Abstract
Background
Vascular continuity is established between a host plant and the root parasite broomrape. It is generally accepted that the direction of vascular continuity results from polar flow of auxin. Our hypothesis was that chemical disruptions of auxin transport and activity could influence the infection of the host by the parasite.
Methods
A sterile system for the routine infection of Arabidopsis thaliana seedlings in Nunc cell culture plates by germinated seeds of Orobanche aegyptiaca was developed. This method permitted a quantitative assay of the rate of host infection. The three-dimensional structure of the vascular contacts was followed in cleared tissue. IAA (indole acetic acid) or substances that influence its activity and transport were applied locally to the host root.
Results
The orientation of the xylem contacts showed that broomrape grafts itself upon the host by acting hormonally as a root rather than a shoot. Local applications of IAA, PCIB (p-chlorophenoxyisobutyric acid) or NPA (naphthylphthalamic acid) all resulted in drastic reductions of Orobanche infection
Conclusions
Broomrape manipulates the host by acting as a sink for auxin. Disruption of auxin action or auxin flow at the contact site could be a novel basis for controlling infection by Orobanche.
Key words: Orobanche aegyptiaca, Arabidopsis thaliana, p-chlorophenoxyisobutyric acid, hormonal flow, host infection, naphthylphthalamic acid, parasitic plants, polarity, vascular connections, xylem
INTRODUCTION
Many of the general features of host infection by parasitic plants, such as broomrape, are well known today, including aspects of parasitic seed germination, attachment to the host and events during the initial penetration of the host by the parasite (Mayer, 2006). A basic understanding of the host–parasite interaction is, however, still lacking. Although the infection of Arabidopsis thaliana by Orobanche sp. has been described (Goldwasser et al., 2002), a simple convenient and quantitative assay of the degree of infection has not been available. One of the obvious features that have not received sufficient attention is the fusion of the vascular systems of host and parasite. Some of the anatomical features of this contact have been described in detail by Dörr and Kollmann (1976, 1995) and Dörr (1996), who clearly showed the connection and plasmatic continuity between host and parasite. Since the localization and orientation of vascular tissues is determined by IAA (indole-3-acetic acid) flow (Sachs, 1981; Berleth and Sachs, 2001; Reinhardt et al., 2003; Scarpella et al., 2006), it could be supposed that IAA flow between the host and the parasite has a role in the infection process. This aspect of the relation between Orobanche and its host has not been studied.
The following reports on an investigation into whether auxin relationships play a role in the precise localization of vascular contacts during the infection of Arabidopsis thaliana plantlets by O. aegyptiaca seedlings. The polarity of the contacts between host and parasite, and the effect of disturbing auxin relationships are described. For this purpose a system was developed in which seedlings of arabidopsis could be routinely infected under sterile conditions and the degree of infection could be quantified.
MATERIALS AND METHODS
Seeds of Orobanche aegyptiaca collected in 2001 from fields near Kfar Yehoshua and Arabidopsis thaliana, ecotype Columbia, were used throughout.
Orobanche seeds were surface-sterilized with 70 % ethanol for 1 min, rinsed three times with deionized water, and then further sterilized with 5 % NaOCl for 5 min and again rinsed three times with sterile deionized water. For conditioning (Bar Nun and Mayer, 1993) the seeds were placed for 6 d at 23 °C on GF/A (Whatman) microfibre filter discs in 5-cm Petri dishes in the dark. All germination treatments were conducted under aseptic conditions. The conditioned seeds were transferred to a 5 ppm solution of the germination stimulant, GR 24 (obtained from Professor B. Zwaneburg, University of Nijmegen) an analogue of strigol (Johnson et al., 1981; Humphrey and Beale, 2006). In the absence of this stimulant, germination was negligible. Germination was examined under a Stereoscopic light microscope.
The Arabidopsis thaliana seeds were sterilized with 70 % ethanol for 1 min, rinsed three times with sterile deionized water, further sterilized with 5 % NaOCl for 5 min and again rinsed three times. The imbibing seeds were chilled for 3 d at 4 °C to improve germination. The seeds were germinated in 9-cm Petri dishes, 0·8 % plant agar (Duchefa Biochemie, Haarlem, The Netherlands) containing half-strength Hoagland nutrient solution. The Petri dishes were placed in a vertical position for 1 week at 23 °C in continuous fluorescent light (3000 lux; approx. 400 µmol m−2 s−1). Germination was 100 %. The arabidopsis seedlings were transferred to sterile Nunc 12-well cell culture plates, containing 0·8 % agar with half-strength Hoagland. At this stage, 40–60 seedlings of Orobanche, 2–3 d old, were spread along the arabidopsis roots.
IAA was applied locally to the arabidopsis roots. The procedure of Pilet and Meuwly (1986) was followed, using Dowex beads loaded with IAA. Twenty milligrams of Dowex 1 × 8 resin beads were slowly stirred in 10 mL solutions of various concentrations of IAA at pH 5·5 for 1 h. The IAA was dissolved in a few drops of ethanol and then diluted with sterile deionized water, as required. Beads stirred only with deionized water were used as a control. Beads with a diameter of 0·3–0·45 mm were then selected for the experiments. From the data of Pilet and Meuwly (1986), the amount of IAA could be calculated to be about 0·17 ng bead−1, equivalent to 0·9 pmole bead−1, when the IAA concentration was 10–4 M. One or two beads loaded with IAA were placed singly on the root, using fine tweezers under the dissecting microscope, about 5 mm from the tip of the root. To test the efficacy of the beads as a source of physiologically activity of auxin, a single bead to the side of vertically oriented primary roots of maize was applied. During the subsequent 24 h, asymmetric inhibition of elongation by IAA from the bead (0·17 ng bead−1) resulted in curvature towards the side were the bead was attached. Beads without IAA did not cause any curvature.
The plates with the arabidopsis and Orobanche plants were examined daily under the stereoscopic microscope. Attachments of the Orobanche to arabidopsis roots and tubercle formation were scored for periods up to 25 d. All operations were carried out under aseptic conditions.
Inhibitors of IAA activity, PCIB (p-chlorophenoxyisobutyric acid), an inhibitor of polar IAA transport, and NPA (naphthylphthalamic acid) were used to disrupt auxin relationships. PCIB, obtained from MP Biochemicals LLS, Aurora, OH, USA, was dissolved in a few drops of ethanol and then diluted with water as required. A small amount, 20 µL, of a 15 µm solution of PCIB was placed in contact with the arabidopsis roots, about 0·5 cm from the root tip. NPA, obtained from Chem Service, West Chester, PA, USA, was dissolved in a drop of DMSO (dimethyl sulfoxide) and then diluted with water as required. Twenty microlitres of a 50 µm or 100 µm solution were applied, as with the PCIB. In all cases, controls were used in which the drops of solvent were diluted with water.
The three-dimensional anatomy of the broomrape-host root interaction was examined in cleared material. Pieces of root, to which Orobanche was attached, were placed in ethanol overnight and then transferred for at least a few hours into 90 % lactic acid, before microscopic observation with transmitted light. The material could be kept in the lactic acid for longer periods.
RESULTS
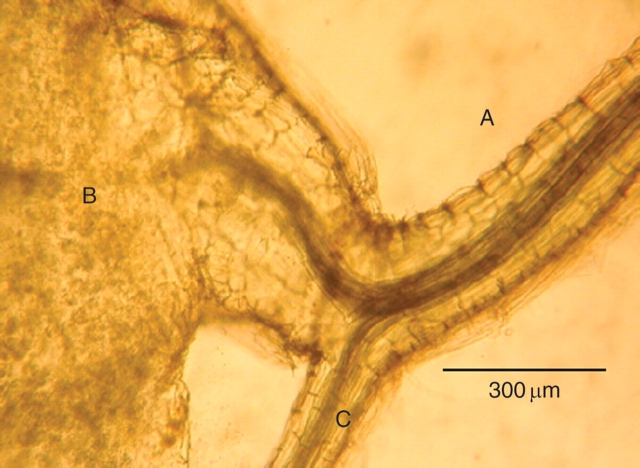
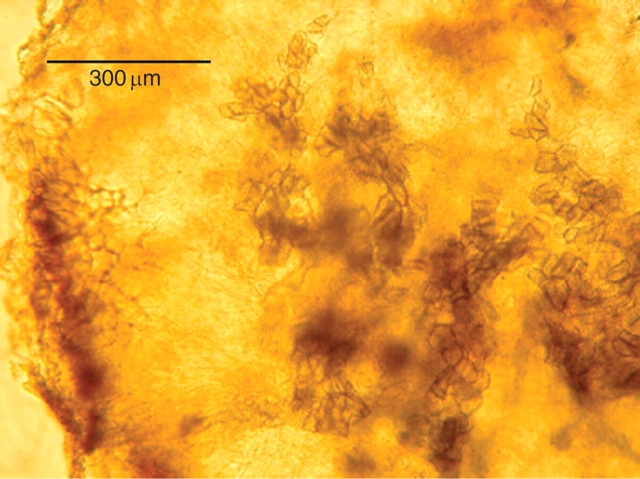
Orobanche tubercles were invariably connected to the host by continuous xylem vessels. The orientation of these vessels, which is indicative of the way their differentiation was induced (Sachs, 1981), was such that they formed direct contacts between parasite and host shoot (Fig. 1). The vascular strands of the xylem emerging from the tubercle of the parasite (Fig. 1, B) turn upwards and are fused with those of the host root (Fig. 1, A) in the direction of the shoot. No such strands are observed towards the host root (Fig. 1, C) in the direction of the root apex. The host root below the infection zone was much thinner (Fig. 1, C) than that above it (Fig. 1, A). With regards to its effect on xylem differentiation, the parasite thus interacts with the host as if it were a root rather than a shoot. In somewhat later stages, when the tubercle had started to form lateral roots, it resembled an unorganized, callus like structure, with scattered relatively unorganized xylem elements (Fig. 2). This structure resembled a callus rich in auxin formed near the roots of wounded tissue, in which callus formation has been induced (Sachs, 1991).
Fig. 1.
Xylem connections between the tubercle of Orobanche and the host root, showing the xylem connection oriented towards the host shoot and the reduced size of the host root past the parasite. A, Part of host root above attachment of the parasite in the direction of the shoot; B, tubercle; C, part of host root below attachment of the parasite, in the direction of the root apex. Note: the vascular strands leading from the tubercle (B) curve upwards to the host root (A) in the direction of the shoot apex. The root below the fusion zone (C) is thinner and no vascular strands join it to the root in the direction of the root apex.
Fig. 2.
Organization of tubercle tissue, showing callus like structure with cell proliferation and scattered partially organized vessels. The outer border of the tubercle is on the left and the vessels are common in the centre and right side of the figure.
An attempt was made to disrupt IAA relationships at the site of infection. The effect of the localized application of IAA, using Dowex beads is shown in Table 1. This added IAA reduced the infection of the host roots. The reduction of infection was dependent on the IAA concentration applied, the effect being dramatic at the highest concentration, 10−3 m IAA. Visual observation of the arabidopsis roots themselves indicated that there was no appreciable inhibition of elongation at the IAA concentrations used. It was impossible to measure their length because the roots curled and penetrated into the agar layer. In order to assay the effect of application of the IAA on root growth a separate experiment was set up, using arabidopsis seedlings grown in Petri dishes and placed vertically to avoid root penetration into the agar. IAA-containing beads were applied. Three days after application of the IAA the root lengths were, in cm ± s.d.: control, 1·71 ± 0·4; 10−4 m IAA 1·66 ± 0·37; 10−3 m IAA, 1·58 ± 0·41. This confirmed that the effect of IAA on root growth was very small, but detectable. The expected enhancement of lateral root initiation by IAA, reported also for arabidopsis (Reed et al., 1998), was observed in the regions between Dowex beads in some plants. PCIB is an antagonist and competitive inhibitor of IAA (MacRae and Bonner, 1953; Oono et al., 2003). Therefore, by applying the inhibitor to Arabidopsis roots, the effect of PCIB on the infection of Arabidopsis by Orobanche was tested (Table 1). PCIB at the concentrations used had no effect on the germination of Orobanche or the development of the host. Yet application of PCIB almost completely inhibited infection of the host.
Table 1.
Effects of application of IAA, PCIB and NPA on the infection of Arabidopsis thaliana roots by Orobanche aegptiaca
| % Host roots infected |
||
|---|---|---|
| Length of treatment (d) | 13 | 25 |
| Control (beads – IAA) | 75 ± 2·0 | 90 ± 3·1 |
| 5 × 10−5m IAA | 62 ± 4 | 79 ± 2·6 |
| 10−4m IAA | 49 ± 3·2 | 57 ± 3·5 |
| 10−3m IAA | 2·8 ± 0·6 | 7 ± 1·2 |
| 15 µm PCIB | 0 | 11 ± 2·1 |
| 50 µm NPA | 11 ± 0·6 | 16 ± 1·4 |
| 100 µm NPA | 4 ± 0 | 12 ± 1·7 |
IAA was applied in Dowex beads and PCIB and NPA were applied locally in solution. Percentage infection was calculated from the number of plants infected from a total of 24 plants. The results are given as the mean of three replicates with s.d.
Similar experiments were carried out using NPA, which is a specific inhibitor of IAA transport (Morris et al., 2004) and whose effect has been studied in arabidopsis (Mattson et al., 1999; Poupart et al., 2005). The localized application of NPA also reduced infection of the host by Orobanche drastically (Table 1). The concentrations of NPA used did not prevent development of the host plants or affect germination and growth of Orobanche.
DISCUSSION
A higher plant parasite must attach itself to the host plant, forming a graft union so as to obtain all the necessary substrates. This graft requires the differentiation of continuous vascular tissues between the host and the parasite. In the case of Orobanche the joining of both the phloem and the xylem of host and parasite has already been demonstrated (Dörr and Kollmann, 1995; Dörr, 1996). The establishment of this continuity requires a transfer of information that co-ordinates the precise location of the vascular differentiation processes in the two plants. This can be expected to depend on the same mechanisms that transfer information within an individual plant (Sachs, 1991; Berleth and Sachs, 2001). The subject of information transfer has not been studied in relation to parasitic infection and it is here that the present work suggests an important conclusion.
Both very old and more recent information about the process of grafting shows that tissue polarity plays a key role in orienting the precise joining of vascular contacts (Vöchting, 1892; Sachs, 1981, 1991) Grafting can occur between two organs, and one of them must have the role of a shoot, acting as an auxin source, and the other the role of a root, which acts as an auxin sink. The difference between these two roles is indicated by the orientation of the vascular contacts: shoots connect to roots while direct contacts between similar organs do not occur (Sachs, 1981). The results shown here are very clear (Fig. 1). Orobanche assumes the role of a root, orienting vascular tissues from the host shoot into itself. Further evidence for this conclusion is the influence of Orobanche on host root development. Action as a root should divert auxin and, presumably, other signals and substrates, inhibiting root development, an inhibition which is readily seen (Fig. 1). If Orobanche were to act as a shoot, on the other hand, auxin would be expected to induce root proliferation in the host, which does not occur.
Vascular continuity depends on auxin flow rather than auxin concentration, which, not being a vector, could not specify directional, polar relations (Sachs, 1981, 1991). The inductive flow follows and maintains the predetermined polarity of the tissue (Sachs, 1981). Formation of vascular tissue in arabidopsis is in agreement with this model of the effect of IAA (Turner and Sieburth, 2003). Therefore in the interaction between arabidopsis and Orobanche it appears that auxin moves from the host's shoot to the developing parasite and xylem formation follows this polar flow of IAA. This is borne out by the observations on the xylem structure in the fusion zone and the fact that xylem continuity is between the tubercle and the host root in the direction of the shoot (Fig. 1). This could account for the present results (Table 1), showing that varied perturbations of auxin relationships prevent parasite development (Table 1). Local sources of auxin can be expected to disrupt the influence of the parasite as a sink for auxin flow. PCIB presumably reduced responses to auxin within the parasite (MacRae and Bonner, 1953; Oono et al., 2003), while NPA disrupted its canalized transport (Mattsson, 1999) within both the host and the parasite.
Root formation, indicative of high auxin concentration, does occur on Orobanche itself, but this occurs after its successful establishment, suggesting a change in its hormonal relations with time. At this later stage of tubercle development the relatively unorganized vessels of the developing parasite also resemble those of a callus in which auxin concentration is high (Fig. 2). This can also be seen in the illustration of the interaction between Orobanche ramosa callus and tomato described by Zhou et al. (2004). The tubercle formed after the Orobanche root has established a connection with the vascular system of the host, in the early stages of infection (Fig. 1), is characterized by extensive internal cell proliferation and disorganized vascular differentiation. Such relatively chaotic development is typical of auxin rich tissue (Sachs, 1991), thus supporting the idea that the parasite is acting as a root-like sink for auxin originating in the host.
The results described here are in accordance with the rather meager information available on the auxin relationships of Orobanche. Slavov et al. (2004) showed that IAA is formed by the germinating seeds of O. ramosa and O. cumana (although at very different levels in the two species). The possible importance of IAA in callus formation by O. ramosa was shown by Batchvarova et al. (1999). Zhou et al. (2004) have also shown that in calli of Orobanche the formation of root-like protrusions are induced by added IAA or combinations of NAA and kinetin, but they did not consider the polarity of auxin flow.
These results could be expected to be relevant and raise questions about other host–parasite relationships. There are, however, very few indications from other plants. Shoot-like activity is also expected of hemiparasites, which take advantage of host roots. In the facultative hemiparasite Triphysaria (Scrophulariaceae) auxin concentration, rather than auxin flow, appeared to be critical for attachment (Tomilov et al., 2004, 2005). These authors showed that haustorial formation by Triphysaria versicolor is associated with the accumulation of IAA and that this IAA effect could be reversibly inhibited by inhibitors of IAA transport such as TIBA or by PCIB.
The present results show that in Orobanche any change in the transport of IAA, caused either by the local application of the auxin itself or disturbance responses to IAA by PCIB or of its polar transport by NPA prevented host infection. It seems therefore that auxin flow from the host to the parasite has an essential role in establishing the linkage between the vascular systems of the host and the parasite, which is essential for successful parasitism of Orobanche on Arabibidopsis.
Parasitic plants and especially Striga and Orobanche pose a severe threat to world agriculture and cause severe losses especially in tropical and sub-tropical regions. No satisfactory way of controlling infection of agricultural crops by broomrape exists at present, and new methods of control are constantly sought. The observation reported here that disruption of the hormonal relationships between host and parasite can prevent infection could open up a new approach to combat infections caused by this parasitic weed.
ACKNOWLEDGEMENT
Sadly Professor T. Sachs did not see the final version of this paper, to which he made such a major contribution.
LITERATURE CITED
- Bar Nun N, Mayer AM. Preconditioning and germination of Orobanche seeds: respiration and protein synthesis. Phytochemistry. 1993;34:39–45. [Google Scholar]
- Batchvarova RB, Slavov SB, Bossolova SN. In vitro culture of Orobanche ramosa. Weed Research. 1999;39:191–197. [Google Scholar]
- Berleth T, Sachs T. Plant morphogenesis: long distance coordination and local patterning. Current Opinion in Plant Biology. 2001;4:57–62. doi: 10.1016/s1369-5266(00)00136-9. [DOI] [PubMed] [Google Scholar]
- Dörr I. New results on interspecific bridges between parasites and their hosts. In: Moreno T, Cubero JI, editors. Advances in parasitic plant research. 1996. pp. 196–201. Congreos y Jornadas, 36/96, Junta de Andalucia, Consejeria de Agricultura y Pessca. [Google Scholar]
- Dörr I, Kollmann R. Strukturelle Grundlagen des Parasitismus bei Orobanche. III. Die Differenzierung des Xylemanschlusses bei. O. crenata. Protoplasma. 1976;89:233–249. [Google Scholar]
- Dörr I, Kollman R. Symplastic sieve element continuity between Orobanche and its host. Botanica Acta. 1995;108:47–55. [Google Scholar]
- Goldwasser Y, Westwood JH, Yoder JI. The use of Arabidopsis to study interactions between parasitic angiosperms and their host plants. In: Somerville CR, Meyerrowitz EM, editors. The arabidopsis book. Rockville, MD: American Society of Plant Biologists; 2002. doi:10.1199/tab.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey AJ, Beale MH. Strigol: biogenesis and physiological activity. Phytochemistry. 2006;67:636–640. doi: 10.1016/j.phytochem.2005.12.026. [DOI] [PubMed] [Google Scholar]
- Johnson AW, Gowda G, Hassanali A, Knox J, Monaco S, Razawi Z. The preparation of synthetic analogues of strigol. Journal of the Chemical Society Perkins Transactions I. 1981;6:1734–1743. [Google Scholar]
- MacRae DH, Bonner J. Chemical structure and antiauxin activity. Physiologia Plantarum. 1953;6:485–510. [Google Scholar]
- Mattsson J, Sung ZR, Berleth T. Response of plant vascular systems to auxin transport inhibition. Development. 1999;126:2979–2991. doi: 10.1242/dev.126.13.2979. [DOI] [PubMed] [Google Scholar]
- Mayer AM. Pathogenesis by fungi and by parasitic plants: similarities and differences. Phytoparasitica. 2006;34:3–16. [Google Scholar]
- Morris DA, Friml J, Zazimalova E. The functioning of hormones in plant growth and development. E.1 The transport of auxins. In: Davies PJ, editor. Plant hormones, biosynthesis, signal transduction, action! Dordrecht: Kluwer Academic publishers; 2004. pp. 437–470. [Google Scholar]
- Oono Y, Ooura C, Rahman A, Aspuria ET, Hayashi K-I, Tanaka A, et al. p-Chlorophenoxyisobutyric acid impaired auxin response in Arabidopsis root. Plant Physiology. 2003;133:1135–1147. doi: 10.1104/pp.103.027847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilet P-E, Meuwly P. Local application of indole-3-acetic acid, by resin beads to intact maize roots. Planta. 1986;169:16–22. doi: 10.1007/BF01369770. [DOI] [PubMed] [Google Scholar]
- Poupart J, Rashotte AM, Muday GK, Waddell CS. The rib1 mutant of Arabodopsis has alterations in indole-3-butyric acid transport, hypocotyls elongation and root architecture. Plant Physiology. 2005;139:1460–1471. doi: 10.1104/pp.105.067967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed RC, Brady SR, Muday GK. Inhibition of auxin movement from the shoot into the root inhibits lateral root development in Arabidopsis. Plant Physiology. 1998;118:1369–1378. doi: 10.1104/pp.118.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt D, Pesce ER, Stieger P, Mandel T, Baltensperger K, Bennett M, et al. Regulation of phyllotaxis by polar auxin transport. Nature. 2003;426:255–260. doi: 10.1038/nature02081. [DOI] [PubMed] [Google Scholar]
- Sachs T. The control of patterned differentiation of vascular tissues. Advances in Botanical Research. 1981;9:151–262. [Google Scholar]
- Sachs T. Pattern formation in plant tissues. Cambridge: Cambridge University Press; 1991. [Google Scholar]
- Scarpella E, Marcos D, Friml J, Berleth T. Control of leaf vascular patterning by polar auxin transport. Genes and Development. 2006;20:2015–1027. doi: 10.1101/gad.1402406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavov S, van Onckelen H, Batchvarova R, Atanassov A, Prinsen E. IAA production during germination of Orobanche spp. seeds. Journal of Plant Physiology. 2004;161:847–853. doi: 10.1016/j.jplph.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Tomilov A, Tomilova N, Yoder JI. In vitro haustorium formation in roots and root cultures of the hemiparasitic plant Triphysaria versicolor. Plant Cell and Organ Culture. 2004;77:257–265. [Google Scholar]
- Tomilov AA, Tomilova NB, Abdallah I, Yoder JI. Localized hormone fluxes and early haustorium formation development in the hemiparasitic plant. Triphysaria versicolor. Plant Physiology. 2005;138:1469–1480. doi: 10.1104/pp.104.057836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner S, Sieburth LE. Vascular patterning. In: Somerville CR, Meyerrowitz EM, editors. The arabidopsis book. Rockville, MD: American Society of Plant Biologists; 2003. doi:10.1199/tab.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vöchting H. Über Transplantation am Pflanzenkörper. Tübingen: Verlag H.Laupp'schen Buchhandlung; 1892. [Google Scholar]
- Zhou WJ, Yoneyama K, Takeuchi Y, Rungmekarat S, Chae SH, Sato D, et al. In vitro infection of host roots by differentiated calli of the parasitic plant. Orobanche. Journal of Experimental Botany. 2004;55:899–907. doi: 10.1093/jxb/erh098. [DOI] [PubMed] [Google Scholar]