Abstract
Cerebrospinal fluid (CSF) has frequently been studied to explore the total metal concentrations in patients with neurodegenerative diseases. Some examples of neurologic diseases include but are not limited to intracerebral hemorrhage, intraventricular hemorrhage, traumatic brain injury, subarachnoid hemorrhage and hydrocephalus. In this study, however, a comprehensive approach was begun using metallomics methods. First, two molecular weight cutoff filters were used to separate CSF constituents by molecular weight. The remaining CSF was then separated with capillary liquid chromatography/normal bore liquid chromatography and analyzed with inductively coupled mass spectrometry (ICPMS). With this ICPMS screening, a possible iron associated protein was suggested by nanoliquid chromatography-CHIP/ion trap mass spectrometry (nanoLC-CHIP/ITMS) identification in conjunction with a Spectrum Mill database search. In this preliminary study, three different types of pooled CSF were partially characterized by their metal (Pb, Mg, Zn, Fe and Cu) containing species with suggestions for fuller studies. Chemical `differences' in the CSF and metal constituents suggests some utility in this analysis for understanding some of the complications observed following subarachnoid hemorrhage.
Keywords: metals, metalloproteins, ICPMS, nanoLC-CHIP/ITMS, stroke, vasospasm, cerebrospinal fluid, CSF
Introduction
Cerebrospinal fluid (CSF) is a secretion product of several different central nervous system (CNS) structures, in particular the ventricular chorioid plexus.1 It surrounds the brain, spinal column, as well as the optic nerve up to and including the optic disk, and as a relatively undiluted draining system from the brain, it reflects several different disorders of the CNS.1 Proteomics has been used to analyze CSF in order to discover disease-associated proteins (biomarkers) and to explain the basic molecular mechanisms that either cause or are a consequence of CNS disorders. Recently, there has been a new area that has been gaining attention in proteomics, and that is the challenge of identifying metal-containing proteins or metalloproteins.2-5 Metalloproteins are thought to play a vital role in biological systems, and to be contributors to neurodegenerative diseases. Many biologically active proteins require specific metals and generally with specific ionization states for their control and or normal function. For this reason, metals in CSF have been measured to evaluate the correlation between their levels and the development of neurodegenerative diseases: trace metals in Parkinson's disease,6 multiple sclerosis,7 Alzheimer's disease;8,9 Cu in Wilson's disease,10 Cr, Cs and Sn in patients with brain tumors11 and Mg in ischemic stroke.12 Total metal content (Fe, Cu, Mn, Zn, Mg, Pb, Al, Cr, Cs) in CSF has been determined by ICPMS,6,7 atomic absorption spectrometry (AAS),7,8 and flameless AAS.
However, more useful than total metal concentrations is to determine metal species, which may lead to better understanding of the functions of metals in CSF, their interactions with each other and the interactions with the protein(s), hence, the reference to this being a “metallomics study,” formed by the availability of metals information in this complex biological environment. Speciation studies of the metals of interest associated with proteins are essential to elucidate the role that the metals play in the structures and the functions of biological compounds in CSF. The most attention in the field of biomedical speciation analysis has been with the essential metals, Fe, Cu, Zn, and toxic metals, Al, Cr, Pb, Cd and Hg. Their speciation has been studied in serum, urine, erythrocytes and breast milk.5 Hall et al. described a method for the determination of Pb-binding ligands in amniotic fluid using high-performance size-exclusion chromatography (HPSEC) coupled with ICPMS.13 Good separation of Pb-binding ligands was obtained and identified with peaks corresponding to ceruloplasmin, prealbumin and a Zn-binding peptide. A study by Bergdah et al. found Pb in at least three molecular weight fractions in human serum.14 The major part of Pb is coincident with Cu and was found to be bound to ceruloplasmin. Of the protein-bound Pb recovered, 80% was reported to be contained in proteins with an apparent molecular mass of ca. 240 kDa, and 20% in proteins with an apparent molecular mass of ca. 45 kDa.14 Godlewska et al. did a study in human serum and found Mg to be associated with both the albumin and globulin fractions but not with transferrin.15 A method for the speciation of protein-binding zinc and copper in human blood serum by chelating resin pretreatment and ICPMS detection was studied by Inagaki et al.16 It was shown by SEC-ICPMS that albumin-Zn and albumin-Cu (loosely bound species) could be selectively removed from serum by adsorption on the Chelex-100 resin, while 2-macroglobulin-Zn and ceruloplasmin-Cu (firmly bound species) remained in the serum.16
Two recent speciation studies in CSF were by Michalke et al., studying Mn speciation in human CSF, using capillary zone electrophoresis (CZE) and size-exclusion chromatography (SEC) coupled with ICPMS.17,18 In these studies, Mn is separated using the two different techniques. Specific metal proteins were not identified.
The preparation of CSF samples also makes studying this sample type difficult. The high salt concentration and the low protein concentration with a large dynamic range in CSF also make detection difficult. Because of their excellent detection capabilities, matrix-assisted laser desorption/ionization (MALDI) and electrospray ionization (ESI) MS have been widely used for protein identification in CSF, after the total separation of proteins by 2-D electrophoresis. However, the digestion of CSF proteins with trypsin before MS analysis compromises the detection of metals associated with CSF proteins, as some of these associations may be weak, therefore, compromised during the sample preparation. For this reason, the CSF is treated gently in this initial study.
CapLC-ICPMS is used for metal determination because it is a sensitive and powerful mass spectrometric technique for the fast and easy detection of metals at trace levels in biological samples.5 Further, using capLC with ICPMS detection is relatively new compared to std. bore LC with ICPMS detection and an important advance with CSF, since it allows the use of strong solvent mobile phases, whereas std. bore LC has limitations of no more than 20% methanol without postcolumn splitting (because this leads to carbon buildup on the cones and lenses of the ICPMS, plasma instability, and eventually the plasma will extinguish). The stronger solvent capability allows better chromatography with high MW separations. An additional novel part of this study is using a commercially available microfludic nanoLC-CHIP/ITMS instrument that was introduced for proteomics analysis in 2005.19-21 This instrument is reliable, easy to use, with good resolution, sensitivity, and good reproducibility for obtaining qualitative information of complex biological samples.22-25
Sample Background
The three types of CSF samples investigated were normal CSF, and samples from patients that had an aneurysmal subarachnoid hemorrhage (SAH). SAH results from a ruptured saccular aneurysm.26 This neurological event can cause death or disability in a significant percentage of patients. For the patients that do survive, rebleeding from the aneurysm and the complications induced by blood in the subarachnoid spaces27-29 are major concerns. The aneurysmal SAH CSF samples are from patients that either had a cerebral vasospasm complication (cerebral vasospasm is a frequent and often fatal complication of hemorrhagic stroke. The putative relevance to the study herein is that the vasospasm is thought to be caused by substances found in the CSF of these patients; this condition is indicated as vas in this study) or did not have the vasospasm complication (indicated as non-vas in this study). Of the SAH patients who survive, as many as 30-40% of the survivors suffer the complication of cerebral vasospasm following the original SAH, and a significant number of the patients with vasospasm will die or have a severely impaired poststroke outcome.27-29 These strokes that occur after a vasospasm are attributable to pathological vasoconstriction of vessels with secondary ischemia and infarction.27,29 Currently, the reason for the cerebral vasospasm is not known.29 Therefore, the goal of this initial study is to begin studies that may ultimately suggest biomarkers to assist in predicting vasospasm. CapLC-ICPMS will be used as a screening tool for any ultimate metal species differences in these different patient populations with, first, an initial attempt at protein identification by utilizing the nanoLC-CHIP/ITMS system as proof of concept.
Metals of Interest
The capability of ICPMS to simultaneously monitor several metals at different concentration levels has allowed the study of Pb, Mg, Zn, Fe and Cu in CSF samples. The metals of interest are listed below. This research is a collaboration of Department of Chemistry scientists with neuroscientists at the University of Cincinnati, Department of Neurology. These metals were chosen by the researchers in the Department of Neurology based on their putative roles in the pathology and complication risk profiles of the patients being investigated. The list of metals below and the reasons of interest for studying them in this patient population are from published and unpublished references.29
1. Lead
Chronically elevated levels of serum Pb have been shown to inhibit vascular endothelial cell proliferation,30 which may impact on recovery of the cerebral vasculature after vasospasm. Pb also increases intravascular superoxide and freeradical damage,31 as well as altering vascular reactivity,32 leading to lead-induced hypertension, developmental disorders, neurotoxicity and death.33
2. Magnesium
Magnesium has long been known to be vasoprotective34,35 due mainly to binding of, and antagonism by, Mg at calcium channels in smooth muscle.36-41 It is used clinically, as magnesium sulfate, to treat preeclampsia.34,42,43 In neurosurgical critical care, it is considered important to keep Mg levels within normal ranges to ensure an appropriate environment for neurologic recovery.44
3. Zinc
Zinc is a cofactor for thousands of mammalian proteins; therefore, Zn toxicity and deficiency have a wide range of physiological manifestations.45,46 Zn is also known to be particularly required by the immune system,45 and recently, it has been found to be essential for normal vascular endothelial function, with both deficiency and toxicity disrupting normal endothelial function leading to hypertension.
4. Copper
Copper is an essential element for the activity of a number of physiologically important enzymes; therefore, enzyme related malfunctions may contribute to severe neurological symptoms.
5. Iron
Fe is a cofactor for many proteins and enzymes and is a metabolite of the HO1-mediated degradation of hemoglobin.47-50 It is toxic in its free form, and a number of serum and CSF proteins are constantly circulating that can bind iron until it is again incorporated into a protein.51 Iron may also act as a pseudoperoxidase (i.e., have antioxidant as well as pro-oxidant capacity).52
This study highlights the differences seen between lead, magnesium, zinc, iron and copper species in CSF using ICPMS as a screening tool with one extension to nanoLC-CHIP-IT/MS for protein identification. These preliminary data are presented with the view that metal speciation in CSF is of vital importance to understand the role of trace metals in patients who have experienced a subarachnoid hemorrhage and its further complications. This type of study will then extend to other patients with neurodegenerative diseases where metals are predicted to play a role and an increase to appropriate sample numbers for assuring statistical validity.
Experimental Section
Chemicals and Standards
HPLC solvents, water and acetonitrile used were of high purity, and were purchased from Burdick and Jackson (Muskegon, MI). Tris-aminomethane was purchased from (Acros Organics, NJ). Acetic acid and ammonium acetate were bought from Fisher Scientific (NJ), and were prepared by dilution of the solid salts with distilled deionized water. CSF was kindly provided by the Department of Neurology at the University of Cincinnati, OH; with all appropriate institutional review board approvals and documentation.
Sample Preparation
The general protocol for this study is to first use chromatography for separating the metal species and then detecting with ICPMS. This allows screening of individual metal species. In this study, three different molecular weight regions of the CSF (MW < 5 kDa, MW 5-50 kDa, MW > 50 kDa) were isolated by ultrafiltration. Reversed phase (RP) chromatography was used because of the range of different hydrophobic properties of CSF proteins, and hopefully separation of metalloproteins. For metals associated with proteins in the <50 kDa range, fast protein liquid chromatography (FPLC) was used. This is the first use of an anion exchange column by Pharmacia Biotech for rapid separation and reproducible profiling of the CSF proteins (to our knowledge, the complete separation mechanism allowing FPLC is still proprietary). In this preliminary study, metal elements were screened using capLC-ICPMS with the goal of studying and monitoring the differences (amount; modifications) of metal moieties associated with CSF peptides and proteins and to develop protocols that would be applicable to the lower molecular weight metabolites. This analytical method was chosen for its effectiveness at targeting metals in metalloproteins plus other species and their association with CSF.
Pooled CSF samples from each of the three different patient populations were studied: normal, nonvasospastic (non-vas), vasospastic (vas) (200 μL from each of four samples to make a total of 800 μL) were prefractionated in three different molecular weight ranges (MW < 5 kDa, MW 5-50 kDa, MW > 50 kDa) by using spin concentrators with cutoff points of 5 and 50 kDa (Agilent, Santa Clara, CA). Vasospasm designation was made using conventional diagnostic criteria.44 In the first step, the CSF samples were loaded into a 50 kDa spin concentrator and spun in a centrifuge (5000g, 20 min at 4 °C) to remove, inasmuch as possible, the most abundant proteins in the CSF such as albumin, transferrin and immunoglobulins. To enrich lower abundant CSF proteins, the eluate of the 50 kDa membrane was passed again through a 5 kDa spin concentrator. The eluted sample was collected as the >5 kDa sample; the retained portion was then the 5-50 kDa sample. The samples were passed through 0.22 μm filters (Agilent Technologies, Santa Clara, CA) prior to LC separation.
Instrumentation and Chromatographic Separation
The ICPMS instrument used was an Agilent 7500ce (Santa Clara, CA) fitted with a MicroMist nebulizer and Scott-type double pass quartz spray chamber. The following ICPMS conditions were used; Rf power, 1550 W; nebulizer carrier gas pressure, 90 psi; sampling depth, 7-8 mm; collision/reaction cell flow rate, 3.8 mL/min He; octopole bias, -18 V; quadrupole bias, -16 V for a net +2 V energy discrimination barrier. Agilent 1100 and 1200 LC systems were used for std. bore and capillary liquid chromatography (Santa Clara, CA), respectively. Both Agilent LC systems, 1100 for normal flow and the 1200 for capillary flow, consist of a binary pump, degasser, chilled autosampler, and a variable wavelength UV detector, so the absorbance of the CSF proteins or other chromophores was recorded at different wavelengths (280, 210, 254 and 460 nm). Thus, the UV molecular and elemental chromatograms were recorded sequentially, but within a very short time of each other. The 1200 capLC system was interfaced to the ICPMS through commercially available PEEK-coated silica tubing. The PEEK tubing from the capLC was connected to the commercially available D5-5 (Cetac Technologies, Omaha, NE) capillary nebulizer. For the preliminary molecular MS study, an Agilent 6300 Series HPLC-CHIP/Ion Trap XCT system was used.
There were two columns investigated for the separation of the metals in CSF using reversed phase chromatography on the capillary LC system (Zorbax and FPLC). The first column used was a Zorbax 300SB C18 column (5 μm, 150 × 0.5 mm) (Agilent, Palo Alto, CA). The injection volume on the capillary system was 8.0 μL and a flow rate of 10.0 μL /min was used with a gradient system. The mobile phase was prepared from two solutions (solvent A, 100% H2O, 0.1% formic acid (FA); B, 90% acetonitrile (ACN), 10% H2O, and 0.1% FA). Gradient elution was performed as follows: A and B (A, 100% H2O, 0.1% FA; B, 90% ACN, 10% H2O, and 0.1% FA), at a flow rate of 10.0 μL/min with the following gradient conditions: 0-5 min, 0% B; 5-25 min, 40% B; 25.01-32 min, 45% B; 32-47 min, 45% B; 47-49 min, 70% B; 49.01-55 min 0% B. The eluate was passed through the UV detector into the ICPMS.
The chromatographic separations of the human CSF proteins with a molecular weight higher than 50 kDa were carried out with anion-exchange fast protein liquid chromatography (FPLC) Mono-Q HR 5/5 column (50 × 5 mm id, Pharmacia Biotech, Uppsala, Sweden) connected to an Agilent 1100 LC pump (Palo Alto, CA). Mobile phases: A, 25 mM Tris-aminomethane/acetic acid, pH 6.5; B, 250 mM ammonium acetate. The CSF proteins were separated by means of a linear gradient of ammonium acetate (0-250 mM in 45 min) buffered by 25 mM Tris-acetic acid (pH 6.5) solution at a flow rate of 1 mL/min with an injection volume of 100 μL.
Results
This is the first study for the above metals in CSF by LC-ICPMS and one of the first speciation studies of metals in CSF, which is unique regarding the vasospastic patient population.
Lead, Magnesium, Zinc, Copper
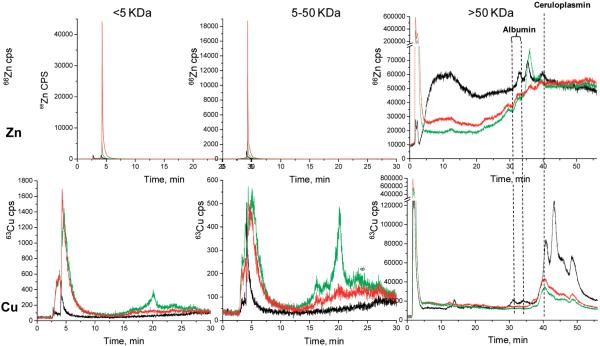
As shown in Figure 1 and Figure 2 the >5 kDa fraction has some differences seen between samples types in the different metals screened. The vas sample has considerably more Zn than either the non-vas or normal samples in the 3-4.5 min peak, where the dead volume comes at about 2.5 min. This is noted for the three MW regions, but the higher MW region has appreciably more structure than the other two and includes some well-known high MW proteins. These chromatograms show some peak identification for metals species associated with prealbumin, albumin, and ceruloplasmin based on retention time matching of pure proteins. Since zinc is consistently higher in the vasospasm patients, this may be important because the immune system can contribute to the generation of toxic metabolites. However, it is unknown at this time where the Zn and the other metals discussed specifically originate.
Figure 1.
LC-ICPMS screening of Cu and Zn in CSF by capLC-ICPMS. CSF has been ultrafiltered into three molecular fractions; <5, 5-50, and >50 kDa.: black line, normal CSF; green line, non-vas; red line, vas.
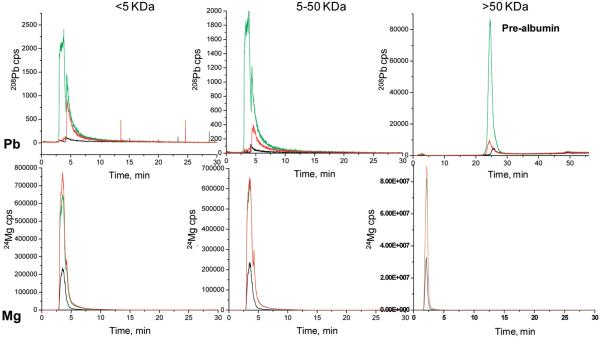
Figure 2.
LC-ICPMS screening of Mg and Pb in CSF by capLC-ICPMS. CSF has been ultrafiltered into three molecular fractions; <5, 5-50, and >50 kDa.: black line, normal CSF; green line, non-vas; red line, vas.
For Cu, non-vas and vas samples appear to be the same in the first half of the chromatogram; however, there is a Cu peak for non-vas around 20 min. For both Zn and Cu, it is important to follow these sample fractions from ICPMS with molecular MS identification techniques, to establish the molecular level differences, at least to some extent, thereby adding important information regarding molecular level changes between diseased or nondiseased states.
All three sample types appear to have Mg in the 3-5 min range. And it can be noted for all the MW regions, Mg clearly predominates in the diseased samples. The normal sample did not have any Pb. The non-vas fraction has a large peak from 3 to 4.5 min that is not seen in the vas sample, and both of the latter have a peak from 4.5 to 6 min. These fractions will then be taken for further evaluation by nanoLC-CHIP/ITMS. Pb appears in the diseased samples at concentrations well above those for the normal sample. At this point, it is at least interesting that the Pb appears in the diseased samples, but not in the normal one, since Pb must be acquired through environmental factors. No conclusions, though, can be suggested until more samples are done.
The 5-50 kDa fractions for each of the 4 metals Pb, Mg, Zn, Cu are the same for this mass region as for the >5 kDa fractions. This is probably due in part to the likelihood that not 100% of all the proteins/compounds in CSF under 5 kDa are spun down through the spin concentrators. Also, there may have been protein decomposition in the sample collection through preparation. SEC is likely a better choice for future work.
The <50 kDa chromatograms are much different than the other two regions. This also is completely different chromatography (anion-exchange, FPLC). As indicated earlier,53 it is not surprising that Cu and Zn match up with the ceruloplasmin and albumin proteins, because ceruloplasmin is known to carry 90% of the copper in human plasma with the other 10% being carried by albumin (human plasma is the closest comparison to CSF that can be found in the literature with respect to electrolyte composition). Further justifying this comparison is the understanding that the SAH patients' blood is added to and mixed with the CSF during the stroke. However, because of relatively inconsistent volumes of blood mixed with the CSF during the SAH, the amounts of these proteins can vary greatly.
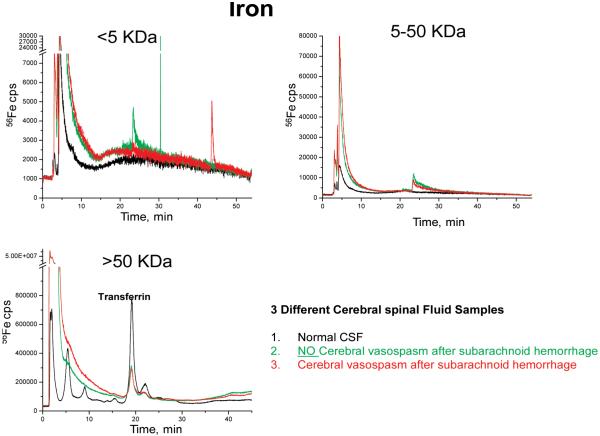
Currently, these results cannot be compared to other speciation studies in CSF, because no studies could be found for a valid comparison. The only speciation study found was using CZE-ICPMS to analyze which of the compounds found in CSF are bound to Mn (and Mn was not studied in this work).17,18 Much more work can be done as a continuation to this study, by determining to which proteins these metals are bound or compounds of which they are a part. As a first step toward this goal, capLC-ICPMS was used to screen for Fe, in the manner indicated above. To extend the study beyond speciation to identify the proteins to which the Fe is bound or associated with, nanoLC-CHIP/ITMS was used. CapLC-ICPMS was used for metal screening, and nanoLC-CHIP/ITMS was used to provide corresponding molecular weight information. Fractions containing an Fe trace from screening by the LC-ICPMS were collected and run separately on nanoLC-CHIP/ITMS followed by database searching with Spectrum Mill.
Iron
Iron showed interesting speciation compared to the other metals. For this proof-of-concept experiment, Fe was used as the metal to be collected and investigated using the method developed. As shown in Figure 3, in the >5 kDa fraction, there were different Fe traces for each of the 3 different sample types. The 5-50 kDa MW fraction had a peak at ~24 min in the vas and non-vas sample, which was not present in the normal sample. The <50 kDa fraction showed the normal sample with the most intense Fe signal corresponding to possible transferrin elution.53 Again, there were no studies reported that investigated speciation of Fe in CSF, but a few were found comparing Fe in human serum. Sariego Muniz et al. used anion-exchange chromatography coupled with double focusing ICPMS for speciation of Fe, Cu and Zn in human serum.54 In this study, some speciation differences were observed between the different study populations, particularly for Fe speciation.54 For that reason, fractions of Fe were collected and run on the nanoLC-CHIP/ITMS for further identification to try and determine if it is possible to identify the proteins with which they are bound or associated.
Figure 3.
LC-ICPMS screening of Fe in three molecular weight fractions.
1. NanoLC-CHIP/ITMS and Protein Identification
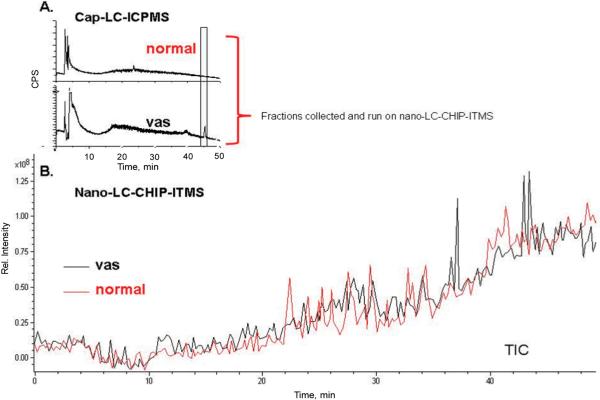
For further characterization for the fractions of interest from LC-ICPMS, the capLC was disconnected from the ICPMS so that fractions could be collected (fractions collected are shown in the rectangle in Figure 4). The CHIP column used for the experiment had the same packing material (Zorbax 300SB C18) as the one used for the ICPMS experiment.
Figure 4.
(A) CapLC-ICPMS screening of Fe in normal and vas samples, with fractions collected shown in box. (B) TIC from nanoLC-CHIP/ITMS of fractions collected.
Database searching was performed using Spectrum Mill (Rev. A. 03.02) (Agilent Technologies, Santa Clara, CA). The selected parameters used for the searches were the following: data searched against the Swiss-Prot database; taxonomy, mammal; enzyme, none specified 2 missed cleavages; mass tolerance of precursor ions and product ions, (±0.8 Da). For database searching with Spectrum Mill, the autovalidation guidelines were adapted from previous studies.55,56 The identified peptides were searched using autovalidation criteria; minimum score for fragmentation of 1+,2+, and 3+ were 8, with a score peak intensity (SPI) of at least 70%. However, for peptides with a 2+ or 3+ fragmentation and SPI of 80+%, a score of greater than 6 was considered.
Shown in Figure 4 is the 56Fe signal for both a pooled sample of vasospastic CSF and normal CSF separated by capLC-ICPMS. The ICPMS trace shows different traces, particularly above 20 min. Both sample types have large peaks in the first 3-6 min, but both show different smaller peaks following, indicating possibly important differences in the diseased versus nondiseased sample types. Figure 4 shows the fractions that were collected for nanoLC-CHIP/ITMS.
The fractions collected from the capLC for the vas and normal CSF samples (Figure 4) were injected into the nanoLCCHIP-ITMS system (the same solvent system was used for both experiments and no additional sample preparation was done).
As shown in Figure 4, and Table 1, from the vasospasm sample, a protein suggested as receptor activity-modifying protein, RAMP 2, was identified by Spectrum Mill at 100% SPI around the retention time that would be expected from a fraction collected from the capLC-ICPMS at 45 min. (From running repeated experiments, the capLC-ICPMS fraction is expected to elute ca. 3 min earlier on the nanoLC-CHIP/ITMS because of chromatographic setup differences.) This result is encouraging because the protein RAMP 2 has been associated with a receptor called adrenomedullin that is one of the key suspects in the mechanism involved in SAH induced vasospasm.57,58 There have been many studies since the discovery of adrenomedullin in 1993 that correlate it with the RAMP and its role in patients with subarachnoid hemorrhage.59 It has been found by Kikumoto et al.57 and Fujioka et al.,60 that plasma concentrations of adrenomedullin are increased in patients that had a subarachnoid hemorrhage, and the level of adrenomedullin correlates with the clinical condition of those patients.61 Fujioka et al. found that CSF levels of adrenomedullin had a positive correlation to vasospasm post SAH.60 Neither a cause and effect nor temporal relationship could be determined in this study. It is thought that the increased vasodilator, adrenomedullin in the CSF, may be indicative of a failure of the vascular smooth muscle to relax properly and, thus, result in higher levels of vasodilators present in the CSF. Wijdicks et al. have reported significant correlation between increased levels of circulating adrenomedullin and the presence of vasospasm after the subarachnoid hemorrhage.62 Patients suffering from a vasospasm had significantly higher levels of adrenomedullin in their CSF than those that had not suffered a vasospasm.60 Elevated plasma adrenomedullin concentrations were also suggested to be the consequence of increased sympathetic activity after subarachnoid hemorrhage.63 Therefore, the identification of the RAMP2 protein from a Fe fraction collected from a subarachnoid hemorrhage patient with the onset of a vasospasm shows the potential of elemental and molecular mass spectrometries for these types of studies, since RAMP2 was only seen in the SAH vasospastic fraction and not in the normal CSF. This result is hopeful and suggests that larger sample sets must be done and compared with pooled and individual samples, before any clinically valid conclusions can be reached.
Table 1.
Partial Results from Figure 4 after Spectrum Mill Data Search
| no. | score | SPI % | sequence | RT min | MH+ (Da) | protein MW (Da) | accession no. | protein name |
|---|---|---|---|---|---|---|---|---|
| 1 | 8.78 | 71.4 | (G)KASALEDQGSNPSSSQDSLHG(A) | 25.2 | 2547.2 | 73038.6 | 109018813 | PREDICTED: protein tyrosine phosphatase |
| 2 | 7.50 | 100.00 | (L)LGAVLKPQESLAQHFPTPDYLNLEGKTLE(E) | 43.7 | 20122.4 | 20122.4 | 47522976 | receptor activity-modifying protein - RAMP 2 |
It is important to characterize the metal binding sites in a metalloprotein, but there are still many challenges. There is no indication of Fe being present in the peptide reported for RAMP2. However, this appears to be the same with another metalloprotein, bovine cytochrome C, as discussed below. With metalloproteins, it is possible that the metal may not appear as part of the return from the spectral database search, complicating the interpretation, since the metal presence then comes only from ICPMS experiments. Also, in this case for the RAMP peptide, the reported sequence does not show any iron, yet it is possible that cysteine (c) and methionine (m) groups (12 residues) as well as oxygen and nitrogen could bind iron that might be lost in the sampling and preparation steps, although this is less likely, given the strength of the metal/sulfur bond. The amino acid sequence for RAMP 2 is:
1 maslraeraa ggprlpatra grpaaltlll llgavlkpqe slaqhfptpd ylnlegktle
61 enyetdaqlc whdykdmds ikkdwcdwal isrpysilqe cleqkadafk lgfpnpwaer
121 iifeahrihf ancslcqptf sdppedvlla mimvpiclip flvtvvwrs kdpeaqt
Kaltashov et al. have reported that transferrin fragmentation in the gas phase did not reveal the presence of any metal-containing fragments, even among the residues forming the metal coordination. Also, no significant difference was seen between apo- and haloprotein, even in the protein fraction containing one of the Fe3+ coordinating residues.64 They attribute this to the fact that the energy requirements (the energy required for dissociation of the metal is less than the energy required to fragment polypeptide bonds) for the dissociation of large polypeptide ions are such that cleavage of a polypeptide bond is always preceded by dissociation of metal cation from the protein, which erases any memory of the protein-metal interaction.64
Precise isentification for those in which the metal is weakly bound or associated is still a problem, plus the data systems may not contain full metalloprotein information. In an unpublished experiment in these laboratories, tryptically digested bovine cytochrome c was run by MALDI-MS. Nine peptides were returned by MASCOT and none of them contained Fe; however, CAQCHTVEK at 1018.2 m/z is the only cysteinecontaining peptide, and binds the Fe primarily through the porphyrin group. When the MW of the Fe porphyrin (including the several different Fe isotopes) is added to 1018.2, the several experimental mass spectral m/z values, reflecting the iron isotope pattern influenced by 13C, are reproduced. There is also the possibility that the collection procedure for the CSF might be contributing to the changes in oxidation states of the metals although this would need to be tested through further experiments.
Conclusions
Using capLC-ICPMS as a screening tool for metals in CSF is an effective and efficient approach for seeing metal differences between different patient populations. Differences were seen between the metals screened and the different sample populations with the metals associated in some way with other molecular entities, such as proteins. When combining capLC-ICPMS for metal screening and nanoLCCHIP/ITMS with molecular weight information in combination with Spectrum Mill, a protein previously linked to SAH patients has been identified. The method developed here, with further study, has the potential to identify metal trace species in CSF, as well as the proteins to which the metal are bound or associated.
Acknowledgment
The authors thank Agilent Technologies for their continuing support of our research by providing us with instrumentation for the University of Cincinnati/Agilent Technologies Metallomics Center of the Americas. We are grateful to the Neurosurgery Group at the University of Cincinnati for allowing us access to the CSF. Thanks go to Dr. Sarath Jayasinghe for the cytochrome c/ MALDI MS information.
References
- (1).Yuan X, Desiderio DM. J. Mass Spectrom. 2005;40:176–181. doi: 10.1002/jms.737. [DOI] [PubMed] [Google Scholar]
- (2).Gómez-Ariza JL, García-Barrera T, Lorenzo F, Bernal V, Villegas MJ, Oliveira V. Anal. Chim. Acta. 2004;524:15–22. [Google Scholar]
- (3).Prange A, Pröfrock D. Anal. Bioanal. Chem. 2005;383:372–389. doi: 10.1007/s00216-005-3420-0. [DOI] [PubMed] [Google Scholar]
- (4).Jakubowski N, Lobinski R, Moens L. J. Anal. At. Spectrom. 2004;19:1–4. [Google Scholar]
- (5).Szpunar J, Lobinski R. Hyphenated Techniques in Speciation Analysis. Royal Society of Chemistry; Cambridge: 2003. [Google Scholar]
- (6).Bocca B, Alimonti A, Petrucci F, Violante N, Sancesario G, Forte G, Senofonte O. Spectrochim. Acta, Part B. 2004;59:559–566. [Google Scholar]
- (7).Melo TM, Larsen C, White LR, Aasly J, Sjobakk TE, Flaten TP, Sonnewald U, Syversen T. Biol. Trace Elem. Res. 2003;93:1–8. doi: 10.1385/BTER:93:1-3:1. [DOI] [PubMed] [Google Scholar]
- (8).Molina JA, Jiménez-Jiménez FJ, Aguilar MV, Meseguer I. J. Neural Transmission. 1998;105:479–488. doi: 10.1007/s007020050071. [DOI] [PubMed] [Google Scholar]
- (9).Kessler H, Pajonk FG, Meisser P, Schneider-Axmann T, Hoffmann K-H, Supprian TH,W, Obeid RM,G, Falkai P, Bayer TA. Journal of Neural Transm. 2006;113:1763–1769. doi: 10.1007/s00702-006-0485-7. [DOI] [PubMed] [Google Scholar]
- (10).Stuerenburg HJ. J. Neural Transm. 2000;107:321–329. doi: 10.1007/s007020050026. [DOI] [PubMed] [Google Scholar]
- (11).El-Yazigi AM,CR, Siqueira EB. Clin. Chem. 1988;34:1084–1086. [PubMed] [Google Scholar]
- (12).Lampl Y, Geva D, Gilad R, Eshel Y, Ronen L, Sarova-Pinhas I. J. Neurol. 1998;245:584–588. doi: 10.1007/s004150050249. [DOI] [PubMed] [Google Scholar]
- (13).Hall GS, Zhu X, Martin EG. Anal. Commun. 1999;36:93–95. [Google Scholar]
- (14).Bergdah IA, Schüt A, Grub A. J. Anal. At. Spectrom. 1996;11:735–738. [Google Scholar]
- (15).Godlewska-Zylkiewicz B, Lesniewska B, Hulanicki A. Anal. Chim. Acta. 1998;385:185–193. [Google Scholar]
- (16).Inagaki K, Mikuriya N, Morita S, Haraguchi H, Nakahara Y, Hattori M, Kinosita T, Saito H. Analyst. 2000;125:197–203. doi: 10.1039/a907088e. [DOI] [PubMed] [Google Scholar]
- (17).Michalke B, Berthele A, Mistriotis P, Ochsenkuehn-Petropoulou M, Halbach S. Electrophoresis. 2007;28:1380–1386. doi: 10.1002/elps.200600686. [DOI] [PubMed] [Google Scholar]
- (18).Michalke B, Halbach S, Berthele A, Mistritiotis P, Ochsenkuehn-Petropoulou M. J. Anal. At. Spectrom. 2007;22:267–272. [Google Scholar]
- (19).Fernandes KG, Montes-Bayón M, González EB, Castillo-Busto E. d., Nóbrega JA, Sanz-Medel A. J. Anal. At. Spectrom. 2005;20:210–215. [Google Scholar]
- (20).Fortier MHB,E, Goodley P, Thibault P. Anal. Chem. 2005;77:1631–1640. doi: 10.1021/ac048506d. [DOI] [PubMed] [Google Scholar]
- (21).Venne K, Bonneil E, Eng K, Thibault P. Anal. Chem. 2005;77:2176–2186. doi: 10.1021/ac048410j. [DOI] [PubMed] [Google Scholar]
- (22).Niñonuevo M, An H, Yin H, Killeen K, Grimm R, Ward R, German B, Lebrilla C. Electrophoresis. 2005;26:3641–3649. doi: 10.1002/elps.200500246. [DOI] [PubMed] [Google Scholar]
- (23).Ninonuevo MR, Park Y, Yin H, Zhang J, Ward RE, Clowers BH, German JB, Freeman SL, Killeen K, Grimm R, Lebrilla CB. J. Agric. Food Chem. 2006;54:7471–7480. doi: 10.1021/jf0615810. [DOI] [PubMed] [Google Scholar]
- (24).Hardouin J, Joubert-Caron R, Caron M. J. Sep. Sci. 2007;30:1482–1487. doi: 10.1002/jssc.200600444. [DOI] [PubMed] [Google Scholar]
- (25).Hardouin J, Duchateau M, Joubert-Caron R, Caron M. Rapid Commun. Mass Spectrom. 2006;20:3236–3244. doi: 10.1002/rcm.2725. [DOI] [PubMed] [Google Scholar]
- (26).Kassell NF. Stroke. 1970;16:562. doi: 10.1161/01.str.16.4.562. [DOI] [PubMed] [Google Scholar]
- (27).Jose I, Suarez MD, Robert W, Tarr MD, Warren R, Selman MD. N. Engl. J. Med. 2006;354:387–396. doi: 10.1056/NEJMra052732. [DOI] [PubMed] [Google Scholar]
- (28).Vindlacheruvu RR MA. Practitioner. 2002;246:608–614. [PubMed] [Google Scholar]
- (29).Loftspring MC, Wurster WL, Pyne-Geithman GJ, Clark J. J. Neurochem. 2007;102:1990–1995. doi: 10.1111/j.1471-4159.2007.04667.x. [DOI] [PubMed] [Google Scholar]
- (30).Fujiwara Y, Kaji T. Toxicology. 1999;133:147–157. doi: 10.1016/s0300-483x(99)00025-6. [DOI] [PubMed] [Google Scholar]
- (31).Ni Z, Hou S, Barton CH, Vaziri ND. Kidney Int. 2004;66:2329–2336. doi: 10.1111/j.1523-1755.2004.66032.x. [DOI] [PubMed] [Google Scholar]
- (32).Webb RC, Winquist RJ, Victery W, Vander AJ. Am. J. Physiol. 1981;241:H211–216. doi: 10.1152/ajpheart.1981.241.2.H211. [DOI] [PubMed] [Google Scholar]
- (33).Purdy RE, Smith JR, Ding Y, Oviesi F, Vaziri ND, Gonick HC. Am. J. Hypertens. 1997;10:997–1003. doi: 10.1016/s0895-7061(97)00108-8. [DOI] [PubMed] [Google Scholar]
- (34).Lazard EM. Am. J. Obs. Gyn. 1933;26:647–656. [Google Scholar]
- (35).Weber A, Herz R, Reiss I. Biochemistry. 1969;8:2266–2271. doi: 10.1021/bi00834a005. [DOI] [PubMed] [Google Scholar]
- (36).Alcock NW, MacIntyre I. Clin. Sci. 1969;22:185–193. [PubMed] [Google Scholar]
- (37).Palaty V. J. Physiol. 1971;218:353–368. doi: 10.1113/jphysiol.1971.sp009622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Russell WE. Eur. J. Biochem. 1973;33:459–466. doi: 10.1111/j.1432-1033.1973.tb02703.x. [DOI] [PubMed] [Google Scholar]
- (39).Carrier O, Hester RK, Jurevics HA, Tenner TE. Blood Vessels. 1976;13:321–337. doi: 10.1159/000158103. [DOI] [PubMed] [Google Scholar]
- (40).Turlapaty PDMV, Altura BM. Eur. J. Biochem. 1978;52:421–423. doi: 10.1016/0014-2999(78)90303-5. [DOI] [PubMed] [Google Scholar]
- (41).Moreland RS, Ford GD. Arch. Biochem. Biophys. 1981;208:325–333. doi: 10.1016/0003-9861(81)90516-6. [DOI] [PubMed] [Google Scholar]
- (42).Horn E. Med. Rev. 1906;32:264–272. [Google Scholar]
- (43).Idama TO, Lindow SW. Br. J. Obs. Gyn. 1998;105:260–268. doi: 10.1111/j.1471-0528.1998.tb10084.x. [DOI] [PubMed] [Google Scholar]
- (44).Pyne-Gaithman G. personal communication. 2007.
- (45).Shull S, Heintz NH, Periasamy M, Manohar M, Janssen YM, Marsh JP, Mossman BT. J. Biol. Chem. 1991;266:24398–24403. [PubMed] [Google Scholar]
- (46).Speich M, Auget JL, Arnaud P. Clin. Chem. 1989;35:833–835. [PubMed] [Google Scholar]
- (47).McCord JM. Semin. Hematol. 1998;35:5–12. [PubMed] [Google Scholar]
- (48).Van Bergen P, Rauhala P, Spooner CM, Chiueh CC. Free Radical Res. 1999;31:631–640. doi: 10.1080/10715769900301201. [DOI] [PubMed] [Google Scholar]
- (49).Wagner KR, Sharp FR, Ardizzone TA, Lu A, Clark JF. J. Cereb. Blood Flow Metab. 2003;23:629–652. doi: 10.1097/01.WCB.0000073905.87928.6D. [DOI] [PubMed] [Google Scholar]
- (50).Zhengyu L, Harada T, London S, Gajdusek C, Mayberg MR. Neurosurgery. 1995;37:1154–1159. doi: 10.1227/00006123-199512000-00015. [DOI] [PubMed] [Google Scholar]
- (51).Durken M, Herrnring C, Finckh B, Nagel S, Nielsen P, Fischer R, Berger HM, Moison RM, Pichlmeier U, Kohlschutter B, Zander AR, Kohlschutter A. Free Radical Biol. Med. 2000;28:887–894. doi: 10.1016/s0891-5849(00)00174-x. [DOI] [PubMed] [Google Scholar]
- (52).Mason MG, Ball AS, Reeder BJ, Silkstone G, Nicholls P, Wilson MT. Appl. Environ. Microbiol. 2001;67:4512–4519. doi: 10.1128/AEM.67.10.4512-4519.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Busto MEC, Montes-Bayon M, Bettmer J, Sanz-Medel A. Analyst. 2008;133:379–384. doi: 10.1039/b715311b. [DOI] [PubMed] [Google Scholar]
- (54).Sariego Muniz C, Marchante Gayon JM, Garcia Alonso JI, Sanz-Medel A. J. Anal. At. Spectrom. 2001;16:587–592. [Google Scholar]
- (55).Vollmer M, Hörth P, Rozing G, Yohann, Grimm R, Hochstrasser D, Sanchez J-C. J. Sep. Sci. 2006;29:499–509. doi: 10.1002/jssc.200500334. [DOI] [PubMed] [Google Scholar]
- (56).Srbek J, Eickhoff J, Effelsberg U, Kraiczek K, van de Goor T, Coufal P. J. Sep. Sci. 2007;30:2046–2052. doi: 10.1002/jssc.200700053. [DOI] [PubMed] [Google Scholar]
- (57).Kikumoto K,KA, Hayashi Y, Minamino N. Inoue. Anesth. Analg. 1998;87:859–863. doi: 10.1097/00000539-199810000-00021. [DOI] [PubMed] [Google Scholar]
- (58).Fujioka M, Nishio K, Sakaki T, Minamino N, Kitamura K. Stroke. 2000;31:3079–3083. doi: 10.1161/01.str.31.12.3079-d. [DOI] [PubMed] [Google Scholar]
- (59).Suarez JI, Tarr RW, Selman WR. N. Engl. J. Med. 2006;354:387–396. doi: 10.1056/NEJMra052732. [DOI] [PubMed] [Google Scholar]
- (60).Fujioka M,NK, Sakaki T, Minamino N, Kitamura K. Stroke. 2000;31:3079–3083. doi: 10.1161/01.str.31.12.3079-d. [DOI] [PubMed] [Google Scholar]
- (61).Kis B, Ueta Y, Busija DW. Peptide Mediators of the Brain Endothelium. Springer; New York: 2007. [Google Scholar]
- (62).Wijdicks EF, Heublein DM., Jr J. Neurosurg. 2001;94:252–256. doi: 10.3171/jns.2001.94.2.0252. [DOI] [PubMed] [Google Scholar]
- (63).Benedict CR LA. Stroke. 1978;9:237–244. doi: 10.1161/01.str.9.3.237. [DOI] [PubMed] [Google Scholar]
- (64).Kaltashov IA, Zhang M, Eyles SJ, Abzalimov RR. Anal. Bioanal. Chem. 2006;386:472–481. doi: 10.1007/s00216-006-0636-6. [DOI] [PubMed] [Google Scholar]