Abstract
Proliferation of dendritic cells (DC) in the spleen is regulated by positive growth signals through the lymphotoxin (LT)-β receptor; however, the countering inhibitory signals that achieve homeostatic control are unresolved. Mice deficient in LTα, LTβ, LTβR, and the NFκB inducing kinase show a specific loss of CD8− DC subsets. In contrast, the CD8α− DC subsets were overpopulated in mice deficient in the herpesvirus entry mediator (HVEM) or B and T lymphocyte attenuator (BTLA). HVEM- and BTLA-deficient DC subsets displayed a specific growth advantage in repopulating the spleen in competitive replacement bone marrow chimeric mice. Expression of HVEM and BTLA were required in DC and in the surrounding microenvironment, although DC expression of LTβR was necessary to maintain homeostasis. Moreover, enforced activation of the LTβR with an agonist Ab drove expansion of CD8α− DC subsets, overriding regulation by the HVEM-BTLA pathway. These results indicate the HVEM-BTLA pathway provides an inhibitory checkpoint for DC homeostasis in lymphoid tissue. Together, the LTβR and HVEM-BTLA pathways form an integrated signaling network regulating DC homeostasis.
Dendritic cells (DC)3 are bone marrow-derived APCs that play a crucial role bridging innate and adaptive immune responses through the activation of naive Ag-specific T cells (1). Expression of CD11c, in addition to the common hemopoietic markers CD11b, F4/80, CD24 (HSA), CD205 (DEC-205), aid in defining DC subpopulations in mouse lymphoid organs (2). Three main subpopulations of CD11chigh expressing DC are present in the mouse spleen, the CD8α+ DC subset, and the CD4+ and a CD8α− CD4− dual negative subsets, the latter two forming the CD8α− DC subset. The CD4+ and CD8α−/4− DC subsets are principally localized in the marginal zone bridging channels, and extend in the red pulp whereas the CD8α+ DC are found in the T cell-rich area in the white pulp (3). Several lines of evidence indicate that DC subsets possess distinct functions, although both CD8α+ and CD8α− DC present Ag to T cells (4, 5). A fourth subset of splenic DC is the plasmacytoid DC (pDC), which expresses low levels of CD11c (B220+ CD11b−) and are distinguished functionally by secretion high levels of type 1 IFNs in response to challenge with viral and bacterial pathogens (6, 7). In the lymph node, two additional DC subsets can be delineated by relatively low expression of CD8, high levels of MHC II with either moderate or high expression of DEC-205 (8).
The pathways regulating the development and homeostasis of DC subpopulations are unresolved (9). Cellular reconstitution studies showed that CD8α+ thymic and splenic DC are derived from early CD4low thymic precursors, leading to the idea that some DC could have a lymphoid origin (10, 11). However, such a pathway seems less likely in view of the observations that common myeloid progenitor cells as well as lymphoid progenitors can differentiate into both CD8α− and CD8α+ DC subsets (12–14). More recent evidence indicates that a resident DC precursor gives rise to conventional CD8− and CD8α subsets independently of pDC (15). Genetic analysis of DC development and homeostasis has revealed distinct genes control the major DC subsets. The CD8α+ DC subset is affected by genetic deficiency in ICSBP (IFN consensus sequence binding factor), also called IRF-8 (IFN response factor-8), Id2 (helix-loop-helix family transcription factor inhibitor DNA binding-2), and Jak3 (Janus tyrosine kinase) (16–18). By contrast, genes involved in the differentiation of the CD8α− DC subset include Ikaros C−/−, transcription factor PU.1, IRF-2 and IRF-4 (IFN regulatory factor), Notch-dependent transcription factor RBP-J, TRAF6 (TNF receptor associated factor-6), and RelB (NFκB) and lymphotoxin-β receptor (LTβR) (19 –26).
Recent evidence indicates the LTβR is a growth regulator of DC in lymphoid tissues (26). TNF does not appear to play this role but does influence DC differentiation in the bone marrow; however, LTα−/−, LTβ−/− and LTβR−/− mice exhibit normal bone marrow DC subsets (27). In contrast, dysregulation of DC in peripheral lymphoid organs is apparent in LT-deficient mice, but was previously thought to be a result of disrupted architecture and loss of chemokines. Recent evidence lessens that possibility and supports the idea that LTβR provides a key signal for self-renewal directly to CD8α− DC subsets (26). Interestingly, RelB is a downstream target of LTβR signaling (28) raising the possibility these molecules function in a common pathway regulating growth of CD8α− DC subsets.
Counter regulatory pathways should exist that operate to limit the growth promoting actions of the LTβR pathway in DC. However, the LTβR is one constituent of a multicomponent system of interconnected signaling pathways, with no defined inhibitory systems that would directly counter regulate signaling. The LTβR binds two ligands, the membrane LTαβ complex and LIGHT (LT-related inducible ligand that competes for glycoprotein D binding to herpesvirus entry mediator on T cells), yet LIGHT also engages the herpesvirus entry mediator (HVEM) (TNFRSF14) (29, 30). The LIGHT-HVEM pathway appears to play a prominent role as a positive cosignaling pathway during T cell activation akin to its TNFR paralogs (e.g., 4-1BB, Ox40, CD27) (31). Adding to the complexity is the recent observation that HVEM engages a non-canonical ligand, B and T lymphocyte attenuator (BTLA) (32). BTLA, a member of the Ig superfamily, is activated by HVEM binding, attenuating Ag receptor signals through an ITIM/ITSM-dependent recruitment of Src homology phosphatase-1 or -2 (33, 34). BTLA and LIGHT bind HVEM at distinct sites (35–37), yet membrane-anchored LIGHT can noncompetitively displace BTLA, suggesting HVEM serves as a molecular switch between positive and inhibitory signaling (35). HVEM-BTLA pathway plays an inhibitory role in regulating T cell proliferation (38–41). The interconnectedness of these cytokines is further underscored by the binding of secreted LTα to both receptors for TNF, in addition to HVEM (29). The multicomponent nature of the LTαβ/LIGHT systems precludes a clear assignment of the pathways involved in regulating DC homeostasis, which is further complicated by the multiple cell types including Ag-activated T and B lymphocytes, NK cells and lymphoid tissue inducer (LTi) cells expressing the various ligands and receptors.
In this study, using mice genetically deficient in the various components of the LTαβ/LIGHT pathways and pharmacological modulation of the LTβR pathway, we demonstrate that LTαβ-LTβR via NFκB-inducing kinase (NIK) is the predominant signaling pathway that positively regulates the growth of CD4+ and CD8α−/4− DC subsets in lymphoid tissues. By contrast, HVEM- and BTLA-deficient mice both show a specific increase in the CD4+ and CD4− CD8α DC subsets, providing a counteracting, inhibitory checkpoint in the accumulation of DC in lymphoid tissues. Together the results indicate homeostasis of DC within lymphoid tissues is achieved by integration of positive and inhibitory signals through the LTαβ-LTβR and HVEM-BTLA pathways.
Materials and Methods
Mice and reagents
C57BL/6 (B6) and LTα−/− were purchased from The Jackson Laboratory. Mice deficient in LTβR−/− (42), LIGHT−/− (43), LTβ−/− (44), LTβ/LIGHT−/− (43), HVEM−/− (45), alymphoplasia (aly) (46), or BTLA−/− (47) were inbred in the C57Bl/6 background. LTβ/LIGHT/HVEM−/− mice were generated by backcrossing the indicated strains and bred at the LIAI. All the knockout mice used in this study were backcrossed for: LTβ, n = 10 generations; LTα, n = 8 and the others n = 5. Sex- and age-matched male and female mice between 7 and 10 wk of age were used in all experiments. Mice were treated with the mouse LTβR-Fc decoy receptor or agonistic anti-LTβR Ab (4H8) by i.p. injection of 100 µg of each reagent every 3 to 4 days. These reagents were prepared as described (48). All breeding and experimental protocols were performed under the approval by LIAI Animal Care Committee.
Cell preparation from lymphoid organs Flow cytometry
Spleens were perfused with balanced salt solution containing collagenase (0.35 mg/ml; CLSIII; Worthington Biochemical), incubated for 30 min at 37°C in HBSS medium containing collagenase (1.4 mg/ml) and further dissociated in 2 mM EDTA saline and passage through a 70 µm nylon mesh filter. Spleen cells were analyzed by flow cytometry with a FACS-Calibur cytofluorometer (BD Bioscience) with FlowJo software (Tree Star). The cells were blocked with anti-FcR 2.4G2 (anti-Fc receptor; BD Pharmingen) and then stained with fluorescein (FITC)-coupled anti-CD11c- (N418), or PE-coupled anti-CD8α (53-6.7), anti-Ly6G (1A8), anti-PDCA-1 (Miltenyi Biotec), PerCp-coupled anti-B220, anti-CD11b (M1/70) or allophycocyanin-coupled anti-CD4 (L3T4), anti-CD11b (M1/70) or anti-F4/80 (BM8) (BD Pharmingen). HVEM and BTLA were detected on cells previously blocked with anti-FcR 2.4G2 by incubation with a rat anti-mouse HVEM (14C1.1) and hamster anti-C57BL/6 BTLA (6A6). Anti-rat IgM-PE or anti-Armenian hamster IgG-PE (BD Pharmingen) were used as the chromophores, respectively. In parallel, rat IgM or hamster IgG Abs were used as controls for nonspecific background staining. The background staining was similar to staining obtained with the specific anti-HVEM or anti-BTLA-specific Abs of cells from HVEM- or BTLA-deficient mice, respectively (data not shown). Cells were gated according to size and scatter to eliminate dead cells and debris from analysis.
BrdU labeling
Mice were administered BrdU (2 mg/mouse) i.p. for a 16 h pulse. Splenocytes were stained with anti-CD4-PE, anti-CD8α-PerCp and anti-CD11c-allophycocyanin. BrdU incorporation was visualized using FITC BrdU detection kit (BD Pharmingen) and flow cytometry.
Bone marrow chimeras
Bone marrow chimeric mice were generated by i.v. transfer of bone marrow cells (5 × 106) from LTβR-deficient or C57BL/6 mice into previously lethally irradiated (9.5 Gy, Cesium) C57BL/6 and LTβR-deficient recipients. Bone marrow was isolated from donor femurs and tibias and depleted of RBC. Eight weeks after reconstitution, flow cytometry was used to analyze DC subsets.
Quantitative PCR
Eight weeks after reconstitution, spleens from LTβR chimera were recovered, snap frozen in liquid nitrogen and total mRNA from spleen was isolated with Trizol (Invitrogen), digested with DNase and reverse transcribed. cDNA was used for real-time PCR on a MX4000 with SyBr Green detection protocol as outlined by the manufacturer. Sequence-specific chemokine primers are available from the corresponding author.
Results
LTαβ-LTβR signaling regulates DC homeostasis
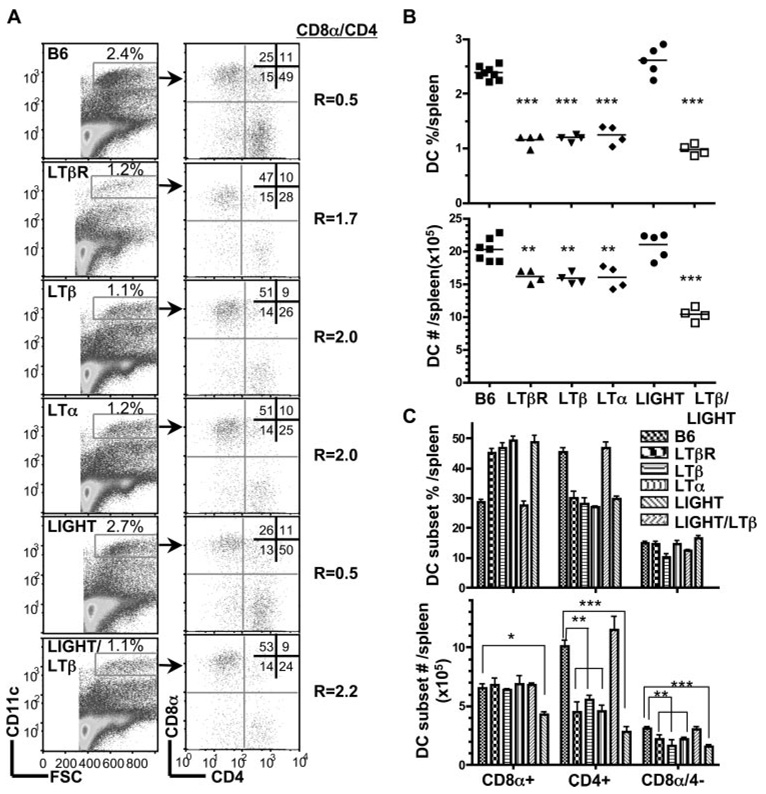
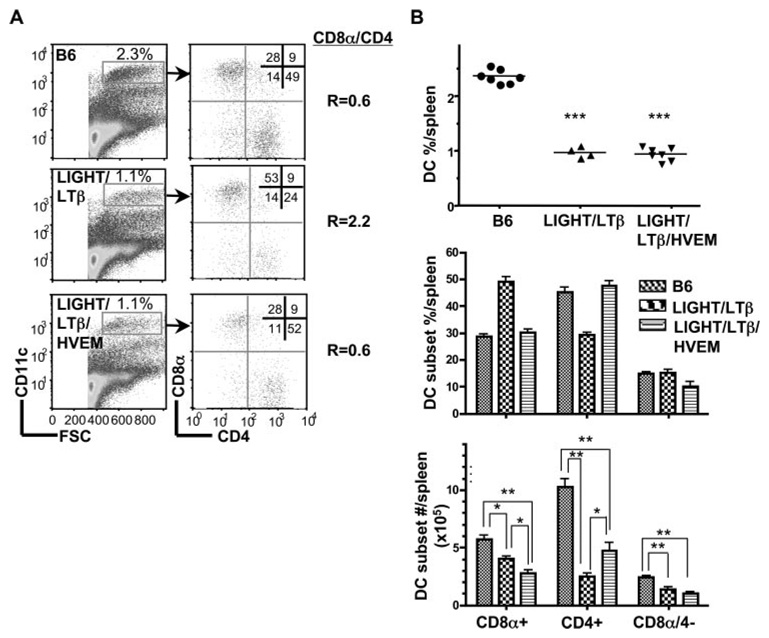
The LTβR utilizes at least two distinct ligands, the heterotrimeric LTα1β2 complex and LIGHT, both of which activate the LTβR. To distinguish the roles of these two ligands in DC homeostasis, C57BL/6 (B6) mice deficient in LTβ (LTβ−/−), LTα (LTα−/−), or LIGHT (LIGHT−/−) were analyzed by flow cytometry for the major DC subsets in the spleen in comparison to wild-type (wt) and LTβR−/− mice. The total number of CD11chigh cells from the spleen was reduced in LTβ−/− and LTα−/− mice when compared with wt B6 mice in a pattern identical to LTβR−/− mice (Fig. 1A). The ratio of CD8α to CD4+ DC subsets in wt B6 mice is 0.5; however, this ratio was inverted in LTβ (2.0), LTα (2.0), and LTβR-deficient mice (1.7). The inverted DC ratio in the LT-deficient mice reflected a specific decrease in the percentage (Fig. 1B) and total numbers of CD4+ and CD8α−/4− DC subsets (Fig. 1C).
FIGURE 1.
LTαβ-LTβR interaction mediates homeostasis of CD8− DC subsets. A, CD11chigh cells in the spleen were analyzed for CD4 and CD8α expression in wt B6, LTβR-, LTβ-, LTα-, LIGHT- and LTβ/LIGHT-deficient mice by flow cytometry as described in Materials and Methods. A representative histogram is shown for each mouse strain. The ratio of CD8α to CD4 DC subsets was calculated from values in the upper left and lower right quadrants. B, The percentage of DC are presented as a fraction of total nucleated splenocytes (top panel) and the total number of DC (bottom panel) in the spleen from the indicated gene deficient mice. Each data point represents an individual animal and the data are pooled from two analyses. C. The percentage (top panel) and total number (bottom panel) of individual CD4+, CD8α, and CD8α−/CD4− DC subsets within the gated CD11chigh DC. The bars are the mean ± SD from at least three mice per group and the data are representative of three independent experiments. In all panels, Student t test evaluation of significance where *, **, and *** denote p < 0.05, p < 0.01, and p < 0.001, respectively, between the indicated groups.
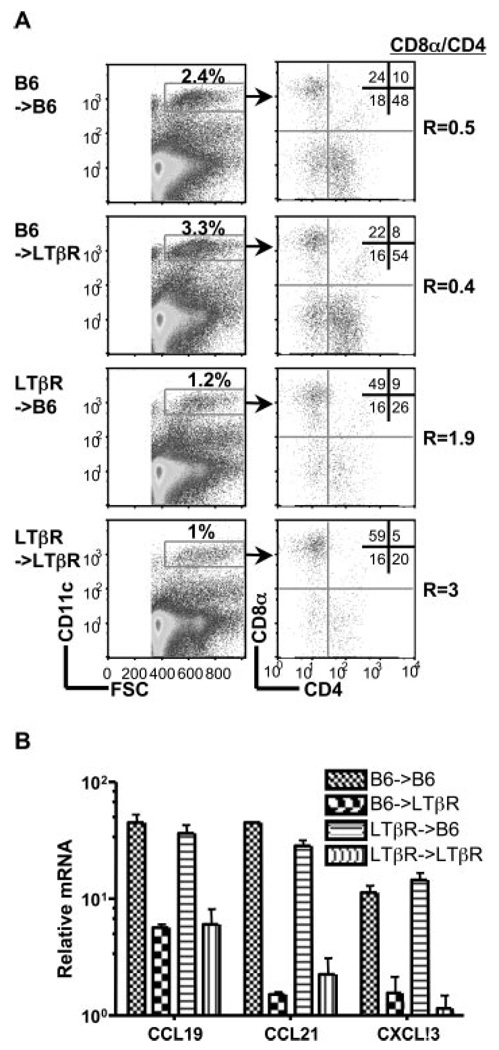
LTβR signaling controls the expression of several chemokines required for the maturation of the splenic architecture, which might effect migration of DC in the adult animal. However, the specific loss of these DC subsets in LTβR−/− mice was independent of the expression of lymphoid organizing chemokines, CCL21 (SLC), CCL19 (ELC), and CXCL13 (BLC) as determined with bone marrow chimeras that isolated LTβR expression in either the bone marrow or the radioresistant stroma (Fig. 2). LTβR−/− recipients reconstituted with wt B6 bone marrow had normal CD4+ and CD8α−/4− DC subsets, yet chemokine levels were as depressed as LTβR−/− mice, whereas in the reciprocal chimeras (LTβR−/−→B6) chemokine expression was normal, but the DC subset ratio was inverted. These results indicate the DC phenotype in LT-deficient mice was not due to impaired maturation of the splenic architecture.
FIGURE 2.
Homeostasis of splenic DC subsets is independent of the lymphoid tissue organizing chemokines. A, Eight weeks after bone marrow reconstitution, splenocytes within the CD11chigh population (gates indicated in figure) were analyzed for expression of CD4 and CD8α. A representative histogram is shown for each group of chimeras and is representative of two independent experiments. The ratio of CD8α to CD4 DC subsets was calculated from values in the upper left and lower right quadrants. B, Quantitative PCR analysis of chemokine mRNA expressed in the spleen from the bone marrow chimeras is presented as the relative amount of indicated mRNA normalized to 18S. Error bars are the mean±SD from at least two mice per group and the data are representative of two independent experiments.
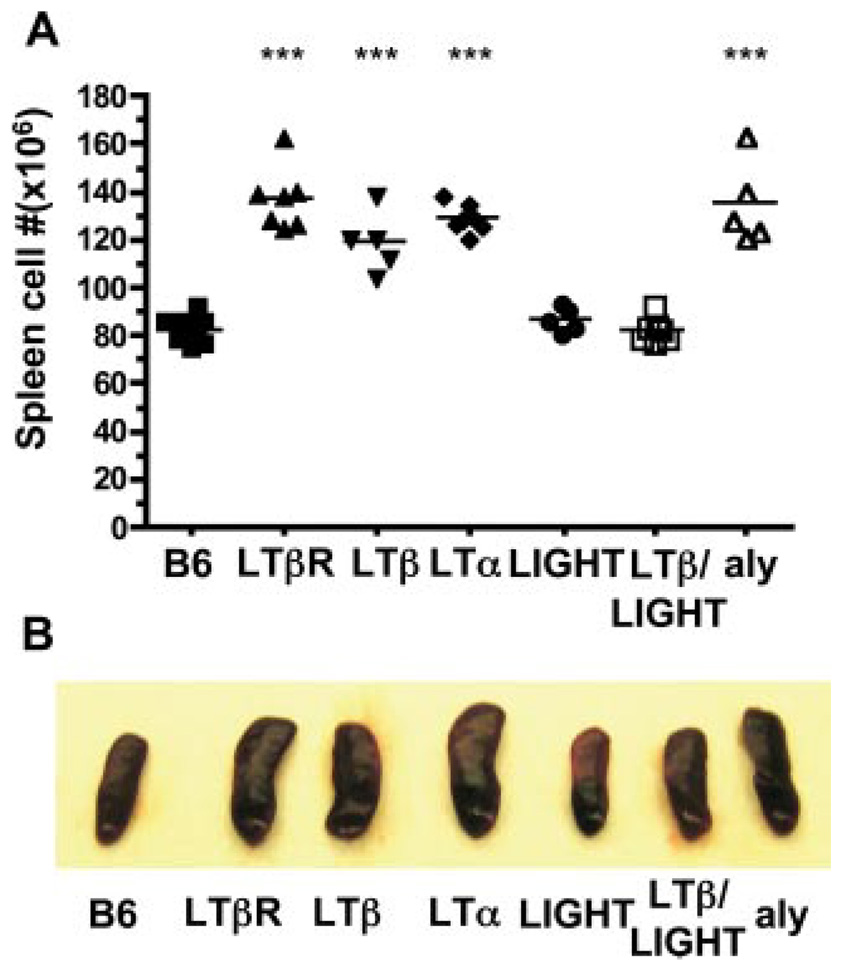
In contrast to LT-deficient mice, genetic deficiency in LIGHT, a second ligand for LTβR, showed normal cellularity in DC and a normal CD8α/CD4 DC ratio (r = 0.5) (Fig. 1). Mice deficient in both LTβ and LIGHT (LTβ/LIGHT−/−) revealed an inverted CD8α/CD4 DC ratio (r = 2.2) suggesting no additional role of LIGHT in controlling the number of DC in the spleen (Fig. 1). However, we observed a difference in the size of the spleen from LTβ and LIGHT/LTβ-deficient mice. An accounting of the cellularity revealed increased number of cells in the spleen of LTβ, LTα, and LTβR-deficient mice relative to wt mice (cellularity increased 45, 56, and 67%, respectively), proportionally effecting all the major lymphoid and myeloid subpopulations, with a corresponding enlargement of the spleen (Fig. 3). Deleting LIGHT in the LTβ−/− mice decreased the total number of cells in the spleen to that of wt mice. Although the total number of cells decreased, which gives the appearance of an additional effect on DC subsets, the ratio of CD8α/CD4 (2.2) remained inverted in the LTβ/LIGHT−/− suggesting that LIGHT was not influencing CD8α DC subset. Previous work attributed the increase in the total number of splenocytes in LT-deficient mice to the lack of peripheral lymphoid organs (42), however, that notion is inconsistent with the observation that lymph nodes and Peyer’s Patches are also missing in the LTβ/LIGHT−/− mice (43). This result also lessens the likelihood that the migration of immediate DC precursor population to the spleen was affected in the LTβ/LIGHT−/− mice (49). That the effect of LIGHT on splenic size and cellularity occurred only in the absence of LTβ suggests that LIGHT counter regulates the LTαβ-LTβR pathway to influence cellularity of the spleen.
FIGURE 3.
Counter regulatory effects of LIGHT and LTαβ-LTβR on splenic size and cellularity. A, The total number of spleen cells were determined in wt B6, LTβR, LTβ-, LTα-, LIGHT-, LTβ/LIGHT-deficient and aly mice. Each point represents the value obtained from an individual animal and the data are from five pooled experiments. B, Representative spleens from mice deficient in LTβR, LTβ, LTα, LIGHT, and LTβ/LIGHT, or mutant aly and wt B6 mice. Student t test significance between the wt B6 and the other groups p < 0.001 (*).
The HVEM-BTLA pathway provides an inhibitory checkpoint for the homeostasis of CD8α− DC subsets
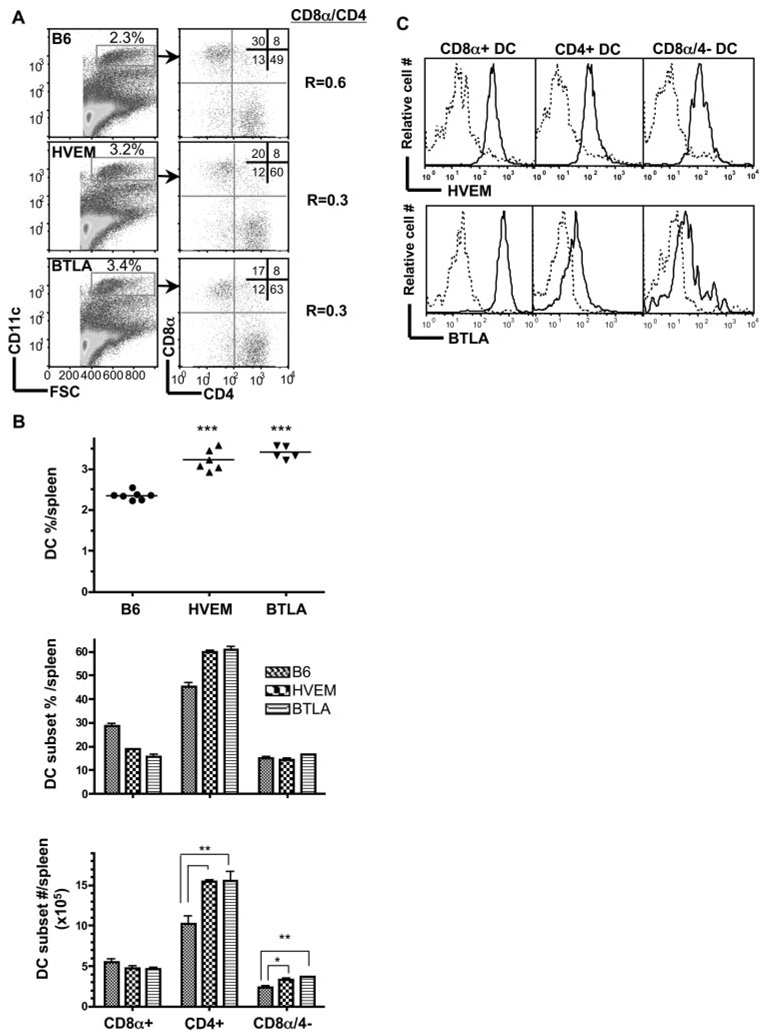
The finding that DC subsets were normal in the LIGHT−/− mice raised the issue of whether HVEM-BTLA pathway was involved in regulating DC homeostasis. Surprisingly in contrast to mice deficient in LTβR signaling, HVEM−/− mice had a significantly higher percentage of DC in the spleen with an altered CD8α/CD4 DC ratio (0.3) reflecting a specific increase in the CD4+ and CD8α−/4− DC subsets compared with wt mice (Fig. 4A). BTLA-deficient mice displayed an identical phenotype to the HVEM−/− mice, with reduced CD8α/CD4 DC ratio (0.3) resulting also from an increase in CD4+ and CD8α−/4− DC subsets when compared with wt mice (Fig. 4B).
FIGURE 4.
HVEM and BTLA counter regulate homeostasis of CD4+ and CD4− CD8α− DC subsets. A, CD11chigh cells gated as indicated were analyzed for CD4 and CD8α expression in wt B6, HVEM- or BTLA-deficient mice. A representative histogram is shown for each mouse strain. The ratio of CD8α to CD4 DC subsets was calculated from values in the upper left and lower right quadrants. B, The percentage of DC as a fraction of total nucleated splenocytes (top panel), the percentage of individual DC subsets (middle panel) and the total number cells in each DC subset (bottom panel) in the spleen from the indicated gene-deficient mice. Each data point represents the value obtained from an individual animal and the data are pooled from two analyses. Bars show the mean ± SD from at least n = three mice per group and the data are representative of three independent experiments. Student’s t test was performed where *, **, and *** denote significance of p < 0.05, p < 0.01, and p < 0.001, respectively, between the indicated groups. C, Flow cytometric analysis of HVEM and BTLA expression by CD8α+, and CD4+ CD8− DC subsets within gated DC from WT (solid line) and HVEM-deficient mice (dashed line) and WT mice (BTLA staining = solid line and ctrl staining = dashed line), respectively.
All of the conventional DC subsets in spleens of B6 mice express HVEM, BTLA (Fig. 4C), and LTβR (26). Interestingly, BTLA expression was detectable on CD4+ and CD8α−/4− subsets but more prominent on CD8α+ DC subset (Fig. 4C). Thus, all DC express the potential to deliver and receive information via the HVEM-BTLA pathway.
DC homeostasis depends on intrinsic expression of the LTβR (26) (Fig. 2) raising the issue of whether HVEM and BTLA expression is required in the hemopoietic or stromal compartments. To test this issue, we generated mixed bone marrow chimeric mice. The results demonstrate that DC from BTLA−/− bone marrow (CD45.2) were much more abundant (6.2-fold) than the DC from wt (CD45.1) in wt recipients, although the CD45.2/CD45.1 ratio of total spleen cells was approximately 1 in reconstituted mice indicating equivalent potential of cells from wt or deficient mice to replenish the spleen (Table I). HVEM−/− DC (CD45.2) also showed an enhanced capacity (4.6-fold) to reconstitute the spleen of wt recipient mice. Reconstitution of the DC pool in recipients deficient in HVEM or BTLA expression moderated the advantage of DC lacking BTLA (wt/BTLA→BTLA; CD45 ratio = 2.0) or HVEM (wt/HVEM→HVEM; CD45 ratio = 2.8) compared with wt recipients suggesting the radioresistant stroma contributes to HVEM-BTLA dependent regulation of DC.
Table I.
DC reconstitution in mixed bone marrow chimeras
| Live cells and CD11chi DC subpopulation | ||||||||
|---|---|---|---|---|---|---|---|---|
| CD45.2/CD45.1 ratioa | ||||||||
| Chimera→recipientb | Total cells | DC-BTLAc | DC-HVEMc | |||||
| BTLA/wt→wt | 1.3 | 6.2 | ||||||
| BTLA/wt→BTLA | 1.0 | 2.0 | ||||||
| HVEM/wt→Wt | 1.2 | 4.6 | ||||||
| HVEM/wt→HVEM | 1.0 | 2.8 | ||||||
| DC Subsets | ||||||||
| CD45.2 (%)d | CD45.1 (%)d | |||||||
| CD8α+ | CD4+ | CD8α−4− | Ratio CD8α/4e | CD8α+ | CD4+ | CD8α4− | Ratio CD8α/4e | |
| wt/BTLA→wt | 19 ± 6 | 62 ± 7 | 14 ± 2 | 0.3 | 16 ± 6 | 65 ± 11 | 19 ± 4 | 0.2 |
| wt/BTLA→BTLA | 22 ± 3 | 53 ± 5 | 18 ± 2 | 0.4 | 22 ± 1 | 56 ± 3 | 20 ± 4 | 0.4 |
| wt/HVEM→wt | 29 ± 2 | 46 ± 4 | 14 ± 0.2 | 0.6 | 19 ± 5 | 56 ± 3 | 25 ± 1 | 0.3 |
| wt/HVEM→HVEM | 29 ± 3 | 48 ± 3 | 13 ± 1 | 0.6 | 19 ± 2 | 58 ± 3 | 21 ± 0.3 | 0.3 |
The two populations of cells expressing allelic forms of CD45 were identified by flow cytometry using the anti-CD45.2 mAb (clone 104). The CD45.2/CD45.1 ratio within the live cell gate was calculated as the sum of the percentage of CD45.2+ cells divided by the sum of percentage of CD45.2− cells.
Mixed bone marrow chimera were made by transferring a 1:1 ratio of CD45.1+ C57BL/6 bone marrow cells and CD45.2+ HVEM- or BTLA-deficient bone marrow cells into lethally irradiated (10 Gy, Cesium) CD45.1+ C57BL/6 (n = 2) or CD45.2+ HVEM- or BTLA-deficient mice (HVEM; BTLA) (n = 3). Spleens from mice were analyzed by flow cytometry 8 wk later.
CD45.2/CD45.1 DC ratio was calculated by determining DC with in the FSC-CD11chi live gate and dividing the sum of the percentage of CD45.2+ DC by the sum of the percentage of CD45.2− DC.
Mean percentage of each DC subset ± SEM for the CD45.2+ and CD45.2− populations.
The CD8α/4 DC subset ratio was the calculated sum of the percentage of CD8α DC divided by the sum of the percentage of CD4 DC.
Analysis of the DC subsets in the mixed chimeras presented a more complex picture on the role of HVEM-BTLA in regulating splenic DC (Table I). The ratio of CD8α/CD4 DC subsets in the CD45.2+ population in the wt or BTLA−/− recipients were nearly equivalent (r=~0.3) and identical to the ratio in BTLA null mice. In contrast, the wt/HVEM mixed chimeras displayed a ratio = 0.6, near wt, that was due to an increase in the CD45.2+ CD8α DC subset. This result suggested that HVEM intrinsically regulates CD8α DC subset. Interestingly, the CD45.1 DC displayed a CD8α/CD4 ratio = 0.3, independent of recipient background, indicating that the absence of HVEM in the CD45.2 DC population can affect the CD8α− subsets in wt DC.
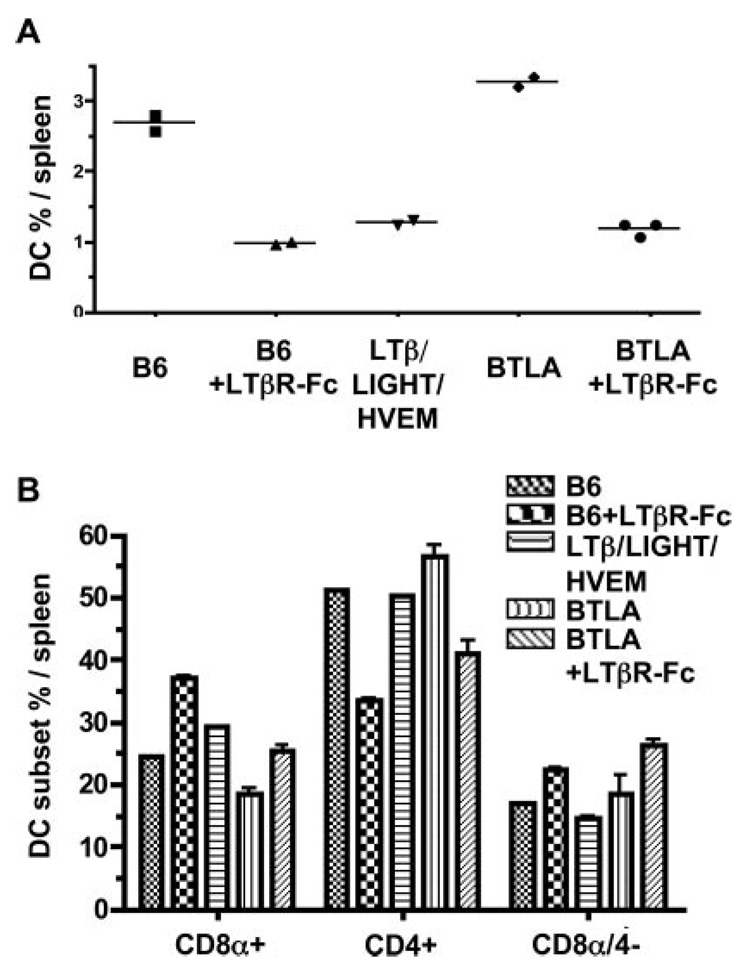
To address whether the inhibitory function of HVEM-BTLA pathway was dominant relative to LTβR signaling, we generated mice deficient in all three “ligands” by crossing LTβ/LIGHT−/− mice with HVEM−/− mice. The percentage and numbers of DC in spleens from the triple deficient mice were similar to the LTβ/LIGHT−/− mice (Fig. 5), but the ratio of CD8α/CD4 DC subsets was comparable to wt (0.6), reflecting a decrease in the CD8α+ subset and an increase in the CD4+ subset (Fig. 5). Although the LTβ/LIGHT−/− mice displayed decreased CD8α+ DC cell numbers because of the relative smaller spleen and cell numbers already discussed, the inclusion of HVEM deficiency further decreased the number of cells in the CD8α+ DC subset, yet the CD4+ subset increased (Fig. 3B) restoring the ratio to that of wt (0.6). This observation was confirmed in BTLA−/− mice treated with the LTβR-Fc decoy, which neutralizes both LIGHT and LTαβ. LTβR-Fc decoy treated BTLA−/− mice exhibited decreased DC cellularity and the subset ratio was altered to r = 0.6, recapitulating the phenotype of the triple deficient mice (Fig. 6). The ability of LTβR-Fc decoy treatment to modulate DC supports the idea that continuous LTβR signaling is required for the homeostasis of CD8α− DC subsets, diminishing the likelihood this phenotype is due to a genetic artifact or a developmentally fixed defect.
FIGURE 5.
LIGHT/LTβ/HVEM mice reveal an LT-independent DC subset with normal CD8α/CD4 subset ratio. A. CD11chigh cells gated as indicated were analyzed for CD4 and CD8α expression in WT B6, LTβ/LIGHT, and LTβ/LIGHT/HVEM-deficient mice as described in the Materials and Methods. A representative histogram is shown for each mouse strain. The ratio of CD8α to CD4 DC subsets was calculated from values in the upper left and lower right quadrants. B, The percentage of DC as a fraction of total nucleated splenocytes (top panel), the percentage of individual DC subsets (middle panel) and the total number cells in each DC subset (bottom panel) in the spleen from the indicated gene deficient mice were calculated from flow data. Each data point represents the value obtained from an individual animal and the data are pooled from two analyses. Bars show the mean ± SD from at least three mice per group, and the data are representative of three independent experiments. Student’s t test was performed where *, **, and *** denote significance of p < 0.05, p < 0.01, and p < 0.001, respectively, between the indicated groups.
FIGURE 6.
DC subsets in LTβR-Fc-treated BTLA−/− mice. A. The percentage of DC in LIGHT/LTβ/HVEM- and BTLA-deficient as well as wt B6 and LTβR-Fc-treated B6 mice are presented as the percentage of total nucleated splenocytes. Mice were treated with the mouse LTβR-Fc fusion protein as described in the methods. B, The percentage of individual CD4+, CD8α, and CD8α/CD4− DC subsets within the gated CD11chigh DC. Each data point represents the value obtained from an individual animal. Bars show the mean ± SD from at least n = 2 mice per group and the data are representative of two independent experiments.
Other hemopoietic cells including pDC, granulocytes, monocytes and macrophages in the spleen were unaffected in the triple deficient mice or in the LTβR-Fc decoy treated BTLA−/− mice (data not shown). Deletion of HVEM in the triple-deficient mice did not alter the size of the spleen or the total number of splenocytes that was observed in the LTβ/LIGHT−/− mice, indicating the HVEM-BTLA pathway does not contribute to this phenotype.
The LTαβ-LTβR and HVEM-BTLA pathways regulate different phases of DC homeostasis
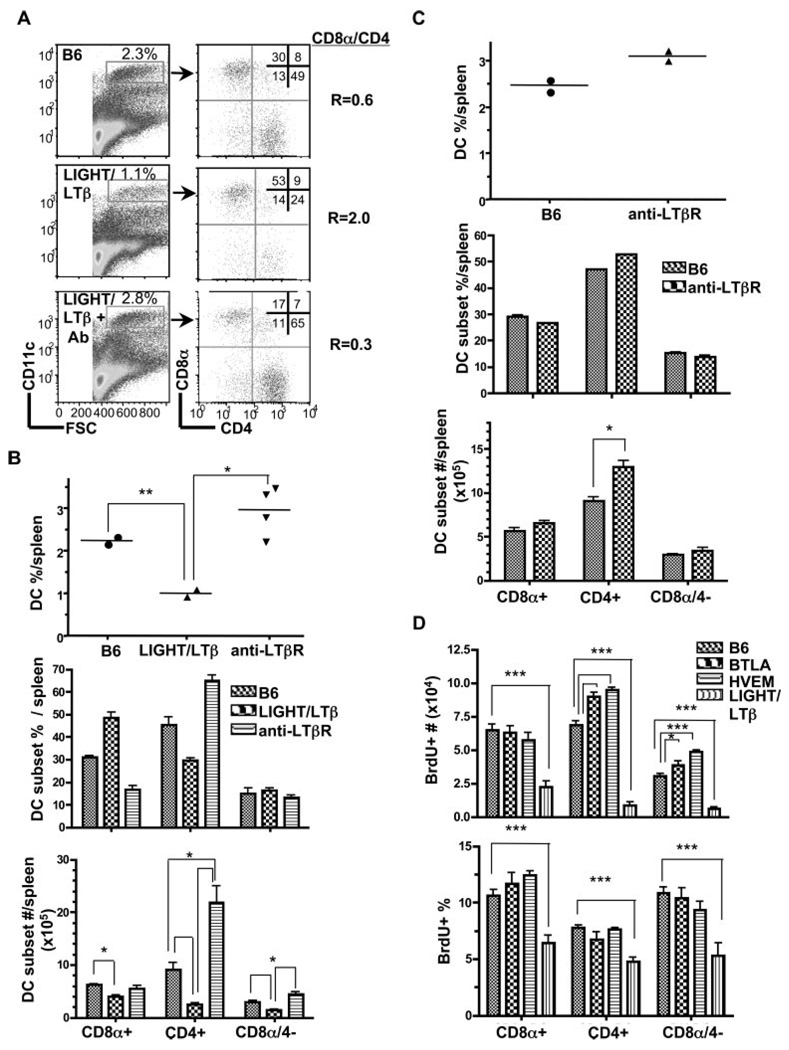
Although the previous results indicated that both LTαβ-LTβR and HVEM-BTLA pathways regulated the same DC subsets, it was not clear whether these pathways converge at a common or distinct phase in DC differentiation. We took a pharmacological approach to address whether enforced activation of the LTβR with an agonist mAb would act in dominant or recessive fashion to HVEM-BTLA. Administration of the anti-LTβR mAb over a 14-day period increased the percentage of splenic DC in LTβ/LIGHT−/− mice with a specific rise in the CD8-subsets to levels that the CD8α/CD4 DC ratio (0.3) exceeded the ratio in wt mice (Fig. 7, A and B), and comparable to mice lacking either HVEM or BTLA (Fig. 4, A and B). A modest effect was observed in the CD8α+ DC subset. Similar results were also obtained in LTα-deficient mice after administration of the agonistic anti-LTβR Ab, however treatment of LTβR−/− mice with the agonist anti-LTβR had no effect on the DC compartment confirming the specificity of this Ab (data not shown). Surprisingly, when the agonist LTβR Ab was administered to wt mice the cellularity of DC in the spleen also increased, as did the percentage of CD8α- DC subsets, with the total number of cells exceeding that of wt (Fig. 7C). The effect of the agonist anti-LTβR was reflected in the increase in the percentage of DC, but not in a major shift in the CD8α/CD4 DC ratio, which is in contrast to the response in LTβ/LIGHT−/− mice. Thus, the effect of the agonist anti-LTβR appeared to override the inhibitory action of HVEM-BTLA, suggesting LTβR signaling is dominant or functions independently of HVEM-BTLA.
FIGURE 7.
Activation of LTβR signaling restores DC homeostasis. A, CD11chigh cells gated as indicated were analyzed for CD4 and CD8α expression in wt B6, LIGHT/LTβ-deficient mice (untreated), and LTβ/LIGHT treated with rat anti-mouse LTβR mAb as described in the Materials and Methods. A representative histogram is shown for each mouse strain. The ratio of CD8α to CD4 DC subsets was calculated from values in the upper left and lower right quadrants. B, The percentage of DC as a fraction of total nucleated splenocytes (top panel), the percentage of individual DC subsets (middle panel) and the total number cells in each DC subset (bottom panel) in the spleen from the indicated gene-deficient or mAb-treated mice. Each data point represents the value obtained from an individual animal. Bars show the mean ± SD from at least n = three mice per group and the data are representative of three independent experiments. Student’s test was performed where * and ** denote a significance of p < 0.05 and p < 0.01, respectively, between the indicated groups. C, The effect of LTβR activation in wt B6 mice on DC subsets. The percentage of DC as a fraction of total nucleated splenocytes (top panel), the percentage of individual DC subsets (middle panel) and the total number cells in each DC subset (bottom panel) in the spleen from wt B6 or rat anti-mouse LTβR treated mice (as in A). Bars show the mean ± SD from at least two mice per group and the data are representative of two independent experiments. Student t test significance between the wt B6 and the other groups p < 0.05 (*). D, Total number (top panel) and frequency (bottom panel) of BrdU+ cells CD8α+, CD4+, and CD8α/CD4− DC in the spleen of wt B6, HVEM-, BTLA- and LIGHT/LTβ-deficient mice treated with BrdU for 16 h. Bars show the mean ± SD from at least three mice per group and the data are representative of two independent experiments. Student’s test was performed where * and *** denote significance of p < 0.05 and p < 0.001, respectively, between the indicated groups.
The proliferation inducing activity of LTβR signaling in the CD8α- DC subsets can be measured by nucleotide (bromideoxyuridine, BrdU) incorporation in dividing DC (26). The division of both mature DC and their immediate precursors are represented in the population during the 16 h BrdU labeling period. The number of cells specifically incorporating BrdU increased in the CD4+ and CD8α-4- DC subsets in HVEM−/− and BTLA−/− mice reflecting a net accumulation of cells in those subsets (Fig. 7D). As expected, dramatically fewer cells incorporated BrdU in LTβ/LIGHT-deficient mice, which is consistent with the corresponding loss in the percentage of proliferating cells in each DC subset (Fig. 7D, bottom panel). However, there was no significant change in the percentage of proliferating cells within each subset in either HVEM−/− or BTLA−/− mice when compared with wt mice. This result indicates that the inhibitory effect of HVEM-BTLA does not impinge on the LTβR-dependent proliferation of DC.
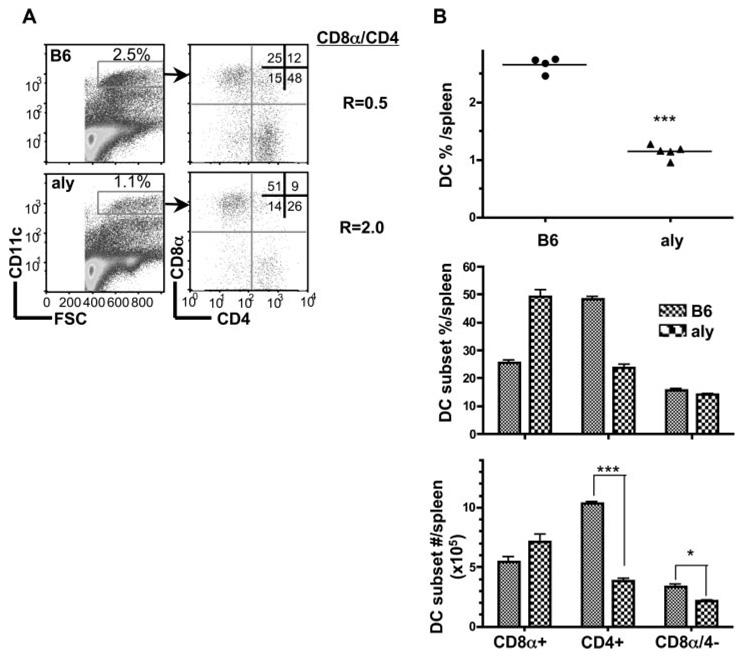
We addressed whether the LTβR and HVEM-BTLA pathways could be distinguished at the level of down stream signaling pathways by examining the noncanonical NFκB pathway. LTβR signaling activates the formation of Rel B/p52 complex by inducing the processing of p100, the precursor form of p52, which is dependent on NIK. HVEM also has the potential to activate the NIK-dependent RelB NFκB pathway (50). Mice harboring a defective NIK gene (alymphoplasia, aly) (46) exhibited a DC profile identical to mice deficient in LTα, LTβ, or LTβR with decreased percentage of CD11chigh DC and an inverted CD8α/CD4+ DC ratio (2.0), specifically reflecting the decreased cellularity of CD4+ and CD8α-4- DC subsets (Fig. 8). Moreover, NIK mutant mice had a larger spleen with increased cellularity, a phenocopy of mice deficient in LTβR, LTα or LTβ (Fig. 3). However, pDC, granulocytes, monocytes and macrophages in aly mice were similar to normal B6 mice (data not shown). The similarity in phenotype of aly mice suggests that NIK acts in a common pathway with LTαβ-LTβR to regulate DC homeostasis. The results further suggest that BTLA is not activating HVEM to engage NIK in regulating DC homeostasis.
FIGURE 8.
Alymphoplasia (NIK mutant) mice have a specific defect in CD8α- DC subsets. A, CD11chigh cells were analyzed for CD4 and CD8α expression from wt B6 and aly mice as described in the Materials and Methods. B, The percentage of DC as a fraction of total nucleated splenocytes (top panel), the percentage of individual DC subsets (middle panel) and the total number cells in each DC subset (bottom panel) in the spleen from wt B6 and aly mice. Each data point represents an individual animal, and the data are pooled from two analyses. Bars show the mean ± SD from at least n = three mice per group and the data are representative of three independent experiments. Student’s test was performed where one and three asterisks denote significance of p < 0.05 and p < 0.001, respectively, between the indicated groups.
Discussion
The identification of the HVEM-BTLA system as an inhibitory checkpoint for the LTαβ-LTβR pathway defines a novel mechanism regulating the homeostatic equilibrium of resident DC populations in lymphoid tissues. The HVEM-BTLA inhibitory pathway primarily impacts the CD8α- DC subsets in the spleen, the same populations that expand in response to LTαβ-LTβR signaling. A majority (~70%) of the resident DC in the adult mouse spleen are under dynamic control by the LTαβ-LTβR and HVEM-BTLA pathways. However, a basal level of DC, with a normal ratio of CD8α to CD4 subsets, was maintained in the spleen in the absence of LTαβ, LIGHT and HVEM indicating a second distinct mechanism operates to control DC populations in the spleen. It is not known if these cells are proliferating. Inhibitory signaling requires expression of HVEM and BTLA in DC and cells in the stromal microenvironment. Together, the LTαβ-LTβR and HVEM-BTLA pathways provide key signals that integrate to achieve homeostasis of DC in lymphoid tissues.
Positive signaling provided through the LTβR controls the proliferation and differentiation of the CD8α- DC subsets or their precursors within peripheral lymphoid tissues (26). Intrinsic expression of the LTβR in hemopoietic compartment was necessary for DC proliferation, and as shown here, LTαβ is the key ligand mediating DC proliferation under homeostatic conditions. The positive signals provided by LTαβ-LTβR pathway specifically increased the number of cells in the CD4+ and CD8α/4- DC subsets (Fig. 1). Moreover, an identical phenotype was observed in mice with mutant NIK (aly) (Fig. 8) or relB (25) implicating the involvement of the NFκB2 processing pathway initiated by LTαβ-LTβR mediates positive signals for DC homeostasis. Restoration of the CD4+ and CD8α-/4- DC subsets in LTβ/LIGHT deficient mice with an agonist anti-LTβR mAb demonstrated that LTβR signaling is sufficient for promoting proliferation and differentiation of the LT-regulated DC subsets. Moreover, the effect of the LTβR-Fc decoy on specific DC subsets demonstrated the dynamic aspect of LTβR signaling required for maintaining DC in the spleen. This result also indicated that the DC defect in LT-deficient mice is not a developmental “fixed” phenotype, as is, for example, the formation of lymph nodes (51). LTβR signaling regulates lymphocyte recirculation across high endothelial venules (52), which could also impact immigration of DC precursors into the spleen (49). The increased splenic cellularity in LT deficient mice probably reflects this alteration of recirculation (Fig. 3), yet the phenotype was corrected in the LTβ/LIGHT double deficient mice, which renders altered immigration to a minor role as a mechanism accounting for LTβR’s function in regulating DC populations.
Mice deficient in either HVEM or BTLA revealed an inhibitory pathway for DC that primarily affected the CD4+ and CD8α-/4- DC subsets, the same subsets dependent on LTβR pathway (Fig. 4). The competitive advantage of HVEM or BTLA deficient DC in repopulating the spleen, a phenotype expected for cells alleviated from an inhibitory pathway, clearly demonstrated the impact of this pathway in restricting DC proliferation and accumulation (Table I). The similarity in this DC phenotype supports the substantial biochemical data that HVEM and BTLA form a signaling pathway (32, 35, 37). Interestingly, the genotype of the stromal cells in the recipient mice modulated the extent that DC competitively repopulated the spleen (e.g., wt/HVEM→wt vs →HVEM). Thus, HVEM and BTLA signals provided by the splenic stromal micro-environment also influence inhibitory signaling that maintains DC homeostasis. Furthermore, wt DC were also impacted in the mixed chimeras reflected by the increased CD8α- DC subsets (ratio = 0.3) independently of recipient background. This effect of HVEM or BTLA deficiency on wt cells is consistent with cellular interactions in trans with neighboring DC that provide inhibitory signaling regulating proliferation and accumulation. Thus, DC interactions with other DC and with the stromal microenvironment provide sources of inhibitory signaling, although the directional flow of signals between these various cell types requires further elucidation.
Evidence that the CD8α+ DC population is subject to regulation by HVEM was found in the competitive repopulation chimera experiment (specific increase in CD8α DC subset r = 0.6) and in the LTβ/LIGHT/HVEM triple deficient mice (Fig. 5B). HVEM deletion by itself had no effect on CD8α DC subset, however in the triple deficient mouse, a specific decrease in the CD8α DC subset occurred relative to LTβ/LIGHT−/− mice, along with an increase in CD4+ DC subset, resetting the CD8α/CD4 subset ratio. The basis of the HVEM phenotype is unclear but is distinct from that observed in BTLA−/− mice. This result could be interpreted as a composite phenotype that includes positive signaling by HVEM promoting CD8α+ subset, and a loss of inhibitory signaling on the CD4+ subset via the HVEM-BTLA pathway, together restoring a normal ratio of CD8α/CD4+ subsets.
The genetic evidence indicates LTαβ-LTβR-NIK-RelB pathway provides positive signals for DC proliferation. The HVEM-BTLA checkpoint in wt and LTβ/LIGHT deficient mice was bypassed with sustained LTβR signaling (agonist anti-LTβR antibody), suggesting the LTβR pathway acts dominantly to HVEM-BTLA (Fig. 7). Additionally, the finding that there was no change in the percentage of BrdU-labeled DC in either HVEM−/− or BTLA−/− mice when compared with wt mice suggests that the inhibitory effect of HVEM-BTLA pathway does not directly impinge on the LTβR driven proliferation. This interpretation seems appropriate in view of the signaling mechanism known for BTLA. The ITIM motif in BTLA is thought to mediate inhibitory signaling through recruitment of SHP1 tyrosine phosphatase, which does not act on substrates targeted by serine kinases NIK or IKKα involved in LTβR activation of RelB. These results indicate LTβR signaling pathway is not directly altered, and in view of BrdU labeling experiment, suggest that HVEM-BTLA pathway may impact a post mitotic phase of DC differentiation, the target of which is unknown.
Different DC subsets appear to influence the quality of T cell responses. CD8α+ DC appear to favor the development proinflammatory TH1 responses because of their potential to produce of high levels of IL-12, whereas CD8α- DC subsets are more potent at inducing TH2 responses (5). However, DC subset directed T cell responses may vary depending on type of costimulation and Ag (53). The CD8α+ DC subpopulation has the capacity to capture apoptotic cell-associated Ags activating CD8+ T cells by cross-priming (54, 55). Thus, the reduction of CD8α- DC subsets may explain in part the alteration of some T cell responses described in LT-deficient mice (56, 57) and enhanced responses in BTLA deficient mice (58, 59). T cell expression of LTαβ impacts the maturation of DC (60). Alteration of LTβR and HVEM-BTLA signaling potential by pharmacological intervention may impact DC subsets required for de novo and persistent Ag presentation, and thus impact the quality of cellular immune responses.
Acknowledgments
We thank Ian Humphreys and Pedro Ruiz for helpful discussions, and Ginelle Patterson, Lisa Hohmann, and Heather Mar for technical assistance.
Footnotes
This work supported in part by grants from the Public Health Service, National Institutes of Health, AI33068, CA69381, AI48073, and AI06789.
Abbreviations used in this paper: BTLA, B and T lymphocyte attenuator; HVEM, herpesvirus entry mediator; ICSBP, IFN consensus sequence binding factor; IRF, IFN response factor; LIGHT, LT-related inducible ligand that competes for glycoprotein D binding to HVEM on T cells; LT, lymphotoxin; NIK, NFκB-inducing kinase; pDC plasmacytoid DC; wt, wild type.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu. Rev. Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 2.Vremec D, Pooley J, Hochrein H, Wu L, Shortman K. CD4 and CD8 expression by dendritic cell subtypes in mouse thymus and spleen. J. Immunol. 2000;164:2978–2986. doi: 10.4049/jimmunol.164.6.2978. [DOI] [PubMed] [Google Scholar]
- 3.Leenen PJ, Radosevic K, Voerman JS, Salomon B, van Rooijen N, Klatzmann D, van Ewijk W. Heterogeneity of mouse spleen dendritic cells: in vivo phagocytic activity, expression of macrophage markers, and subpopulation turnover. J. Immunol. 1998;160:2166–2173. [PubMed] [Google Scholar]
- 4.Maldonado-Lopez R, De Smedt T, Michel P, Godfroid J, Pajak B, Heirman C, Thielemans K, Leo O, Urbain J, Moser M. CD8α+ and CD8α− subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J. Exp. Med. 1999;189:587–592. doi: 10.1084/jem.189.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maldonado-Lopez R, Moser M. Dendritic cell subsets and the regulation of Th1/Th2 responses. Semin. Immunol. 2001;13:275–282. doi: 10.1006/smim.2001.0323. [DOI] [PubMed] [Google Scholar]
- 6.Asselin-Paturel C, Boonstra A, Dalod M, Durand I, Yessaad N, Dezutter-Dambuyant C, Vicari A, O’Garra A, Biron C, Briere F, Trinchieri G. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat. Immunol. 2001;2:1144–1150. doi: 10.1038/ni736. [DOI] [PubMed] [Google Scholar]
- 7.Martin P, Del Hoyo GM, Anjuere F, Arias CF, Vargas HH, Fernandez LA, Parrillas V, Ardavin C. Characterization of a new subpopulation of mouse CD8α+ B220+ dendritic cells endowed with type 1 interferon production capacity and tolerogenic potential. Blood. 2002;100:383–390. doi: 10.1182/blood.v100.2.383. [DOI] [PubMed] [Google Scholar]
- 8.Henri S, Vremec D, Kamath A, Waithman J, Williams S, Benoist C, Burnham K, Saeland S, Handman E, Shortman K. The dendritic cell populations of mouse lymph nodes. J. Immunol. 2001;167:741–748. doi: 10.4049/jimmunol.167.2.741. [DOI] [PubMed] [Google Scholar]
- 9.Ardavin C. Origin, precursors and differentiation of mouse dendritic cells. Nat. Rev. Immunol. 2003;3:582–590. doi: 10.1038/nri1127. [DOI] [PubMed] [Google Scholar]
- 10.Ardavin C, Wu L, Li CL, Shortman K. Thymic dendritic cells and T cells develop simultaneously in the thymus from a common precursor population. Nature. 1993;362:761–763. doi: 10.1038/362761a0. [DOI] [PubMed] [Google Scholar]
- 11.Wu L, Li CL, Shortman K. Thymic dendritic cell precursors: relationship to the T lymphocyte lineage and phenotype of the dendritic cell progeny. J. Exp. Med. 1996;184:903–911. doi: 10.1084/jem.184.3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Traver D, Akashi K, Manz M, Merad M, Miyamoto T, Engleman EG, Weissman IL. Development of CD8α-positive dendritic cells from a common myeloid progenitor. Science. 2000;290:2152–2154. doi: 10.1126/science.290.5499.2152. [DOI] [PubMed] [Google Scholar]
- 13.Manz MG, Traver D, Miyamoto T, Weissman IL, Akashi K. Dendritic cell potentials of early lymphoid and myeloid progenitors. Blood. 2001;97:3333–3341. doi: 10.1182/blood.v97.11.3333. [DOI] [PubMed] [Google Scholar]
- 14.Wu L, D’Amico A, Hochrein H, O’Keeffe M, Shortman K, Lucas K. Development of thymic and splenic dendritic cell populations from different hemopoietic precursors. Blood. 2001;98:3376–3382. doi: 10.1182/blood.v98.12.3376. [DOI] [PubMed] [Google Scholar]
- 15.Naik SH, Metcalf D, van Nieuwenhuijze A, Wicks I, Wu L, O’Keeffe M, Shortman K. Intrasplenic steady-state dendritic cell precursors that are distinct from monocytes. Nat. Immunol. 2006;7:663–671. doi: 10.1038/ni1340. [DOI] [PubMed] [Google Scholar]
- 16.Aliberti J, Schulz O, Pennington DJ, Tsujimura H, Reis e Sousa C, Ozato K, Sher A. Essential role for ICSBP in the in vivo development of murine CD8α+ dendritic cells. Blood. 2003;101:305–310. doi: 10.1182/blood-2002-04-1088. [DOI] [PubMed] [Google Scholar]
- 17.Hacker C, Kirsch RD, Ju XS, Hieronymus T, Gust TC, Kuhl C, Jorgas T, Kurz SM, Rose-John S, Yokota Y, Zenke M. Transcriptional profiling identifies Id2 function in dendritic cell development. Nat. Immunol. 2003;4:380–386. doi: 10.1038/ni903. [DOI] [PubMed] [Google Scholar]
- 18.Yamaoka K, Min B, Zhou YJ, Paul WE, O’Shea J. Jak3 negatively regulates dendritic cell cytokine production and survival. Blood. 2005;106:3227–3233. doi: 10.1182/blood-2005-02-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu L, Nichogiannopoulou A, Shortman K, Georgopoulos K. Cell-autonomous defects in dendritic cell populations of Ikaros mutant mice point to a developmental relationship with the lymphoid lineage. Immunity. 1997;7:483–492. doi: 10.1016/s1074-7613(00)80370-2. [DOI] [PubMed] [Google Scholar]
- 20.Guerriero A, Langmuir PB, Spain LM, Scott EW. PU.1 is required for myeloid-derived but not lymphoid-derived dendritic cells. Blood. 2000;95:879–885. [PubMed] [Google Scholar]
- 21.Ichikawa E, Hida S, Omatsu Y, Shimoyama S, Takahara K, Miyagawa S, Inaba K, Taki S. Defective development of splenic and epidermal CD4+ dendritic cells in mice deficient for IFN regulatory factor-2. Proc. Natl. Acad. Sci. USA. 2004;101:3909–3914. doi: 10.1073/pnas.0400610101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caton ML, Smith-Raska MR, Reizis B. Notch-RBP-J signaling controls the homeostasis of CD8− dendritic cells in the spleen. J. Exp. Med. 2007;204:1653–1664. doi: 10.1084/jem.20062648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suzuki S, Honma K, Matsuyama T, Suzuki K, Toriyama K, Akitoyo I, Yamamoto K, Suematsu T, Nakamura M, Yui K, Kumatori A. Critical roles of interferon regulatory factor 4 in CD11bhigh CD8α− dendritic cell development. Proc. Natl. Acad. Sci. USA. 2004;101:8981–8986. doi: 10.1073/pnas.0402139101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobayashi T, Walsh PT, Walsh MC, Speirs KM, Chiffoleau E, King CG, Hancock WW, Caamano JH, Hunter CA, Scott P, Turka LA, Choi Y. TRAF6 is a critical factor for dendritic cell maturation and development. Immunity. 2003;19:353–363. doi: 10.1016/s1074-7613(03)00230-9. [DOI] [PubMed] [Google Scholar]
- 25.Wu L, D’Amico A, Winkel KD, Suter M, Lo D, Shortman K. RelB is essential for the development of myeloid-related CD8α- dendritic cells but not of lymphoid-related CD8α+ dendritic cells. Immunity. 1998;9:839–847. doi: 10.1016/s1074-7613(00)80649-4. [DOI] [PubMed] [Google Scholar]
- 26.Kabashima K, Banks TA, Ansel KM, Lu TT, Ware CF, Cyster JG. Intrinsic lymphotoxin-β receptor requirement for homeostasis of lymphoid tissue dendritic cells. Immunity. 2005;22:439–450. doi: 10.1016/j.immuni.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 27.Abe K, Yarovinsky FO, Murakami T, Shakhov AN, Tumanov AV, Ito D, Drutskaya LN, Pfeffer K, Kuprash DV, Komschlies KL, Nedospasov SA. Distinct contributions of TNF and LT cytokines to the development of dendritic cells in vitro and their recruitment in vivo. Blood. 2003;101:1477–1483. doi: 10.1182/blood.V101.4.1477. [DOI] [PubMed] [Google Scholar]
- 28.Dejardin E, Droin NM, Delhase M, Haas E, Cao Y, Makris C, Li ZW, Karin M, Ware CF, Green DR. The lymphotoxin-β receptor induces different patterns of gene expression via two NF-κB pathways. Immunity. 2002;17:525–535. doi: 10.1016/s1074-7613(02)00423-5. [DOI] [PubMed] [Google Scholar]
- 29.Mauri ND, Ebner R, Montgomery RI, Kochel KD, Cheung TC, Yu G-L, Ruben S, Murphy M, Eisenbery RJ, Cohen GH, et al. LIGHT: a new member of the TNF superfamily and lymphotoxin α are ligands for herpes-virus entry mediator. Immunity. 1998;8:21–30. doi: 10.1016/s1074-7613(00)80455-0. [DOI] [PubMed] [Google Scholar]
- 30.Ware CF. Network communications: lymphotoxins, LIGHT, and TNF. Annu. Rev. Immunol. 2005;23:787–819. doi: 10.1146/annurev.immunol.23.021704.115719. [DOI] [PubMed] [Google Scholar]
- 31.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu. Rev. Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 32.Sedy JR, Gavrieli M, Potter KG, Hurchla MA, Lindsley RC, Hildner K, Scheu S, Pfeffer K, Ware CF, Murphy TL, Murphy KM. B and T lymphocyte attenuator regulates T cell activation through interaction with herpesvirus entry mediator. Nat. Immunol. 2005;6:90–98. doi: 10.1038/ni1144. [DOI] [PubMed] [Google Scholar]
- 33.Gavrieli M, Murphy KM. Association of Grb-2 and PI3K p85 with phosphotyrosile peptides derived from BTLA. Biochem. Biophys. Res. Commun. 2006;345:1440–1445. doi: 10.1016/j.bbrc.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 34.Chemnitz JM, Lanfranco AR, Braunstein I, Riley JL. B and T lymphocyte attenuator-mediated signal transduction provides a potent inhibitory signal to primary human CD4 T cells that can be initiated by multiple phosphotyrosine motifs. J. Immunol. 2006;176:6603–6614. doi: 10.4049/jimmunol.176.11.6603. [DOI] [PubMed] [Google Scholar]
- 35.Cheung TC, Humphreys IR, Potter KG, Norris PS, Shumway HM, Tran BR, Patterson G, Jean-Jacques R, Yoon M, Spear PG, et al. Evolutionarily divergent herpesviruses modulate T cell activation by targeting the herpesvirus entry mediator cosignaling pathway. Proc. Natl. Acad. Sci. USA. 2005;102:13218–13223. doi: 10.1073/pnas.0506172102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Compaan DM, Gonzalez LC, Tom I, Loyet KM, Eaton D, Hymowitz SG. Attenuating lymphocyte activity: the crystal structure of the BTLA-HVEM complex. J. Biol. Chem. 2005;280:39553–39561. doi: 10.1074/jbc.M507629200. [DOI] [PubMed] [Google Scholar]
- 37.Gonzalez LC, Loyet KM, Calemine-Fenaux J, Chauhan V, Wranik B, Ouyang W, Eaton DL. A coreceptor interaction between the CD28 and TNF receptor family members B and T lymphocyte attenuator and herpesvirus entry mediator. Proc. Natl. Acad. Sci. USA. 2005;102:1116–1121. doi: 10.1073/pnas.0409071102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murphy KM, Nelson CA, Sedy JR. Balancing co-stimulation and inhibition with BTLA and HVEM. Nat. Rev. Immunol. 2006;6:671–681. doi: 10.1038/nri1917. [DOI] [PubMed] [Google Scholar]
- 39.Krieg C, Boyman O, Fu YX, Kaye J. B and T lymphocyte attenuator regulates CD8+ T cell-intrinsic homeostasis and memory cell generation. Nat. Immunol. 2007;8:162–171. doi: 10.1038/ni1418. [DOI] [PubMed] [Google Scholar]
- 40.Hurchla MA, Sedy JR, Gavrielli M, Drake CG, Murphy TL, Murphy KM. B and T lymphocyte attenuator exhibits structural and expression polymorphisms and is highly induced in anergic CD4+ T cells. J. Immunol. 2005;174:3377–3385. doi: 10.4049/jimmunol.174.6.3377. [DOI] [PubMed] [Google Scholar]
- 41.Truong W, Hancock WW, Anderson CC, Merani S, Shapiro AM. Coinhibitory T-cell signaling in islet allograft rejection and tolerance. Cell Transplant. 2006;15:105–119. doi: 10.3727/000000006783982160. [DOI] [PubMed] [Google Scholar]
- 42.Futterer A, Mink K, Luz A, Kosco-Vilbois MH, Pfeffer K. The lymphotoxin β receptor controls organigenesis and affinity maturation in peripheral lymphoid tissues. Immunity. 1998;9:59–70. doi: 10.1016/s1074-7613(00)80588-9. [DOI] [PubMed] [Google Scholar]
- 43.Scheu S, Alferink J, Potzel T, Barchet W, Kalinke U, Pfeffer K. Targeted disruption of LIGHT causes defects in costimulatory T Cell activation and reveals cooperation with lymphotoxin β in mesenteric lymph node genesis. J. Exp. Med. 2002;195:1613–1624. doi: 10.1084/jem.20020215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alimzhanov MB, Kuprash DV, Kosco-Vilbois MH, Luz A, Turetskaya RL, Tarakhovsky A, Rajewsky K, Nedospasov SA, Pfeffer K. Abnormal development of secondary lymphoid tissues in lymphotoxin β-deficient mice. Proc. Natl. Acad. Sci. USA. 1997;94:9302–9307. doi: 10.1073/pnas.94.17.9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y, Subudhi SK, Anders RA, Lo J, Sun Y, Blink S, Wang Y, Wang J, Liu X, Mink K, et al. The role of herpesvirus entry mediator as a negative regulator of T cell-mediated responses. J. Clin. Invest. 2005;115:711–717. doi: 10.1172/JCI200522982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shinkura R, Kitada K, Matsuda F, Tashiro K, Ikuta K, Suzuki M, Kogishi K, Serikawa T, Honjo T. Alymphoplasia is caused by a point mutation in the mouse gene encoding NF-κB-inducing kinase. Nat. Genet. 1999;22:74–77. doi: 10.1038/8780. [DOI] [PubMed] [Google Scholar]
- 47.Watanabe N, Gavrieli M, Sedy JR, Yang J, Fallarino F, Loftin SK, Hurchla MA, Zimmerman N, Sim J, Zang X, et al. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat. Immunol. 2003;4:670–679. doi: 10.1038/ni944. [DOI] [PubMed] [Google Scholar]
- 48.Banks TA, Rickert S, Benedict CA, Ma L, Ko M, Meier J, Ha W, Schneider K, Granger SW, Turovskaya O, et al. A lymphotoxin-IFN-β axis essential for lymphocyte survival revealed during cytomegalovirus infection. J. Immunol. 2005;174:7217–7225. doi: 10.4049/jimmunol.174.11.7217. [DOI] [PubMed] [Google Scholar]
- 49.Liu K, Waskow C, Liu X, Yao K, Hoh J, Nussenzweig M. Origin of dendritic cells in peripheral lymphoid organs of mice. Nat. Immunol. 2007;8:578–583. doi: 10.1038/ni1462. [DOI] [PubMed] [Google Scholar]
- 50.Hauer J, Puschner S, Ramakrishnan P, Simon U, Bongers M, Federle C, Engelmann H. TNF receptor (TNFR)-associated factor (TRAF) 3 serves as an inhibitor of TRAF2/5-mediated activation of the noncanonical NF-κB pathway by TRAF-binding TNFRs. Proc. Natl. Acad. Sci. USA. 2005;102:2874–2879. doi: 10.1073/pnas.0500187102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rennert PD, James D, Mackay F, Browning JL, Hochman PS. Lymph node genesis is induced by signaling through the lymphotoxin β receptor. Immunity. 1998;9:71–80. doi: 10.1016/s1074-7613(00)80589-0. [DOI] [PubMed] [Google Scholar]
- 52.Drayton DL, Bonizzi G, Ying X, Liao S, Karin M, Ruddle NH. IκB kinase complex α kinase activity controls chemokine and high endothelial venule gene expression in lymph nodes and nasal-associated lymphoid tissue. J. Immunol. 2004;173:6161–6168. doi: 10.4049/jimmunol.173.10.6161. [DOI] [PubMed] [Google Scholar]
- 53.Skokos D, Nussenzweig MC. CD8- DCs induce IL-12-independent Th1 differentiation through δ 4 notch-like ligand in response to bacterial LPS. J. Exp. Med. 2007;204:1525–1531. doi: 10.1084/jem.20062305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.den Haan JM, Lehar SM, Bevan MJ. CD8+ but not CD8− dendritic cells cross-prime cytotoxic T cells in vivo. J. Exp. Med. 2000;192:1685–1696. doi: 10.1084/jem.192.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schulz O, Reis e Sousa C. Cross-presentation of cell-associated antigens by CD8α+ dendritic cells is attributable to their ability to internalize dead cells. Immunology. 2002;107:183–189. doi: 10.1046/j.1365-2567.2002.01513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kumaraguru U, Davis IA, Deshpande S, Tevethia SS, Rouse BT. Lymphotoxin α [minus/minus] mice develop functionally impaired CD8+ T cell responses and fail to contain virus infection of the central nervous system. J. Immunol. 2001;166:1066–1074. doi: 10.4049/jimmunol.166.2.1066. [DOI] [PubMed] [Google Scholar]
- 57.Lund FE, Partida-Sanchez S, Lee BO, Kusser KL, Hartson L, Hogan RJ, Woodland DL, Randall TD. Lymphotoxin-α-deficient mice make delayed, but effective, T and B cell responses to influenza. J. Immunol. 2002;169:5236–5243. doi: 10.4049/jimmunol.169.9.5236. [DOI] [PubMed] [Google Scholar]
- 58.Han P, Goularte OD, Rufner K, Wilkinson B, Kaye J. An inhibitory Ig superfamily protein expressed by lymphocytes and APCs is also an early marker of thymocyte positive selection. J. Immunol. 2004;172:5931–5939. doi: 10.4049/jimmunol.172.10.5931. [DOI] [PubMed] [Google Scholar]
- 59.Tao R, Wang L, Han R, Wang T, Ye Q, Honjo T, Murphy TL, Murphy KM, Hancock WW. Differential effects of B and T lymphocyte attenuator and programmed death-1 on acceptance of partially versus fully MHC-mismatched cardiac allografts. J. Immunol. 2005;175:5774–5782. doi: 10.4049/jimmunol.175.9.5774. [DOI] [PubMed] [Google Scholar]
- 60.Summers-DeLuca LE, McCarthy DD, Cosovic B, Ward LA, Lo CC, Scheu S, Pfeffer K, Gommerman JL. Expression of lymphotoxin-αβ on antigen-specific T cells is required for DC function. J. Exp. Med. 2007;204:1071–1081. doi: 10.1084/jem.20061968. [DOI] [PMC free article] [PubMed] [Google Scholar]