Abstract
Leiomyomas are benign uterine tumors considered to arise from transformation of myometrial cells. What initiates the conversion of myometrial cells into leiomyoma is unknown, however cytogenetic analysis often shows occurrence of nonrandom chromosomal abnormalities that may account for their establishment. It is clear that ovarian steroids are essential for leiomyoma growth, and local expression of many autocrine/paracrine mediators serving as key regulators of cell-cycle progression, cellular hypertrophy, extracellular matrix accumulation, and apoptosis appear to play central roles in this capacity. However, the stability of the expression of these genes represents the hallmarks of leiomyoma establishment, growth, and regression. With the emergence of microRNA (miRNA) as a key regulator of gene expression stability, in this review we present evidence for the expression and potential regulatory functions on miRNAs in leiomyoma with particular emphasis on the expression of their selective target genes whose products influence various cellular activities critical to pathogenesis of leiomyomas.
Keywords: Leiomyoma, myometrium, microRNA, ovarian steroids, regulation
Leiomyomas are benign uterine tumors that develop during the reproductive years and are suppressed after menopause. Clinical observations and epidemiologic studies have estimated that the lifelong risk of developing leiomyomas is ~70%, with a higher risk among African Americans compared with that of other ethnic groups (for review, see refs. 1, 2). Despite limited potential of becoming malignant, the presence of symptomatic leiomyomas account for more than one third of all the hysterectomies performed annually in the United States alone.1
Leiomyomas are thought to develop from cellular transformation of myometrial cells; however, the identity of the factor(s) and molecular mechanism of their actions that contribute toward this process are unknown. Evidence exists supporting the potential involvement of genomic instability, affecting several genes including estrogen and progesterone receptors, with an increased risk of leiomyoma development.3–6 Several genomic and proteomic studies have also provided evidence for altered molecular environment of leiomyomas compared with that of myometrium as a possible causative marker of their growth and regression.7–11 Included among these factors are several oncogenic and tumor suppressor genes that may participate in transformation of myometrial cells into leiomyomas. Moreover, local expression of a large number of autocrine/paracrine mediators, including growth factors, cytokines, chemokines, angiogenic and inflammatory response mediators, proteases, extracellular matrix (ECM), and their receptors may account for leiomyoma growth. Although the biological significance of many of these genes in the pathophysiology of leiomyoma remains to be established, they are known to regulate events such as cell growth and differentiation, apoptosis, and ECM turnover that are critical to leiomyoma growth and regression. However, the stability of the expression of these genes to allow for their optimal and timely expression is fundamental for implementation of their specific biological functions in normal cellular activities, and their instability is directly linked to establishment and progression of various disorders.
Gene expression at the transcriptional and translational levels serves as a critical step in determining the optimal and timely availability of gene products, and the outcome of their associated cellular activities. In this capacity, the recent discovery of microRNAs (miRNAs) and their functional analysis has revealed their importance as key regulators of gene expression stability.12–17 As a member of the non–protein-coding small RNA family, miRNAs are transcribed from genes scattered at multiple locations in all chromosomes except the Y chromosome.14 Interestingly, ~50% of the miRNA genes are found in clusters throughout the genome, indicating their potential co-transcription and coordinated expression as polycistronic primary transcripts.18,19 After transcription from their respective genes and several processing steps, resulting in the generation of 70- to 90-nucleotide (nt) precursor miRNAs (pre-miR-NAs), they are transported into the cytoplasm and cleaved by Dicer into ~19- to 24-nt mature miRNAs.20–23 The mature miRNAs through complementary interactions with the 3′ untranslated region (3′ UTR) of target genes regulate their expression at transcriptional and translational levels.20–23 The specificity of the interaction of miRNAs with target genes is primarily dictated by the “seed” region located at residues 2 to 8 at their 5′ ends.20–23 Well over 1500 miRNAs have been identified or predicted in mammalians, including in humans. As a result of redundancy within the seed region of miRNAs, the predictive algorithms have suggested that up to a third of all genes may contain putative single or multiple binding sites.19 Although the biological significance of many miRNAs remains to be elucidated, recent functional studies have implicated their expression with developmental processes, cell-cycle progression and apoptosis, as well as inflammation and immune regulation.14,19,20,22,23 Altered expression of some miRNAs has also been associated with several disorders, including cellular transformation and tumorigenesis in multiple cancers.13,14,19 Interestingly, > 50% of miRNA genes are located at chromosomal fragile sites often altered in several human cancers.14 Recent identification of the expression of a large number of miRNAs in leiomyomas also implies their key regulatory functions in many cellular activities central to establishment and later growth and regression of leiomyomas. Here we will provide an overview of the expression and possible role for some of these miRNAs in regulating the expression of genes important to cellular activities associated with pathogenesis of leiomyomas.
miRNA EXPRESSION PROFILE IN LEIOMYOMAS
Based on current information in the database (http://microna.sanger.ac.uk/; http://www.targetscan.org/; http://pictar.bio.nyu.edu/), many putative miRNAs have been identified and/or predicted in the genome of different species, including in mammalians. In humans, around 580 distinct miRNA sequences have been identified and more than 1000 have been predicted19,24 that may regulate up to one third of protein-coding and possibly non–protein-coding genes.24 Use of microarray technology has allowed profiling of the expression of a large number of miRNAs in various cells and tissues under normal and disease conditions (see other articles in this issue of the journal).25–27
Using this approach, three recent studies, including our own, have reported the expression profile of a few hundred miRNAs in leiomyomas and matched myometrium, as well as their isolated smooth muscle cells.28–30 The study by Wang and colleagues identified 45 miRNAs whose expression was significantly altered in leiomyomas, with let-7 family, miR-21, miR-23b, miR-29b, and miR-197 representing the most unregulated miRNAs in leiomyoma compared with myometrium.30 Marsh and colleagues also reported an altered expression of 46 miRNAs with 19 overexpressed and 27 underexpressed in leiomyomas compared with myometrium, including miR-21, miR-34a, miR-125b, miR-139, and miR-323.28 Based on global normalization, we identified 91 miRNAs, with a progressive decline either in the level of their expression and/or numbers in leiomyomas compared with that in myometrium.29 We also identified a further decline in the number and level of expression of these miRNAs in isolated myometrial smooth muscle cells (MSMCs) and leiomyoma smooth muscle cells (LSMCs) compared with that of their original tissues.29 Based on the level of expression and predicted target genes important to leiomyomas, we selected miR-20a, miR-21, miR-23a, miR-23b, miR-26a, miR-18a, miR-206, miR-181a, and miR-142–5p and validated their expression in these tissues and cells.29 Collectively, the result of these studies revealed that a selective number of miRNAs are aberrantly expressed in leiomyomas compared with myometrium.28–30
To gain more of the identity and possible function of these miRNAs, we performed a comparative analysis of the miRNAs reported as differentially expressed in leiomyomas in these studies.28–30 The analysis revealed 27 miRNAs as commonly expressed at least in two of these studies (Table 1). Based on the predictive algorithm data sets (see http://microrna.sanger.ac.uk; http://www.targetscan.org/; http://pictar.bio.nyu.edu/), most of the genes targeted by these miRNAs are functionally associated with cell-cycle regulation, differentiation, mobility, and apoptosis, as well as inflammatory response and ECM turnover (Fig. 1). In addition, four of these miRNAs are also predicted to target estrogen and progesterone receptor genes. Although the expression and regulation of some of the genes has been identified in leiomyomas, whether they are regulated by these specific miRNAs and/or other miRNAs in leiomyoma and myometrial cells remains to be established. The task of dissecting the regulatory function of each miRNA on its predicted target gene(s) is rather complex, because of the specificity of the miRNA seed region, in that a single miRNA can potentially target the expression of hundreds of genes, or a single gene could be the potential target of many different miRNAs.17,31,32 In addition, the extent of target gene regulation depends on the degree of complementary sequence homology of genes with their corresponding miRNAs.12,32
Table 1.
Commonly Identified miRNAs in Leiomyomas
| miRNAs | Chromosome Locus | Predicted Target Genes | Function |
|---|---|---|---|
| let7a-1/7d | 9q22.32 | HMGA2, TSC1, IRS | Apoptosis, cell-cycle progression |
| let7b | 22q13.1 | HMGA2 | Apoptosis, cell-cycle progression |
| let7c | 21q21.1 | HMGA2 | Apoptosis, cell-cycle progression |
| let7e | 19q13.41 | HMGA2 | Apoptosis, cell-cycle progression |
| miR100/125b | 11q24.1 | NF-κB, TNF-α | Inflammation |
| miR125a | 19q13.41 | lin-28 | Neuronal differentiation |
| miR143/145 | 5q32 | ERK5, MAPK7 | Tumor suppressor |
| miR144 | 17q11.2 | Caspase-3 | Apoptosis, tumor suppressor |
| miR149 | 2q37.3 | CASP2, Bcl2L2 | Apoptosis |
| miR150 | 19q13.33 | CASP10, PRKCA | Apoptosis |
| miR16–1 | 13q14.2 | BCL2 | Apoptosis, tumor suppressor |
| miR195/324 | 17p13.1 | FGF2 | Mitogenic and angiogenic |
| miR197 | 1p13.3 | BRCA1 | Tumor suppressor |
| miR199a*-2 | 1q24.3 | ITGB8 | Cell-extracellular matrix interactions |
| miR203 | 14q32.33 | MYB | Oncogene |
| miR21 | 17q23.2 | PTEN, TPM1 | Apoptosis, hypertrophy |
| miR215 | 1q41 | ESR1 | Transcription factor |
| miR23a/23b | 19p13.12 | TGIF | Transcription factor |
| miR24-1 | 9q22.32 | BCL2L11, TCF7 | Apoptosis |
| miR26a-1 | 3p22.3 | HMGA2 | Apoptosis, cell-cycle progression |
| miR27a | 19p13.12 | TSC1, RXR, RAR, IGF1 | Tumor suppressor |
| miR29b | 7q32.3 | TRAF4, COL1A2 | Inflammation, extracellular matrix |
| miR29c | 1q32.2 | TRAF4, COL1A2 | Inflammation, extracellular matrix |
| miR30a | 6q13 | PLAGL2, RAR | Oncogene |
| miR34a | 1p36.32 | CDK4, CDK6, E2F3 | Apoptosis, tumor suppressor |
| miR376b | 14q32.31 | TNFRSF25, CAPN3 | Apoptosis |
| miR99a | 21q21.1 | HOXA1, FGFR3 | Development, differentiation |
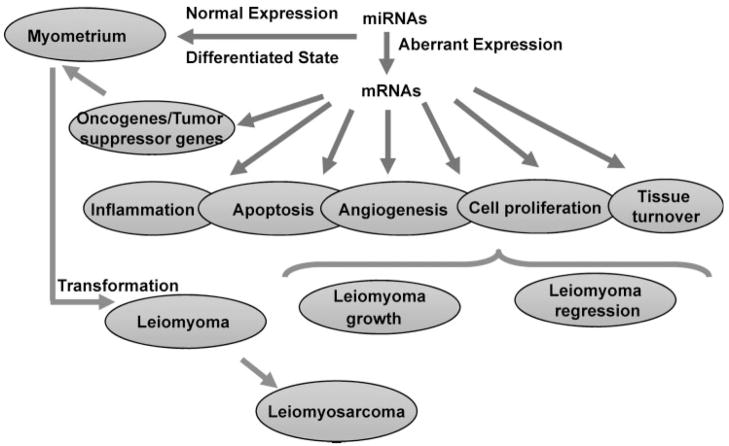
Figure 1.
Schematic presentation of proposed influence of miRNA expression and regulatory functions on myometrial and leiomyoma gene expression. The products of these genes are involved in regulating various cellular activities, including inflammatory response, apoptosis, angiogenesis, cell growth, differentiation, and tissue turnover, processes that result in leiomyoma growth and fibrotic characteristic. Additionally, aberrant expression of some of these miRNAs and altered expression of oncogenes/tumor suppressor genes may also result in myometrial cellular transformation into leiomyomas and later into leiomyosarcoma, although the latter rarely occur. However, it is yet to be determined if altered expression of miRNAs results in unregulated processes that lead to leiomyoma establishment and growth or if activities such as inflammation, or myometrial cellular transformation into leiomyomas results in altered expression of miRNAs and thus the expression of their target genes.
Several miRNAs including the let-7 family have been predicted to target the expression of genes with oncogenic and tumor suppressor activities, such as high mobility group (HMG) genes.33 Wang et al demonstrated that let-7b targets the expression of high mobility group A2 (HMGA2) in LSMCs.30 Our preliminary results also indicate that the expression of transforming growth factor β (TGF-β) receptor type II is the target of miR-21 in LSMCs.34 TGF-β is a key profibrotic cytokine that mediates its biological activities by binding to TGF-β receptor (TGF-βR) types I to III, of which types I and II are transmembrane proteins with a cytoplasmic serine/threonine kinase domain.35–37 TGF-β and TGF-β receptors as well as their intracellular signaling pathways are overexpressed in leiomyoma compared with that in myometrium. The consequence of a lower expression of miR-21 in leiomyoma and LSMCs compared with that in myometrium might represent a loss of one of the regulatory mechanisms resulting in unregulated expression of TGF-β receptor and increased TGF-β activities. The biological functions attributed to TGF-βs include cellular hypertrophy, ECM turnover, and angiogenesis, key processes that are central to various fibrotic disorders, including leiomyomas.35–40 Because a large number of genes are predicted targets of let-7 and miR-21, their altered expression in leiomyomas compared with that in myometrium could have a significant regulatory implication on the outcome of leiomyoma growth and regression. However, it is not yet established whether altered expression or mechanisms regulating their target genes differentiate leiomyomas from myometrium. We are currently investigating the mechanism(s) by which miR-21 alters the expression of TGF-β type II receptor and other predicted target genes in leiomyoma and myometrium.
miRNA AND CELLULAR TRANSFORMATION
Cellular transformation of MSMCs or myometrial connective tissue fibroblasts is considered to result in the establishment of leiomyomas; however, transformation of leiomyomas into leiomyosarcoma is very rare. Factors and molecular mechanisms implementing their actions that account for such cellular transformation are currently unknown, although genetic and epigenetic alterations are considered to initiate malignant transformation in most human cancers. Accumulated evidence supports key roles for several oncogenes in cellular transformation processes, and significant progress has been made toward understanding their mechanisms of action.41 Recent reports have provided evidence linking several oncogene and tumor suppressor gene networks with regulatory function of miRNAs. Among a selective number of miRNAs that emerged to serve in this capacity are let-7 family, miR-17–92 cluster, miR-372–373, miR-155/BIC, and miR-15–16,18,42–44 which is frequently deleted in several patients with chronic lymphocytic leukemia.18,41 The ability of these miRNAs to serve as oncogenic or tumor suppressors is due to their regulatory function on genes whose products influence cell-cycle progression and apoptotic signaling.13,43,44
Interestingly, the expression of several miRNAs, including miR-17–5p, miR-155, miR-15, miR-16, and let-7 family, was altered in a LSMC culture spontaneously transformed (T-LSMC) in our laboratory and in SK-LMS-1, a leiomyosarcoma cell line, compared with that in MSMC and LSMC. We also identified an altered expression of miR-20a, miR-21, miR-23a, miR-23b, miR-26a, miR-18a, miR-206, miR-181a, and miR-142–5p in these cells.29 These miRNAs are predicted to target the expression of genes involved in cell-cycle regulation and may also target the ovarian steroid receptor genes. These results raise the possibility that these miRNAs, some with the ability to initiate cellular transformation by targeting protooncogenes and tumor suppresser genes (Fig. 1), effect myometrial cell transformation into leiomyomas and possibly into cancerous phenotype, although its occurrence is very rare. Evidence suggests that genomic instability, affecting several genes including estrogen and progesterone receptors, is associated with an increased risk of leiomyoma development. As such, it would be of interest to correlate these genomic sites with sites harboring miRNAs genes that are also reported to be subject to frequent deletion in other disorders.14 Such association may further assist identifying possible mechanisms for aberrant expression and contributions of miRNAs to pathogenesis of leiomyomas.
miRNA AND STEM CELL REGULATION
Adult stem cells have been identified in almost all the tissues examined and appear to possess a long-term replicative potential with the capacities of self-renewal and multilineage differentiation. The adult stem cells are considered to participate in various normal tissue organizations under highly regulated processes, and alterations in their microenvironment may contribute to events leading to tumorigenesis.45 This is due to a high degree of similarity between somatic stem cells and cancer cells, including their fundamental ability for self-renewal and differentiation.45–47 A recent study has also identified the presence of adult stem cells in human myometrium.48 Given these common attributes, miRNAs through differential regulation of specific genes, including protooncogenes and tumor suppressor genes, could functionally play a critical role in myometrial stem cell transformation into leiomyoma. Several studies have identified several miRNAs in association with specific stem cell types, or their differentiation process into adipocyte, cardiac, neural, and hematopoietic lineages.49 Noticeable among them are miR-181, miR-124, miR-128, miR-23, and the miR-15a–miR-16 clusters.45–47,49 Although the expression and possible regulatory functions of these miRNAs has been ascribed to stem cell biology and their differentiation, it is yet to be established whether they have any role in the expression of genes that facilitate myometrial stem cells to undergo transformation into leiomyoma (Fig. 1).
ROLE OF ETHNICITY AND miRNA EXPRESSION
It is estimated that the lifelong risk of developing leiomyomas is ~70%, with a higher risk among African Americans compared with that of other ethnic groups. Several studies have associated an increased risk of developing leiomyomas, including among African Americans, with genomic instability affecting the expression of several genes.3,4,6,11 Comparative assessment of gene expression also indicates an influence of ethnicity on overall gene expression profile in leiomyomas.10,11,50 Using tissue microarray, an immunohistochemistry study has reported an altered intensity of immunostaining of several proteins in leiomyomas among African Americans compared with that of other ethnic groups.51 In a recent genomic and proteomic study, we also identified an altered expression of several genes and proteins in leiomyomas of African Americans compared with that of whites.11 It has also been suggested that the levels rather than the ethnic-specific expression of these genes and proteins account for the difference between leiomyomas and possibly myometrium in African Americans compared with that in whites. Although posttranscriptional and translational regulations may account for these differences, regulatory function of miRNAs could also influence their differential expression in leiomyomas, including their ethnic-dependent expression.
The study by Wang and colleagues provided support for the potential role of miRNAs in gene expression regulation in leiomyomas based on ethnicity.30 The results identified an altered expression of several miRNAs, including miR-23a/b, let-7s, miR-145, miR-197, miR-411, and miR-412, in leiomyomas from African Americans compared with those from whites. Leiomyomas from other ethnic groups (Asian and Hispanic) exhibited miRNA expression in between that of African American and white women.30 We also found an altered expression of miR-23a and miR-23b in leiomyomas from African American compared with that in whites and showed their regulation by ovarian steroids.29 The biological implication of ethnic-dependent expression of these and other miRNAs and their target genes requires detailed investigation. However, these miRNAs are predicted to target the expression of many genes involved in several cellular activities such as cell-cycle regulation, inflammatory reaction, apoptosis, and ECM turnover that are critical in pathogenesis of leiomyomas.
The study by Wang and colleagues also found a size-dependent expression of miRNAs in leiomyomas.30 A few studies have also indicated differences in gene expression profile of leiomyomas based on size and location of tissue biopsies collected for analysis. Interestingly, larger leiomyomas are observed to be more symptomatic and occur more frequently among African Americans compared with that in other ethnic groups. Despite their growth in size, larger leiomyomas often undergo degeneration, specifically at the center. As such, analysis of genes and/or miRNAs expression in leiomyomas based on location (periphery vs. center) must be considered to avoid misleading information associating growth in size of leiomyomas with their expression profiles. However, validating and verifying the expression of genes targeted by the miRNAs commonly identified in leiomyomas would allow for better understanding of their regulatory function based on ethnicity and molecular mechanisms that promote leiomyoma growth and degenerative processes.
REGULATORY FUNCTION OF OVARIAN STEROIDS ON miRNA EXPRESSION
It is quite clear that ovarian steroids and their receptors play central roles in leiomyoma growth because of their establishment and rapid growth during hyperestrogenic states and regression with menopause. Local estrogen biosynthesis has also been suggested to play a role in promoting leiomyoma growth.1 However, evidence exists indicating limited difference in the level of estrogen and progesterone receptors between myometrium and leiomyomas and the low level of local estrogen biosynthesis in leiomyoma implies a limited biological significance compared with ovarian-derived estrogens.1 Clinical studies have also produced conflicting results regarding the effect of progesterone or synthetic progestins on leiomyoma growth.1 However, under in vitro conditions, ovarian steroids influence the expression of several genes with growth-promoting activities in leiomyoma and myometrial primary cell cultures.1
Because of the growth dependency of leiomyomas on ovarian steroids, creating a hypoestrogenic state by gonadotropin-releasing hormone agonist (GnRHa) therapy often results in leiomyoma regression.1 GnRHa therapy causes a reduction in uterine/leiomyomas steroid receptors content, volume, arteriole size, and blood flow. Daily administration of antiprogesterone (mifepristone; RU-486) also induces a significant decrease in leiomyomas/uterine volume.1 RU-486 causes a significant reduction in leiomyoma progesterone receptor content without affecting the estrogen receptor levels.1 Clinical studies have also found that selective progesterone receptor modulators, asoprisnil (J867) and CDB-2914, have beneficial effects on leiomyoma regression, further supporting the role of progesterone and its receptor in leiomyoma growth.1,52,53 Under in vitro conditions, RU-486, ZK98299, and CDB-2914 also altered the rate of DNA synthesis and growth factors and proteases expression in MSMC and LSMC primary cultures.54–56 Although both MSMCs and LSMCs equally express progesterone receptors A and B, J867 and CDB-2914 have been reported not to have any effects on the expression of these genes in MSMCs.55
Considerable evidence exists implicating menstrual cycle–dependent expression of a large number of genes in myometrium and leiomyomas and their regulation by ovarian steroids in myometrial and leiomyoma cells under in vitro conditions.7,10,38,57–59 Recent studies, including our own, also suggest a regulatory function for ovarian steroids in the expression of miRNAs.29,30 Wang et al identified differential expression of a group of miRNAs in leiomyomas and myometrium during the proliferative compared with the secretory phase of the menstrual cycle and during inactive cycles supporting possible involvement of ovarian steroids in their expression.30 Because the miRNAs expression profiling in our study used leiomyomas and myometrium from the early to mid secretory phase of the menstrual cycle, a progesterone-dominated period, we used primary culture of MSMCs and LSMCs and provided direct evidence for the influence of ovarian steroids on the expression of a selective number of miRNAs.29 We found that 17β estradiol (E2) and medroxyprogesterone acetate (MPA) differentially regulated the expression of these miRNAs in a cell-dependent manner.29 For instance, treatment of these cells with E2 inhibited whereas MPA enhanced the expression of miR-21 and miR-26a in MSMCs and LSMCs, respectively. The expression of these miRNAs was also the target of regulatory actions of estrogen antagonist ICI-182780 and progesterone antagonist RU-486, respectively. These treatments either alone or after co-treatments with their respective sex steroids induced both an inhibitory and enhancing effect on the expression of these miRNAs in MSMCs and LSMCs.29 These results indicate complex but important regulatory functions for the ovarian steroids in the expression of miRNAs. However, detailed analysis is required to correlate the expression of these miRNAs and their target genes to determine if both are subject to regulation by the ovarian steroids. Such a regulatory activity may result in differential expression of miRNAs in leiomyoma and myometrium, a mechanism that could serve in regulating the expression of many of the target genes influencing leiomyoma growth or regression as seen with their selective estrogen and progesterone receptor modulators.
POSSIBLE FUNCTION OF miRNAs IN LEIOMYOMA
Among the miRNAs identified as differentially expressed in leiomyomas and myometrium are let-7 family, miR-21, miR-23b, miR-29b, miR-197, miR-34a, miR-125b, and miR-26a (Table 1).28–30 Based on the predictive algorithm data sets (see http://microrna.sanger.ac.uk; http://www.targetscan.org/; http://pictar.bio.nyu.edu/) and evidence obtained in other cell types, most of these miRNA target genes are functionally associated with cell-cycle progression, cell differentiation, mobility and apoptosis, cell-cell communication, transformation and tumorigenesis, as well as inflammatory response and ECM turnover (Fig. 2).16,27,42,48
Figure 2.
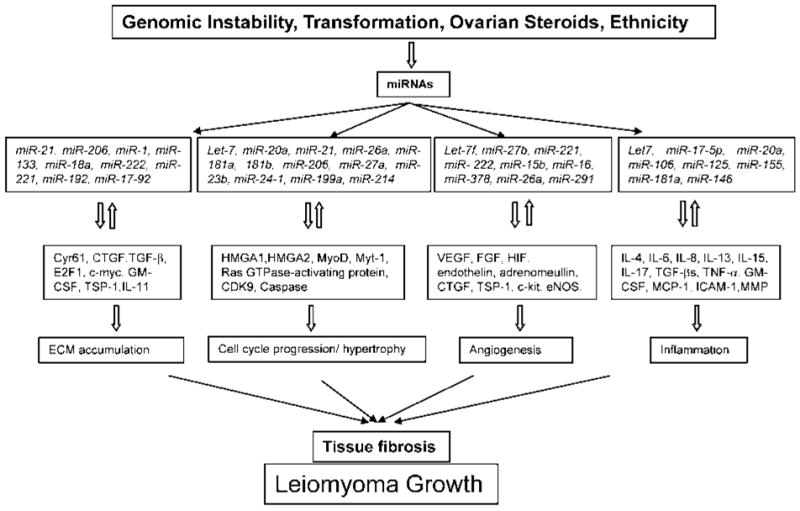
Proposed summary of the influence of gene instability, transformation, ovarian steroids, and ethnicity on miRNA expression and pathogenesis of leiomyoma. Genetic alterations, cellular transformation of myometrial cells, ovarian steroids, and ethnic differences may effect the expression of miRNAs and their aberrant expression alter the stability of their target genes. The product of some of these genes may in turn regulate the expression of miRNAs, which through a feedback mechanism influence various cellular activities including tissue turnover, cell-cycle progression, hypertrophy, angiogenesis, and inflammation resulting in tissue fibrosis, a characteristic of leiomyoma during growth.
miRNAs as Regulator of Cell Growth and Apoptosis
A combination of mitotic activities, alterations in cellular hypertrophy, and ECM accumulation plays a major role in leiomyoma growth.7,38,58–61 The rate of mitotic index, which reflects cellular proliferation, is estimated to be as high as 40 mitotic figures/100 high-power fields in leiomyomas during the luteal phase and significantly higher in tumors from younger (ages 30 to 34 years) than from older (ages 45 to 49 years or 50 to 54 years) women.62,63 During the proliferative phase, leiomyomas and myometrium have similar mitotic index, which increases in women who received progestin therapy compared with that in untreated or estrogen/progestin treated groups. However, the mitotic index in leiomyomas was relatively low to account for their rapid growth and conversely a rapid decrease in their size with GnRHa and RU-486 therapies. GnRHa and RU-486 therapies often cause leiomyoma regression, possibly by enhancing cellular apoptosis,64,65 ECM degradation, and reduced cellular hypertrophy. Leiomyomas can undergo extensive growth with mitotic index less than1062,63 implying the importance of other cellular activities for growth in size of leiomyoma. Alterations in cellular hypertrophy and ECM accumulation are known to play a major role in progression of various fibrotic disorders.66
Evidence suggests that overexpression of miR-20a and miR-21 is inversely associated with apoptosis and malignant cellular transformation in several cancer cells.27,67–70 Analysis of many cancer-derived cell lines for sequence variations in 15 miRNAs for tumor-associated mutations has implicated miR-26a in these processes.14,68 In contrast, elevated expression of miR-181 has been associated with cellular differentiation and establishment of muscle phenotype,71 and miR-206 expression has been identified in estrogen receptor α (ERα)-negative MB-MDA-231 cells, but not ERα-positive MCF-7 cells72 (see the article by Adams et al in this issue of the journal). Because leiomyomas express low levels of miR-21 and miR-206 compared with that in myometrium, their differential expression may result in reprogramming of the expression of their specific target genes that influence growth of leiomyomas.
Among the genes predicted as the target of miR-26a are HMGA1 and HMGA2, expressed at very high levels in various tumors, including leiomyomas, and considered to serve in cellular events leading into tumorigenesis.30,73 In thyroid carcinomas, the expression of miR-26a was inversely correlated with HMGA1 and HMGA2 expression, which are highly elevated in these tumors. Overexpression of miR-26a in thyroid cancer cells suppressed HMGA1 and HMGA2 expression and consequently inhibited their proliferation.74 HMGA2 is also predicted as one of the genes targeted by the let-7 family, and overexpression of let-7b in leiomyoma cells resulted in suppression of HMGA2 expression.30 Furthermore, increased expression of miR-26a has been considered to promote myogenesis as overexpression of miR-26a in murine myocytes increased creatine kinase activity, an enzyme that markedly increases during myogenesis, and upregulated myoD and myogenin mRNA expression levels.75
Human cytochrome P450 (CYP) 1B1, an enzyme involved in estrogen metabolism, has been identified as one of the genes targeted by miR-27b, which is located on human chromosome 9q22.1 and clusters with miR-23b and miR-24–1. A low level of miR-27b expression in MCF-7 cells has been inversely associated with CYP1B1 expression and overexpression of miR-27b resulting in inhibition of CYP1B1 expression.76 Leiomyomas expressed lower levels of miR-27b, miR-23b, and miR-24 compared with that in myometrium suggesting their potential function in regulating estrogen metabolism and promoting leiomyoma cell growth. Additionally, miR-27a facilitates cancer cell proliferation through suppression of Myt-1, which blocks cell-cycle progression at G2-M phases.77 Marsh et al reported an upregulation of miR-27a,30 suggesting possible regulation of genes involved in leiomyoma cell proliferation. However, we detected a lower level of miR-27a expression in leiomyomas compared with that in myometrium,29 which could be due to the menstrual cycle–dependent expression. If miR-27a expression is differentially regulated during the menstrual cycle, the result could influence the expression of genes targeting cell-cycle progression in leiomyoma, which undergoes different growth pattern during the menstrual cycle.
Several members of the let-7 family have also been reported to promote terminal differentiation and reentry into the cell cycle.78 These events are essential components of tissue turnover that occur during fibrosis, and aberrant expression of let-7 in leiomyomas may result in altered expression of genes with similar functional activities. Hypertrophy is also critical in leiomyoma growth in size and tissue turnover,11,79 and several miRNAs such as miR-21 and miR-18b have been found to function in that capacity.80 Inhibition of miR-21 and miR-18b expression, which were differentially expressed in rat cardiomyocytes, resulted in the induction of hypertrophy, including increased cell size and expression of hypertrophic markers, such as α-actinin and α-actin.80 Blocking the expression of these miRNAs with short double-stranded RNAs further indicated that miR-21 acts as a negative regulator of hypertrophy.80 Furthermore, antisense-mediated depletion of overexpressed miR-21 also resulted in prevention of neointimal lesion formation.81 Under in vitro conditions, a similar approach resulted in decreased cell proliferation and increased cell apoptosis of vascular smooth muscle cells obtained from these lesions compared with that of normal differentiated cells.81 In addition, the muscle-specific miR-1 was downregulated after aortic constriction-induced hypertrophy in a mouse model. Overexpression of miR-1 in cardiac myocytes inhibited the expression of predicted growth-related target genes, including Ras GTPase-activating protein, cyclin-dependent kinase 9, fibronectin, and Ras homo-log enriched in brain (Rheb), in addition to protein synthesis and cell size.82 Other miRNAs that displayed a greater change during cellular hypertrophy were miR-199a, miR-199a*, miR-199b, miR-21, and miR-214.82 In summary, these results suggest that miRNAs, by targeting the expression of genes functionally associated with cell-cycle progression, differentiation, apoptosis, and hypertrophy, could have far-reaching influence on leiomyoma growth and regression.
miRNAs and Tissue Remodeling
Tissue remodeling is critical to the progression of fibrotic disorders and modulation of ECM, adhesion molecules, and proteases expression, and phenotypic changes toward a myofibroblastic phenotype are essential components of this process.39,83–85 We and others have identified the expression of several genes in this category in leiomyoma and myometrium and their isolated smooth muscle cells, including fibronectin, collagens, decorin, versican, fibromodulin, several members of the integrin family, as well as proteolytic enzymes and their physiologic inhibitors.7,8,10,50,86,87 Of particular interest are elevated expression of decorin, vimentin, fibulin, thrombostatin, and fibromodulin in leiomyoma because of their ability to bind TGF-β and control TGF-β autocrine/paracrine actions, a mechanism considered to regulate TGF-β availability and the outcome of tissue fibrosis.36,37,40,83,88 Leiomyoma is believed to derive from transformation of myometrial connective tissue fibroblast and/or smooth muscle cells, and the expression of vimentin in leiomyoma/LSMC imply that these cells have adopted a myofibroblastic characteristic. Although granulation tissue myofibroblasts are derived from local fibroblasts, other cell types including smooth muscle cells have the potential of acquiring a myofibroblastic phenotype.84,85 Various cytokines, including granulocyte monocyte colony stimulating factor (GM-CSF), interleukin (IL) 11, and TGF-β participate in transformation of fibroblasts into myofibroblastic phentotype.84,85 Despite the importance of tissue turnover in pathophysiology of tissue fibrosis, little is known about the extent of ECM expression, regulation, and differences that may contribute to the fibrotic characteristic of leiomyoma.
The mechanism of how miRNAs function in regulating the expression of genes related to tissue turnover has not been investigated, although many genes in this category are predicted targets of their regulatory functions. As indicated, miR-21 appears to be involved in cellular processes that are important to neointimal lesion formation.81 Because leiomyomas display many features of fibrotic tissue disorders, a lower expression of miR-21 in LSMCs and leiomyoma vascular smooth muscle cells may function in a fashion observed in neointimal lesions.81 Our preliminary observation that miR-21 targets the expression of TGF-β receptor is of further interest as unregulated expression of various components of the TGF-β system is associated with altered expression of several ECM, proteases, and other ECM-related genes.8,11,38,79,86,87,89 It has been reported that miRNAs may be involved in the mechanism of TGF-mediated collagen regulation involving miR-192, which is increased by TGF-β.90 Downregulation of two related miRNAs, miR-206 and miR-1, has also been reported to inhibit the expression of connexin 43 (Cx43) during myoblast differentiation.91 Several connexins including Cx43 are expressed in leiomyomas, and their expression, including the expression of miR-206, are under the regulatory function of ovarian steroids.8,11,29,62,79 In addition, the expression of several muscle-specific miRNAs, such as miR-1, miR-206, and miR-133a, is also altered in response to stresses in adult skeletal muscle, with a significant increase in miR-206 and downregulation of miR-1 and miR-133a.92
The miR-17–92 cluster contains miR-17–5p, miR-17–3p, miR-18a, miR-19a, miR-20a, miR-19b, and miR-92–1, and miR-18 and miR-19 have been found to target the expression of connective tissue growth factor (CTGF) and thrombospondin-1 (TSP-1), respectively.93 The biological significance of miRNA-mediated CTGF regulation is due to the ability of CTGF in mediating the profibrotic actions of TGF-β in tissue fibrosis.40,94,95 However, we have reported that the expression of CTGF and TGF-β is inversely correlated in leiomyomas, although TGF-β induced CTGF expression in LSMCs under in vitro condition.94 Interestingly, miR-18 and miR-19 are equally expressed in leiomyoma and myometrium, but their potential influence on the expression of CTGF and other target genes may result in regulation of various cellular functions critical to tissue turnover in leiomyoma.
CTGF is a member of the cystine-rich secreted family (CCN), which includes cysteine-rich, angiogenic inducer 61 (CYR61)—known to modulate cell migration, angiogenesis, and ECM production.95 CYR61 is a predicted target of miR-221 and miR-222, which are downregulated in myometrium compared with that in leiomyoma. A lower expression of CYR61 in leiomyomas compared with that in myometrium96 could also be due to overexpression of these miRNAs. TSP-1 is known to play an important role in a variety of biologic processes, including cell-cell and cell-matrix interactions, antiangiogenic activity, and activation of latent TGF-β.97 Similar to CTGF, TSP-1 is expressed at significantly lower levels in leiomyoma compared with that in myomterium.11 Notably, TSP-1 and CTGF have been found as targets of miR-17–92 regulatory functions. miR-17–5p and miR-20a have also been associated with downregulation of transcription factor, E2F1, with miR-20a acting as an activator of c-myc,98 which acts as transcriptional regulator of several ECM-related genes.
miRNAs and Angiogenesis
Angiogenesis is considered to play a key role in pathogenesis of leiomyoma. Angiogenesis is regulated by a balance between the expression of many factors with enhancing and inhibitory activities in several cellular events that regulate vascular growth. Leiomyomas express several factors with angiogenic properties including vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), hypoxia-induced factor (HIF), endothelin, and adrenomedullin.65 In addition, factors with antiangiogenic properties, such as TSP-1, are also expressed in leiomyoma. The expression of many of these proangiogenic and antiangiogenic factors is regulated by ovarian steroids as well as many locally expressed gene products.99 Because the stability and balance of the expression of these angiogenic and antiangiogenic factors are important in leiomyoma growth, miRNAs could play key regulatory functions in the outcome of angiogenesis in leiomyoma.
Among the miRNAs functionally identified to regulate the expression of angiogenic factors are let-7 family, miR-27b, miR-221, miR-222, miR-26a, miR-291, miR-15, and miR-16.98,100 Drosha and Dicer, which regulate miRNAs biosynthesis, have also been shown to play a critical role in endothelial sprouting and network formation by targeting the expression of miRNAs such as let-7 family, miR-21, miR-221, miR-222, miR-26a, miR-27b, and miR-29a.101 Inhibition of let-7f and miR-27b significantly reduced sprout formation in vitro, and Dicer knockdown through small interference RNA (siRNA) treatment significantly blocked angiogenesis in vivo in part through upregulation of TSP-1 expression involving Akt pathway.101 Overexpression of miR-221 and miR-222 in endothelial cells also resulted in inhibition of angiogenesis by blocking tube formation and migration by decreasing c-kit, specific receptor for stem cell factor.102 miR-221 and miR-222 indirectly reduced the expression of endothelial nitric oxide synthase (eNOS), which is angiogenic and a contributor to other endothelial cell functions.101 Leiomyomas express miR-221 and miR-222, but their expression is lower compared with that in matched myometrium, suggesting a promotion of angiogenesis possibly involving many of the above mediators. Leiomyomas also express lower levels of miR-15b and miR-16. Among the predicted targets of miR-15b and miR-16 is VEGF, a key proangiogenic mediator.103
Hypoxia, which promotes angiogenesis, has been shown to reduce miR-15b and miR-16 expression.103 Given the importance of hypoxia in angiogenesis, a hypoxic condition resulting in reduction of these miRNAs may contribute toward angiogenesis and leiomyoma growth. Another miRNA, miR-378, which has been considered to serve as a regulator of angiogenic mediators, is also downregulated in leiomyoma. Mice injected with miR-378–transfected cells formed much larger tumors, with larger blood vessels, compared with that in vector-transfected cells, implying a role for miR-378 in blood vessel formation.104 Leiomyomas also express several antiangiogenic genes,105 whose expression could be subjected to regulatory functions of miRNAs. Currently, there is no direct evidence implicating the regulatory function of miRNAs in the expression of angiogenic and antiangiogenic genes in leiomyoma. However, the results generated in other systems have opened the field of miRNAs research and their possible involvement in angiogenesis in leiomyoma. As such, several miRNAs candidates through differential regulation of angiogenic and antiangiogenic genes may influence leiomyoma growth and regression.
miRNAs and Inflammation
Inflammatory response and the regulating molecules are essential for normal tissue homeostasis; however, its unregulated progression could result in several disorders, including tissue fibrosis. We have proposed that a local inflammatory response mediated through individual and combined actions of several inflammatory and profibrotic mediators results in initiation and progression of fibrosis in leiomyoma.8,38 We and others identified the expression of several genes with proinflammatory and profibrotic activities in leiomyomas, supporting their functions in leiomyomas (for review, see Refs. 8, 38, 58). Among the inflammatory and profibrotic mediators with such characteristics are several cytokines and chemokines such as interleukin family, IL-4, IL-6, IL-8, IL-13, IL-15, IL-17, TGF-βs, tumor necrosis factor (TNF)-α, GM-CSF, and monocyte chemoattractant protein-1 (MCP-1).8,38,58 These cytokines are classified as type 1/type 2 related subsets, and their predominance toward the type 2 direction is considered to result in inflammatory/immune responses leading to progression of tissue fibrosis.8,38,58 In addition, elevated expression of some of these mediators leads to unregulated cellular apoptosis, angiogenesis, and cell survival, possibly through the activation of fms-related tyrosine kinase 1 (Flt-1), Fas, Fas ligand, cyclin D1, cyclin-dependent kinase inhibitor (CDKN) 1A, 1B, 2B, 1C, tumor protein 53 (p53), platelet-derived growth factor (PDGF), VEGF, tissue factor, eicosanoids, intercellular adhesion molecule 1, fibronectin, urokinase-type plasminogen activator, and matrix metalloproteinases.7,8,38 The expression of many of these genes has been documented in myometrium with altered expression in leiomyomas.7,11,38,79,94 The individual and combined actions of many of these factors also participate in other cellular activities that collectively result in progression of inflammation and fibrotic disorders, depending on their optimal and timely expression and regulation.
Gene expression stability is a key to the outcome of inflammatory response and its resolution. Several miRNAs, specifically, let7, miR-17–5p, miR-20a, miR-106a, miR-125b, miR-146, and miR-155, have been identified to influence the expression of inflammatory and immune response mediators.106–108 Among the proinflammatory genes identified as targets of these miRNAs are several of the above genes that are expressed in leiomyomas.8,11,38,58,79 The expression of these miRNAs has been identified in myometrium and leiomyomas as well as in their isolated smooth muscle cells.28–30 The expression of several of these miRNAs, more specifically miR-125b and miR-155, is required for proper development of inflammatory- and immune-related cells.109–114 The exposure of macrophages to proinflammatory cytokines, such as TNF-α, resulted in altered expression of miR-125b and miR-155, and over-expression of miR-155 in macrophages or in mice resulted in increased TNF-α production after endotoxin exposure.114 Macrophages, T cells, and mast cells are present, and their populations are higher in leiomyomas compared with that in myometrium.38,58 These observations imply that miR-125 and miR-155, and possibly other miRNAs, may potentially regulate the expression of inflammatory- and immune-related genes in leiomyomas. However, it is unclear if inflammation causes the expression of specific miRNAs or if unregulated expression of miRNAs results in activation of inflammation-associated genes during an inflammatory response.
Other miRNAs, including overexpression of miR-17–5p, miR-20a, and miR-106a, have been reported to inhibit the differentiation and maturation of monocytes.115 These miRNAs are predicted to regulate several genes including TGF-β and TGF-β receptors family, a major cytokine with anti-inflammatory activities that differentially regulates proteases, ECM, and adhesion molecules expression resulting in progression of tissue fibrosis.66 In addition, miR-181a expression is increased in mature T cells116 and found to regulate the expression of antiapoptotic proteins, such as BCL2.117 We identified the expression of miR-181a in myometrium and leiomyomas and showed that its expression is differentially regulated by the ovarian steroids in their isolated smooth muscle cells.29 These results suggest that miRNAs and their differential expression may serve to promote inflammatory response and through regulation of profibrotic genes enhance the fibrotic nature of leiomyomas.
CONCLUSIONS
During the past two decades, specifically with the advancement in gene expression profiling, the expression of a large number of genes has been identified in leiomyoma and myometrium. Although the biological significance of a vast number of these genes remains to be elucidated, these investigations provided evidence for the complexity of the leiomyoma microenvironment and differences in genes as compared to the myometrium. As such, the stability of the expression of these genes is fundamental for implementation of their specific biological functions resulting in normal cellular activities in myometrium or abnormalities associated with leiomyomas. It is reasonable to assume that one or a group of these gene products, including several oncogenes, are responsible for cellular transformation of myometrial cells into leiomyoma. Additionally, the product of many other genes, including a large number of growth factors, cytokines, chemokines, angiogenic and inflammatory response mediators, proteases, ECM, and their receptors are known to regulate events such as cell growth and differentiation, apoptosis, and ECM turnover that are critical to leiomyoma growth and regression. The menstrual cycle regulation and ethnic-dependent expression of some of these genes may also influence the stability of the expression of these genes.
Gene expression regulation at transcriptional and translational levels is critical in determining the optimal and timely availability of gene products for implementing their biological functions. Since their discovery a few years ago, it has become clear that miRNAs play an importance regulatory function in gene expression stability, and their expression in leiomyomas and myometrium implies such functions. Accumulated evidence indicates the potential involvement of genomic instability as a possible mechanism of leiomyoma establishment and growth. Because miRNAs are transcribed from genes scattered at multiple locations in all chromosomes with the exception of the Y chromosome and many miRNA genes are found in clusters, such genomic instability leiomyomas could affect miRNA genes. As such, correlating these unstable genomic sites with regions harboring miRNAs genes, which are also subject to frequent deletion in other disorders, may allow for identification of a possible mechanism for aberrant expression and contributions of miRNAs to pathogenesis of leiomyomas.
With respect to biological implication of expression of miRNAs in leiomyoma, at the present time we could only present a speculative assessment of their roles in several cellular activities that are important in leiomyoma pathogenesis. As such, detailed functional assessment of genes targeted by the differentially expressed miRNAs regulating cell-cycle progression, cellular hypertrophy, ECM accumulation, and apoptosis, which are key to leiomyoma growth, is needed. It is also necessary to investigate the menstrual cycle–dependent and ethnic-dependent expression of these miRNAs. This is because leiomyomas occur more frequently, grow more rapidly, and are more symptomatic in African Americans compared with that in other ethnic groups, and various hormonal therapies have been used for medical management of leiomyoma growth without detailed information about their local actions. We showed that miRNAs are a target of regulatory function of ovarian steroids, but how these hormonal therapies regulate the expression of miRNAs remains to be investigated. Because one miRNA may target many genes and one gene may be targeted by many miRNAs, achieving the task of identification of target genes of miRNAs would be difficult, but the results provide promising information useful for therapeutic management of leiomyoma through specific gene expression targeting.
Acknowledgments
We would like to acknowledge the contribution of Dr. Qun Pan in the initial study of miRNA profiling in these tissues and Drs. Wally Mclean, Stan Williams, John Davis, Shireen Madani, and Jill Roscoe for assisting us during the past years with collection of the tissues used in our research in this field. We thank the many colleagues whose research has contributed to the concepts in this review and apologize for not being able to reference all their work. This work was supported by NIH grant HD37432.
ABBREVIATIONS
- 3′UTR
3′untranslated region
- BCL2
B cell lymphoma 2
- CDKN
cyclin-dependent kinase inhibitor
- CTGF
connective tissue growth factor
- Cx43
connexin 43
- CYP1B1
cytochrome P450 1B1
- CYR61
cysteine-rich, angiogenic inducer 61
- E2 17β
estradiol
- ECM
extracellular matrix
- eNOS
endothelial nitric oxide synthase
- ERα
estrogen receptor α
- FGF
fibroblast growth factor
- Flt-1
fms-related tyrosine kinase 1 (VEGF receptor)
- GM-CSF
granulocyte monocyte colony stimulating factor
- GnRHa
gonadotropin-releasing hormone agonist
- HIF
hypoxia-induced factor
- HMGA1
high mobility group A1
- HMGA2
high mobility group A2
- IL
interleukin
- LSMC
leiomyoma smooth muscle cell
- MCP-1
monocyte chemoattractant protein-1
- miRNA
microRNA
- MPA
medroxyprogesterone acetate
- MSMC
myometrial smooth muscle cell
- p53
tumor protein 53
- PDGF
platelet-derived growth factor
- PR
progesterone receptor
- Rheb
Ras homolog enriched in brain
- TGF-β
transforming growth factor β
- TNF
tumor necrosis factor
- TSP-1
thrombospondin-1
- VEGF
vascular endothelial growth factor
Footnotes
Emerging Role of MicroRNAs in Reproductive Medicine; Guest Editor, Nasser Chegini, Ph.D.
References
- 1.Parker WH. Etiology, symptomatology, and diagnosis of uterine myomas. Fertil Steril. 2007;87:725–736. doi: 10.1016/j.fertnstert.2007.01.093. [DOI] [PubMed] [Google Scholar]
- 2.Wise LA, Palmer JR, Stewart EA, et al. Age-specific incidence rates for self-reported uterine leiomyomata in the Black Women’s Health Study. Obstet Gynecol. 2005;105:563–568. doi: 10.1097/01.AOG.0000154161.03418.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al Hendy A, Salama SA. Catechol-O-methyltransferase polymorphism is associated with increased uterine leiomyoma risk in different ethnic groups. J Soc Gynecol Investig. 2006;13:136–144. doi: 10.1016/j.jsgi.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Denschlag D, Bentz EK, Hefler L, et al. Genotype distribution of estrogen receptor-alpha, catechol-O-methyltransferase, and cytochrome P450 17 gene polymorphisms in Caucasian women with uterine leiomyomas. Fertil Steril. 2006;85:462–467. doi: 10.1016/j.fertnstert.2005.07.1308. [DOI] [PubMed] [Google Scholar]
- 5.Villanova FE, Andrade PM, Otsuka AY, et al. Estrogen receptor alpha polymorphism and susceptibility to uterine leiomyoma. Steroids. 2006;71:960–965. doi: 10.1016/j.steroids.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Lobel MK, Somasundaram P, Morton CC. The genetic heterogeneity of uterine leiomyomata. Obstet Gynecol Clin North Am. 2006;33:13–39. doi: 10.1016/j.ogc.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 7.Luo X, Ding L, Xu J, et al. Leiomyoma and myometrial gene expression profiles and their responses to gonadotropin-releasing hormone analog therapy. Endocrinology. 2005;146:1074–1096. doi: 10.1210/en.2004-1384. [DOI] [PubMed] [Google Scholar]
- 8.Luo X, Ding L, Xu J, et al. Gene expression profiling of leiomyoma and myometrial smooth muscle cells in response to transforming growth factor-beta. Endocrinology. 2005;146:1097–1118. doi: 10.1210/en.2004-1377. [DOI] [PubMed] [Google Scholar]
- 9.Quade BJ, Wang TY, Sornberger K, et al. Molecular pathogenesis of uterine smooth muscle tumors from transcriptional profiling. Genes Chromosomes Cancer. 2004;40:97–108. doi: 10.1002/gcc.20018. [DOI] [PubMed] [Google Scholar]
- 10.Tsibris JC, Segars J, Coppola D, et al. Insights from gene arrays on the development and growth regulation of uterine leiomyomata. Fertil Steril. 2002;78:114–121. doi: 10.1016/s0015-0282(02)03191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan Q, Luo X, Chegini N. Genomic and proteomic profiling I: leiomyomas in African Americans and Caucasians. Reprod Biol Endocrinol. 2007;5:34. doi: 10.1186/1477-7827-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 13.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 14.Calin GA, Croce CM. MicroRNAs and chromosomal abnormalities in cancer cells. Oncogene. 2006;25:6202–6210. doi: 10.1038/sj.onc.1209910. [DOI] [PubMed] [Google Scholar]
- 15.Engels BM, Hutvagner G. Principles and effects of microRNA-mediated post-transcriptional gene regulation. Oncogene. 2006;25:6163–6169. doi: 10.1038/sj.onc.1209909. [DOI] [PubMed] [Google Scholar]
- 16.Jovanovic M, Hengartner MO. miRNAs and apoptosis: RNAs to die for. Oncogene. 2006;25:6176–6187. doi: 10.1038/sj.onc.1209912. [DOI] [PubMed] [Google Scholar]
- 17.Zeng Y. Principles of micro-RNA production and maturation. Oncogene. 2006;25:6156–6162. doi: 10.1038/sj.onc.1209908. [DOI] [PubMed] [Google Scholar]
- 18.Calin GA, Pekarsky Y, Croce CM. The role of microRNA and other non-coding RNA in the pathogenesis of chronic lymphocytic leukemia. Best Pract Res Clin Haematol. 2007;20:425–437. doi: 10.1016/j.beha.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Ku G, McManus MT. Behind the scenes of a small RNA gene-silencing pathway. Hum Gene Ther. 2008;19:17–26. doi: 10.1089/hum.2007.1226. [DOI] [PubMed] [Google Scholar]
- 20.Mineno J, Okamoto S, Ando T, et al. The expression profile of microRNAs in mouse embryos. Nucleic Acids Res. 2006;34:1765–1771. doi: 10.1093/nar/gkl096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morita S, Horii T, Kimura M, et al. One Argonaute family member, Eif2c2 (Ago2), is essential for development and appears not to be involved in DNA methylation. Genomics. 2007;89:687–696. doi: 10.1016/j.ygeno.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Murchison EP, Stein P, Xuan Z, et al. Critical roles for Dicer in the female germline. Genes Dev. 2007;21:682–693. doi: 10.1101/gad.1521307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Carroll D, Mecklenbrauker I, Das PP, et al. A Slicer-independent role for Argonaute 2 in hematopoiesis and the microRNA pathway. Genes Dev. 2007;21:1999–2004. doi: 10.1101/gad.1565607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu Z, Jian Z, Shen SH, et al. Global analysis of microRNA target gene expression reveals that miRNA targets are lower expressed in mature mouse and Drosophila tissues than in the embryos. Nucleic Acids Res. 2007;35:152–164. doi: 10.1093/nar/gkl1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roldo C, Missiaglia E, Hagan JP, et al. MicroRNA expression abnormalities in pancreatic endocrine and acinar tumors are associated with distinctive pathologic features and clinical behavior. J Clin Oncol. 2006;24:4677–4684. doi: 10.1200/JCO.2005.05.5194. [DOI] [PubMed] [Google Scholar]
- 26.Zhao JJ, Hua YJ, Sun DG, et al. Genome-wide microRNA profiling in human fetal nervous tissues by oligonucleotide microarray. Childs Nerv Syst. 2006;22:1419–1425. doi: 10.1007/s00381-006-0173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Volinia S, Calin GA, Liu CG, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A. 2006;103:2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marsh EE, Lin Z, Yin P, et al. Differential expression of microRNA species in human uterine leiomyoma versus normal myometrium. Fertil Steril. 2008;89:1771–1776. doi: 10.1016/j.fertnstert.2007.05.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pan Q, Luo X, Chegini N. Differential expression of microRNAs in myometrium and leiomyomas and regulation by ovarian steroids. J Cell Mol Med. 2008;12:227–240. doi: 10.1111/j.1582-4934.2007.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Wang T, Zhang X, Obijuru L, et al. A micro-RNA signature associated with race, tumor size, and target gene activity in human uterine leiomyomas. Genes Chromosomes Cancer. 2007;46:336–347. doi: 10.1002/gcc.20415. [DOI] [PubMed] [Google Scholar]
- 31.Brennecke J, Stark A, Russell RB, et al. Principles of microRNA-target recognition. PLoS Biol. 2005;3:e85. doi: 10.1371/journal.pbio.0030085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grimson A, Farh KK, Johnston WK, et al. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol Cell. 2007;27:91–105. doi: 10.1016/j.molcel.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Belge G, Meyer A, Klemke M, et al. Upregulation of HMGA2 in thyroid carcinomas: a novel molecular marker to distinguish between benign and malignant follicular neoplasias. Genes Chromosomes Cancer. 2008;47:56–63. doi: 10.1002/gcc.20505. [DOI] [PubMed] [Google Scholar]
- 34.Pan Q, Luo X, Chegini N. Regulation and function analysis of mir-21 in leiomyoma and myometrium smooth muscle cells as well as in transformed leiomyoma and leiomyosarcoma cells. Reprod Sci. 2008;15:158A. [Google Scholar]
- 35.Gauldie J, Bonniaud P, Sime P, et al. TGF-beta, Smad3 and the process of progressive fibrosis. Biochem Soc Trans. 2007;35:661–664. doi: 10.1042/BST0350661. [DOI] [PubMed] [Google Scholar]
- 36.Rahimi RA, Leof EB. TGF-beta signaling: a tale of two responses. J Cell Biochem. 2007;102:593–608. doi: 10.1002/jcb.21501. [DOI] [PubMed] [Google Scholar]
- 37.Schmierer B, Hill CS. TGFbeta-SMAD signal transduction: molecular specificity and functional flexibility. Nat Rev Mol Cell Biol. 2007;8:970–982. doi: 10.1038/nrm2297. [DOI] [PubMed] [Google Scholar]
- 38.Chegini N. Gene expression and hormonal response. In: Brosens I, editor. Uterine Leiomyomata: Pathogenesis and Management. London, UK: Taylor & Francis; 2005. pp. 41–67. [Google Scholar]
- 39.Jenkins G. The role of proteases in transforming growth factor-beta activation. Int J Biochem Cell Biol. 2008;40:1068–1078. doi: 10.1016/j.biocel.2007.11.026. [DOI] [PubMed] [Google Scholar]
- 40.Leask A. Targeting the TGFbeta, endothelin-1 and CCN2 axis to combat fibrosis in scleroderma. Cell Signal. 2008;40:1068–1078. doi: 10.1016/j.cellsig.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 41.Cho WC. OncomiRs: the discovery and progress of microRNAs in cancers. Mol Cancer. 2007;6:60. doi: 10.1186/1476-4598-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cimmino A, Calin GA, Fabbri M, et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci U S A. 2005;102:13944–13949. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He L, He X, Lowe SW, et al. microRNAs join the p53 network—another piece in the tumour-suppression puzzle. Nat Rev Cancer. 2007;7:819–822. doi: 10.1038/nrc2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.He X, He L, Hannon GJ. The guardian’s little helper: microRNAs in the p53 tumor suppressor network. Cancer Res. 2007;67:11099–11101. doi: 10.1158/0008-5472.CAN-07-2672. [DOI] [PubMed] [Google Scholar]
- 45.Sales KM, Winslet MC, Seifalian AM. Stem cells and cancer: an overview. Stem Cell Rev. 2007;3:249–255. doi: 10.1007/s12015-007-9002-0. [DOI] [PubMed] [Google Scholar]
- 46.Foshay KM, Gallicano GI. Small RNAs, big potential: the role of microRNAs in stem cell function. Curr Stem Cell Res Ther. 2007;2:264–271. doi: 10.2174/157488807782793781. [DOI] [PubMed] [Google Scholar]
- 47.Togel F, Westenfelder C. Adult bone marrow-derived stem cells for organ regeneration and repair. Dev Dyn. 2007;236:3321–3331. doi: 10.1002/dvdy.21258. [DOI] [PubMed] [Google Scholar]
- 48.Mori A, Nakayama K, Suzuki J, et al. Analysis of stem cell factor for mast cell proliferation in the human myometrium. Mol Hum Reprod. 1997;3:411–418. doi: 10.1093/molehr/3.5.411. [DOI] [PubMed] [Google Scholar]
- 49.Lakshmipathy U, Hart RP. Concise review: microRNA expression in multipotent mesenchymal stromal cells. Stem Cells. 2008;26:356–363. doi: 10.1634/stemcells.2007-0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ahn WS, Kim KW, Bae SM, et al. Targeted cellular process profiling approach for uterine leiomyoma using cDNA microarray, proteomics and gene ontology analysis. Int J Exp Pathol. 2003;84:267–279. doi: 10.1111/j.0959-9673.2003.00362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wei JJ, Chiriboga L, Arslan AA, et al. Ethnic differences in expression of the dysregulated proteins in uterine leiomyomata. Hum Reprod. 2006;21:57–67. doi: 10.1093/humrep/dei309. [DOI] [PubMed] [Google Scholar]
- 52.Chwalisz K, Perez MC, Demanno D, et al. Selective progesterone receptor modulator development and use in the treatment of leiomyomata and endometriosis. Endocr Rev. 2005;26:423–438. doi: 10.1210/er.2005-0001. [DOI] [PubMed] [Google Scholar]
- 53.Levens ED, Potlog-Nahari C, Armstrong AY, et al. CDB-2914 for uterine leiomyomata treatment: a randomized controlled trial. Obstet Gynecol. 2008;111:1129–1136. doi: 10.1097/AOG.0b013e3181705d0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chegini N, Ma C, Tang XM, et al. Effects of GnRH analogues, ‘add-back’ steroid therapy, antiestrogen and antiprogestins on leiomyoma and myometrial smooth muscle cell growth and transforming growth factor-beta expression. Mol Hum Reprod. 2002;8:1071–1078. doi: 10.1093/molehr/8.12.1071. [DOI] [PubMed] [Google Scholar]
- 55.Xu Q, Ohara N, Chen W, et al. Progesterone receptor modulator CDB-2914 down-regulates vascular endothelial growth factor, adrenomedullin and their receptors and modulates progesterone receptor content in cultured human uterine leiomyoma cells. Hum Reprod. 2006;21:2408–2416. doi: 10.1093/humrep/del159. [DOI] [PubMed] [Google Scholar]
- 56.Ohara N, Morikawa A, Chen W, et al. Comparative effects of SPRM asoprisnil (J867) on proliferation, apoptosis, and the expression of growth factors in cultured uterine leiomyoma cells and normal myometrial cells. Reprod Sci. 2007;14:20–27. doi: 10.1177/1933719107311464. [DOI] [PubMed] [Google Scholar]
- 57.Al Hendy A, Salama S. Gene therapy and uterine leiomyoma: a review. Hum Reprod Update. 2006;12:385–400. doi: 10.1093/humupd/dml015. [DOI] [PubMed] [Google Scholar]
- 58.Chegini N. Implication of growth factor and cytokine networks in leiomyomas. In: Hill J, editor. Cytokines in Human Reproduction. New York, NY: John Wiley & Sons; 2000. pp. 133–159. [Google Scholar]
- 59.Walker CL, Stewart EA. Uterine fibroids: the elephant in the room. Science. 2005;308:1589–1592. doi: 10.1126/science.1112063. [DOI] [PubMed] [Google Scholar]
- 60.Catherino W, Salama A, Potlog-Nahari C, et al. Gene expression studies in leiomyomata: new directions for research. Semin Reprod Med. 2004;22:83–90. doi: 10.1055/s-2004-828614. [DOI] [PubMed] [Google Scholar]
- 61.Nowak RA. Identification of new therapies for leiomyomas: what in vitro studies can tell us. Clin Obstet Gynecol. 2001;44:327–334. doi: 10.1097/00003081-200106000-00019. [DOI] [PubMed] [Google Scholar]
- 62.Andersen J. Factors in fibroid growth. Baillieres Clin Obstet Gynaecol. 1998;12:225–243. [PubMed] [Google Scholar]
- 63.Kawaguchi K, Fujii S, Konishi I, et al. Mitotic activity in uterine leiomyomas during the menstrual cycle. Am J Obstet Gynecol. 1989;160:637–641. doi: 10.1016/s0002-9378(89)80046-8. [DOI] [PubMed] [Google Scholar]
- 64.Martel KM, Ko AC, Christman GM, et al. Apoptosis in human uterine leiomyomas. Semin Reprod Med. 2004;22:91–103. doi: 10.1055/s-2004-828615. [DOI] [PubMed] [Google Scholar]
- 65.Maruo T, Ohara N, Wang J, et al. Sex steroidal regulation of uterine leiomyoma growth and apoptosis. Hum Reprod Update. 2004;10:207–220. doi: 10.1093/humupd/dmh019. [DOI] [PubMed] [Google Scholar]
- 66.Chegini N. Peritoneal molecular environment, adhesion formation and clinical implication. Front Biosci. 2002;7:e91–e115. doi: 10.2741/A911. [DOI] [PubMed] [Google Scholar]
- 67.Sylvestre Y, De Guire V, Querido E, et al. An E2F/miR-20a autoregulatory feedback loop. J Biol Chem. 2007;282:2135–2143. doi: 10.1074/jbc.M608939200. [DOI] [PubMed] [Google Scholar]
- 68.Diederichs S, Haber DA. Sequence variations of microRNAs in human cancer: alterations in predicted secondary structure do not affect processing. Cancer Res. 2006;66:6097–6104. doi: 10.1158/0008-5472.CAN-06-0537. [DOI] [PubMed] [Google Scholar]
- 69.Meng F, Henson R, Lang M, et al. Involvement of human micro-RNA in growth and response to chemotherapy in human cholangiocarcinoma cell lines. Gastroenterology. 2006;130:2113–2129. doi: 10.1053/j.gastro.2006.02.057. [DOI] [PubMed] [Google Scholar]
- 70.Si ML, Zhu S, Wu H, et al. miR-21-mediated tumor growth. Oncogene. 2007;26:2799–2803. doi: 10.1038/sj.onc.1210083. [DOI] [PubMed] [Google Scholar]
- 71.Naguibneva I, Ameyar-Zazoua M, Polesskaya A, et al. The microRNA miR-181 targets the homeobox protein Hox-A11 during mammalian myoblast differentiation. Nat Cell Biol. 2006;8:278–284. doi: 10.1038/ncb1373. [DOI] [PubMed] [Google Scholar]
- 72.Adams BD, Furneaux H, White BA. The micro-ribonucleic acid (miRNA) miR-206 targets the human estrogen receptor-alpha (ERalpha) and represses ERalpha messenger RNA and protein expression in breast cancer cell lines. Mol Endocrinol. 2007;21:1132–1147. doi: 10.1210/me.2007-0022. [DOI] [PubMed] [Google Scholar]
- 73.Visone R, Pallante P, Vecchione A, et al. Specific microRNAs are downregulated in human thyroid anaplastic carcinomas. Oncogene. 2007;26:7590–7595. doi: 10.1038/sj.onc.1210564. [DOI] [PubMed] [Google Scholar]
- 74.Berlingieri MT, Pierantoni GM, Giancotti V, et al. Thyroid cell transformation requires the expression of the HMGA1 proteins. Oncogene. 2002;21:2971–2980. doi: 10.1038/sj.onc.1205368. [DOI] [PubMed] [Google Scholar]
- 75.Wong CF, Tellam RL. microRNA-26a targets the histone methyltransferase Enhancer of Zeste homolog 2 during myogenesis. J Biol Chem. 2008;283:9836–9843. doi: 10.1074/jbc.M709614200. [DOI] [PubMed] [Google Scholar]
- 76.Tsuchiya Y, Nakajima M, Takagi S, et al. MicroRNA regulates the expression of human cytochrome P450 1B1. Cancer Res. 2006;66:9090–9098. doi: 10.1158/0008-5472.CAN-06-1403. [DOI] [PubMed] [Google Scholar]
- 77.Mertens-Talcott SU, Chintharlapalli S, Li X, et al. The oncogenic microRNA-27a targets genes that regulate specificity protein transcription factors and the G2-M checkpoint in MDA-MB-231 breast cancer cells. Cancer Res. 2007;67:11001–11011. doi: 10.1158/0008-5472.CAN-07-2416. [DOI] [PubMed] [Google Scholar]
- 78.Johnson CD, Esquela-Kerscher A, Stefani G, et al. The let-7 microRNA represses cell proliferation pathways in human cells. Cancer Res. 2007;67:7713–7722. doi: 10.1158/0008-5472.CAN-07-1083. [DOI] [PubMed] [Google Scholar]
- 79.Luo X, Pan Q, Liu L, et al. Genomic and proteomic profiling II: comparative assessment of gene expression profiles in leiomyomas, keloids, and surgically-induced scars. Reprod Biol Endocrinol. 2007;5:35. doi: 10.1186/1477-7827-5-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tatsuguchi M, Seok HY, Callis TE, et al. Expression of microRNAs is dynamically regulated during cardiomyocyte hypertrophy. J Mol Cell Cardiol. 2007;42:1137–1141. doi: 10.1016/j.yjmcc.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ji R, Cheng Y, Yue J, et al. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of MicroRNA in vascular neointimal lesion formation. Circ Res. 2007;100:1579–1588. doi: 10.1161/CIRCRESAHA.106.141986. [DOI] [PubMed] [Google Scholar]
- 82.Sayed D, Hong C, Chen IY, et al. MicroRNAs play an essential role in the development of cardiac hypertrophy. Circ Res. 2007;100:416–424. doi: 10.1161/01.RES.0000257913.42552.23. [DOI] [PubMed] [Google Scholar]
- 83.Chegini N. TGF-b System: the principal pro-fibrotic mediator of peritoneal adhesion formation. Semin Reprod Med. 2008;26:298–312. doi: 10.1055/s-0028-1082388. [DOI] [PubMed] [Google Scholar]
- 84.Hinz B, Phan SH, Thannickal VJ, et al. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170:1807–1816. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127:526–537. doi: 10.1038/sj.jid.5700613. [DOI] [PubMed] [Google Scholar]
- 86.Levens E, Luo X, Ding L, et al. Fibromodulin is expressed in leiomyoma and myometrium and regulated by gonadotropin-releasing hormone analogue therapy and TGF-beta through Smad and MAPK-mediated signalling. Mol Hum Reprod. 2005;11:489–494. doi: 10.1093/molehr/gah187. [DOI] [PubMed] [Google Scholar]
- 87.Luo X, Levens E, Williams RS, et al. The expression of Abl interactor 2 in leiomyoma and myometrium and regulation by GnRH analogue and transforming growth factor-beta. Hum Reprod. 2006;21:1380–1386. doi: 10.1093/humrep/del011. [DOI] [PubMed] [Google Scholar]
- 88.ten Dijke P, Arthur HM. Extracellular control of TGFbeta signalling in vascular development and disease. Nat Rev Mol Cell Biol. 2007;8:857–869. doi: 10.1038/nrm2262. [DOI] [PubMed] [Google Scholar]
- 89.Ding L, Xu J, Luo X, et al. Gonadotropin releasing hormone and transforming growth factor beta activate mitogen-activated protein kinase/extracellularly regulated kinase and differentially regulate fibronectin, type I collagen, and plasminogen activator inhibitor-1 expression in leiomyoma and myometrial smooth muscle cells. J Clin Endocrinol Metab. 2004;89:5549–5557. doi: 10.1210/jc.2004-0161. [DOI] [PubMed] [Google Scholar]
- 90.Kato M, Zhang J, Wang M, et al. MicroRNA-192 in diabetic kidney glomeruli and its function in TGF-beta-induced collagen expression via inhibition of E-box repressors. Proc Natl Acad Sci U S A. 2007;104:3432–3437. doi: 10.1073/pnas.0611192104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Anderson C, Catoe H, Werner R. MIR-206 regulates connexin43 expression during skeletal muscle development. Nucleic Acids Res. 2006;34:5863–5871. doi: 10.1093/nar/gkl743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McCarthy JJ, Esser KA. Micro RNA-1 and microRNA-133a expression are decreased during skeletal muscle hypertrophy. J Appl Physiol. 2007;102:306–313. doi: 10.1152/japplphysiol.00932.2006. [DOI] [PubMed] [Google Scholar]
- 93.Dews M, Homayouni A, Yu D, et al. Augmentation of tumor angiogenesis by a Myc-activated microRNA cluster. Nat Genet. 2006;38:1060–1065. doi: 10.1038/ng1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Luo X, Ding L, Chegini N. CCNs, fibulin-1C and S100A4 expression in leiomyoma and myometrium: inverse association with TGF-beta and regulation by TGF-beta in leiomyoma and myometrial smooth muscle cells. Mol Hum Reprod. 2006;12:245–256. doi: 10.1093/molehr/gal015. [DOI] [PubMed] [Google Scholar]
- 95.Leask A, Abraham DJ. All in the CCN family: essential matricellular signaling modulators emerge from the bunker. J Cell Sci. 2006;119:4803–4810. doi: 10.1242/jcs.03270. [DOI] [PubMed] [Google Scholar]
- 96.Sampath D, Zhu Y, Winneker RC, et al. Aberrant expression of Cyr61, a member of the CCN (CTGF/Cyr61/Cef10/NOVH) family, and dysregulation by 17 beta-estradiol and basic fibroblast growth factor in human uterine leiomyomas. J Clin Endocrinol Metab. 2001;86:1707–1715. doi: 10.1210/jcem.86.4.7423. [DOI] [PubMed] [Google Scholar]
- 97.Bein K, Odell-Fiddler ET, Drinane M. Role of TGF-beta1 and JNK signaling in capillary tube patterning. Am J Physiol Cell Physiol. 2004;287:C1012–C1022. doi: 10.1152/ajpcell.00101.2004. [DOI] [PubMed] [Google Scholar]
- 98.Coller HA, Forman JJ, Legesse-Miller A. “Myc’ed messages”: myc induces transcription of E2F1 while inhibiting its translation via a microRNA polycistron. PLoS Genet. 2007;3:e146. doi: 10.1371/journal.pgen.0030146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hyder SM, Huang JC, Nawaz Z, et al. Regulation of vascular endothelial growth factor expression by estrogens and progestins. Environ Health Perspect. 2000;108(Suppl 5):785–790. doi: 10.1289/ehp.00108s5785. [DOI] [PubMed] [Google Scholar]
- 100.Kuehbacher A, Urbich C, Dimmeler S. Targeting microRNA expression to regulate angiogenesis. Trends Pharmacol Sci. 2008;29:12–15. doi: 10.1016/j.tips.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 101.Kuehbacher A, Urbich C, Zeiher AM, et al. Role of Dicer and Drosha for endothelial microRNA expression and angiogenesis. Circ Res. 2007;101:59–68. doi: 10.1161/CIRCRESAHA.107.153916. [DOI] [PubMed] [Google Scholar]
- 102.Poliseno L, Tuccoli A, Mariani L, et al. MicroRNAs modulate the angiogenic properties of HUVECs. Blood. 2006;108:3068–3071. doi: 10.1182/blood-2006-01-012369. [DOI] [PubMed] [Google Scholar]
- 103.Hua Z, Lv Q, Ye W, et al. MiRNA-directed regulation of VEGF and other angiogenic factors under hypoxia. PLoS One. 2006;1:e116. doi: 10.1371/journal.pone.0000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lee DY, Deng Z, Wang CH, et al. MicroRNA-378 promotes cell survival, tumor growth, and angiogenesis by targeting SuFu and Fus-1 expression. Proc Natl Acad Sci U S A. 2007;104:20350–20355. doi: 10.1073/pnas.0706901104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Weston G, Trajstman AC, Gargett CE, et al. Fibroids display an anti-angiogenic gene expression profile when compared with adjacent myometrium. Mol Hum Reprod. 2003;9:541–549. doi: 10.1093/molehr/gag066. [DOI] [PubMed] [Google Scholar]
- 106.Chen CZ, Lodish HF. MicroRNAs as regulators of mammalian hematopoiesis. Semin Immunol. 2005;17:155–165. doi: 10.1016/j.smim.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 107.Li SC, Tang P, Lin WC. Intronic microRNA: discovery and biological implications. DNA Cell Biol. 2007;26:195–207. doi: 10.1089/dna.2006.0558. [DOI] [PubMed] [Google Scholar]
- 108.Lodish HF, Zhou B, Liu G, et al. Micromanagement of the immune system by microRNAs. Nat Rev Immunol. 2008;8:120–130. doi: 10.1038/nri2252. [DOI] [PubMed] [Google Scholar]
- 109.O’Connell RM, Taganov KD, Boldin MP, et al. MicroRNA-155 is induced during the macrophage inflammatory response. Proc Natl Acad Sci U S A. 2007;104:1604–1609. doi: 10.1073/pnas.0610731104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rodriguez A, Vigorito E, Clare S, et al. Requirement of bic/microRNA-155 for normal immune function. Science. 2007;316:608–611. doi: 10.1126/science.1139253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Costinean S, Zanesi N, Pekarsky Y, et al. Pre-B cell proliferation and lymphoblastic leukemia/high-grade lymphoma in E(mu)-miR155 transgenic mice. Proc Natl Acad Sci U S A. 2006;103:7024–7029. doi: 10.1073/pnas.0602266103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Meng F, Henson R, Wehbe-Janek H, et al. The microRNA let-7a modulates interleukin-6-dependent STAT-3 survival signaling in malignant human cholangiocytes. J Biol Chem. 2007;282:8256–8264. doi: 10.1074/jbc.M607712200. [DOI] [PubMed] [Google Scholar]
- 113.Meng F, Wehbe-Janek H, Henson R, et al. Epigenetic regulation of microRNA-370 by interleukin-6 in malignant human cholangiocytes. Oncogene. 2008;27:378–386. doi: 10.1038/sj.onc.1210648. [DOI] [PubMed] [Google Scholar]
- 114.Tili E, Michaille JJ, Cimino A, et al. Modulation of miR-155 and miR-125b levels following lipopolysaccharide/TNF-alpha stimulation and their possible roles in regulating the response to endotoxin shock. J Immunol. 2007;179:5082–5089. doi: 10.4049/jimmunol.179.8.5082. [DOI] [PubMed] [Google Scholar]
- 115.Fontana L, Pelosi E, Greco P, et al. MicroRNAs 17–5p-20a-106a control monocytopoiesis through AML1 targeting and M-CSF receptor upregulation. Nat Cell Biol. 2007;9:775–787. doi: 10.1038/ncb1613. [DOI] [PubMed] [Google Scholar]
- 116.Li QJ, Chau J, Ebert PJ, et al. miR-181a is an intrinsic modulator of T cell sensitivity and selection. Cell. 2007;129:147–161. doi: 10.1016/j.cell.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 117.Neilson JR, Zheng GX, Burge CB, et al. Dynamic regulation of miRNA expression in ordered stages of cellular development. Genes Dev. 2007;21:578–589. doi: 10.1101/gad.1522907. [DOI] [PMC free article] [PubMed] [Google Scholar]