SUMMARY
The mitochondrial replicative DNA helicase is an essential cellular protein that shows high similarity with the bifunctional primase-helicase of bacteriophage T7, the gene 4 protein (T7 gp4). The N-terminal primase domain of T7 gp4 comprises seven conserved sequence motifs, I, II, III, IV, V, VI, and an RNA polymerase basic domain. The putative primase domain of metazoan mitochondrial DNA helicases has diverged from T7 gp4 and in particular, the primase domain of vertebrates lacks motif I, which comprises a zinc binding domain. Interestingly, motif I is conserved in insect mtDNA helicases. Here, we evaluate the effects of overexpression in Drosophila cell culture of variants carrying mutations in conserved amino acids in the N-terminal region, including the zinc binding domain.
Overexpression of alanine substitution mutants of conserved amino acids in motifs I, IV, V and VI and the RNA polymerase basic domain results in increased mtDNA copy number as is observed with overexpression of the wild type enzyme. In contrast, overexpression of three N-terminal mutants W282L, R301Q and P302L that are analogous to human autosomal dominant progressive external ophthalmoplegia mutations results in mitochondrial DNA depletion, and in the case of R301Q, a dominant negative cellular phenotype. Thus whereas our data suggest lack of a DNA primase activity in Drosophila mitochondrial DNA helicase, they show that specific N-terminal amino acid residues that map close to the central linker region likely play a physiological role in the C-terminal helicase function of the protein.
Keywords: mitochondria, mitochondrial DNA, replisome, primase, helicase, autosomal dominant progressive external ophthalmoplegia
1. INTRODUCTION
Mitochondrial DNA (mtDNA) helicase, also known as Twinkle, is essential for the replication of mtDNA [1, 2]. mtDNA helicase shows high amino acid sequence similarity with bacteriophage T7 gene 4 protein (T7 gp4), which comprises an N-terminal primase domain, a C-terminal helicase domain and an intervening linker region (Fig. 1). Similar to T7 gp4, the mtDNA helicase forms a hexamer [1, 3–5]. A detailed comparative sequence analysis of the primase domain suggests that in many eukaryotes, mtDNA helicase may also have a primase function, though most metazoans may not because they lack some critical amino acids [6]. T7 gp4 and mtDNA helicase share seven conserved sequence motifs in the N-terminal primase region: I, II, III, IV, V, VI, and an RNA polymerase (RNAP) basic domain [6]. Motif I is a zinc binding domain and contributes to the recognition of the trinucleotide template sequence and its transfer to the primase active site for primer synthesis, whereas the roles of motifs II and III are less clear. The RNAP basic domain is homologous to the basic region found in many RNA polymerases and contributes to phosphodiester bond formation. In T7 gp4, some amino acid residues in the RNAP basic domain are essential for phage growth [7]. Motifs lV–VI form a topoisomerase-primase (TOPRIM) fold also found in topoisomerases [8]. In the T7 gp4 TOPRIM fold, five acidic residues create an acidic patch central to the active site, and are essential for primer synthesis and the extension of oligoribonucleotides [9, 10]. In many eukaryotic mtDNA helicases these motifs are well conserved, whereas in metazoan mtDNA helicases, some essential acidic residues in the TOPRIM fold are missing, suggesting loss of primase activity [6]. Moreover, in the zinc binding domain of most metazoan mtDNA helicases, only the third of four cysteine residues is conserved with the exception of insects [6]. Interestingly, in insects including fly, mosquito and silkworm, all four cysteine residues are conserved (Fig. 1).
Fig. 1. Sequence alignment and location of amino acid substitution mutations in the N-terminal domain of Drosophila mtDNA helicase.
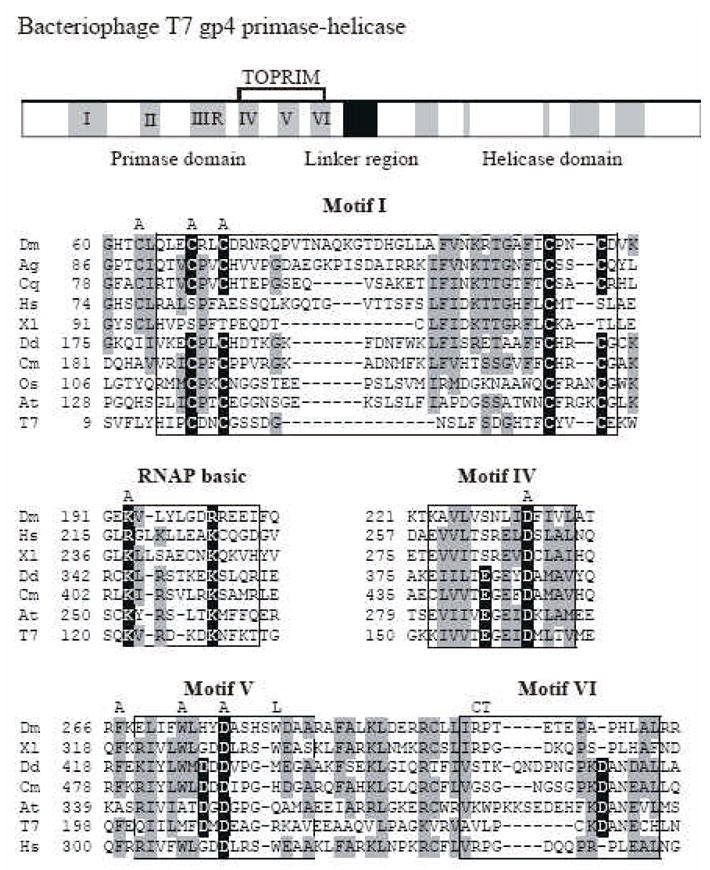
The upper panel shows a schematic diagram of the sequence organization of the bacteriophage T7 primase-helicase. The five amino acid sequence motifs common to prokaryotic primases are indicated in black numbers. Sequence alignment of relevant regions are shown below with mtDNA helicases of the fly (Dm), African malaria mosquito (Ag), Southern house mosquito (Cq), frog (Xl), man (Hs), amoeba (Dd), red alga (Cm), Rice (Os), mouse-ear cress (At) and bacteriophage T7 gp4 (T7). Data are taken from Gray et al. [6]. Amino acid residues highlighted in black are critical residues in T7 gp4, and those that are conserved in mtDNA helicases are indicated in gray. The positions of the alanine substitutions in the Drosophila mtDNA helicase used in this study are shown above the alignment as “A”. The positions of the human adPEO mutations that we analyzed in Drosophila are shown above the alignment in single letter code as “L”, “C” and “T”.
Mutations in human mtDNA helicase can cause autosomal dominant progressive external ophthalmoplegia (adPEO), which shows multiple mtDNA deletions or depletion in specific tissues such as the central nervous system and skeletal muscle [5, 11–15]. Recently, we have shown that overexpression of Drosophila protein variants carrying amino acid substitutions in the helicase domain and linker region that are equivalent to human adPEO mutations results in the depletion of mtDNA in cultured cells [1]. To date, nearly 20 adPEO mutations have been found in mtDNA helicase, and most of these map within the linker region and helicase domain. Furthermore, adPEO mutations within the N-terminal domain of mtDNA helicase lie close to the linker region. The function of the N-terminal remains unclear. Recently, Falkenberg’s group showed that deletions in the N-terminal domain of human Twinkle decrease the efficiency of single stranded DNA binding and helicase activityin vitro [3]. Here, we extend our physiological studies in Drosophila by investigating the importance of the N-terminal domain in d-mtDNA helicase. We evaluate the effects of overexpression in Drosophila cell culture of variants carrying mutations in the N-terminal domain, including mutants equivalent to human adPEO mutations.
2. MATERIALS AND METHODS
Generation and Induction of Stable Cell Lines
Drosophila Schneider S2 cells were cultured at 25°C in Drosophila Schneider Medium (Invitrogen) supplemented with 10% fetal bovine serum. Cells were subcultured to 5×106 cells/ml every third day. Cells were transfected using Effecten (Qiagen). Hygromycin-resistant cells were selected with 200 μg/ml hygromycin. Cells were passed at least five times in hygromycin-containing medium and then cultured in standard medium. The cell lines were grown to a density of 3×106/ml and then treated with 0.2 mM CuSO4 to induce high-level expression from the metallothionein promoter.
Immunoblotting
Total cellular protein (20 μg per lane) was fractionated by SDS-PAGE in 9% gels and transferred to nitrocellulose filters. Filters were preincubated for 1 h with 5% skim milk in phosphate-buffered saline (PBS), followed by incubation for 1 h with d-mtDNA helicase antibody (1:20 ml in PBS containing 0.1% Tween 20). Filters were washed four times with PBS containing 0.1% Tween 20, incubated for 1 h with horseradish peroxidase-conjugated anti-rabbit IgG (BIO-RAD), and washed with PBS containing 0.1% Tween 20. Protein bands were visualized using ECL Western blotting reagents (Amersham Biosciences). Rabbit polyclonal antibody against the Drosophila ATPase β subunit was provided by Rafael Garesse (CSIC-UAM, Madrid). We established two independent cell lines from each of the wild type and mutant constructs, and performed two independent analyses for all of the cell lines. Quantitation was performed using the Kodak 1D program software. We found that there is little variation among the two independent cell lines, and the quantitation presented in Figs. 2, 3, 4 shows the average of the duplicate analyses from one of the two cell lines for each construct. All of these data vary less than 15% and are thus reliable.
Fig. 2. Expression of Drosophila mtDNA helicase mutants analogous to human adPEO mutations in Schneider cells.
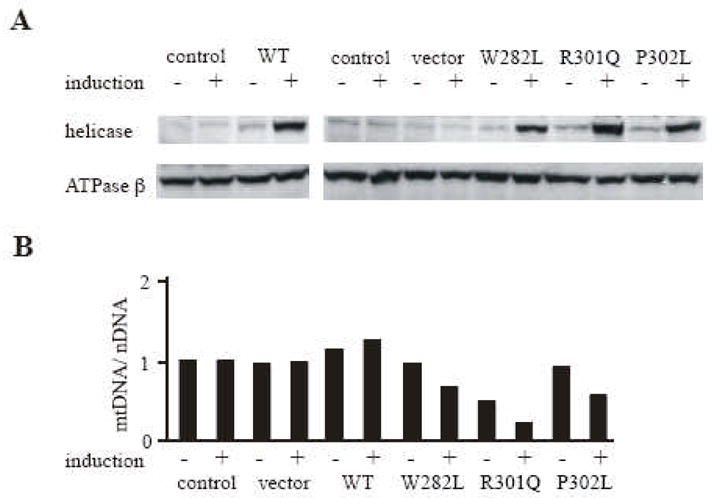
Schneider cells with no plasmid (control) or carrying pMt/Hy (vector), pMt/WT/Hy (WT), pMt/W282L/Hy (W282L), pMt/R301Q/Hy (R301Q) or pMt/P302L/Hy (P302L) were cultured for 14 days in the presence or absence of 0.2 mM CuSO4. A, Protein extracts (20 μg) were fractionated by 9 % SDS-PAGE, transferred to nitrocellulose filters and probed with affinity-purified rabbit antiserum against d-mtDNA helicase or antiserum against d-ATPase β was indicated. B, Effect of overexpression of the deletion mutants on mtDNA copy number. Total mtDNA (10 μg) was extracted from Schneider cells (control) or Schneider cells carrying pMt/Hy (vector), pMt/WT/Hy WT ( ), pMt/W282L/Hy (W282L), pMt/R301Q/Hy (R301Q) or pMt/P302L/Hy (P302L) that were cultured for 14 days in the presence of 0.2 mM CuSO4. DNA was digested with XhoI, fractionated in a 0.7 % agarose/TBE gel, and then blotted to a nylon membrane. The membrane was hybridized with a radiolabeled probe for CytB, and then stripped and re-hybridized with radiolabeled probe for the histone gene cluster. The relative mtDNA copy number was quantitated as described under Methods.
Fig. 3. Expression in Schneider cells of Drosophila mtDNA helicases carrying mutations in the putative zinc binding domain.
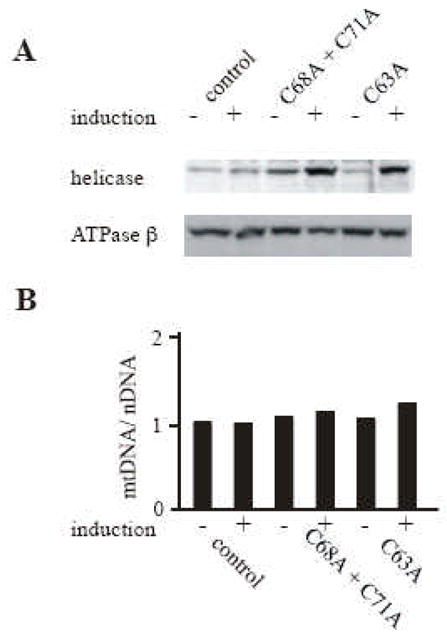
Schneider cells containing no plasmid (control) or carrying pMt/C68A + C71A/Hy (C68A + C71A), or pMt/C63A/Hy (C63A) were cultured for 14 days in the absence or presence of 0.2 mM CuSO4. A, Immunoblot analysis of d-mtDNA helicase and d-ATPase β was carried out as described in the legend to Fig. 2. B, mtDNA abundance was determined as described in the legend to Fig. 2.
Fig. 4. Expression in Schneider cells of Drosophila mtDNA helicases carrying mutations in the putative TOPRIM fold and RNAP basic domain.
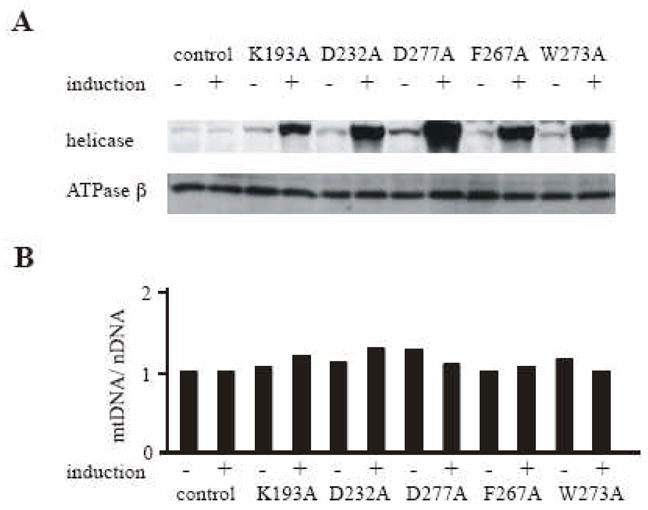
Schneider cells containing no plasmid (control) or carrying pMt/K193A/Hy (K193A), pMt/D232A/Hy ( D232A ), pMt/D277A/Hy (D277A), pMt/F267A/Hy (F267A), or pMt/W273A/Hy (W273A) were cultured for 14 days in the absence or presence of 0.2 mM CuSO4. A, Immunoblot analysis of d-mtDNA helicase and d-ATPase β was carried out as described in the legend to Fig. 2. B, mtDNA abundance was determined as described in the legend to Fig. 2.
Protein Crosslinking Analysis
Crosslinking analysis was performed as described previously [16]. 1×108 cells that were induced with 0.2 mM CuSO4 for 7 days were washed twice with PBS and resuspended in 200 μl crosslinking buffer (PBS containing 1% formaldehyde). After incubation for 5 min at room temperature, 200 μl of quenching solution (PBS containing 250 mM glycine, 10 mM EDTA and 4% SDS) was added and the samples were mixed thoroughly. 20 μl of the protein solution was fractionated by 6% SDS-PAGE and transferred to nitrocellulose filters. Immunoblot analysis was performed as described above.
Southern Blotting
Genomic DNA was purified from Drosophila Schneider S2 cells by standard methods. DNA (10 μg per lane) was cleaved with XhoI, fractionated on a 0.7% agarose/TBE gel and transferred to Hybond-N+ nylon membrane (Amersham Biosciences). Hybridization was performed as described previously [16–18]. Filters were washed three times for 10 min at room temperature with 2 × SSC containing 0.1% SDS, once for 30 min at 65 °C with 0.2 × SSC containing 0.1% SDS, and then analyzed with a PhosphorImager. Blots were probed with radiolabeled DNAs for the mitochondrial gene Cytb and the nuclear histone gene cluster. The ratio of the signals for these two genes was used to determine the relative copy number of mtDNA. The Southern blot experiments shown in Figs. 2–4 were performed twice with each of the two independent cell lines carrying each plasmid construct, including the control (no plasmid), vector only, wild type, and each of the mutant d-mtDNA helicases. The data presented represent one such experiment, and quantitation is provided for the duplicate experiments from one of the two cell lines. All of the comparable data for each construct vary by less than 15%.
Preparation of Inducible Plasmids Expressing d-mtDNA Helicase Variants
The construction of the plasmid pMt/WT/Hy was performed as described previously [1]. The expression vectors carrying mutant d-mtDNA helicases were prepared by Quick-Change mutagenesis, or by PCR with Pfu DNA polymerase. A typical PCR was carried out in a 50 μl reaction mixture with 50 ng of pMt/WT/Hy and 2 units of Pfu DNA polymerase. A specific primer pair was used for each mutant as follows: C63A: 5′-GACGGACACACGgcCTTGCAGCTGG -3′ and 5′-CCAGCTGCAAGgcCGTGTGTCCGTC -3′; C68A+C71A: 5′-CAGCTGGAAgcTCGCCTCgcCGATCGCAATAG -3′ and 5′-CTATTGCGATCGgcGAGGCGAgcTTCCAGCTG -3′; K193A: 5′-GTGGTTGGCGAAgcGGTGTTATATCTG -3′ and 5′-CAGATATAACACCgcTTCGCCAACCAC -3′; D232A: 5′-GCGGTTCTAGTCAGCAATCTAATTGcCTTTATTGTCCTTGCCACACAG -3′ and 5′-CTGTGTGGCAAGGACAATAAAGgCAATTAGATTGCTGACTAGAACCGC - 3′; F267A: 5′-GCCTTGGAACGCgcCAAGGAGCTCATC -3′ and 5′-GATGAGCTCCTTGgcGCGTTCCAAGGC -3′; W273A: 5′-GAGCTCATCTTTgcGTTGCACTACGATG -3′ and 5′-CATCGTAGTGCAACgcAAAGATGAGCTC -3′; D277A: 5′-CTCATCTTTTGGTTGCACTACGcTGCCAGTCACAGCTGGGATGCAGCTAG - 3′ and 5′-CTAGCTGCATCCCAGCTGTGACTGGCAgCGTAGTGCAACCAAAAGATGAG - 3′; W282L: 5′-CAGTCACAGCTtGGATGCAGCTAG -3′ and 5′-CTAGCTGCATCCaAGCTGTGACTG -3′; R301Q: 5′-GCCTGTTAATCCaACCCACTGAGAC -3′ and 5′-GTCTCAGTGGGTtGGATTAACAGGC -3′ ; P302L: 5′-GTTAATCCGACtCACTGAGACGG -3′ and 5′-CCGTCTCAGTGaGTCGGATTAAC -3′. Lower case letters indicate the positions of modified nucleotide residues.
3. RESULTS
3.1. Overexpression in Schneider Cells of Drosophila mtDNA Helicase Variants Analogous to Human adPEO mutants in the N-terminal Domain
To date, nearly 20 adPEO mutations have found in human mtDNA helicase; most of these mutations map in the linker region and the helicase domain, though some are found in N-terminal region (Fig. 1). To evaluate the effects of the human adPEO mutations in N-terminal region, we constructed metallothionein-inducible plasmids expressing wild type d-mtDNA helicase and variants carrying W282L, R301Q, and P302L amino acid substitutions, which are analogous to the W315L, R334Q, and P335L mutations found in human adPEO patients, respectively (Fig. 1). The constructs were transfected into Schneider cells, and we established two independent stable cell lines for each. After 14 days of incubation in the absence and presence of 0.2 mM CuSO4, immunoblot analysis indicated increases in the range of 1.3 to 2- and 7 to 12-fold in the levels of the various d-mtDNA helicases, respectively, relative to the control cell lines, whereas there was no significant change in the level of ATP synthase β subunit used as a control (Fig. 2A). Crosslinking analysis of the mutants as described under Methods showed that all are capable of forming hexamers (data not shown). The effects of overexpression of the mutants on mtDNA maintenance were examined. To determine the mtDNA copy number, total cellular DNA was isolated, cleaved with XhoI and analyzed by Southern blot (see Methods). Blots were hybridized sequentially with probes for the nuclear histone gene cluster as a control and the mitochondrial gene CytB. Relative mtDNA copy number was determined from the ratio of CytB hybridization to histone gene cluster hybridization. After 14 days of induction, the relative mtDNA copy number was increased 1.2 fold in cells overexpressing the wild type enzyme as compared to the control cells (Fig. 2B). In cells overexpressing the R301Q mutation, the relative mtDNA copy number was reduced to 48% and 22% of the control cells, correlating with the 2- and 7-fold overexpression of R301Q in the absence and presence of induction, respectively (Fig. 2B). Meanwhile, cells overexpressing the W282L and P302L mutants, showed modest effects. In these mutant-expressing cells, the relative mtDNA copy number was unchanged in the absence of CuSO4, but after 14 days of induction, the relative mtDNA copy number was decreased to 66% and 56% of the control, respectively. In the absence of copper, we observed no effects on cell growth or viability in any of the cell lines. After 5 weeks of induction, R301Q expressing cells grew very poorly and produced a lethal phenotype. In contrast, copper-treatment had no adverse effects on the growth of cells carrying the wild type, W282L and P302L mtDNA helicases (data not shown). Multiple mtDNA deletions represent the molecular hallmark of adPEO mutations. To detect such multiple deletions, we used a sensitive PCR-based approach as described previously [6], but we failed to detect any mtDNA deletions in any of the cell lines (data not shown).
3.2. Overexpression of Zinc Binding Domain Mutants of Drosophila mtDNA Helicase in Schneider Cells
Amino acids C68, C71, C102, and C105 constitute a putative Cys4 zinc-binding motif in d-mtDNA helicase (Fig. 1). To investigate the importance of this putative zinc binding domain, we constructed a metallothionein-inducible plasmid expressing mtDNA helicase carrying a double substitution of C68A and C71A. These amino acids were selected because they are well conserved in most eukaryotes including Drosophila and related species, but are not conserved in vertebrates (Fig. 1). The construct was transfected into Schneider cells and we established two stable cell lines. After 14 days of incubation in the absence and presence of 0.2 mM CuSO4, immunoblot analysis indicated increases in the range of 1.2 to 2- and 5 to 7-fold in the levels of the variant d-mtDNA helicase relative to the control cell lines (Fig. 3A). The ATP synthase β subunit probed as a control showed no significant change in expression level. Crosslinking analysis of the double mutant as described under Methods showed that the double mutant was capable of forming hexamers (data not shown). The effect of overexpression of the d-mtDNA helicase variant on mtDNA maintenance was evaluated by Southern blot analysis as described above. After 14 days of induction, relative mtDNA copy number was increased 1.2 fold in cells overexpressing the wild type enzyme as compared to the control cells (Fig. 2B). Similarly, overexpression of C68A + C71A mutant resulted in a 1.1 fold increase in relative mtDNA copy number as compared to the control cells. Because we did not observe a dominant negative phenotype with this double mutant, we investigated a potential role for Cys63. C63 is well conserved in metazoans, but is not conserved in other mtDNA helicases (Fig. 1). We reasoned that because C63 is 5 amino acids away from C68, and might serve as one of the constituent amino acids of the zinc binding domain instead of C71; notably, the distance between the two relevant cysteines is 2 or 4 amino acids in higher plants (Fig. 1). We thus constructed a vector expressing the variant carrying C63A and transfected it into Schneider cells. After 14 days of induction, the C63A mutant was over expressed 5-fold and relative mtDNA copy number was increased by 1.2 fold relative to the control (Fig. 3B). We conclude that the overexpression of alanine substitution mutants of cysteine residues in the putative zinc binding domain do not interfere with mtDNA replication in Schneider cells.
3.3. Overexpression of TOPRIM Fold and RNAP Basic Motif Mutants of Drosophila mtDNA Helicase in Schneider Cells
To evaluate the potential contributions of amino acids contributing to the TOPRIM fold and RNAP basic motif we constructed metallothionein-inducible plasmids expressing d-mtDNA helicase variants carrying K193A, D232A, and D277A substitution. K193 is analogous to K122 in T7 gp4, and was demonstrated to be essential for phosphodiester bond catalysis [7, 19]. Furthermore, D232 and D277 are analogous to D161 and D209 in the primase active site of T7 gp4, and are essential for Mg2+ mediated binding and hydrolysis of NTPs [9, 10]. The constructs were transfected into Schneider cells, and we established two independent stable cell lines for each. After 14 days of incubation in the absence and presence of 0.2 mM CuSO4, immunoblot analysis indicated increases in the range of 1.5 to 3- and 10 to 20-fold in the levels of the various d-mtDNA helicases, respectively, relative to the control cell lines, whereas there was no significant change in the level of ATP synthase β subunit (Fig. 4A). Crosslinking analysis of the mutants as described under Methods showed that all were capable of forming hexamers (data not shown). In each of the mutants, overexpression resulted in increases of 1.0 to 1.3-fold of the mtDNA copy number relative to the control cells, values that are comparable to the wild type overexpressing cells. Moreover, we established and examined cell lines expressing variants carrying alanine substitutions F267A and W273A. F267 and W273 are also well conserved in eukaryotic mtDNA helicases. Similar to other primase active site mutants, overexpression yielded the relative mtDNA copy number in increases of 1.0 to 1.2-fold (Fig. 4B), and failed to yield a dominant negative phenotype.
4. DISCUSSION
The N-terminal domain of Drosophila mtDNA helicase shares homology with the primase domain of T7 gp4, and whereas some critical residues for primase activity are missing in d-mtDNA helicase, most of those within the six conserved primase motifs are well conserved [6]. To evaluate the physiological importance of these conserved residues, we established Drosophila Schneider cell lines expressing mutant forms. Surprisingly, none of the alanine substitution mutants in the zinc binding motif, the RNAP basic motif, or motifs IV or V showed dominant negative phenotypes. In contrast, overexpression of three mutants analogous to human adPEO mutations in motif V (W282L) and motif VI (R301Q and P302L) resulted in depletion of mtDNA copy number and in the case of R301Q, a dominant negative cellular phenotype. Although these residues are not well conserved among T7 gp4 and mtDNA helicases, R301Q shows a severe depletion of mtDNA similar to the helicase active site mutants we described in our previous report [1], and P302L and W282L show a moderate reduction. Interestingly, one T7 gp4 mutant, G116D, which localizes to motif III, is defective only in helicase activity [20]. Thus, as with this T7 gp4 mutation, these adPEO mutants may be defective in helicase rather than primase activity. This possibility is corroborated by the fact that all of the adPEO mutants in the N-terminal domain lie close to linker region, and it has been shown recently that some human recombinant mutants are defective in helicase activity or hexamerization in vitro [21]. We found that our Drosophila adPEO mutants form hexamers, so they might exhibit defects in helicase activity per se. However, in the case of the W282L and P302L mutants, >50% of mtDNA copy number is maintained in cells under induced conditions, where the typical molecular ratio of endogenous to exogenous mtDNA helicase polypeptides is 1:9. If the mutants form hexamers with the same efficiency as the wild type polypeptides, >85% of the hexamers should contain either zero or one wild type protomer. Distinct from R301Q, it is likely that hexamers carrying only W282L or P302L mutant protomers or one wild type protomer retain substantial helicase activity. This is in contrast to the d-mtDNA helicase active site mutants and other adPEO mutations in linker region and helicase domain that we reported earlier [1], which showed only a severe mtDNA depletion and dominant negative cellular phenotype, or no molecular or cellular phenotype whatsoever. In the case of the N-terminal region mutations, the moderate phenotype may correspond to a helicase modulator y function and/or to changes in the efficiency and/or stability of hexamer formation. Similar to our earlier mutants, none of the three d-mtDNA helicase N-terminal adPEO mutants show multiple mtDNA deletions as is observed in various human tissues.
In T7 gp4, the zinc binding domain and its constituent conserved cysteine residues are essential for primase activity and phage growth [22, 23]. In eukaryotic mtDNA helicases except for some metazoans, all four cysteines are well conserved. In most metazoans, only the third cysteine is conserved, while in some insects including fly, mosquito and silkworm, all four cysteine residues are conserved. We found that the overexpression of a putative zinc finger mutant, C68A + C71A in Drosophila results in an increase in mtDNA that is equivalent to that observed with overexpression of wild type d-mtDNA helicase. Similarly, the C63A mutant did not show a dominant negative phenotype. We tested the possibility that C63 might replace C68 as a component of the zinc binding domain because the distance between the two cysteines is 2 and 4 in higher plants (Fig. 1). Altogether our data suggests that these cysteine residues may not be essential for d-mtDNA helicase function.
The RNAP basic motif is proposed to contribute to nucleotide binding in T7 gp4 [7, 19]. In mtDNA helicases, two basic residues, K193 and R200, that are analogous to K122 and K128 in T7 gp4, respectively, are well conserved and are critical for primer synthesis in the phage enzyme [7, 19]. However, overexpression of d-mtDNA helicase carrying the K193A mutation did not result in a dominant negative phenotype.
Motifs IV–VI constitute the TOPRIM fold in T7 gp4 in which five residues, E157, D161, D207, D209 and D237 form an acidic patch and bind two Mg2+ ions for catalysis. Three of these, E157, D207, and D209, are perfectly conserved in proteins that contain a TOPRIM fold [9, 10]. In d-mtDNA helicase, acidic residues corresponding to E157, D209 and D237 are missing, so it is not thought to have primase activity. However, D232 and D277, analogous to D161 and D209, respectively, are conserved, and these are perfectly conserved in mtDNA helicases and T7 gp4. Nonetheless, the overexpression of D232A and D277A did not yield dominant negative phenotypes. Similarly, F267 and W273 in d-mtDNA helicase are relatively conserved in motif V of mtDNA helicases and T7 gp4, and overexpression of F267A and W273A also failed to yield dominant negative phenotypes. Consistent with our physiological data, Falkenberg’s group showed recently that a recombinant human protein carrying a deletion of residues 1–314 of the N-terminal domain, which removes motifs I–IV and half of motif V, still retains helicase activity in vitro [3]. Thus, in the absence of evidence for a primase activity either in vivo or in vitro or a requirement for residues N-terminal to motif V in helicase activity, it remains unclear what is the reason for conservation of N-terminal amino acids shared between mtDNA helicases and prokaryotic primases. However, we note that mutations in specific conserved residues of the T7 gp4 protein that abolish primase activity in vitro, do not interfere with replication in the presence of wild type T7 gp4 in vivo [7, 9, 22]. Though unlikely given the high level of overexpression we documented, it remains possible that a situation similar to that in T7 contributes to a lack of a dominant negative phenotype in the Drosophila cell lines, thus warranting more detailed physiological and biochemical studies.
Acknowledgments
This work was supported by NIH grant GM45295 to L.S.K.
Abbreviations
- mtDNA
mitochondrial DNA
- d-
Drosophila
- adPEO
autosomal dominant progressive external ophthalmoplegia
- T7 gp4
bacteriophage T7 gene 4 protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Matsushima Y, Kaguni LS. Differential phenotypes of active site and human autosomal dominant progressive external ophthalmoplegia mutations in Drosophila mitochondrial DNA helicase expressed in Schneider cells. J Biol Chem. 2007;282:9436–9444. doi: 10.1074/jbc.M610550200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tyynismaa H, Sembongi H, Bokori-Brown M, Granycome C, Ashley N, Poulton J, Jalanko A, Spelbrink JN, Holt IJ, Suomalainen A. Twinkle helicase is essential for mtDNA maintenance and regulates mtDNA copy number. Hum Mol Genet. 2004;13:3219–3227. doi: 10.1093/hmg/ddh342. [DOI] [PubMed] [Google Scholar]
- 3.Farge G, Holmlund T, Khvorostova J, Rofougaran R, Hofer A, Falkenberg M. The N-terminal domain of TWINKLE contributes to single-stranded DNA binding and DNA helicase activities. Nucleic Acids Res. 2008;36:393–403. doi: 10.1093/nar/gkm1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ziebarth TD, Farr CL, Kaguni LS. Modular architecture of the hexameric human mitochondrial DNA helicase. J Mol Biol. 2007;367:1382–1391. doi: 10.1016/j.jmb.2007.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spelbrink JN, Li FY, Tiranti V, Nikali K, Yuan QP, Tariq M, Wanrooij S, Garrido N, Comi G, Morandi L, Santoro L, Toscano A, Fabrizi GM, Somer H, Croxen R, Beeson D, Poulton J, Suomalainen A, Jacobs HT, Zeviani M, Larsson C. Human mitochondrial DNA deletions associated with mutations in the gene encoding Twinkle, a phage T7 gene 4-like protein localized in mitochondria. Nat Genet. 2001;28:223–231. doi: 10.1038/90058. [DOI] [PubMed] [Google Scholar]
- 6.Shutt TE, Gray MW. Twinkle, the mitochondrial replicative DNA helicase, is widespread in the eukaryotic radiation and may also be the mitochondrial DNA primase in most eukaryotes. J Mol Evol. 2006;62:588–599. doi: 10.1007/s00239-005-0162-8. [DOI] [PubMed] [Google Scholar]
- 7.Lee SJ, Richardson CC. Essential lysine residues in the RNA polymerase domain of the gene 4 primase-helicase of bacteriophage T7. J Biol Chem. 2001;276:49419–49426. doi: 10.1074/jbc.M108443200. [DOI] [PubMed] [Google Scholar]
- 8.Aravind L, Leipe DD, Koonin EV. Toprim--a conserved catalytic domain in type IA and II topoisomerases, DnaG-type primases, OLD family nucleases and RecR proteins. Nucleic Acids Res. 1998;26:4205–4213. doi: 10.1093/nar/26.18.4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee SJ, Richardson CC. Acidic residues in the nucleotide-binding site of the bacteriophage T7 DNA primase. J Biol Chem. 2005;280:26984–26991. doi: 10.1074/jbc.M504817200. [DOI] [PubMed] [Google Scholar]
- 10.Kato M, Ito T, Wagner G, Richardson CC, Ellenberger T. Modular architecture of the bacteriophage T7 primase couples RNA primer synthesis to DNA synthesis. Mol Cell. 2003;11:1349–1360. doi: 10.1016/s1097-2765(03)00195-3. [DOI] [PubMed] [Google Scholar]
- 11.Virgilio R, Ronchi D, Hadjigeorgiou GM, Bordoni A, Saladino F, Moggio M, Adobbati L, Kafetsouli D, Tsironi E, Previtali S, Papadimitriou A, Bresolin N, Comi GP. Novel Twinkle (PEO1) gene mutations in mendelian progressive external ophthalmoplegia. J Neurol. 2008;255:1384–1391. doi: 10.1007/s00415-008-0926-3. [DOI] [PubMed] [Google Scholar]
- 12.Sarzi E, Goffart S, Serre V, Chretien D, Slama A, Munnich A, Spelbrink JN, Rotig A. Twinkle helicase (PEO1) gene mutation causes mitochondrial DNA depletion. Ann Neurol. 2007;62:579–587. doi: 10.1002/ana.21207. [DOI] [PubMed] [Google Scholar]
- 13.Copeland WC. Inherited Mitochondrial Diseases of DNA Replication. Annu Rev Med. 2007;59:131–146. doi: 10.1146/annurev.med.59.053006.104646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spinazzola A, Zeviani M. Disorders of nuclear-mitochondrial intergenomic signaling. Gene. 2005;354:162–168. doi: 10.1016/j.gene.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 15.Kaguni LS. DNA polymerase gamma, the mitochondrial replicase. Annu Rev Biochem. 2004;73:293–320. doi: 10.1146/annurev.biochem.72.121801.161455. [DOI] [PubMed] [Google Scholar]
- 16.Matsushima Y, Farr CL, Fan L, Kaguni LS. Physiological and biochemical defects in carboxyl-terminal mutants of mitochondrial DNA helicase. J Biol Chem. 2008;283:23964–23971. doi: 10.1074/jbc.M803674200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsushima Y, Adan C, Garesse R, Kaguni LS. Drosophila mitochondrial transcription factor B1 modulates mitochondrial translation but not transcription or DNA copy number in Schneider cells. J Biol Chem. 2005;280:16815–16820. doi: 10.1074/jbc.M500569200. [DOI] [PubMed] [Google Scholar]
- 18.Matsushima Y, Garesse R, Kaguni LS. Drosophila mitochondrial transcription factor B2 regulates mitochondrial DNA copy number and transcription in schneider cells. J Biol Chem. 2004;279:26900–26905. doi: 10.1074/jbc.M401643200. [DOI] [PubMed] [Google Scholar]
- 19.Lee SJ, Richardson CC. Interaction of adjacent primase domains within the hexameric gene 4 helicase-primase of bacteriophage T7. Proc Natl Acad Sci U S A. 2002;99:12703–12708. doi: 10.1073/pnas.202471499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Washington MT, Rosenberg AH, Griffin K, Studier FW, Patel SS. Biochemical analysis of mutant T7 primase/helicase proteins defective in DNA binding, nucleotide hydrolysis, and the coupling of hydrolysis with DNA unwinding. J Biol Chem. 1996;271:26825–26834. doi: 10.1074/jbc.271.43.26825. [DOI] [PubMed] [Google Scholar]
- 21.Korhonen JA, Pande V, Holmlund T, Farge G, Pham XH, Nilsson L, Falkenberg M. Structure-function defects of the TWINKLE linker region in progressive external ophthalmoplegia. J Mol Biol. 2008;377:691–705. doi: 10.1016/j.jmb.2008.01.035. [DOI] [PubMed] [Google Scholar]
- 22.Kusakabe T, Richardson CC. The role of the zinc motif in sequence recognition by DNA primases. J Biol Chem. 1996;271:19563–19570. doi: 10.1074/jbc.271.32.19563. [DOI] [PubMed] [Google Scholar]
- 23.Mendelman LV, Beauchamp BB, Richardson CC. Requirement for a zinc motif for template recognition by the bacteriophage T7 primase. EMBO J. 1994;13:3909–3916. doi: 10.1002/j.1460-2075.1994.tb06702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


