Abstract
Background and Aims
The small leafy succulent shrub Halocnemum strobilaceum occurs in saline habitats from northern Africa and Mediterranean Europe to western Asia, and it is a dominant species in salt deserts such as those of north-west China. The effects of temperature, light/darkness and NaCl salinity were tested on seed germination, and the effects of salinity were tested on seed germination recovery, radicle growth and radicle elongation recovery, using seeds from north-west China; the results were compared with those previously reported on this species from ‘salt steppes’ in the Mediterranean region of Spain.
Methods
Seed germination was tested over a range of temperatures in light and in darkness and over a range of salinities at 25 °C in the light. Seeds that did not germinate in the NaCl solutions were tested for germination in deionized water. Seeds from which radicles had barely emerged in deionized water were transferred to NaCl solutions for 10 d and then back to deionized water for 10 d to test for radicle growth and recovery.
Key Results
Seeds germinated to higher percentages in light than in darkness and at high than at low temperatures. Germination percentages decreased with an increase in salinity from 0·1 to 0·75 m NaCl. Seeds that did not germinate in NaCl solutions did so after transfer to deionized water. Radicle elongation was increased by low salinity, and then it decreased with an increase in salinity, being completely inhibited by ≥2·0 m NaCl. Elongation of radicles from salt solutions <3·0 m resumed after seedlings were transferred to deionized water.
Conclusions
The seed and early seedling growth stages of the life cycle of H. strobilaceum are very salt tolerant, and their physiological responses differ somewhat between the Mediterranean ‘salt steppe’ of Spain and the inland cold salt desert of north-west China.
Key words: Halocnemum strobilaceum, halophyte, inland salt desert, radicle growth, radicle growth recovery, seed germination, seed germination recovery
INTRODUCTION
Plants can be divided into two broad groups with respect to salt tolerance: halophytes (salt tolerant) and glycophytes (salt intolerant). Halophytes can grow and complete their life cycle in saline environments, whereas glycophytes cannot (Ungar, 1991). The responses of seeds of halophytes to salt differ from those of non-halophytes. Thus, compared with glycophytes, seeds of halophytes germinate at higher salinities, and they maintain viability even under extreme salinity or osmotic stress, recovering and germinating when the water potential of the medium increases (Ungar, 1995).
Germination of halophytes in the field is controlled by several environmental factors, in particular light (Gutterman, 1993; Huang and Gutterman, 1998, 1999a), temperature (Badger and Ungar, 1989) and salinity (Ungar, 1995; Khan et al., 2002). A survey of the light and temperature requirements for germination of 91 halophytes of salt marshes and salt deserts included information on the light/dark requirements for germination of 23 species and on the optimum temperature requirements for germination of all 91 species. Seeds of four species required light for germination, four germinated to higher percentages in light than in dark, 13 germinated equally well in light and dark, and two germinated to higher percentages in dark than in light. The optimum temperature for germination ranged from 5 to 35/25 °C, and the mean optimum was about 21 °C (Baskin and Baskin, 1998).
Halophyte species vary in their tolerance to salinity during seed germination (Ungar, 1995; Khan et al., 2002). Seeds of halophytes can recover the capacity to germinate after exposure to salt stress that inhibits their germination (Woodell, 1985). Further, following pre-treatment of seeds of halophytes with salt solutions, the rate and percentage of germination often increase after they are transferred to fresh water (Katembe et al., 1998). Germination of Limonium stocksii (Plumbaginaceae) seeds was only 5 % at 0·5 m NaCl, but ungerminated seeds germinated to nearly 100 % after they were transferred to distilled water (Zia and Khan, 2004). Osmotic pre-treatment significantly stimulated germination recovery of seeds of two Atriplex species (Chenopodiaceae), and their germination was 90 % 2 d after transfer to distilled water (Katembe et al., 1998). However, whereas germination of Halogeton glomeratus (Chenopodiaceae) seeds was 45–85 % in fresh water after they were soaked in low salinity solutions, it was only 0–8 % for seeds given a high salinity pre-treatment (Khan et al., 2001).
Halocnemum strobilaceum (Chenopodiaceae) occurs in saline habitats from northern Africa and Mediterranean Europe to western Asia (Liu, 1985). In China, this species is distributed mainly in the north-west provinces of Xinjiang and Gansu. It is an ecologically important semi-shrub in saline soils on the plain in front of the south slopes of Tianshan Mountain, Xinjiang. In this area, H. strobilaceum is one of the dominant or co-dominant halophytic species in saline lowlands and on the shores of salt lakes, margins of alluvial fans and salt crusts of fluvial plains (Liu, 1985). In highly saline patches or on shores of salt lakes, H. strobilaceum often forms monodominant communities or co-occurs with Kalidium foliatum (Chenopodiaceae) and Salicornia europaea (Chenopodiaceae). In slightly saline habitats, it is commonly found in association with other species such as Halostachys caspica (Chenopodiaceae), Tamarix hispida, T. ramosissima (Tamaricaceae), Atriplex verrucifera and Lycium ruthenicum (Solanaceae) (Wu, 1980). Halocnemum strobilaceum is very tolerant to salinity, and thus plants of this species can grow naturally in soil with a total salt content of 38 % (soil dry-weight basis) in the 0–10 cm soil depth layer and of 29 % in the 10–30 cm layer. They can even survive in soil covered by a 5–10 cm layer of salt crust (Wu, 1980).
Although the effect of salinity on seed germination of H. strobilaceum from ‘salt steppes’ in the Mediterranean region of south-eastern Spain has been investigated by Pujol et al. (2000, 2001), there are few studies on the responses of this species to other environmental factors such as temperature and light (Song et al., 2006). Most importantly, different ecotypes of the same species occurring in different habitats may have different adaptation strategies to the special environmental conditions during seed germination and seedling growth in their local sites. To this end, the present study investigated the seed germination ecophysiology of H. strobilaceum in salt deserts of China. The specific goals of this research were to (a) test the effect of temperature, light/dark and salinity on seed germination and of salinity on seed germination recovery, radicle growth and radicle elongation recovery using seeds collected from the temperate cold desert of north-west China; and (b) compare the responses of seeds of the geographically widespread H. strobilaceum from the cold desert of north-west China with those from the Mediterranean region of Spain reported by Pujol et al. (2000, 2001).
MATERIALS AND METHODS
Seeds and field site description
Seeds of H. strobilaceum (Pall.) M. Bieb. are oblate and brown with small dense protuberances on the surface. The embryo is fully developed and located in a peripheral position, rarely encircling the endosperm. Thus, the type of seed produced by H. strobilaceum is peripheral (Martin, 1946). The mean (±s.e.) mass of four groups of 1000 seeds each was 86·25 ± 4·23 mg, and the mean ( ± s.e.) seed diameter (n = 20) was 0·66 ± 0·02 mm.
Mature seeds were collected in November 2004, from plants of H. strobilaceum growing in a salt desert near the Fukang Desert Ecological Station of the Xinjiang Institute of Ecology and Geography, Chinese Academy of Sciences (87 °45′–88 °05′E; 43 °45′–44 °30′N; 460 m a.s.l.). This area is a temperate inland desert in the hinterland of the Eurasian continent. The site has typical desert vegetation, alkali-saline grey desert soil and a continental climate. Mean annual temperature is 6·6 °C and mean temperatures of the coldest (January) and hottest (July) months are −17 and 25·6 °C, respectively. Annual precipitation (including rain and snow) is 164 mm, 66 % of which occurs in spring and summer, and in winter snow covers the ground to a depth of only 3–29 mm. Annual potential evaporation is >2000 mm. Salts accumulate on the soil surface in the dry season; however, salt concentration is diluted by water from melting snow in spring and by rain in summer (Li, 1990).
Soil samples collected from ten randomly chosen areas within the study population of H. strobilaceum in November 2004 and analysed by the residue drying quality measure (Bao, 2000) had mean (±s.e.) total soil salinities in the 0–2, 2–5 and 5–10 cm soil layers of 0·65 ± 0·03 %, 1·66 ± 0·06 % and 1·83 ± 0·07 %, which are equal to NaCl concentrations of 0·11 ± 0·005, 0·28 ± 0·010 and 0·31 ± 0·012 m, respectively. Since there were few or no differences between the effects of NaCl, Na2SO4, MgCl2 and MgSO4 on germination of H. strobilaceum in the present study (unpublished data) or in that of Pujol et al. (2000), data only for NaCl are presented. Another reason for considering only NaCl is that apparently it is the major component of salinity in the deserts of north-west China (Fan et al., 1993; Tobe et al., 2000). Minimum and maximum soil salinities in the study site were 0·02 and 0·88 m NaCl, respectively.
Seeds were incubated in 50 mm diameter Petri dishes on three layers of Whatman No. 1 filter paper moistened with 2·5 ml of deionized water or with different concentrations of NaCl solution. Petri dishes were sealed by plastic film to prevent evaporation. An emerged radicle was the criterion for germination (Côme, 1982).
Effects of temperature and light on germination
Seeds that had been stored dry at 4 °C for 1 month were incubated at 5, 10, 15, 20, 25, 30 and 35 °C in constant fluorescent light (100 µmol m−2 s−1, 400–700 nm; hereafter light) and in constant darkness. Germination in the light was monitored every 24 h, at which time germinated seeds were counted and removed from the Petri dishes. After 15 d, when no additional seeds had germinated for 5 d, experiments in light were terminated, and seed germination in darkness was checked first and only at this time.
Effects of salinity on germination and recovery
The effects of 0·0 (deionized water control), 0·1, 0·2, 0·3, 0·4, 0·5, 0·75, 1·0, 2·0, 4·0 and 6·0 m concentrations of NaCl on germination of seeds (stored dry at 4 °C for 3 months) were tested at 25 °C in the light. Germination was monitored every 24 h, and germinated seeds were counted and removed from the dishes. The time taken for germination percentages of all the replicates to reach 50 % was recorded as TG50 (Tobe et al., 2000). Final germination percentages were calculated after incubation for 15 d.
Ungerminated seeds from the 15 d NaCl pre-treatments were rinsed three times in deionized water and then incubated in deionized water. Seed germination recovery was monitored every 24 h, at which time germinated seeds were counted and removed from the dishes. The time taken for germination recovery percentages of all the replicates to reach 50 % was recorded as TGr50. Final germination recovery percentages were calculated after 15 d of incubation using the following formula:
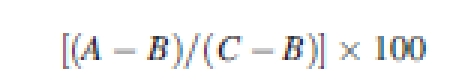 |
where A is the number of seeds germinated in salt solutions plus those that recovered to germinate in the deionized water, B is the number of seeds germinated in salt solutions and C is the total number of seeds tested (Khan and Ungar, 1984). Final germination was recorded as (A/C) × 100.
Effects of salinity on radicle growth and recovery
Seeds stored dry at 4 °C for 3 months were incubated initially in deionized water at 25 °C in the light. When the radicles had barely emerged (<1·0 mm), 20 of these young seedlings were transferred into Petri dishes containing 0·0 (deionized water control), 0·1, 0·2, 0·3, 0·4, 0·5, 0·75, 1·0, 2·0 and 3·0 m NaCl solutions. Seedling incubation was terminated after 10 d and mean radicle length recorded. Then, seedlings incubated in different NaCl concentrations for 10 d were rinsed three times with deionized water and transferred to filter paper saturated with deionized water for an additional 10 d, at which time final radicle lengths of the seedlings were measured.
Data analysis
Seed germination percentage and radicle length were expressed as mean ±s.e. One-way or two-way analysis of variance (ANOVA; P < 0·05) was used to compare treatment effects (Sokal and Rohlf, 1995). If ANOVA showed significant effects, Tukey's test was used to determine differences among treatments.
RESULTS
Effects of temperature and light on germination
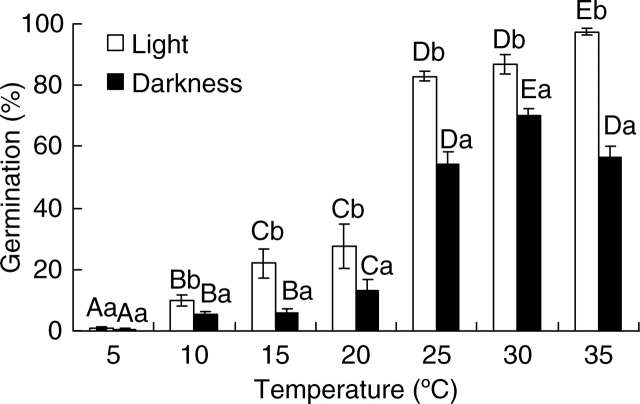
After 15 d of incubation, the germination percentage over the 10–35 °C temperature range was significantly higher in light than in darkness (P < 0·001) (Fig. 1). Germination percentages in the light and in darkness increased significantly with the increase in temperature (P < 0·001). Although seeds germinated to a high percentage at 35 °C, seedlings were distorted, or they died. A two-way ANOVA showed that germination was significantly affected by temperature, light and their interaction (Table 1).
Fig. 1.
Effect of temperature and light on germination of Halocnemum strobilaceum seeds after 15 d of incubation in deionized water. Different upper case letters indicate significant differences in germination percentage across the range of temperatures for seeds incubated in the light and for those incubated in the dark, and different lower case letters indicate significant differences between seeds incubated in the light and darkness at a given temperature. Values are means ± s.e.
Table 1.
Two-way ANOVA analysis of effects of temperature, light and their interactions on seed germination of Halocnemum strobilaceum
| Source of variance | d.f. | SS | MS | F-value | P-value |
|---|---|---|---|---|---|
| Temperature | 5 | 443·107 | 88·621 | 207·167 | <0·001 |
| Light | 1 | 21·870 | 21·870 | 51·125 | <0·001 |
| Temperature × light | 5 | 9·890 | 1·978 | 4·624 | 0·002 |
Effects of salinity on germination and recovery
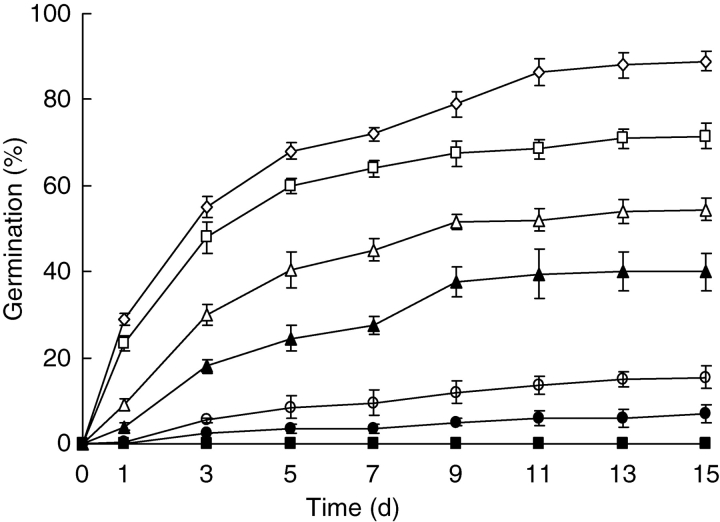
Germination was significantly affected by salinity (P < 0·001). As salinity increased from 0·0 to 0·5 m NaCl, the rate and percentage of germination decreased, and it was significantly inhibited by higher salinities (Fig. 2). No seeds germinated at salinities of ≥0·75 m. At NaCl concentrations of 0–0·3 m, TG50 increased, i.e. germination rate decreased. Seed germination percentage did not reach 50 % (TG50) at salinities exceeding 0·3 m until after 15 d of incubation (Table 2). As the pre-treatment concentration of salinity increased, the percentage and rate of germination recovery increased, and for all salinities germination recovery percentages were significantly higher than that of the control (P < 0·001). Even seeds pre-treated with 6·0 m NaCl recovered. Germination recovery did not differ significantly among seeds transferred from 2·0–6·0 m NaCl solutions to deionized water (P > 0·05).
Fig. 2.
Effect of NaCl concentration on germination of Halocnemum strobilaceum seeds incubated at 25 °C in the light for 15 d. (1) Open diamond, deionized water (control); (2) open square, 0·1 m NaCl; (3) open triangle, 0·2 m NaCl; (4) filled triangle, 0·3 m NaCl; (5) open circle, 0·4 m NaCl; (6) filled circle, 0·5 m NaCl; (7) filled square, 0·75 and 1·0 m NaCl. Values are means ± s.e.
Table 2.
Effects of NaCl on germination and its recovery in deionized water of seeds of Halocnemum strobilaceum
| Seed germination ( % ± s.e.) |
|||||
|---|---|---|---|---|---|
| Salinity (m) | Initial solution, B/C × 100 | Recovery* [(A – B)/(C – B)] × 100 | Final†, A/C × 100 | TG50 (d) | TGr50 (d) |
| 0 | 89·0 ± 1·3a | 17·5 ± 6·8a | 91·5 ± 1·7a | 3 | >15 |
| 0·1 | 73·5 ± 4·9b | 43·8 ± 4·8b | 84·5 ± 3·8b | 4 | >15 |
| 0·2 | 54·5 ± 3·0c | 58·8 ± 8·6c | 79·5 ± 2·6cde | 12 | >15 |
| 0·3 | 40·0 ± 4·4d | 62·8 ± 7·0c | 76·0 ± 5·9def | >15 | 4 |
| 0·4 | 15·5 ± 2·8e | 68·3 ± 4·1c | 73·5 ± 2·6f | >15 | 4 |
| 0·5 | 7·0 ± 1·7e | 73·5 ± 2·6cd | 75·5 ± 1·0f | >15 | 4 |
| 0·75 | 0 | 74·5 ± 1·0d | 74·5 ± 1·0f | >15 | 3 |
| 1·0 | 0 | 77·0 ± 1·7d | 77·0 ± 1·7ef | >15 | 3 |
| 2·0 | 0 | 88·0 ± 2·8e | 88·0 ± 2·8ab | >15 | 3 |
| 4·0 | 0 | 85·5 ± 2·4e | 85·5 ± 2·4 b | >15 | 2 |
| 6·0 | 0 | 84·5 ± 2·8e | 84·5 ± 2·8bcd | >15 | 2 |
* Recovery is the percentage of ungerminated seeds in the NaCl pre-treatment solution that germinated after transfer to deionized water [(A – B)/(C – B)] × 100, where A is the sum of the number of seeds germinated in the salt solutions plus the number that recovered to germinate in deionized water, B is the number of seeds germinated in the initial salt solutions and C is the total number of seeds tested.
† Final germination was recorded as (A/C) × 100, where A is the sum of the number of seeds germinated in salt solutions and of those that recovered to germinate in the deionized water, and C is the total number of seeds tested.
Values followed by the same letter do not differ significantly.
Effects of salinity on radicle growth and recovery
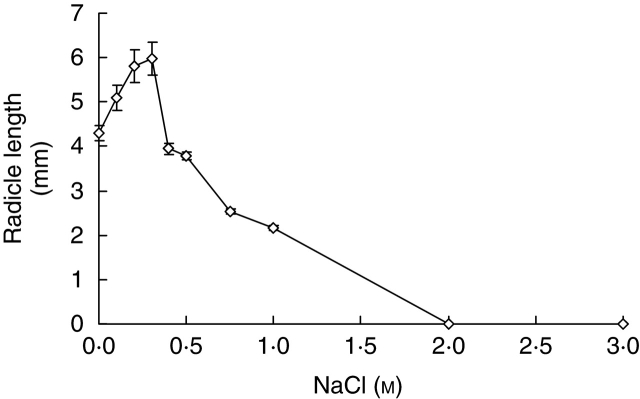
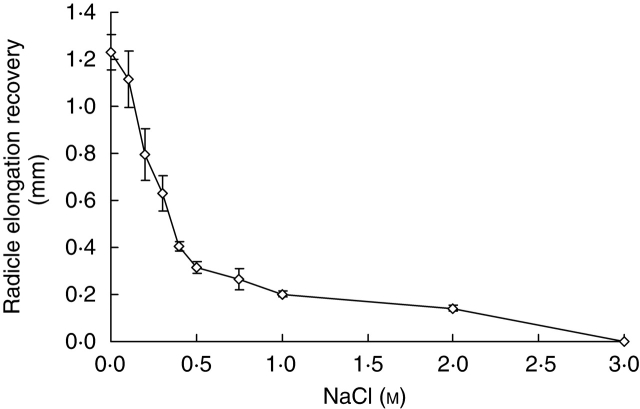
Concentrations of 0·1–0·3 m NaCl significantly increased radicle elongation (P < 0·001), whereas concentrations of 0·75–3·0 m significantly inhibited it (P < 0·001). No radicle elongation occurred at NaCl concentrations of ≥2·0 m (Fig. 3). Radicle growth recovered after seedlings were transferred to deionized water. However, elongation recovery decreased with the increase in pre-treatment salinities. Seedlings from solutions ≥3·0 m NaCl showed no capacity for recovery (Fig. 4).
Fig. 3.
Effect of NaCl concentration on radicle elongation of Halocnemum strobilaceum at 25 °C in the light after 10 d of incubation. Values are means ± s.e.
Fig. 4.
Radicle elongation recovery in Halocnemum strobilaceum after transfer from different concentrations of NaCl to deionized water. Radicle length recovery was recorded after 10 d of incubation in deionized water at 25 °C in the light. Values are means ± s.e.
DISCUSSION
In the life cycle of a plant, seeds have the highest resistance to extreme environmental stresses, whereas seedlings are most susceptible, and this is especially true for desert species (Gutterman, 1993). Therefore, successful establishment of a plant population is dependent on the adaptive aspects of seed germination and of early seedling growth. Germination of many halophytes occurs when the combination of photoperiod, temperature regime, soil water and soil salinity is optimal for plant growth (Naidoo and Naicker, 1992; Gutterman, 1993; Zia and Khan, 2004). Thus, for example, seeds of perennial plants in the Artemisietum herbae-albae plant community type of the Negev Desert, Israel, germinate en masse only once in several years (Evenari and Gutterman, 1976).
Germination percentages of H. strobilaceum seeds were higher in light than in darkness at all temperatures tested, indicating that a considerable portion of the seed population is light sensitive. This characteristic of H. strobilaceum is similar to that of the desert shrubs Artemisia monosperma (Asteraceae) (Huang and Gutterman, 1998), A. sphaerocephala (Huang and Gutterman, 1999b) and A. ordosica (Huang and Gutterman, 2000). The light requirement for germination of H. strobilaceum seeds ensures that they will germinate successfully on or near the soil surface when other conditions are suitable for seedling emergence. If buried at soil depths to which light cannot penetrate, they may become part of a persistent soil seed bank. Song et al. (2006) reported that H. strobilacerum seeds could germinate to high percentages when they were incubated in continuous darkness. However, they monitored germination daily but do not say if seeds were examined under a green ‘safe light’ or under ‘white light’. If the latter, then they did not incubate seeds in ‘continuous’ darkness. In which case, the seeds of H. strobilaceum probably were light stimulated as they were being checked daily for germination (Baskin and Baskin, 1998). This could explain why seeds of H. strobilaceum germinated to higher percentages in the dark in the Song et al. (2006) study than they did in the present study. Further, even a green ‘safe light’ can satisfy the light requirement of some positively photoblastic seeds (Walck et al., 2000).
Seeds of H. strobilaceum germinated to >50 % only at 25, 30 and 35 °C, and at each of these temperatures they germinated to a higher percentage in light than in darkness. These responses are similar to those of seeds of some salt marsh and salt desert species, i.e. the best conditions for germination are high temperatures in light (Baskin and Baskin, 1998). In north-west China, seeds of H. strobilaceum mature in November, when the average temperature is <0 °C and thus too low for germination (Fig. 1). In the field, seeds of this species germinate primarily from April to July. Thus, seed germination begins in spring, when soil moisture is high, due to snow melt, and average temperatures are 0–10 °C. However, in light, only 5–35 % of the seeds of H. strobilaceum germinated even at 10–20 °C, whereas >80 % of the seeds germinated at 25–35 °C, which is coincident with temperatures in its natural habitat in July. In this summer rainy season, rainwater washes away salts on the soil surface and at shallow depths. Thus, seeds heretofore prevented from germinating can germinate under this optimal combination of low salinity, high soil moisture and high temperatures. The ability of seeds of H. strobilaceum to germinate in spring to early summer, when soil moisture is available for seedling establishment, is a life history/ecophysiological adaptation of this species to its dry saline desert habitats.
Halophyte seeds respond to salinity in two ways: (a) germination is inhibited by high salinity, but seeds do not lose their viability; and (b) salinity stress delays germination but does not induce dormancy or kill the seeds (Ungar, 1978, 1995; Levitt, 1980). Seeds of Salicornia rubra and Halogeton glomeratus even germinated in a 1·0 m NaCl solution (Khan et al., 2000, 2001), and Salicornia bigelovii seeds did so in a 0·856 m NaCl solution (Rivers and Weber, 1971). However, seeds of Salicornia pacifica var. utahensis germinated to only 3 % in a 0·856 m NaCl solution (Khan and Weber, 1986), and those of Allenrolfea occidentalis (Chenopodiaceae) did not germinate when salinity exceeded 0·8 m NaCl (Gul and Weber, 1999). Although germination of H. strobilaceum seeds decreased with increase in salinity, 7 % of them germinated in a 0·5 m NaCl solution. Song et al. (2006) also found that only a low percentage of H. strobilaceum seeds germinated at 0·5 m NaCl. Cold stratification caused a significant increase in germination of H. strobilaceum seeds incubated at 0·2, 0·4 and 0·6 m NaCl at 25 °C in the light (Qu, 2006). In general, the difference between germination percentages of seeds at low salinities and that in deionized water is insignificant, but they gradually decrease with further increase of salinity (Khan and Ungar, 1997; Katembe et al., 1998; Gul and Weber, 1999; Khan et al., 2000; Huang et al., 2003).
Seeds of most halophytes exposed to salinities that inhibit germination will recover and germinate after they are transferred to distilled water. Further, final germination percentages are even increased by salt pre-treatment in most species (Khan and Ungar, 1997; Gul and Weber, 1999; Khan et al., 2000; Huang et al., 2003). For example, seeds of Allenrolfea occidentalis recovered to germinate rapidly and to high percentages after they were transferred from high salinity solutions to distilled water (Gul and Weber, 1999). The recovery germination percentage was about 20 % in Haloxylon ammodendron seeds pre-soaked in a 0·2 m NaCl solution, but about 56 % in those pre-soaked in solutions of 0·8–1·4 m NaCl (Huang et al., 2003). However, seed germination of some species is permanently inhibited by high salinity. For instance, seeds of Zygophyllum simplex (Zygophyllaceae) could hardly recover to germinate after they were transferred from a high salinity solution to distilled water (Khan and Ungar, 1997). In the present experiments, ungerminated seeds of H. strobilaceum germinated well after they were transferred from solutions of high salinity to deionized water. Germination recovery increased gradually with increase of pre-treatment salinity and was quite high even for seeds pre-treated in a 6·0 m NaCl solution, which is near the molarity of a saturated solution of NaCl. Thus, seeds of H. strobilaceum are well adapted to saline habitats via a high capacity for germination recovery.
The mean salinity of soil collected at ten sites in the present study area ranged from 0·11 to 0·31 m at a 0–10 cm depth, and minimum and maximum soil salinity across all sites were 0·02 and 0·88 m NaCl, respectively. Wu (1980) reported that plants of H. strobilaceum are highly tolerant to salinity, and they can grow naturally in soil with a total salt concentration of 38 % (equal to 6·5 m NaCl) in the 0–10 cm soil layer and of 29 % (equal to 5·0 m NaCl) in the 10–30 cm depth layer. They even can survive in soil covered by a 5–10 cm layer of salt crust. In sites where the soil salinity is ≤0·5 m NaCl, seeds of H. strobilaceum can germinate successfully. However, in sites where soil salinity exceeds 0·5 m NaCl, seeds do not germinate, and thus they may remain in the soil seed bank until the salt is diluted by water from melting snow or until precipitation, temperature and light are not limiting for germination. As such, soil salinity could act as a ‘rain gauge’ for seed germination (Gutterman, 1993), in which case it would play an important ecological role in the successful establishment of H. strobilaceum populations.
Seedlings of halophytes can develop in solutions with low concentrations of salts; however, radicle growth may be greatly retarded by high salinity (Malcolm et al., 2003). As expected, growth of H. strobilaceum radicles was increased by low salinities (≤0·3 m) and decreased by high salinities (≥0·5 m). These physiological responses are similar to those of Annona muricata and A. squamos (Annonaceae), in which inhibition of radicle elongation was significantly related to increase in salinity (Passos et al., 2005). Tobe et al. (2000) found that although 0·1–0·2 m NaCl solutions did not affect the viability of Kalidium caspicum seeds, they promoted mortality of radicles. However, young roots of H. strobilaceum have high salinity tolerance (up to 1·0 m) and recovery ability (up to 2·0 m). Thus, for successful establishment of H. strobilaceum seedlings, the high salinity on the soil surface and at shallow soil depths needs to be diluted by precipitation or by melt water from snow. During the rainy season in spring and summer, the salinity on the soil surface would be decreased by precipitation, and then seeds could germinate successfully and seedlings become established. In the desert area near the Dead Sea, seedlings of Mesembryanthemum nodiflorum (Aizoaceae) appear in the winter, only after several rains, which dilute the salt accumulated during the summer from the upper layer of the soil. The seeds of this halophyte do not germinate in high salinity, and seedlings appear mainly in depressions and runnels, where more water penetrates into the soil (Gutterman, 1980/81).
Germination of seeds of H. strobilaceum from both north-west China and southeastern Spain decreased with increase in salinity. However, seeds from southeastern Spain could germinate at a higher salinity [0·86 m NaCl (Pujol et al., 2001)] than those from north-west China (0·5 m NaCl). Germination recovered rapidly after seeds of H. strobilaceum from both Spain and China were transferred to fresh water, but the recovery rate of seeds from southeastern Spain (TGr50 = 2 d) (Pujol et al., 2000) was a little faster than that of seeds from north-west China (TGr50 = 3–4 d). Growth of seedlings of H. strobilaceum from both southeastern Spain and north-west China was stimulated at low salinities [0·17–0·51 m NaCl, southeastern Spain (Pujol et al., 2001) and ≤0·3 m NaCl, north-west China] but completely inhibited at higher salinities [≥0·68 m NaCl, southeastern Spain (Pujol et al., 2001) and ≥0·75 m NaCl, north-west China]. Thus, tolerance to salinity of seedlings from southeastern Spain apparently is higher than that of those from north-west China.
In north-west China, H. strobilaceum occurs in inland salt deserts, where the amount and frequency of precipitation are highly unpredictable, resulting in periods of salt and drought stress. The ability of H. strobilaceum seeds to germinate at low salinity and to recover from high salinity, and of seedlings to grow in low salinity, survive at high salinity and resume growth after the soil solution is diluted by water is an adaptation to its saline desert habitat. When salinity is high in the dry season, seeds do not germinate, but they do germinate when salinity is diluted by precipitation. Although evaporation following precipitation will decrease soil water potential, radicles of H. strobilaceum can continue to elongate. When evaporation and soil salinity are low, radicle elongation is even accelerated. Thus, following an increase in soil moisture, seedlings of H. strobilaceum can resume growth and their roots can grow to a sufficient soil depth to become established before the next rain occurs. Clearly, then, the seed germination and early seedling growth stages of the life cycle of H. strobilaceum are parts of the adaptive strategy of this species that allows it to inhabit harsh saline conditions such as those in the deserts of north-west China.
ACKNOWLEDGEMENTS
This study was funded by the Knowledge Innovation project from the Chinese Academy of Sciences (KZCX2-YW-431) and the National Natural Science Foundation (30570281, 30330130) of PR China. Fukang Desert Ecological Station of the Xinjiang Institute of Ecology and Geography, Chinese Academy of Sciences, provided assistance for collection of seeds and soil samples.
LITERATURE CITED
- Badger KS, Ungar IA. The effects of salinity and temperature on the germination of the inland halophyte Hordeum jubatum. Canadian Journal of Botany. 1989;67:1420–1425. [Google Scholar]
- Bao SD. Soil chemistry and agriculture analysis. Beijing: China Agriculture Press; 2000. (in Chinese) [Google Scholar]
- Baskin CC, Baskin JM. Seeds: ecology, biogeography, and evolution of dormancy and germination. San Diego: Academic Press; 1998. [Google Scholar]
- Côme D. Germination. In: Mazliak P, editor. Croıssance et développement. Physiologie végétale. II. Paris: Hermann; 1982. pp. 129–225. [Google Scholar]
- Evenari M, Gutterman Y. Observations on the secondary succession of three plant communities in the Negev desert, Israel. I. Artemisietum herbae albae. In: Hommage J, Chouard P, editors. Etudes de biologie vegetale. Paris: CNRS, Gif-sur-Yvette; 1976. pp. 57–86. [Google Scholar]
- Fan Z, Chang Q, Tian C, Yabuki S, Okada A, Liu C. Genesis and characteristics of salt-affected soils in Tarim Basin. Proceedings of the Japan–China international symposium on the study of the mechanism of desertification; Science and Technology Agency; 1993. pp. 219–226. [Google Scholar]
- Gul B, Weber DJ. Effect of salinity, light and temperature on germination in Allenrolfea occidentalis. Canadian Journal of Botany. 1999;77:240–246. [Google Scholar]
- Gutterman Y. Annual rhythm and position effect in the germinability of Mesembryanthemum nodiflorum. Israel Journal of Botany. 29:93–97. 1980/81. [Google Scholar]
- Gutterman Y. Seed germination in desert plants. Adaptations of desert organisms. Berlin: Springer-Verlag; 1993. [Google Scholar]
- Huang ZY, Gutterman Y. Artemisia monosperma achene germination in sand: effects of sand depth, sand/water content, cyanobacterial sand crust and temperature. Journal of Arid Environments. 1998;38:27–43. [Google Scholar]
- Huang ZY, Gutterman Y. Water absorption by mucilaginous achenes of Artemisia monosperma: floating and germination affected by salt concentrations. Israel Journal of Plant Sciences. 1999;a 47 [Google Scholar]
- Huang ZY, Gutterman Y. Germination of Artemisia sphaerocephala (Asteraceae) occurring in the sandy desert areas of Northwest China. South African Journal of Botany. 1999;b 65 [Google Scholar]
- Huang ZY, Gutterman Y. Comparison of germination strategies of Artemisia ordosica with its two congeners from deserts of China and Israel. Acta Botanica Sinica. 2000;42:71–80. [Google Scholar]
- Huang ZY, Zhang XS, Zheng GH, Gutterman Y. Influence of light, temperature, salinity and storage on seed germination of Haloxylon ammodendron. Journal of Arid Environments. 2003;55:453–464. [Google Scholar]
- Katembe WJ, Ungar IA, Mitchell JP. Effect of salinity on germination and seedling growth of two Atriplex species. Annals of Botany. 1998;82:167–175. [Google Scholar]
- Khan MA, Ungar IA. Seed polymorphism and germination responses to salinity stress in Atriplex triangularis Willd. Botanical Gazette. 1984;145:487–494. [Google Scholar]
- Khan MA, Ungar IA. Effect of thermoperiod on recovery of seed germination of halophytes from saline conditions. American Journal of Botany. 1997;84:279–283. [PubMed] [Google Scholar]
- Khan MA, Weber DJ. Factors influencing seed germination in Salicornia pacifica var. utahensis. American Journal of Botany. 1986;73:1163–1167. [Google Scholar]
- Khan MA, Gul B, Weber DJ. Germination responses of Salicornia rubra to temperature and salinity. Journal of Arid Environments. 2000;45:207–214. [Google Scholar]
- Khan MA, Gul B, Weber DJ. Seed germination characteristics of Halogeton glomeratus. Canadian Journal of Botany. 2001;79:1189–1194. [Google Scholar]
- Khan MA, Gul B, Weber DJ. Seed germination in the Great Basin halophyte Salsola iberica. Canadian Journal of Botany. 2002;80:650–655. [Google Scholar]
- Levitt J. Responses of plants to environmental stress. 2nd edn. New York: Academic Press; 1980. [Google Scholar]
- Li SG. Natural conditions and the establishment basis of Fukang ecosystematic observation and experiment station of Chinese Academy of Science. Arid Zone Research. 1990;7:3. (in Chinese) [Google Scholar]
- Liu YX. Flora in desertis reipublicae popularum sinarum. I. Beijing: Science Press; 1985. (in Chinese) [Google Scholar]
- Malcolm CV, Lindley VA, O'Leary JW. Halophyte and glycophyte salt tolerance at germination and the establishment of halophyte shrubs in saline environments. Plant and Soil. 2003;253:171–185. [Google Scholar]
- Martin AC. The comparative internal morphology of seeds. The American Midland Naturalist. 1946;36:513–660. [Google Scholar]
- Naidoo G, Naicker K. Seed germination in the coastal halophytes Triglochin bulbosa and Triglochin striata. Aquatic Botany. 1992;42:217–229. [Google Scholar]
- Passos VM, Santana NO, Gama FC, Oliveira JG, Azevedo RA, Vitória AP. Growth and ion uptake in Annona muricata and A. squamosa subjected to salt stress. Biologia Plantarum. 2005;49:285–288. [Google Scholar]
- Pujol JA, Calvo JF, Ramírez-Díaz L. Recovery of germination from different osmotic conditions by four halophytes from southeastern Spain. Annals of Botany. 2000;85:279–286. [Google Scholar]
- Pujol JA, Calvo JF, Ramírez-Díaz L. Seed germination, growth, and osmotic adjustment in response to NaCl in a rare succulent halophyte from southeastern Spain. Wetlands. 2001;21:256–264. [Google Scholar]
- Qu XX. Adaptive strategies of the three halophytic species in Xinjiang in their seed germination and early seedling growth stage. Beijing: Institute of Botany, Chinese Academy of Sciences; 2006. MS thesis, Laboratory of Quantitative Vegetation Ecology (in Chinese with English abstract) [Google Scholar]
- Rivers WG, Weber DJ. The influence of salinity and temperature on seed germination in Salicornia bigelovii. Physiologia Plantarum. 1971;24:73–75. [Google Scholar]
- Sokal RR, Rohlf EJ. Biometry. 3rd edn. San Francisco, CA: Freeman; 1995. [Google Scholar]
- Song J, Feng G, Zhang FS. Salinity and temperature effects on germination for three salt-resistant euhalophytes, Halostachys caspica, Kalidium foliatum and Halocnemum strobilaceum. Plant and Soil. 2006;279:201–207. [Google Scholar]
- Tobe K, Li X, Omasa K. Seed germination and radicle growth of a halophyte. Kalidium caspicum. Annals of Botany. 2000;85:391–396. [Google Scholar]
- Ungar IA. Halophyte seed germination. The Botanical Review. 1978;44:235–255. [Google Scholar]
- Ungar IA. Ecophysiology of vascular halophytes. Boca Raton, FL: CRC Press; 1991. [Google Scholar]
- Ungar IA. Seed germination and seed-bank ecology in halophytes. In: Kigel J, Galili G, editors. Seed development and germination. New York: Marcel Dekker; 1995. pp. 599–628. [Google Scholar]
- Walck JL, Baskin JM, Baskin CC. Increased sensitivity to green light during transition from conditional dormancy to nondormancy in seeds of three species of Solidago (Asteraceae) Seed Science Research. 2000;10:495–499. [Google Scholar]
- Woodell SRJ. Salinity and seed germination patterns in coastal plants. Vegetatio. 1985;61:223–229. [Google Scholar]
- Wu ZY. Chinese vegetation. Beijing: Science Press; 1980. (in Chinese) [Google Scholar]
- Zia S, Khan MA. Effect of light, salinity, and temperature on seed germination of Limonium stocksii. Canadian Journal of Botany. 2004;82:151–157. [Google Scholar]