Summary
The internalization of activated receptor tyrosine kinases (RTKs) by endocytosis and their subsequent down regulation in lysosomes plays a critical role in regulating the duration and intensity of downstream signaling events. Uncoupling of the RTK cMet from ligand-induced degradation was recently shown to correlate with sustained receptor signaling and increased cell tumorigenicity, suggesting that the corruption of these endocytic mechanisms could contribute to increased cMet signaling in metastatic cancers. To understand how cMet signaling for normal cell growth is controlled by endocytosis and how these mechanisms are dysregulated in metastatic cancers, we developed flow cytometry-based assays to examine cMet internalization.
Keywords: cMet, endocytosis, flow cytometry, hepatocyte growth factor, Internalin B, receptor tyrosine kinase
1. Introduction
Receptor tyrosine kinases (RTKs) are a family of single-pass transmembrane proteins that control a wide variety of cellular events in multicellular organisms including cell proliferation, differentiation, survival, and migration. Escape from the negative regulatory mechanisms that normally inactivate RTK signaling, promote neoplastic growth and cell transformation (1–6). Tumor cell growth and metastasis are the result of increased signaling from multiple receptors, including the cMet RTK. cMet activation by binding to its physiological ligand hepatocyte growth factor (HGF) or to the Internalin B (InlB) protein of Listeria monocytogenes, promotes receptor endocytosis and triggers the activation of multiple downstream signaling cascades (7–10). In normal cells, cMet signaling is critical for cell growth, motility, and organ development, as well as their regenerative response to acute injury (reviewed in ref. 11). Conversely, dys-regulated cMet signaling has been shown to promote cell proliferation, increased cell motility, and invasive morphogenetic responses correlating closely with the increased metastatic potential of several human cancers (reviewed in refs. 11 and 12). Receptor internalization from the cell surface represents a key first step in the inactivation process, by targeting the internalized receptor for lysosomal degradation. Corruption of these mechanisms contributes to prolonged cMet signaling (5,6,13) suggesting that defects in cMet endocytic trafficking could be a contributing factor to increased cMet levels and signaling in some human metastatic cancers.
Flow cytometry is a qualitative and rapid method for counting, examining, and sorting the fluorescent characteristics of single cells. Flow cytometry offers several advantages for examining the endocytic trafficking properties of RTKs over several techniques including confocal microscopy, (co)immunoprecipitations, and cell surface biotinylation assays. First, flow cytometry is a rapid, high-throughput approach that can be used to quantify thousands of events in a subpopulation of cells. Second, it measures the signal intensity of all cells within a population and is therefore less susceptible to investigator bias. Third, flow cytometry allows the study of rare cell subsets that comprise too small of a population for analysis by conventional biochemical methods. Finally, the parametric nature of flow cytometry makes it amenable for measuring multiple events simultaneously within individual cells of heterogeneous populations. This chapter describes two flow cytometry-based assays we have developed for measuring residual cell surface levels of cMet in response to ligand and the direct internalization of cMet/ligand complexes under steady-state conditions.
2. Materials
2.1. Cell Culture
Cell line: Human mammary epithelial cells (T47D) stably expressing full-length human cMet (T47D/cMet; a generous gift of M. Park, McGill University) are maintained in Dulbecco's Modified Eagle's Medium (DMEM) containing 10% fetal bovine serum (FBS), supplemented with 400 μg/mL G418 as explained elsewhere (7,8).
Phosphate-buffered saline (PBS): To make a 10×PBS stock solution, dissolve 80 g NaCl, 2 g KCl, 11.5 g Na2HPO4, and 2 g KH2PO4 in distilled water and adjust the final volume to 1 L, store at room temperature. PBS is prepared using the 10×PBS stock diluted in distilled water and stored at room temperature.
FBS: aliquot and store at −20°C.
Fluorescence-activated cell sorting (FACS) buffer: PBS prechilled to 4°C and supplemented with 2% FBS immediately before use. Prechill to 4°C before use.
Acid stripping buffer: DMEM supplemented with 0.2% bovine serum albumin (BSA) and with the pH adjusted to pH 3.5 using HCl.
4% Formaldehyde: A 4% formaldehyde solution in PBS is prepared immediately before use from a 16% formaldehyde stock (product no. 18505, Ted Pella, Inc.).
5 mM ethylenediamine-tetra-acetic acid (EDTA)-PBS: Calcium free PBS (Mediatech, Inc. Cat. no. 21−031-CV) supplemented with 5 mM EDTA pH 8.0.
Goat anti-HGF receptor (HGFR): R&D Systems Inc. (Cat. no. AF276), aliquot and store at −20°C.
Chicken anti-goat Alexa488-conjugated secondary antibody: Invitrogen (Cat. no. A21467), aliquot and store at 4°C.
Recombinant human HGF(rhHGF): Pepro Tech Inc. (Cat. no. 100−39), aliquot and store at −20°C.
2.2. Preparation and Labeling of Recombinant InlB
Recombinant InlB: pKI22 expressing recombinant InlB containing an N-terminal His6 tag was expressed in BL21 DE3 cells and isolated by Ni+ chromatography as previously described (10). A protein fraction enriched in recombinant InlB was desalted (D-Salt Dextran, Pierce), purified by ion-exchange chromatography (HiTrap SP Amersham Pharmacia) and eluted with PBS containing 0.4 to 0.5 M NaCl (10). Pure recombinant InlB was Amicon concentrated to 2 to 3 mg/mL of protein (BCA Assay, Pierce), exchanged into PBS and stored in aliquots at −80°C.
Alexa488-antibody labeling kit: from Invitrogen Corp. (Cat.no. A-20181), store at −80°C.
P6 micro bio-spin chromatography columns: Bio-Rad Laboratories (Cat.no. 732−6221), store at 4°C.
Cover slips: VWR, selected micro-cover glasses 12 mm round, no. 1 thickness (Cat. no. 48366−251).
Nunclon*Δ 4-well sterile tissue culture dishes (VWR, Cat. no. 62407−068).
0.45 M sucrose in DMEM: solution is made by dissolving 7.7 g of sucrose (Fisher Scientific, Cat. no. S5−3) in 50 mL of DMEM without serum.
Alexa594-Transferrin (Tfn): molecular probes (Invitrogen, Cat. no. T-13343).
Glass slides: FISHER finest Premium Microscope Slides Frosted 3″× 1″× 1 mm (Cat. no. 12−544−3).
Mounting reagent: FluorSave™ Reagent (Calbiochem, Cat. no. 345789).
Zeiss LSM 510 Meta microscope (Carl Zeiss, Inc.).
3. Methods
In this section, we describe two flow cytometry-based assays to examine cMet internalization. We previously reported that the Listeria protein InlB mimics HGF-induced cMet internalization through clathrin-coated pits and receptor degradation in lysosomes (7). Accordingly, InlB is an effective ligand to use with HGF to examine cMet endocytosis under normal conditions, a prerequisite for studies that examine the role of receptor endocytosis as a determinant of cMet signaling in metastatic cancers. To obtain reproducible and reliable information, it is often necessary to utilize complementary approaches that distinguish receptor internalization from ligand uptake. The first method we describe measures the relative amount of residual cMet remaining on the cell surface following ligand-induced receptor internalization. Following treatment with ligand for predetermined times, the cells are removed from the plates and stained under nonpermeable conditions using an antibody specific for the extracellular domain of cMet.
The second method examines the internalization of fluorescently labeled HGF or InlB under steady-state conditions. In this assay, serum starved cells are incubated at 37°C in media containing fluorescently labeled ligand to trigger the internalization of receptor/ligand complexes. Noninternalized ligand is removed from the cell surface using an acid-washing protocol, the cells are then harvested and processed for flow cytometry. The level of internalized fluorescently labeled ligand is used as an indicator for receptor internalization. Conditions for ligand labeling with the fluorescent dye Alexa488 are described.
3.1. Internalization Assay Using cMet Cell Surface Staining
All of the solutions required for cell treatment are prepared in advance as required. T47D/cMet cells are allowed to adhere to 60-mm plates at a density of 6× 105 cells/plate for each data point (see Note 1). Additional control plates for negative controls are also required (see step 12).
The following day when the cells are 80% confluent, the cells are serum starved for 5 h by incubation at 37°C in prewarmed DMEM (without serum; see Note 2).
To initiate receptor internalization, the cells are incubated in prewarmed DMEM containing 100 ng/mL HGF or InlB at 37°C for 5, 10, or 15 min. One plate is incubated in DMEM without ligand for 15 min at 37°C as a control.
Receptor internalization is halted by shifting the cells to ice followed by two washes in ice-cold PBS for 10 min each at 4°C with gentle shaking.
Noninternalized ligand is stripped from the cell surface using freshly prepared, ice-cold acid stripping buffer (DMEM/0.2% BSA, pH 3.5 adjusted with HCl), three times for 5 min each on a shaking platform (see Note 3). The cells are then washed with ice-cold PBS three times for 5 min each with gentle shaking.
Incubate the cells in prechilled FACS buffer containing 3μg/mL of goat anti-HGFR antibody at 4°C for 1 h (see Notes 4 and 5).
After washing the cells three times with ice-cold PBS for 5 min each on a shaking platform, the cells are incubated in prechilled FACS buffer containing 8 μg/mL of the appropriate Alexa488-conjugated secondary antibody for 1 h on ice.
Following three 5-min washes with ice-cold PBS, the cells are removed from each plate using 1 mL of 5 mM EDTA-PBS prewarmed to 37°C for 10 min (see Notes 6 and 7).
Transfer each cell suspension to individually labeled microfuge tubes and pellet the cells by centrifugation at 1,100g for 3 min at 4°C.
Following three washes in ice-cold FACS buffer, resuspend the cells in FACS buffer at a density of 106 cells per milliliter. If the sample will not be analyzed immediately, add an equal volume of 4% formaldehyde to fix the cells and store in the dark at 4°C.
Prior to analysis by flow cytometry using a BD FACSCanto™ instrument, the fixed cells are washed three times with ice-cold FACS buffer by centrifugation at 1,100g for 2 min each. Typically 20,000 cells are acquired for each time point (see Note 8).
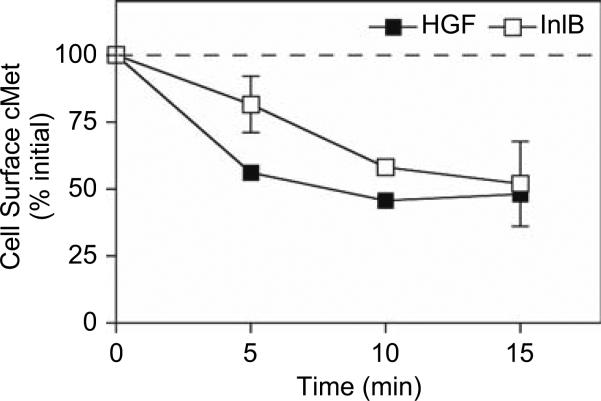
Data analysis to quantify changes in the mean surface receptor fluorescence values was performed using FACS DIVA software version 5.0.1 (BD Biosciences). The mean fluorescence intensity (MFI) of the cells for each data set is compared to negative control plates stained with either the secondary antibody only or with a nonspecific primary antibody and the secondary antibody. The relative percentage of residual cell surface cMet at each time point (tx) is calculated relative to the MFI of cells without internalization (t0) as (MFI tx – MFI control antibody only/MFI t0 – MFI control antibody only) × 100. An example of the results is shown in Fig. 1.
Fig. 1.
cMet internalization in response to HGF or InlB. T47D/cMet cells were left untreated with ligand or incubated in media containing 100 ng/mL of HGF or InlB for 5, 10, or 15 min at 37°C. The cells were chilled to 4°C and cMet remaining on the cell surface was specifically labeled with anti-cMet antibody using non permeabilized conditions and quantified by flow cytometry. Results represent the mean fluorescence intensities (MFI) normalized to untreated control cells under each experimental condition from duplicate experiments. Values represent the means for each data set with standard error. The results indicate that treatment with HGF or InlB results in a decrease in surface cMet levels, consistent with receptor internalization.
3.2. Conjugation of Alexa488 to HGF and InlB
Add 200 μL of a 2 mg/mL solution of InlB or HGF (see Note 9) with 20 uL of freshly prepared 1.0 mM sodium bicarbonate to increase the pH of the reaction mixture to approx 8.0 for optimal ligand labeling.
Transfer the entire reaction mixture to a single reaction vial containing the appropriate, unconjugated fluorophore. Although we typically use Alexa488 for our labeling studies; we have also had good results using Alexa594.
Incubate the reaction for 1 h at room temperature in the dark with occasional shaking, followed by an overnight incubation in the dark at 4°C mixing end-over-end (see Note 10).
The following morning, a P6 micro bio-spin column (Bio-Rad, Hercules) is pre-equilibrated with PBS by washing three times with 500 μL of PBS by centrifugation at 1,100g for 2 min. Transfer the pre-equilibrated tube to a new prelabeled microfuge tube.
The labeling reaction must be clarified by centrifugation at 10,000g for 10 min at 4°C to remove excess aggregates. The resulting supernatant from the labeling reaction is then carefully transfered to the pre-equilibrated P6 micro bio-spin column and centrifuged at 1,100g for 4 min at room temperature. The combination of a clarification step with the spin column ensures the removal of unconjugated dye that would otherwise contribute to nonspecific, high background fluorescence.
The fluorescently labeled ligand is recovered in the column eluent. Dilute a sample of the labeled ligand and measure the absorbance of the conjugated solution at 280 nm and 494 nm in a cuvette with a 1 cm path length. The concentration of incorporated label into the ligand is determined using the following equation: Protein concentration (M) = [A280-(A494 × 0.11)] × dilution factor/molecular extinction coefficient. The molecular extinction coefficient × for HGF and InlB is measured using the following equation: ε = A280/cl where c is the concentration of the protein (moles/L) and l is the length of the light-absorbing samples (cm).
The labeled ligand is aliquoted and stored at −80°C (see Note 11).
3.3. Confocal Microscopy to Alexa488-Labeling
Cover slips that have been precleaned with 70% ethanol under sterile conditions are placed into 4-well plates in preparation for cell plating. When the cover slips have air dried, plate duplicate sets of T47D/cMet cells at a density of 5× 104 cells per well and allow them to adhere overnight.
The following morning cells are serum starved for 5 h at 37°C by incubation with prewarmed DMEM (without serum).
One set of cover slips is washed with prewarmed serum free DMEM for 15 min at 37°C. The second set of cover slips is incubated in prewarmed DMEM containing 0.45 M sucrose for 15 min at 37°C to produce a hypertonic condition that inhibits endocytosis (see Note 12).
The appropriate cover slips are then incubated in prewarmed DMEM with or without 0.45 M sucrose supplemented with 100 ng/mL Alexa488-labeled InlB and 5 μg/mL Alexa594-Tfn for 15 min at 37°C(see Note 13).
The plate containing the cover slips is rapidly transferred to ice and washed three times with ice-cold PBS for 5 min each on a shaking platform to remove excess ligand.
The PBS is gently removed by aspiration and 500 μL of 4% formaldehyde is added to each well for 10 min at room temperature.
Following fixation the formaldehyde is discarded (into a hazardous waste container) and the cells are washed three times at room temperature with PBS to remove residual formaldehyde.
Each cover slip is removed from the well using forceps and carefully air dried to remove excess solution. One drop of FluorSave™ Reagent is placed on a clean glass slide and the cover slip is inverted into it so that the surface with the attached cells is directly in contact with the mounting reagent.
The slides are allowed to set overnight in the dark to avoid photobleaching of the fluorophore and stored at 4°C in the dark prior to imaging.
Images are captured using a Zeiss LSM 510 Meta confocal microscope and a 63x Oil objective. An example of the results is shown in Fig. 2.
Fig. 2.
Alexa488-labeled InlB internalization is blocked by hypertonic shock. T47D/cMet were cells preincubated in DMEM (− hypertonic) or containing 0.45 M sucrose (+ hypertonic) for 15 min at 37°C. The cells were then incubated in the corresponding media supplemented with 100 ng/mL of Alexa488-labeled InlB and 5 μg/mL Alexa 594-labeled Transferrin (Tfn) for 15 min at 37°C and then processed for confocal microscopy. Representative examples are shown (scale, 10 μm). Hypertonic treatment results in the colocalization of InlB and Tfn on the cell surface (closed arrows) consistent with the inhibition of cMet internalization. Conversely, internalized InlB colocalizes with internalized Tfn (open arrows) in non hypertonic cells and appear as punctate spots reminiscent of early endosomes.
3.4. Steady-State Internalization of Fluorescently Labeled Ligand
T47D/cMet cells are allowed to adhere onto 60-mm plates at a density of 6 × 105 cells/plate. The cells are serum starved for 5 h by incubation at 37°C in DMEM only.
The following morning, the cells are incubated in prewarmed DMEM supplemented with 100 ng/mL Alexa 488-labeled HGF or Alexa 488-labeled InlB at 37°C for 5, 10, or 15 min to promote cMet internalization. Controls include serum starved cells that were treated with media lacking ligand.
The cells are rapidly placed on ice to halt receptor trafficking and then washed three times with ice-cold PBS for 5 min each on a shaking platform to remove excess ligand.
Noninternalized ligand is removed from the cell surface using three, 5 min washes in ice-cold acid stripping buffer followed by three 5 min washes in ice-cold PBS. Plates are routinely covered with aluminum foil during the washes to minimize bleaching of the fluorophore.
The washed cells are then removed from each plate by incubation in 1 mL of prewarmed 5 mM EDTA-PBS at 37°C for 10 min and transferred into prelabeled microfuge tubes.
The cells are recovered by centrifugation at 1100g for 3 min at 4°C, followed by three washes in ice-cold FACS buffer.
The cells are then gently resuspended in FACS buffer to a density of 106 cells per milliliter by trituration. If the sample will not be analyzed immediately, the cells are fixed by the addition of an equal volume of 4% formaldehyde and stored in the dark at 4°C.
The MFI of the cells is typically measured by flow cytometry using a BD FACSCanto instrument and FACS DIVA software version 5.0.1 (BD Biosciences). Fixed cells are first washed with ice-cold FACS buffer three times as described above prior to analysis. Typically 20,000 cells are acquired for each time point. To quantify changes in the value of internalized MFI (MFIint), the MFI of the cells at each time point (tx) is compared to control cells that were untreated with ligand (MFIControl) and calculated using the following equation: MFIint = MFItx – MFIControl (see Note 14). An example of the results is shown in Fig. 3.
Fig. 3.
Internalization of Alexa488-labeled InlB. T47D/cMet cells were treated in DMEM with or without 100 ng/mL of Alexa488-labeled InlB at 37°C for the indicated times. Following incubation the cells were chilled to 4°C, noninternalized ligand was removed by acid washing and the cells processed for flow cytometry. Results represent the internal mean fluorescence intensity (MFIint) normalized to untreated control cells under each experimental condition from duplicate experiments. Values represent the means for each data set with standard error. The results show a rapid increase in internal InlB fluorescence consistent with the internalization of the cMet receptor in response to InlB.
4. Notes
Cell density is a critical determinant for the success of this approach. High cell densities could affect the efficiency of ligand binding to the receptor and hence downstream cell-signaling responses. For example, cell density has been shown to affect the internalization of the epidermal growth factor receptor (EGFR) and the formation of the endocytic Cbl-endophilin complex in HEK 293 cells (14).
Serum starvation has the advantage that it can synchronize cell-signaling responses and receptor internalization events. Since the optimal time for serum starvation varies considerably between different cell lines and for different receptor systems, this step needs to be empirically determined. Growth hormones such as HGF and EGF normally exist in serum resulting in high levels of basal receptor activation and hence receptor internalization in cells cultured in media supplemented with serum. While growth factors such as EGF can be readily removed from sera by dialysis, the large size of HGF necessitates the use of cell incubations under serum-free conditions. We routinely access the efficacy of our serum starvation conditions for different cell lines using Western analysis with antibodies specific for two key tyrosine residues in the cMet kinase domain (Y1234, Y1235) that are phosphorylated in response to ligand binding (7,8,10). Another potential concern is crosstalk between different RTK systems. For example, in human hepatocellular (HepG2 and HuH7) and pancreatic (DAN-G) carcinoma cells activation of the EGFR by EGF also results in the transactivation and internalization of cMet (15). Accordingly, it is best to test for receptor crosstalk by Western analysis prior to experimentation.
Acid stripping cells by washing with low pH solutions readily dissociates most ligands from their receptors (4,13,16). However the optimal acid stripping conditions for removing cell surface associated ligand without damaging the cells must be empirically determined for each cell line and for each receptor/ligand combination. Some cell types do not tolerate low pH washes (e.g., pH 3.5). In these situations, we have washed cells using solutions with a slightly higher pH (e.g., pH 4.0) for longer time periods, taking care to monitor the response of the cells morphologically. We routinely monitor the effectiveness of the acid washing conditions for the removal of cell surface associated fluorescent ligand by confocal microscopy or flow cytometry.
Although fetal bovine sera or goat sera are generally good blocking reagents for these types of studies, it is preferable to use serum from the same species as the fluorophore-conjugated secondary antibody as a blocking reagent, to reduce any nonspecific binding events and lower background fluorescence accordingly. The use of BSA as a blocking agent may introduce other nonspecific binding sites that could increase background fluorescence.
The primary and secondary antibodies should be used at saturating concentrations to ensure complete labeling of cell surface cMet. The working concentration for each antibody is determined empirically. It is important to note that the saturating concentration of each antibody may vary between cell lines due to differences in the cell surface levels of cMet.
We routinely use 5 mM EDTA-PBS to detach adherent cells from plates and preserve the integrity of the cell surface receptors. However, some tightly adherent lines require the use of 0.25% Trypsin-EDTA in HBSS (Cellgro, Cat. no. 25−053-CI) for detachment. Since trypsin is a protease, trypsin treatment will result in the proteolytic cleavage of the extracellular domain of cMet and hence will result in reduced levels of cell surface staining. In these situations, we use flow cytometry to monitor the uptake of fluorescently labeled ligand as an alternative approach (see Subheading 3.4.).
Alternatively, ice-cold 5 mM EDTA-PBS can be used to harvest the cells prior to staining. This approach has the advantage that it utilizes less antibody during the labeling reaction. Following isolation, the cells are pelleted in microfuge tubes by centrifugation at 1100g for 2 min at 4°C and resuspended in FACS buffer at a concentration of 106 cells per milliliter prior to antibody staining. We routinely confirm cell density on a sample of the cells using a hemocytometer. If the cell density is too great it will affect the efficiency of subsequent cell surface staining. In this situation, the cells can be diluted to the correct cell density using additional FACS buffer. If the cell density is insufficient, the cells can be concentrated by centrifugation and resuspended in a smaller volume of FACS buffer.
Live cells can be distinguished from dead cells using the cell impermeant stain propidium iodide (PI). When bound to DNA the fluorescence of PI is enhanced 20 to 30 fold. Accordingly, dead cells and not live cells will stain strongly with PI. Incubate cells with 5μg/mL PI (use 1 mg/mL stock solution diluted into FACS buffer) at room temperature for 15 min immediately prior to flow cytometry analysis.
Purified proteins must be resuspended in a buffer free of ammonium ions or primary amines to avoid competition with the amine groups of the reactive dye and inefficient protein labeling.
Under our ligand-labeling conditions, we estimate that each molecule of ligand is labeled with four to six molecules of dye without any apparent loss in biological activity. Since the kinetics of the labeling reaction could vary for different proteins, it is important to empirically determine the optimal labeling conditions for each protein. Over labeling could result in loss of protein function, reduce the affinity of the protein for its target or cause protein aggregation and hence nonspecific binding.
We have found that the conjugates are stable for at least 6 mo when aliquoted and stored at −80°C. We do not add sodium azide to the conjugates as this may confer some cell toxicity in subsequent experiments.
The treatment of cells with hypertonic media inhibits clathrin-mediated receptor internalization (17). Although its precise mechanism of action remains unclear, hypertonic shock has been shown to cause a decrease in the size and number of clathrin-coated pits at the plasma membrane (17). It is important to note that the inhibitory effect of hypertonic media on endocytosis is rapidly reversible. Thus ligand must be added to the cells in hypertonic media in order to obtain optimal inhibition of receptor endocytosis.
Since Tfn and its receptor are internalized exclusively via clathrin-coated pits, they are routinely used as specific markers for this endocytic route.
These methods can be used to study the roles of different molecules in RTK internalization by transfecting plasmids expressing dominant negative mutant proteins. Optimally it is best to use compatible fluorescent markers to identify the subpopulation of transfected cells that do not interfere with the fluorophores chosen to monitor ligand internalization or receptor retention on the cell surface. When studying a rare event in the cell population, we often increase the resuspension volume of the sample to compensate for the 200 μL void volume associated with the BD FACSCanto™ instrument.
Acknowledgments
This work was supported by grants from the National Science Foundation (IBN-343739) and the National Institutes of Health (CA-112605 and CA-119075) to L.A.E. We would like to thank Mark Griffin in the UTMB at Galveston Flow Cytometry and Cell Sorting Core Facility and Marta Lorinczi for their technical assistance.
References
- 1.Booden MA, Eckert LB, Der CJ, Trejo J. Persistent signaling by dysregulated thrombin receptor trafficking promotes breast carcinoma cell invasion. Mol Cell. 2004;24:1990–1999. doi: 10.1128/MCB.24.5.1990-1999.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oosterhoff JK, Kuhne LC, Grootegoed JA, Blok LJ. EGF signalling in prostate cancer cell lines is inhibited by a high expression level of the endocytosis protein REPS2. Int. J. Cancer. 2005;113:561–567. doi: 10.1002/ijc.20612. [DOI] [PubMed] [Google Scholar]
- 3.Li L, Cohen SN. Tsg101: a novel tumor susceptibility gene isolated by controlled homozygous functional knockout of allelic loci in mammalian cells. Cell. 1996;85:319–329. doi: 10.1016/s0092-8674(00)81111-3. [DOI] [PubMed] [Google Scholar]
- 4.Longva KE, Blystad FD, Stang E, Larsen AM, Johannessen LE, Madshus IH. Ubiquitination and proteasomal activity is required for transport of the EGF receptor to inner membranes of multivesicular bodies. J. Cell. Biol. 2002;156:843–854. doi: 10.1083/jcb.200106056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peschard P, Fournier TM, Lamorte L, et al. Mutation of the c-Cbl TKB domain binding site on the Met receptor tyrosine kinase converts it into a transforming protein. Mol. Cell. 2001;8:995–1004. doi: 10.1016/s1097-2765(01)00378-1. [DOI] [PubMed] [Google Scholar]
- 6.Peschard P, Park M. Escape from Cbl-mediated downregulation: a recurrent theme for oncogenic deregulation of receptor tyrosine kinases. Cancer Cell. 2003;3:519–523. doi: 10.1016/s1535-6108(03)00136-3. [DOI] [PubMed] [Google Scholar]
- 7.Li N, Xiang GS, Dokainish H, Ireton K, Elferink LA. The Listeria protein internalin B mimics hepatocyte growth factor-induced receptor trafficking. Traffic. 2005;6:459–473. doi: 10.1111/j.1600-0854.2005.00290.x. [DOI] [PubMed] [Google Scholar]
- 8.Shen Y, Naujokas M, Park M, Ireton K. InIB-dependent internalization of Listeria is mediated by the Met receptor tyrosine kinase. Cell. 2000;103:501–510. doi: 10.1016/s0092-8674(00)00141-0. [DOI] [PubMed] [Google Scholar]
- 9.Veiga E, Cossart P. Listeria hijacks the clathrin-dependent endocytic machinery to invade mammalian cells. Nature Cell Biol. 2005;7:894–900. doi: 10.1038/ncb1292. [DOI] [PubMed] [Google Scholar]
- 10.Ireton K, Payrastre B, Cossart P. The Listeria monocytogenes protein InlB is an agonist of mammalian phosphoinositide 3-kinase. J. Biol. Chem. 1999;274:17025–17032. doi: 10.1074/jbc.274.24.17025. [DOI] [PubMed] [Google Scholar]
- 11.Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nature Rev. Mol. Cell Biol. 2003;4:915–925. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- 12.Gao CF, Vande Woude GF. HGF/SF-Met signaling in tumor progression. Cell Res. 2005;15:49–51. doi: 10.1038/sj.cr.7290264. [DOI] [PubMed] [Google Scholar]
- 13.Abella JV, Peschard P, Naujokas MA, et al. Met/Hepatocyte growth factor receptor ubiquitination suppresses transformation and is required for Hrs phosphorylation. Mol. Cell Biol. 2005;25:9632–9645. doi: 10.1128/MCB.25.21.9632-9645.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmidt MH, Furnari FB, Cavenee WK, Bogler O. Epidermal growth factor receptor signaling intensity determines intracellular protein interactions, ubiquitination, and internalization. Proc. Natl. Acad. Sci. USA. 2003;100:6505–6510. doi: 10.1073/pnas.1031790100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischer OM, Giordano S, Commoglio PM, Ulrich A. Reactive oxygen species mediate Met receptor transactivation by G protein-coupled receptors and the epidermal growth factor receptor in human carcinoma cells. J. Biol. Chem. 2004;279:28970–28978. doi: 10.1074/jbc.M402508200. [DOI] [PubMed] [Google Scholar]
- 16.Klausner RD, Ashwell G, van Renswoude J, Harford JB, Bridges KR. Binding of apotransferrin to K562 cells: explanation of the transferrin cycle. Proc Natl Acad Sci USA. 1983;80:2263–6. doi: 10.1073/pnas.80.8.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heuser JE, Anderson RGW. Hypertonic Media Inhibit Receptor-mediated Endocytosis by Blocking Clathrin-coated Pit Formation. J.Cell Biol. 1989;108:389–400. doi: 10.1083/jcb.108.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]