Abstract
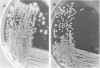
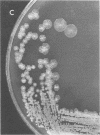
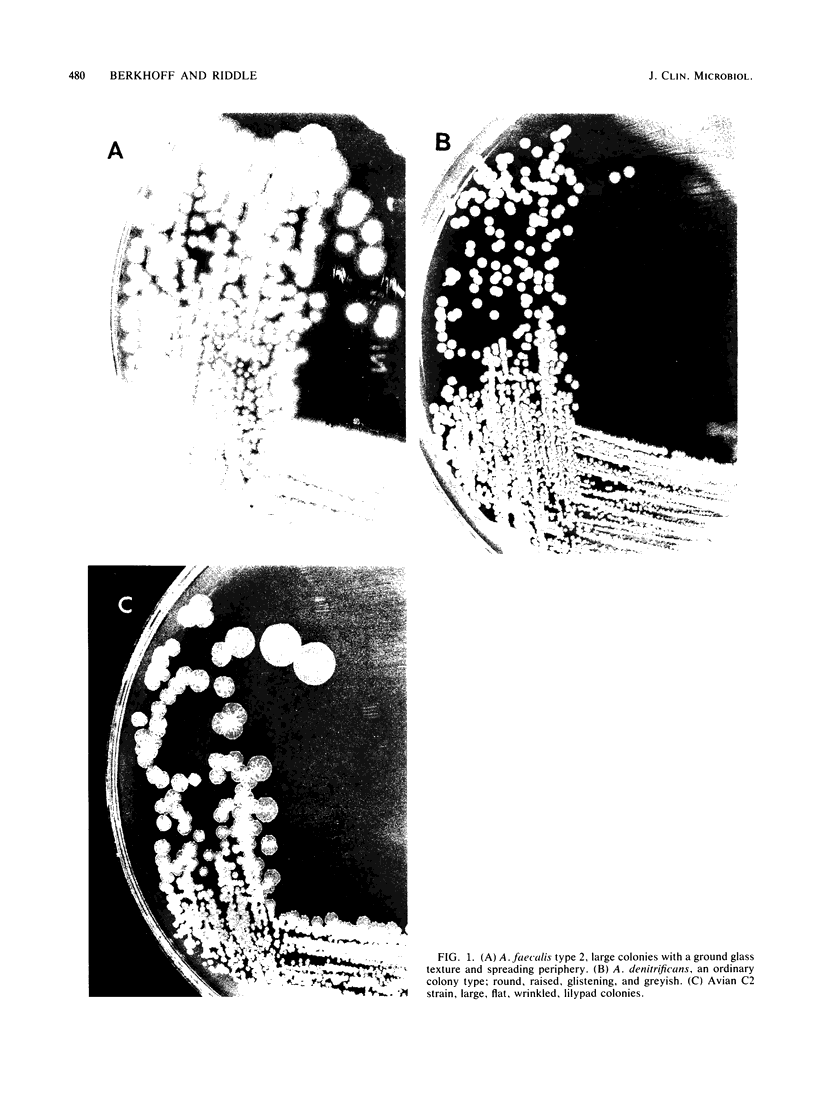
Although standard biochemical tests used for the identification of Alcaligenes spp. revealed only minor differences, the oxidative low-peptone technique clearly differentiated between Alcaligenes-like bacteria of avian origin and Alcaligenes spp. reference strains. Based on their colonial morphology, biochemical profiles, and hemagglutination, the Alcaligenes-like bacteria of avian origin were further divided into two subgroups, C1-T1 and C2-T2. Colonies of subgroup C1-T1 were nondescript, round, raised, glistening, translucent, greyish, and about 2 mm in diameter. Colonies of subgroup C2-T2 were off-white, flat, dry and wrinkled, generally round, and resembled tiny lily pads. Biochemical profiles by the oxidative low-peptone method showed the C1-T1 subgroup alkalinizing only three substrates (citrate, acetate, and succinate), whereas the C2-T2 subgroup alkalinized eight substrates (citrate, acetate, butyrate, itaconate, malonate, saccharate, succinate, and M-tartrate). Subgroup C1-T1 agglutinated human, chicken, and turkey erythrocytes, whereas subgroup C2-T2 did not. The recognition of these two subgroups within the Alcaligenes-like bacteria of avian origin is important, since it may explain the differences seen in pathogenicity among isolates.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berkhoff H. A., McCorkle F. M., Jr, Brown T. T. Pathogenicity of various isolates of Alcaligenes faecalis for broilers. Avian Dis. 1983 Jul-Sep;27(3):707–713. [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr, Tjoa W. Hemagglutination of human group A erythrocytes by enterotoxigenic Escherichia coli isolated from adults with diarrhea: correlation with colonization factor. Infect Immun. 1977 Nov;18(2):330–337. doi: 10.1128/iai.18.2.330-337.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes L. Rapid flagella stain. J Clin Microbiol. 1981 Apr;13(4):807–809. doi: 10.1128/jcm.13.4.807-809.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R., Riley P. S., Hollis D. G., Weaver R. E., Krichevsky M. I. Characterization of some groups of gram-negative nonfermentative bacteria by the carbon source alkalinization technique. J Clin Microbiol. 1981 Jul;14(1):39–47. doi: 10.1128/jcm.14.1.39-47.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto L. A., Pickett M. J. Rapid method for identification of gram-negative, nonfermentative bacilli. J Clin Microbiol. 1976 Jun;3(6):566–575. doi: 10.1128/jcm.3.6.566-575.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rarick H. R., Riley P. S., Martin R. Carbon substrate utilization studies of some cultures of Alcaligenes denitrificans, Alcaligenes faecalis, and Alcaligenes odorans isolated from clinical specimens. J Clin Microbiol. 1978 Sep;8(3):313–319. doi: 10.1128/jcm.8.3.313-319.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimler R. B., Simmons D. G. Differentiation among bacteria isolated from turkeys with coryza (rhinotracheitis). Avian Dis. 1983 Apr-Jun;27(2):491–500. [PubMed] [Google Scholar]