gamma 2 messenger RNA expression in the early stages of adipogenesis. Cell Growth & Differentiation. 1999;10:43–48. [PubMed] [Google Scholar]
- Smas CM, Sul HS. Pref-1, a protein containing EGF-like repeats, inhibits adipocyte differentiation. Cell. 1993;73:725–734. doi: 10.1016/0092-8674(93)90252-l. [DOI] [PubMed] [Google Scholar]
- Starkey KJ, Janezic A, Jones G, Jordan N, Baker G, Ludgate M. Adipose thyrotropin receptor expression is elevated in Graves' and thyroid eye diseases ex vivo and indicates adipogenesis in progress in vivo. Journal of Molecular Endocrinology. 2003;30:369–380. doi: 10.1677/jme.0.0300369. [DOI] [PubMed] [Google Scholar]
- Tang QQ, Otto TC, Lane MD. Mitotic clonal expansion: a synchronous process required for adipogenesis. PNAS. 2003;100:44–49. doi: 10.1073/pnas.0137044100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tontonoz P, Hu E, Devine J, Beale EG, Spiegelman BM. PPAR2 regulates adipose expression of the phosphoenolpyruvate carboxykinase gene. Molecular and Cellular Biology. 1995;15:351–357. doi: 10.1128/mcb.15.1.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderpump MPJ, Tunbridge WMG, French JM, Appleton D, Bates D, Clark F, Grimley J Evans, Hasan M, Rodgers H, Tunbridge F, et al. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham Survey. Clinical Endocrinology. 1995;43:55–68. doi: 10.1111/j.1365-2265.1995.tb01894.x. [DOI] [PubMed] [Google Scholar]
- Vassart G, Dumont JE. The thyrotropin receptor and the regulation of thyrocyte function and growth. Endocrine Reviews. 1992;13:596–611. doi: 10.1210/edrv-13-3-596. [DOI] [PubMed] [Google Scholar]
- Wang HY, Malbon CC. Gs alpha inhibition of adipogenesis is via SYK. Journal of Biological Chemistry. 1999;274:32159–32166. doi: 10.1074/jbc.274.45.32159. [DOI] [PubMed] [Google Scholar]
- Wang HY, Watkins DC, Malbon CC. Antisense oligodeoxynucleotides to Gs proteinα-subunit sequence accelerate differentiation of fibroblasts to adipocytes. Nature. 1992;358:334–337. doi: 10.1038/358334a0. [DOI] [PubMed] [Google Scholar]
- Zaballos MA, Garcia B, Santisteban P. Gβγ dimers released in response to TSH activate phosphoinositide 3 kinase and regulate gene expression in thyroid cells. Molecular Endocrinology. 2008;16:342–352. doi: 10.1210/me.2007-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Odom DT, Koo S-H, Conkright MD, Canettieri G, Best J, Chen H, Jenner R, Herbolsheimer E, Jacobsen E, et al. Genome-wide analysis of cAMP-response element binding protein occupancy, phosphorylation, and target gene activation in human tissues. PNAS. 2005;102:4459–4464. doi: 10.1073/pnas.0501076102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Baker G, Janus D, Paddon C, Fuhrer D, Ludgate M. Biological effects of thyrotropin receptor activation on human orbital preadipocytes. Investigative Ophthalmology & Visual Science. 2006;47:5197–5203. doi: 10.1167/iovs.06-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]