Abstract
Background and Aims
Exposure of plants to ethylene can influence a spectrum of developmental processes including organ senescence and abscission. The aim of this study was to examine the role of the gaseous regulator in Nicotiana sylvestris plants exhibiting a silenced or constitutive ethylene response.
Methods
Transgenic N. sylvestris plants were generated that either ectopically expressed the Arabidopsis mutant ethylene receptor ETR1-1 or the tomato EIN3-like (LeEIL1) gene. Highly expressing homozygous lines were selected and the time-course of development, from germination to organ senescence, was studied.
Key Results
Fifty percent of the homozygous Pro35S:ETR1-1 lines examined showed a high susceptibility to collapse prior to flowering, with plant death occurring within a few days of leaf wilting. The time-course of leaf senescence in the remaining Pro35S:ETR1-1 lines was visibly arrested compared to wild type (negative segregant) plants and this observation was reaffirmed by chlorophyll and protein analysis. Petal necrosis was also delayed in Pro35S:ETR1-1 lines and corolla abscission did not take place. When senescence of Pro35S:ETR1-1 plants did take place this was accompanied by leaf bleaching, but tissues remained fully turgid and showed no signs of collapse. A single Pro35S:LeEIL1 line was found to exhibit consistently accelerated leaf and flower senescence and precocious flower bud shedding.
Conclusions
These observations support a role for ethylene in regulating a spectrum of developmental events associated with organ senescence and tissue necrosis. Furthermore, the transgenic lines generated during this study may provide a valuable resource for exploring how senescence processes are regulated in plants.
Key words: Nicotiana sylvestris, ethylene, senescence, chlorophyll, flower abscission, etr1-1, necrosis, pathogenesis
INTRODUCTION
The gaseous plant hormone ethylene is a key regulator of plant growth and development (Osborne, 1990). Processes affected by exposure to ethylene include: stem and root elongation, flower initiation, organ senescence and abscission, and fruit ripening (Abeles et al., 1992). The capacity of ethylene to influence developmental events in vivo has been proposed to be the consequence of changes in production of, and/or sensitivity to, the gas. In support of the former, it has been well documented that elevated ethylene biosynthesis accompanies the ripening of climacteric fruit, the senescence and shedding of organs, and the imposition of both abiotic or biotic stress on plant tissues (Kieber, 1997). Whilst the mechanism that dictates sensitivity to a plant hormone is unclear, certain cells have been classified as target cells for the gas (Osborne and McManus, 2005) and there is some evidence that a possible explanation for this observation could lie at the receptor level (Payton et al., 1996).
Exposure of Arabidopsis plants to ethylene induces premature yellowing of the oldest leaves (Smart, 1994). Grbic and Bleecker (1995) carried out a detailed study of the process and showed that the gas specifically promoted the transcription of senescence-associated genes whilst down-regulating the expression of genes associated with photosynthesis. The role of ethylene in senescence-related processes in vivo has been explored with the aid of Arabidopsis mutants such as etr1 and ein2 that are insensitive to the gas. Both mutants have been shown to exhibit a delay in the onset of leaf senescence when compared to wild type (Grbic and Bleecker, 1995). Moreover, Oh et al. (1997) identified a mutant exhibiting an arrested rate of senescence, as determined by declining chlorophyll content and photosynthetic efficiency, which proved to be allelic to the ein2 locus. A delay in floral organ abscission in etr1 and ein2 plants has been reported by several groups (Ecker, 1995; Bleecker and Patterson, 1997; Kende and Zeevaart, 1997) implicating ethylene also in the timing of organ shedding.
The impact of maintaining a constitutive ethylene response on the timing of developmental events such as senescence has been less well investigated. The Arabidopsis ctr1 mutant experiences a continuous activation of the ethylene response pathway. Kieber et al. (1993) reported that although the morphology of ctr1 plants can be phenocopied by treatment of wild-type plants with exogenous ethylene, the timing of leaf senescence was similar in both mutant and wild type material. Furthermore, transgenic tomato plants that over-produce ethylene did not show an increased rate of leaf senescence relative to the control (Lanahan et al., 1994).
A constitutive ethylene response has also been achieved by over-expression of the transcription factors downstream of CTR1 in the ethylene response pathway (Chao et al., 1997; Solano et al., 1998). The Arabidopsis ETHYLENE INSENSITIVE3 (EIN3) protein is a nuclear-localized component of the ethylene signal-transduction pathway with DNA-binding activity. Loss-of-function mutations in this protein result in ethylene insensitivity. EIN3 and other EIN3-like genes (EILs) are important players in ethylene signalling, since the ein3 eil1 double loss-of-function mutant shows complete insensitivity to ethylene (Alonso et al., 2003), and a high level of expression of EIN3 or EIL1 has been shown to confer constitutive ethylene phenotypes in all developmental stages in a WT or ein2 mutant background (Alonso et al., 1999). Although leaf senescence has not been linked with EIN3 or EIL1, various studies have reported an effect of these mutations on plant development. Tieman et al. (2001) demonstrated that reduced EIL expression in tomato (LeEIL1), affects ethylene responses, including leaf epinasty, flower abscission, flower senescence and fruit ripening. In contrast, over-expression of LeEIL1 in the ethylene insensitive tomato Never-ripe background partially rescued the delayed-ripening phenotype in fruits (Chen et al., 2004).
The dominant nature of the etr1 mutation in Arabidopsis lead Wilkinson et al. (1997) to examine the consequences of over-expressing the mutant ETR1 gene in heterologous species under the direction of the viral 35S promoter. This study revealed that the hormone recognition and response pathways were highly conserved in plant species and that both tomato and petunia plants could be rendered ethylene-insensitive using this approach. Moreover, the transgenic material exhibited marked delays in events such as fruit ripening, flower senescence and abscission (Wilkinson et al., 1997). Subsequently, ethylene insensitivity was conferred on Nicotiana tobaccum plants using the same strategy, leading to the discovery by Knoester et al., (1998) that ethylene perception was required for basic defence-related gene expression but not for hypersensitivity to tobacco mosaic virus. The authors also reported that this material exhibited delayed flower senescence and abscission.
The aim of this study was to examine the role of ethylene in the regulation of leaf senescence in the diploid species Nicotiana sylvestris. The approach adopted was to generate transgenic plants exhibiting a silenced ethylene response by ectopically expressing the Arabidopsis ETR1-1 gene or expressing the tomato EIN3-like gene (LeEIL1) to confer a constitutive response to the gas. In addition, other developmental processes affected by ethylene, such as flower senescence and floral organ abscission, were studied using the material.
MATERIALS AND METHODS
Plant material and growth conditions
Seeds of Nicotiana sylvestris were surface-sterilized and placed on MS media plates before incubation at 4 °C in the dark for 1–3 d. After this time the plates were maintained at 20 °C under constant light to enable germination to take place. Seedlings were transferred to soil at approximately 4 weeks old and grown in a growth room phytotron unit at 22 °C and a 15-h photo-period provided by 250 µmol m−2 s−1 globe (Son-T) lighting. Nicotiana sylvestris leaf samples were frozen in liquid nitrogen after collection and stored in a freezer at –70 °C if not used immediately.
Marking of Nicotiana sylvestris flowers
Nicotiana sylvestris flowers were tagged on the day the corolla limb opened fully. Floral senescence was determined by measuring the time from full corolla opening until the collapse of the corolla limb and the complete necrosis of the floral tube.
Leaf senescence experiments
The N. sylvestris homozygous lines used in the leaf senescence experiments were R8 and R31, which correspond to material transformed with the mutant Arabidopsis ETR1-1 gene. Lines e1, e13, e17 and e24 refer to plants transformed with the tomato EIL gene (EIN3). Leaf number relates to the position of the leaf relative to the whole plant with Leaf 1 being the oldest. Measurements were not possible in some cases when a leaf was extensively necrotic. Leaf material was collected from plants grown in a population of at least five plants for each line. The time at which the first flower was produced was chosen as an index point to represent the approximate age of the plant. Therefore, the age of the plants at the time of sample collection were described as ‘days before’ and ‘days after’ flowering.
Transgenic lines of N. sylvestris and wild type (negative segregant) plants were allowed to flower and the leaves to senesce naturally. Chlorophyll levels were measured non-destructively with a hand-held automated chlorophyll SPAD meter (SPAD-502; Minolta, Japan) at ten different areas around the leaf blade and the averaged readings from two leaves from different individuals of the same line were pooled. Chlorophyll and protein were extracted from leaf disc samples collected from the central position of the leaf blade. A reading of chlorophyll consisted of a pooled measurement of four single leaf disc samples from two leaves of different individuals of the same line.
For detached-leaf experiments, fully expanded green and healthy leaves (from position 7 on the plant) were excised at the junction between the petiole and the stem 15 d before flowering. The excised petiole surface was rapidly submerged into 50 mL distilled water contained in a 250-mL beaker. Individual detached leaves were maintained in a growth room at 22 °C under a 15 h photoperiod. Chlorophyll determinations were carried out using a SPAD meter, as described previously, over a period of 15 d or until it was no longer possible to carry out further analysis.
Plasmid construction and plant transformation
The Agrobacterium tumefaciens LBA4404 strain and plasmid vector pBIN19 used in the transformation to produce EIN3 over-expression plants were donated by Dr G. P. Chen (School of Biosciences University of Nottingham). The strain ABI and vector pCD2 used in the transformation to produce mutant ETR1-1 plants were obtained from Professor H. Klee (Florida University, Gainsville, FL). The transformations of Nicotiana sylvestris were carried out under the supervision of Dr Martin Maunders and Dr Dawn Carter at Advanced Technology Cambridge (ATC).
The two youngest leaves were removed by a scalpel blade from 6-week-old plants. The plants were grown in soil in a growth chamber at 18 °C and a photoperiod of 16 h light and 8 h dark. The excised leaves were sterilized in 8 % (v/v) bleach for 10 min and then rinsed repeatedly with sterile distilled water.
Colonies of A. tumefaciens strain ABI and LBA4404 were grown on APM agar plates [0·5 % (w/v) yeast extract, 0·05 % (w/v) casamino acid, 0·8 % (w/v) mannitol, 0·2 % (w/v) (NH4)2SO4, 0·5 % (w/v) NaCl, pH 6·6, with bacteriological agar added to 0·8 % (w/v) prior to autoclaving] with appropriate antibiotics (75 mg mL−1 spectinomycin and 100 mg mL−1 kanamycin for ABI, and 500 mg mL−1 streptomycin and 50 mg mL−1 kanamycin for LBA4404) at 29 °C in the dark for 3 d. A single colony was selected and inoculated into 10 mL of APM medium with the appropriate antibiotics and incubated in the dark at 29 °C with shaking at 200 r.p.m.
A sterile cork borer no·6 was used to punch leaf discs along the smaller veins. The leaf discs were transformed by immersing in 25 mL of overnight A. tumefaciens suspension (OD600 = 0·6–0·8) and mixed for approximately 2 min at room temperature. Explants were blotted dry using several layers of sterile Whatman No·1 filter paper and then placed on non-selective MS B0·5 N0·05 media [Murashige and Skoog medium, 0·5 mgL−1 of 6-benzyl amino purine (BAP) and 1-naphthyl acetic acid (NAA) at 0·05 mgL−1] in 9-cm Petri dishes with ten discs per plate. Non-co-cultivated discs were also prepared the same way to act as control material. Sealed plates were then placed in culture room at 18 °C and a photoperiod of 16 h light and 8 h dark.
After 2 d, explants were transferred on to selective MS BN plates with antibiotics (kanamycin, 100 mg mL−1) and claforan (500 mg mL−1) and incubated as above. The explants were transferred to fresh media every 2 weeks until calli and shoots developed. The transformed plants were cultivated in media with different concentrations of claforan to eliminate the presence of A. tumefaciens after the transformation and to promote callus growth and shoot regeneration. One callus/shoot was excised from each disc and placed on LS B0·5 C500 medium (Linsmaier and Skoog medium with 0·5 mg L−1 of BAP and 500 mg mL−1 claforan) in a 150-mL jar. When a dominant shoot from each clump was large enough, it was removed from the surrounding material and placed on LS C250 (Linsmaier and Skoog medium and 250 mg mL−1 claforan) media. After 7 d, the shoot top was further cleaned and placed on LS medium without claforan. The shoot base was retained in LS C250 for the growth of a second shoot, from which a copy of the transgenic plant was obtained.
Genomic PCR and RT-PCR analysis
Plant genomic DNA or total RNA was extracted from leaf tissues using DNeasy or RNeasy Plant Kits (Qiagen, Epsom, UK), respectively, according to the manufacturer's instructions. The concentration of the nucleic acids in solution was determined using a NanoDrop ND-1000 UV-Vis Spectrophotometer. RNA was also visualized on a 1 % (w/v) agarose gel. Two-to-three micrograms of total RNA was used to synthesize cDNA using the SuperScript II RNase H RT method (Invitrogen, Carlsbad, USA). One microlitre of cDNA was included in a 25 µL PCR reaction using Red Hot DNA polymerase (ABgene, Crawley, UK). The PCR programme used was: 94 °C for 50 s, followed by 35 cycles of 94 °C for 50 s; 60 °C for 45 s; 72 °C for 1 min; and a final elongation step at 72°C for 10 min. The sequences of the primers used were: 35SFor. 5′-AACTGCAGACTATCCTTCGCAAGAC-3′; ETRRev. 5′CAGCGACCACCTCCCCTAGC-3′; EIN3Rev. 5′AGCAGACACTTCCACTTCCTTCAG-3′. All PCR products were analysed using electrophoresis.
Chlorophyll determination of plant tissues
Frozen tissue samples were homogenized to a fine powder in 1·5 mL Eppendorf tubes containing liquid nitrogen and washed with 1 mL of ice-cold 80 % (v/v) acetone. Samples were vortexed for 15 s and then subjected to centrifugation (13 000 g, 10 min at 4 °C); the bleached leaf tissue collected at the bottom of each Eppendorf tube was then disturbed and the samples centrifuged again until the chlorophyll content of the tissue had dissolved completely in acetone. The chlorophyll concentration in the supernatant was assessed at wavelengths of 663 nm and 646 nm using a Cecil CE2041 spectrophotometer. The total chlorophyll concentration of the samples was calculated using the formula as described in Inskeep and Bloom (1985) and expressed as the weight of chlorophyll (μg) per unit fresh weight of the tissue (mg).
Leaf chlorophyll content was also estimated non-destructively using a hand-held automated chlorophyll SPAD meter. The readings were made by attaching the SPAD sensor at five randomly selected locations on individual leaves. The mean of these readings was calculated and measurements repeated for three other leaves of identical age for each sample.
Protein extraction
Soluble proteins were extracted by re-suspending the pellet retained from chlorophyll quantification in 50 mL of ice-cold fresh protein extraction buffer [98·79 mL protein buffer stock (60 mm Tris–HCl, pH 8·0, 500 mm NaCl and 10 mm EDTA), 10 mm β-mercaptoethanol and 0·1 mm phenylmethylsulfonyl fluoride]. A volume of 5 mL of the supernatant was removed after centrifugation (10 000 g, 10 min, 4 °C) and added to 995 mL of 20 % (v/v) Bradford's solution for protein determination using the BioRad Protein Assay Programme (BioRad Laboratories, Munchen, Germany).
Statistical analysis
Where appropriate, data were subject to an analysis of variance test (ANOVA) to determine statistical significance.
RESULTS
Generation of transgenic Nicotiana sylvestris lines expressing ETR1-1 or LeEIL1
To study the effect of ethylene sensitivity on leaf senescence, transgenic material was generated by transforming Nicotiana sylvestris with the mutant Arabidopsis ETR1-1 gene or the tomato EIN3-like gene (LeEIL1), each under the control of the cauliflower mosaic virus 35S promoter. Three independent transformations were performed which produced 63 transgenic N. sylvestris lines containing the LeEIL1 construct and 45 lines containing the ETR1-1 construct. The presence of the transgene was confirmed by PCR in putative transformants (data not shown).
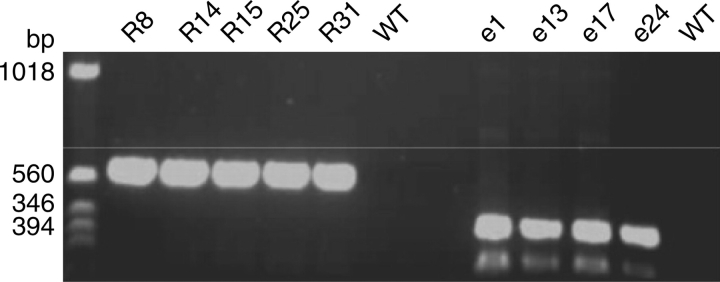
Expression of the transgene was determined by RT-PCR (see Fig. 1) using RNA extracted from leaf tissue, and five Pro35S:ETR1-1 (R8, R14, R15, R25, R31) and four Pro35S:LeEIL1 (e1, e13, e17, e24) lines were selected for further analysis and used to generate homozygous material. Each line exhibited a 3 : 1 ratio of antibiotic resistance when progeny were grown on kanamycin plates, indicating that they were generated by the insertion of the transgene at a single site in the genome. The negatively segregating lines were used as wild type (WT) controls in all subsequent experiments.
Fig. 1.
RT-PCR analysis of wild type (WT), Pro35S:ETR1-1 (R8, R14, R15, R25 and R31) and Pro35S:LeEIL1 (e1, e13, e17 and e 24) Nicotiana sylvestris lines. RNA was extracted from mature leaves and used to generate cDNA. Amplification was achieved using ETR1-1- (R8–WT) or LeEIL1- (e1–WT) specific primers.
Phenotypes of transgenic plants
Seeds from WT, and Pro35S:ETR1-1 or Pro35S:LeEIL1 transgenic lines were germinated on MS plates and the seedlings were transferred to soil after a week. No differences could be observed in either the onset or rate of germination between the transgenic lines and the WT. At 3 weeks after germination, Pro35S:ETR1-1 plants had significantly larger leaves than either WT or Pro35S:LeEIL1 lines. At this stage, the average length of leaf number 5 for Pro35S:ETR1-1 plants was 15·9 cm compared with 10·3 cm for WT (P < 0·01) and 9·8 cm for Pro35S:LeEIL1 (P < 0·01). There was no significant difference between the average length of WT and Pro35S:LeEIL1 leaves (data not shown).
At approximately 5 weeks after germination, some Pro35S:ETR1-1 lines showed a high susceptibility to collapse. The initial symptom was wilting of the leaf blades, with plant death occurring within a few days. WT and Pro35S:LeEIL1 plants grown under identical conditions did not exhibit such symptoms. The death rate was the highest in lines R25 and R14, with 65 % and 50 %, respectively, of plants grown exhibiting this phenotype. Mortality of immature plants within lines R8 and R31 plants was only 5 %. Some individual plants remained healthy throughout development in all Pro35S:ETR1-1 lines.
Visual time course of leaf senescence
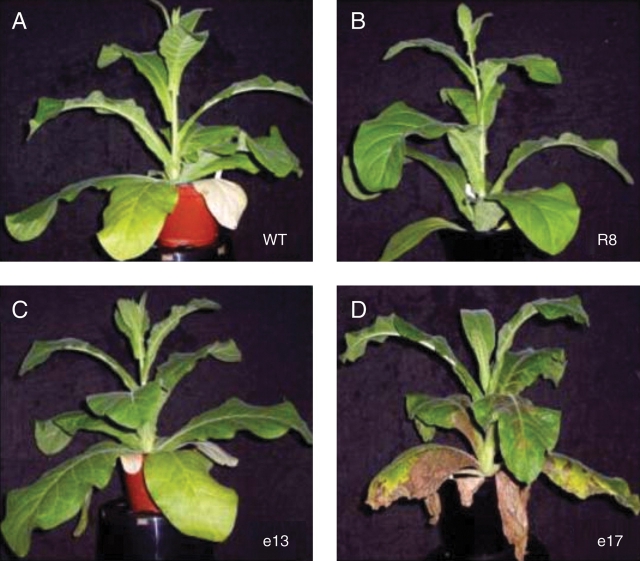
The time at which the first flower was produced was chosen as an index point to represent the age of the plant. The age of the plants at the time of sample collection was described as ‘days before’ or ‘days after’ flowering. A difference in the time-course of leaf senescence between the transgenic lines and WT of the same age could be seen at 10 d before flowering. At this time, yellowing of the lower leaves was apparent in both WT and Pro35S:LeEIL1 plants (see Fig. 2A–C), with leaves of line e17 showing extensive senescence and necrosis that had progressed to the higher leaves. In contrast, the lower leaves on plants from Pro35S:ETR1-1 line R8 remained green and healthy (Fig. 2D).
Fig. 2.
Development of Nicotiana sylvestris plants: wild type, WT (A); Pro35S:ETR1-1 R8 (B); and Pro35S:LeEIL1 e13 (C) and e17 (D). Plants were photographed at 10 d before flowering.
At 5 d before flowering leaf senescence was extensive in the lower leaves of WT and all the Pro35S:LeEIL1 plants, while leaves at a similar position in the Pro35S:ETR1-1 plants showed no visible yellowing (Fig. 3). At 1 d after flowering, the lower leaves on WT and Pro35S:LeEIL1 plants had become completely necrotic with leaf senescence progressing upwards to the intermediate part of the plant. In Pro35S:ETR1 plants, some yellowing of the lower leaves was apparent; however, neither wilting nor tissue necrosis was evident (Fig. 4A).
Fig. 3.
Development of Nicotiana sylvestris plants: wild type, WT (A); Pro35S:ETR1-1 R8 (B) and R31 (C); and Pro35S:LeEIL1 e1 (D) and e13 (E). Plants were photographed at 5 d before flowering.
Fig. 4.

Development of Nicotiana sylvestris plants: wild type (WT), Pro35S:LeEIL1 (e1), Pro35S:ETR1-1 (R8 and R31), at (A) 1 d and (B) 15 d after flowering.
At 15 d after flowering, all the lower leaves had become necrotic and most of the intermediate leaves on WT and Pro35S:LeEIL1 plants showed extensive senescence, which subsequently advanced to the uppermost leaves. In Pro35S:ETR1-1 plants, even those leaves at the lowest position, which had undergone almost total chlorophyll loss, retained their turgidity (Fig. 4B). At 70 d after flowering bleaching of Pro35S:ETR1-1 plants was complete; however, even at this stage leaves showed no visible signs of wilting (data not shown).
Only a single Pro35S:LeEIL1 line (e17) showed accelerated senescence in comparison with WT. This phenomenon was apparent at 10 d before flowering (Fig. 2), and continued to later stages of development. At 25 d after flowering, whilst the uppermost WT leaves remained green, e17 plants showed total necrosis (data not shown).
Quantification of leaf senescence
To quantify the time-course of leaf senescence, chlorophyll concentration was used as a measure of the photosynthetic capacity of intact and detached leaves. Chlorophyll concentration was measured non-destructively using a SPAD meter in successive leaves of the transgenic and WT lines during natural development and then quantified by acetone extraction. In addition, protein content was determined in the leaf samples.
The SPAD analysis of leaves from plants at 4 d after flowering revealed that the lower leaves (1–4) of Pro35S:ETR1-1 lines R8 and R31 retained a detectable amount of chlorophyll, whereas in Pro35S:LeEIL1 lines e1, e13 and WT, they had become completely necrotic. In leaves 5–7 a differential in chlorophyll concentration between Pro35S:ETR1-1 and Pro35S:LeEIL1/WT lines remained evident, although in Pro35S:ETR1-1 line R8 this disappeared by Leaf 9. In the upper leaves (10–13), chlorophyll concentration was comparable in all lines (see Supplementary Information, available online).
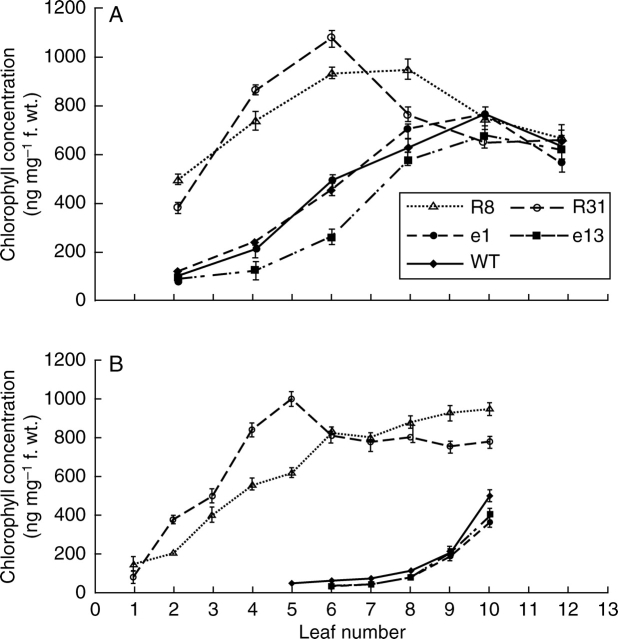
A similar pattern was observed in leaf chlorophyll concentrations measured by acetone extraction on the day of flowering and 15 d after flowering. At the onset of flowering, Pro35S:ETR1-1 lines R8 and R31 showed significantly higher chlorophyll concentration in the lower and intermediate leaves (2, 4 and 6) in comparison with Pro35S:LeEIL1 lines e1, e13, and WT (Fig. 5A). By 15 d after flowering, Pro35S:LeEIL1 lines e1, e13 and WT leaves had lost the majority of chlorophyll in all their leaves, with the level barely measurable in leaf numbers 5 and 6. In contrast, the chlorophyll concentration in leaves 7–10 in Pro35S:ETR1-1 lines R8 and R31 was substantially higher and both lines still retained a measurable concentration in the lowest leaves (Fig. 5B).
Fig. 5.
Leaf chlorophyll concentration in wild type(WT), Pro35S:LeEIL1 (e1 and e13) and Pro35S:ETR1-1 (R8 and R31) Nicotiana sylvestris plants at (A) the onset of flowering, and (B) 15 d after flowering. Chlorophyll was extracted in 80% (v/v) acetone and determined spectrophotometrically. Leaves were labelled such that leaf 1 is the oldest and leaf 12 the youngest. Values are means ± s.e. (n = 3).
Soluble protein concentrations measured at the day of flowering and 15 d after flowering showed a similar pattern to that of the chlorophyll analysis, indicating that a much higher amount of protein, compared to WT material, was retained in leaves from Pro35S:ETR1-1 lines R8 and R31 over the time-course of leaf development (Fig. 6A, B).
Fig. 6.
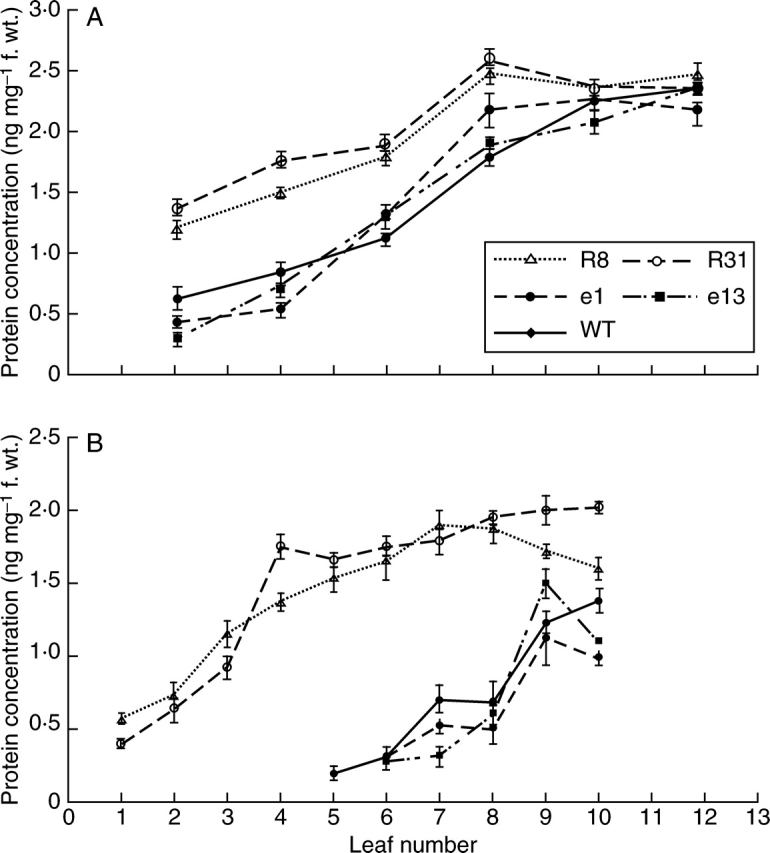
Leaf protein concentration in wild type (WT), Pro35S:LeEIL1 (e1 and e13) and Pro35S:ETR1-1 (R8 and R31) Nicotiana sylvestris plants at (A) the onset of flowering, and (B) 15 d after flowering. Leaves were labelled such that leaf 1 is the oldest and leaf 12 the youngest. Values are means ± s.e. (n = 3).
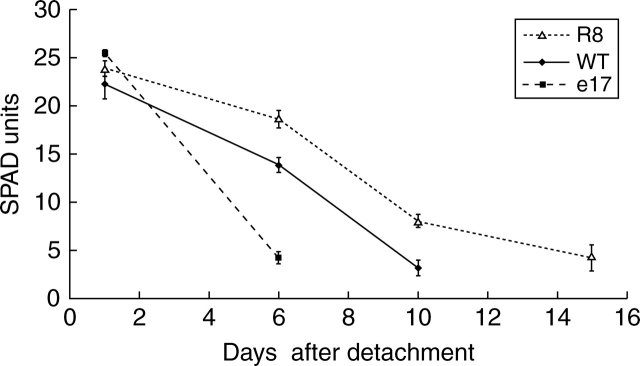
As the Pro35S:LeEIL1 line e17 exhibited extremely premature leaf senescence, a time-course of the process, compared to WT and Pro35S:ETR1-1 plants, was carried out using fully expanded detached leaves excised from plants 15 d before flowering. Figure 7 shows that within 6 d of excision chlorophyll levels, as determined by SPAD analysis, had fallen by 80 % in leaves of line e17 plants while those in WT plants had dropped by only half this amount. The decline of chlorophyll in detached leaves of Pro35S:ETR1-1 line R8 was less than 20 % over this period. By 10 d after excision over 80 % of the chlorophyll had been lost in WT plants, while this level of chlorophyll was not reached in R8 plants until 5 d later.
Fig. 7.
Time course of leaf chlorophyll concentration in detached leaves from wild type (WT), Pro35S:LeEIL1 (e17), and Pro35S:ETR1-1 (R8) Nicotiana sylvestris plants at 15 d before flowering. Chlorophyll in leaf 7 was measured using a SPAD meter. Values are means ± s.e. (n = 10).
Flowering and the time-course of flower senescence
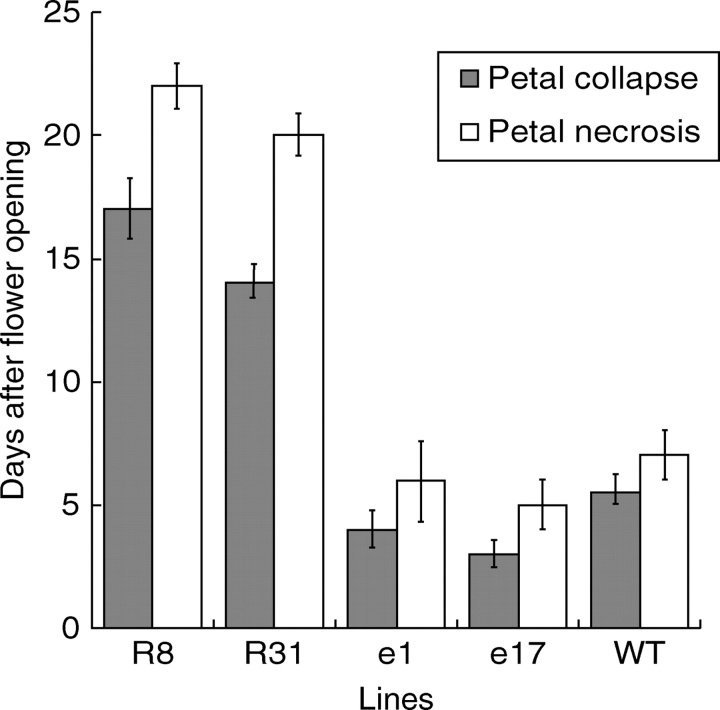
The onset of flowering was accelerated in Pro35S:ETR1-1 plants by approximately 1 week and delayed by approximately 1·5 weeks in Pro35S:LeEIL1 plants compared with the WT. A significant delay in floral senescence was also observed in the Pro35S:ETR1-1 plants in comparison with WT. In this study, floral senescence was characterized by two parameters that occurred during floral development in all lines. Firstly, petal collapse (PC), as the number of days after opening until the petal loses turgidity. Secondly, petal necrosis (PN), as the number of days after opening until necrosis of the petal tube was complete.
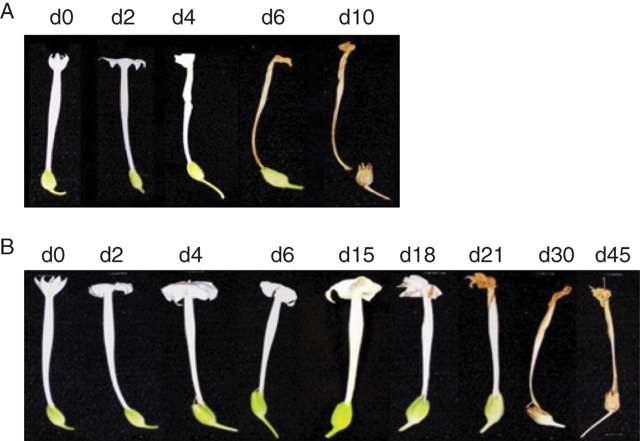
Within 4 d after opening (d4), petals from WT plants had lost turgidity and collapsed and by d6 the flowers had desiccated and become necrotic (Fig. 8A). In contrast, petals from the Pro35S:ETR1-1 line R8 remained fully turgid for up to 18 d after opening and did not become completely necrotic until 30 d after opening (Fig. 8B). Floral abscission was also abolished in the Pro35S:ETR1-1 plants, with the petal tube remaining firmly attached until it was split open by the growing ovary in the later stages of organ development. Other floral organs such as the style and the anther filaments did not abscise in Pro35S:ETR1-1 plants even after the ovary was completely dry and ready for seed dispersal (d45). In contrast, WT floral organ abscission occurred at 10 d after flower opening (d10). The time-course of petal collapse (PC) and petal necrosis (PN) was measured in flowers from two Pro35S:ETR1-1 lines (R8 and R31) and two Pro35S:LeEIL1 lines (e1 and e17) in comparison with WT. There was no significant difference between the timing of PN in the two Pro35S:LeEIL1 lines and WT, although PC was accelerated in e17. Both Pro35S:ETR1-1 lines (R8 and R31) showed a significant delay in PC and PN compared with WT (P < 0·01; Fig. 9).
Fig. 8.
Time course of floral development in (A) wild type and (B) Pro35S:ETR1-1 (R8) Nicotiana sylvestris plants. Day of opening designated as d0. Scale bar (d45) = 1·2 cm.
Fig. 9.
Time course of petal collapse and petal necrosis in wild type (WT), Pro35S:LeEIL1 (e1 and e17) and Pro35S:ETR1-1 (R8 and R31) Nicotiana sylvestris plants. Values are means ± s.e. (n = 15).
An additional phenotype related to the abscission process was observed in the Pro35S:LeEIL1 line e17, with numerous young flower buds being shed prematurely. This phenomenon resulted in only a few flowers from this line surviving to produce seeds.
DISCUSSION
The results presented in this study demonstrate that Nicotiana sylvestris plants expressing the dominant mutant ethylene receptor gene ETR1-1 from Arabidopsis exhibit a substantial delay in both the onset and progression of leaf and flower senescence. Whilst the chlorophyll and protein content of the oldest leaves on a WT plant declined substantially after flowering, the timing of this observation was significantly delayed in Pro35S:ETR1-1 N. sylvestris plants. In contrast, the expression of the tomato EIN3-like gene (LeEIL1) in N. sylvestris plants did not alter consistently the progression of senescence, although a single Pro35S:LeEIL1 line (e17) exhibited accelerated leaf senescence and petal collapse in addition to precocious flower-bud shedding. Another phenotypic characteristic that was observed in this study was the propensity for some Pro35S:ETR1-1 lines to exhibit extreme susceptibility to tissue collapse resulting in the rapid death of the entire plant.
Ethylene perception involves a family of receptors that trigger a signal transduction cascade (Bleecker and Schaller, 1996). The complexity of the system has been partially delineated by the discovery of dominant and recessive mutations that lock the plant in an ethylene-insensitive or constitutively responsive state. The ability of the mutant Arabidopsis ETR1-1 receptor gene to confer ethylene insensitivity in heterologous plant systems has been utilized by Wilkinson et al. (1997) and Knoester et al. (1998) to generate transgenic petunia, tomato and tobacco plants with a reduced capacity to respond to the gas. These plants showed a number of phenotypes associated with altered ethylene perception, including delayed petal senescence and floral organ abscission, non-ripening fruit and reduced resistance to pathogens. In the present study, N. sylvestris plants were transformed with the mutant Arabidopsis ETR1-1 gene driven by the viral 35S promoter to examine, in detail, the role of ethylene during natural leaf and flower senescence. In addition, transgenic plants, ectopically expressing the tomato EIN3-like gene (LeEIL1), were generated in an attempt to produce tobacco material exhibiting symptoms of constitutive ethylene responsiveness, as EIN3 has been shown to function as a positive regulator of ethylene responses throughout plant development (Tieman et al., 2001).
Four Pro35S:ETR1-1 and five Pro35S:LeEIL1 homozygous lines were selected for further analysis due to their high levels of expression of the respective transgenes. Over 50 % of mature plants from two of the Pro35S:ETR1-1 lines (R14 and R25) exhibited signs of spontaneous leaf wilt, tissue collapse and necrosis prior to, or immediately after, flowering. Although no disease analysis was carried out on this material, it has been well documented that elevated ethylene production is an early response by a plant to the perception of pathogen attack and is involved in the induction of defence reactions (Boller, 1991; Lund et al., 1998). Plants insensitive to the gas may thus become more susceptible to pathogen attack. Knoester et al. (1998) reported that Nicotiana tobaccum plants transformed with the Arabidopsis ETR1-1 gene showed a large number of premature plant deaths. The authors found that the transformed plants were impaired in their expression of basic pathogenesis-related (PR) defence genes, and subsequently became susceptible to non- or weakly pathogenic soil fungi. Interestingly, the Arabidopsis etr1-1 mutant has not been reported to show such symptoms.
Only two out of the four N. sylvestris lines studied exhibited this extreme behaviour even though the dominant nature of the mutant ETR1-1 gene should render all lines ethylene insensitive. In both previous studies with transgenic etr1-1 plants, lines with an intermediate sensitivity could be identified (Wilkinson et al., 1997; Knoester et al., 1998), indicating that the magnitude of phenotypic responses observed in the transgenic plants may be influenced by factors such as the positional effect of the transgene.
Due to the high incidence of the ‘sudden death’ response in Pro35S:ETR1-1 lines R14 and R25, the time-course of leaf senescence was studied in detail in lines R8 and R31. A delay in the onset of older-leaf yellowing, immediately prior to entering the reproductive phase, could be detected in plants from both lines prior to flowering and this became increasingly evident in the days after flowering had taken place. By 15 d after flowering the lower leaves of WT plants showed signs of severe collapse and necrosis; however, at this time, whilst bleaching of lamina at equivalent positions in both lines R8 and R31 had taken place, the tissues remained fully turgid. Indeed, in these Pro35S:ETR1-1 lines there were few signs of tissue collapse and browning even 70 d after flowering. These observations suggest that ethylene may have a dual role during leaf senescence, functioning both as a catalyst of the process and a regulator of events such as necrosis that take place downstream from its initiation.
The visual observations of differential leaf yellowing were confirmed using more analytical approaches. SPAD analysis revealed that a reduction in chlorophyll of the seven oldest leaves, in WT compared with Pro35S:ETR1-1 lines, was apparent within 4 d of flowering. This distinction could be identified at the onset of flowering if levels of extractable leaf chlorophyll were determined and by 15 d after flowering chlorophyll was barely detectable in these leaves from WT plants, whilst concentrations remained high in lines R8 and R31. These differences between WT and Pro35S:ETR1-1 lines were replicated if extractable protein contents were measured. These results demonstrate that N. sylvestris plants expressing the dominant mutant ethylene receptor gene ETR1-1 from Arabidopsis exhibit a substantial delay in both the onset and progression of leaf senescence.
Nicotiana sylvestris plants incorporating a construct for over-expressing the tomato EIN3-like gene (LeEIL1) were also generated in order to examine the possible effects of this transgene on development and to act as a comparison with the ethylene-insensitive lines. The effects of over-expressing the downstream components of the ethylene-response pathways have been infrequently examined. Although Bleecker et al. (1988) and Zacarias and Reid (1990) demonstrated that ethylene can accelerate the senescence of mature whole plants and mature rosette leaves, studies of mutants exhibiting a constitutively active ethylene response pathway have failed to detect an acceleration of leaf senescence compared with WT plants (Guzmán and Ecker, 1990; Kieber et al., 1993). In this investigation, only one of the five Pro35S:LeEIL1 homozygous lines studied (e17) showed a detectable difference in the timing of leaf and flower senescence compared with WT. In this line leaf senescence was markedly accelerated, with yellowing and necrosis of the oldest leaves being apparent even 10 d before flowering and petal collapse taking place on average over 2 d earlier than in WT plants. Accelerated chlorophyll loss in line e17 could also be detected in detached leaves compared with WT plants, with Pro35S:ETR1-1 lines exhibiting a delay in leaf yellowing under these conditions.
It has been proposed that ethylene may be an effective inducer of senescence only in tissues that have reached a particular developmental age (Grbic and Bleecker, 1995). Tieman et al. (2001) further suggested that the dependence of the ethylene response on the developmental stage of a tissue indicates a mechanism to regulate differential sensitivity to ethylene throughout development. It is possible that the e17 phenotype is the result of a positional effect of transgene insertion that brings about a dislocation between factors associated with developmental age and the ethylene signalling process, and that this culminates in an activation of the senescence pathway. However, the observation that e17 also exhibits other ethylene-associated phenotypes, such as the premature shedding of flower buds, suggests that it is more likely that the phenotype is the consequence of an enhancement in the ethylene response. The lack of a consistent phenotype in other LeEIL1 lines could therefore be the result of a failure in the transgenic protein to be expressed at sufficient levels to induce a response, perhaps as a result of co-suppression by the endogenous tobacco EIL-like genes. A quantitative study on the expression of the LeEIL1 protein in each line might help to resolve this issue.
The transgenic lines generated in this study may present an opportunity to further dissect the pathways that regulate specific aspects of senescence. For example, the striking phenotypic differences over the time-course of development between WT plants and Pro35S:ETR1-1 transgenic lines demonstrate that the necrosis response may be uncoupled from other leaf senescence syndromes. The delayed chlorophyll degradation may be attributed to ethylene insensitivity, but the lack of necrosis response may be the result of a perturbation in programmed cell death or the effect of interference in the action of other plant hormones, such as jasmonic and salicylic acids. In contrast to the delayed leaf senescence phenotype in Pro35S:ETR1-1 lines, this study has also demonstrated that the constitutive activation of the downstream ethylene response pathway by over-expressing the LeEIL1 transgene may lead to an accelerated leaf senescence and early tissue-necrosis phenotype. Mutants or transgenic lines that exhibit an accelerated leaf-senescence phenotype are uncommon due to the complex nature of the senescence process, which is likely to involve several redundant regulatory pathways. A detailed biochemical and molecular study of senescence in line e17 could prove extremely valuable in highlighting additional genes and pathways important for the process.
In conclusion, this study provides evidence to support the hypothesis that ethylene plays an important role in the timing of senescence processes in plants. Transgenic N. sylvestris plants expressing the Arabidopsis ETR1-1 gene, to bring about ethylene insensitivity, exhibit substantially delayed leaf and flower senescence and a cessation in floral organ abscission. Whilst only a single transgenic N. sylvestris line containing the ectopically expressed tomato EIN3-like gene displays a modified phenotype, this exhibits features consistent with enhanced ethylene sensitivity, including accelerated leaf and flower senescence and precocious flower-bud abscission. The transgenic lines generated in this study may provide a useful platform with which to further explore the regulation of senescence processes in plants.
SUPPLEMENTARY INFORMATION
Supplementary information is available online at http://aob.oxfordjournals.org/ and shows leaf chlorophyll concentration in wild type, Pro35S:LeEIL1 and Pro35S:ETR1-1 Nicotiana sylvestris plants at 4 d after flowering.
LITERATURE CITED
- Abeles FB, Morgan PW, Saltveit ME. Ethylene in plant biology. 2nd edn. New York: Academic Press, Inc; 1992. [Google Scholar]
- Alonso JM, Hirayama T, Roman G, Nourizadeh S, Ecker JR. EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis thaliana. Science. 1999;284:2148–2152. doi: 10.1126/science.284.5423.2148. [DOI] [PubMed] [Google Scholar]
- Alonso JM, Stepanova AN, Solano R, Wisman E, Ferrari S, Ausubel FM, Ecker JR. Five components of the ethylene-response pathway identified in a screen for weak ethylene-insensitive mutants in Arabidopsis. Proceedings of the National Academy of Sciences USA. 2003;100:2992–2997. doi: 10.1073/pnas.0438070100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleecker AB, Patterson SE. Last exit: senescence, abscission and meristem arrest in Arabidopsis. The Plant Cell. 1997;9:1169–1179. doi: 10.1105/tpc.9.7.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleecker AB, Schaller GE. The mechanism of ethylene perception in plants. Plant Physiology. 1996;111:653–660. doi: 10.1104/pp.111.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleecker AB, Estelle MA, Kende H. Insensitivity to ethylene conferred by a dominant mutation in Arabidopsis thaliana. Science. 1988;241:1086–1089. doi: 10.1126/science.241.4869.1086. [DOI] [PubMed] [Google Scholar]
- Boller T. Ethylene in pathogenesis and disease resistance. In: Mattoo AK, Suttle JC, editors. The plant hormone ethylene. Boca Raton, FL: CRC Press; 1991. pp. 293–314. [Google Scholar]
- Chao Q, Rothenberg M, Solano R, Roman G, Tezaghi W, Ecker JR. Activation of the ethylene gas response pathway in Arabidopsis by the nuclear protein ETHYLENE-INSENSITIVE3 and related proteins. Cell. 1997;89:1133–1144. doi: 10.1016/s0092-8674(00)80300-1. [DOI] [PubMed] [Google Scholar]
- Chen G, Alexander L, Grierson D. Constitutive expression of EIL-like transcription factor partially restores ripening in the ethylene-insensitive Nr tomato mutant. Journal of Experimental Botany. 2004;55:1491–1497. doi: 10.1093/jxb/erh168. [DOI] [PubMed] [Google Scholar]
- Ecker JR. The ethylene signal transduction pathway in plants. Science. 1995;268:667–675. doi: 10.1126/science.7732375. [DOI] [PubMed] [Google Scholar]
- Grbic V, Bleecker A. Ethylene regulates the timing of leaf senescence in Arabidopsis. The Plant Journal. 1995;8:595–602. [Google Scholar]
- Guzmán P, Ecker JR. Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. The Plant Cell. 1990;2:513–523. doi: 10.1105/tpc.2.6.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inskeep M, Bloom P. Chlorophyll quantitation by spectophotometry. Plant Physiology. 1985;77:483–485. doi: 10.1104/pp.77.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kende H, Zeevart JAD. The five ‘classical’ plant hormones. The Plant Cell. 1997;9:1197–1210. doi: 10.1105/tpc.9.7.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieber JJ. The ethylene response pathway in Arabidopsis. Annual Review of Plant Physiology and Plant Molecular Biology. 1997;48:277–296. doi: 10.1146/annurev.arplant.48.1.277. [DOI] [PubMed] [Google Scholar]
- Kieber JJ, Rothenberg M, Roman G, Feldmann KA, Ecker J R. CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the Raf-family of protein kinases. Cell. 1993;72:427–441. doi: 10.1016/0092-8674(93)90119-b. [DOI] [PubMed] [Google Scholar]
- Knoester M, van Loon LC, van den Heuvel J, Bol JF, Linthorst HJB. Ethylene-insensitive tobacco lacks non-host resistance to soil-born fungi. Proceedings of the National Academy of Sciences USA. 1998;95:1933–1937. doi: 10.1073/pnas.95.4.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanahan B, Yen HC, Giovannoni JJ, Klee HJ. The Never-ripe mutation blocks ethylene perception in tomato. The Plant Cell. 1994;6:521–530. doi: 10.1105/tpc.6.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund ST, Stall RE, Klee HJ. Ethylene regulates the susceptible response to pathogen infection in tomato. The Plant Cell. 1998;10:371–382. doi: 10.1105/tpc.10.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SA, Park J-H, Lee GI, Paek KH, Park SK, Nam HG. Identification of three genetic loci controlling leaf senescence in Arabidopsis thaliana. The Plant Journal. 1997;12:527–535. doi: 10.1046/j.1365-313x.1997.00527.x. [DOI] [PubMed] [Google Scholar]
- Osborne DJ. Ethylene formation, cell types and differentiation. In: Flores HE, Arteca RN, Shannon JC, editors. Polyamines and ethylene: biochemistry physiology, and interactions. Baltimore, MD: American Society of Plant Physiologists; 1990. pp. 203–215. [Google Scholar]
- Osborne DJ, McManus M. Hormones, signals and target cell in plant development. Cambridge: Cambridge University Press; 2005. [Google Scholar]
- Payton S, Fray RG, Brown S, Grierson D. Ethylene receptor expression is regulated during fruit ripening, flower senescence and abscission. Plant Molecular Biology. 1996;31:1227–1231. doi: 10.1007/BF00040839. [DOI] [PubMed] [Google Scholar]
- Smart CM. Gene expression during leaf senescence. New Phytologist. 1994;126:419–448. doi: 10.1111/j.1469-8137.1994.tb04243.x. [DOI] [PubMed] [Google Scholar]
- Solano R, Stepanova A, Chao Q, Ecker JR. Nuclear events in ethylene signaling: a transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE-RESPONSE-FACTOR 1. Genes and Development. 1998;12:3703–3714. doi: 10.1101/gad.12.23.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieman DM, Ciardi JA, Taylor MG, Klee HJ. Members of the tomato LeEIL gene family are functionally redundant and regulate ethylene responses throughout plant development. The Plant Journal. 2001;26:47–58. doi: 10.1046/j.1365-313x.2001.01006.x. [DOI] [PubMed] [Google Scholar]
- Wilkinson JQ, Lanahan MB, Clark DG, Bleeker AB, Chang C. A dominant mutant receptor from Arabidopsis confers ethylene insensitivity in heterologous plants. Nature Biotechnology. 1997;15:444–447. doi: 10.1038/nbt0597-444. [DOI] [PubMed] [Google Scholar]
- Zacarias L, Reid MS. The role of growth regulators in the senescence of Arabidopsis thaliana leaves. Physiologia Plantarum. 1990;80:549–554. [Google Scholar]