Abstract
Background
The biomechanical behaviour of plant cells depends upon the material properties of their cell walls and, in many cases, it is necessary that these properties are quite specific. Additionally, physiological regulation may require that target cells responding to hormonal signals or environmental factors are able to modulate these characteristics.
Argument
This paper uses a rheological analysis of creep of elongating sunflower (Helianthus annuus) sunflower hypocotyls to demonstrate that the mechanical behaviour of plant cell walls is complex and involves multiple layered processes that can be distinguished from one another by the time-scale over which they lead to a change in tissue dimensions, their sensitivity to pH and temperature, and their responses to changes in spatial arrangement of the cell wall brought about by treatment with high Mr PEG. Furthermore, it appears possible to regulate individual rheological processes, with limited effect on others, in order to modulate growth without affecting tissue structural integrity. It is proposed that control of the water content of the cell wall and therefore the space between cell wall polymers may be one mechanism by which differential regulation of cell wall biomechanical properties is achieved. This hypothesis is supported by evidence showing that enzyme extracts from growing tissues can cause swelling in cell wall fragments in suspension.
Implications
The physiological implications of this complexity are then considered for growing tissues, stomatal guard cells and abscission cells. It is noted that, in each circumstance, a different combination of mechanical properties is required and that differential regulation of properties affecting behaviour over different time-scales is often necessary.
Key words: Helianthus annuus, cell wall, rheology, growth, stomata, abscission
INTRODUCTION: SPACE AND TIME
Space
It has been established that the physical properties of synthetic polymers are substantially affected by spatial constraint of the macromolecular components, so that behaviour of plastics can be controlled by addition of low molecular weight molecules known as plasticizers to maintain separation (Ward and Hadley, 1993). Thompson (2005) noted that spacing within plant cell walls, particularly between cellulose microfibrils, may be of similar importance. This was suggested by the finding that incorporation of pectins and various hemicelluloses into cellulose pellicles produced by Acetobacter resulted in composites which were weaker and more extensible than pellicles of pure cellulose (Chanliaud and Gidley, 1999; Whitney et al., 1999). In the plant cell wall, microfibril separation may be affected by a number of factors including cross-linking polysaccharides tethering microfibrils together or holding them apart (or indeed a balance between the two) and spacing by interpenetrating polysaccharides. However, the water content of the wall will also have a substantial effect on separations. This has two important consequences:
The cell wall matric potential and the relationship between matric potential and water content may be important in determining cell wall properties.
The importance of cell wall spacing can be tested by alteration of matric potential using polyethylene glycol (PEG). PEG with Mw > 4000 cannot penetrate plant cell walls (Carpita et al., 1979), and so treatment with PEG 6000 in solution alters their water potential by reducing their water content.
Thompson (2005) demonstrated that PEG solutions with osmotic pressures of 3·0 MPa substantially reduced the long-term creep of frozen and thawed sunflower hypocotyls at pH 5·0 and similar behaviour has been found using solutions with an osmotic pressure of 1·0 MPa.
Time
In the field of rheology, it is also understood that the mechanical properties observed are dependent upon the time frame of observation. One important element of this is that the dividing line between solid and fluid behaviour is not absolute, with behaviour becoming more and more fluid over longer time-scales. This is described using the ‘Deborah number’, which is the ratio of the relaxation time of the material to the observation time; the smaller the value, the more fluid the behaviour observed (Scott Blair, 1969). It is named for a line from the Song of Deborah in Judges 5, which can be translated as ‘the mountains flowed before the Lord’ (although the King James Bible uses the less apposite ‘melted’). Over geological time-scales many rocks can be treated as fluids.
A good example of the importance of this idea in plant physiology is tissue growth, when the plant's structure must exhibit solid behaviour over short time-scales, but behave in a more fluid fashion over longer periods for the cell walls to stretch and allow permanent extension.
This paper will show that characterization of plant cell walls involves a number of separate rheological elements acting over different time-scales and which are differentially affected by cell wall water content, heat treatment and pH suggesting that multiple physical or chemical processes are involved. Examples of particular tissues or cells where correct function depends upon specific control of individual biomechanical elements will then be considered.
EFFECTS OF CELL WALL SPACING ON CREEP OF GROWING TISSUE OVER A RANGE OF TIME-SCALES
Rheological analysis of creep of growing tissue provides a good example of biomechanical behaviour involving multiple cell wall processes manifesting over different time-scales and exhibiting different responses to cell wall spacing, pH and boiling.
The effect of cell wall spacing on creep of frozen and thawed etiolated sunflower (Helianthus annuus) hypocotyls was investigated by using PEG solutions to modify the cell wall water content. The effect on boiled tissue segments was also investigated to determine whether any of the rheological elements were mediated by endogenous enzyme activity. Because the segments had either been frozen and thawed or boiled, there should be no confounding effects caused by changes in cell turgor.
Etiolated seedlings (‘Giant Yellow’, Suttons) were grown in covered pots of water-saturated perlite for 5 d at 30 ºC (perlite from Silvaperl, Gainsborough, UK). Segments (20 mm) from the top of the hypocotyls were longitudinally bisected using a hand-held razor blade, frozen with freezing spray (R.A. Lamb, Eastbourne, UK) or boiled for 120 s in distilled water and then dropped into 10 mM MES buffer containing 5 mm KCl, 1 mm CaCl2 and 0·27 g PEG g–1 water (to give π = 1·0 MPa). Segments were then pressed between microscope slides covered in absorbent paper using a 2 kg weight for 60 s and returned to the PEG solution for 10 min.
Creep of the segments was measured using a constant load extensiometer as described by Thompson (2001). Segments were securely clamped into the bottom of reservoirs of buffer with approx. 8 mm exposed and the exact length was measured using a magnifying eyepiece with a graticule. Initial force was <0·004 N and was increased in 0·098 N increments during the course of the experiment. Segments were initially bathed in buffer containing PEG to give a known osmotic pressure or equivalent control buffer with no PEG, and the applied force was increased first to 0·098 N for 90 min and then to 0·196 N for a further 240–360 min. After this period, the initial buffer was drained from the tube and gently replaced with control buffer (if the initial buffer contained PEG) or buffer containing PEG (if the segment was initially bathed in control buffer). For each increase in the applied force the relative length was calculated using the ‘true strain’, i.e. relative length = Ln(Lt/L0) + 1.
Figure 1 illustrates the effects of increasing or decreasing water potential by 1·0 MPa on creep of hypocotyl segments. When water potential was increased, within a few min the tissue began to extend more rapidly. Although creep rates subsequently decreased, they did so slowly and were still substantially greater than they had been at a water potential of −1·0 MPa several hours later. Interestingly, the opposite exchange also caused a rapid increase in creep rate, but this only lasted for 30–45 min before establishment of a near-constant rate of extension considerably lower than that at higher water potential. It is worth noting that, on some occasions, immediately after increases in water potentials there was a slight contraction of segments before creep rates began to rise, and it seems likely that this was an inversion of the short rapid extension following a reduction in water potential. Changes appeared to be reversible as segments returned to control buffer several hours after they had been transferred to a water potential of −1·0 MPa responded in the same way as segments for which the first exchange was from a water potential of −1·0 MPa to zero and vice versa. No effect was observed when buffer containing an osmoticum that can penetrate the cell wall (glucose) giving π = 1·0 MPa was used, suggesting that the changes in creep rate were due to cell wall water content and not water potential per se [although Edelmann (1995) has observed an effect with this type of treatment].
Fig. 1.
An illustration of typical examples of the effects of increasing and decreasing cell wall water content by replacing buffer containing PEG 6000 to give π = 1·0 MPa with control buffer or the opposite exchange on creep of cell walls of frozen and thawed hypocotyls of etiolated 5-d-old sunflower seedlings.
Modelling creep of plant tissue
Rheological models describing the effects of increasing or decreasing water potential on cell wall properties were then used to break extension down into different components operating over different time-scales and compared to determine whether individual components were affected by changes in cell wall water content in different ways.
Extension was modelled by fitting experimental data time series recorded over 240 min to equations describing rheological elements or combinations of rheological elements by non-linear regression by minimization of squares of residuals. The effectiveness of models was determined by calculating values for time series equivalent to the experimental data using fitted model parameters and determining correlation with the experimental data.
Previously, it has been reported that extension of plant tissues after a step increase in stress using a constant load extensiometer conforms closely to eqn (1) (Thompson, 2001):
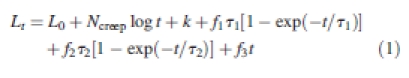 |
1 |
This model is composed of a number of different elements and modelled extension generally corresponds with observed extension with r2 > 0·999. Although increases and decreases in water content are different in nature to stress increases, their effects exhibited some surprising correspondences with eqn (1) that may shed light upon the different elements of the model and their interaction, and so a short description of the elements of the model is given below.
Log-time element
Extension due to this element at time t is given by Ncreeplog t and is most important in the early stages of extension following any instantaneous length increase, as described by Büntemeyer et al. (1998). A correction is also required in conjunction with this element because it cannot be extrapolated backwards to t = 0 and this is a component of the parameter k, the other being instantaneous elastic extension. An unfortunate consequence of this is that the model cannot be used to obtain an accurate value for instantaneous elasticity.
Kelvin elements 1 and 2
These exhibit exponentially decreasing extension rates (Scott Blair, 1969) and their length at time t is given by fτ[1 − exp(−t/τ)] where f is the initial extension rate of the element and τ is the retardation time (the time required for f to decrease by a factor of e). Creep of tomato fruit epidermis after stress increases involved two Kelvin elements: Kelvin element 1 with a retardation time of 10–20 min (τ1) and Kelvin element 2 with a retardation time of 70–120 min (τ2), so that Kelvin element 1 is important in short-term behaviour and Kelvin element 2 affects long-term extension. Sunflower hypocotyls exhibited very similar behaviour except that τ1 and τ2 were both slightly shorter.
Viscous flow element
This is a simple element describing extension at a constant rate for a constant load. The extension at time t is given by ft where f is the extension rate (or flow rate).
In Thompson (2001) it was found that f2 and f3 (which define creep over the longer time-scales corresponding to plant growth) were increased at reduced pH. f1 was also increased at lower pH but to a lesser degree than f2 and f3. The effects of pH on f1, f2 and f3 were all abolished by boiling. The log-time element and apparent instantaneous elastic extension were independent of boiling and pH.
Modelling the effects of altered cell wall spacing on creep
It was found that creep after cell wall water content was increased was best described by eqn (2) (treating the time at which the control buffer was introduced as τ = 0; r2 was generally >0·99):
 |
2 |
The time constants for the two Kelvin elements were of the same order of magnitude as observed after a step increase in applied force (e.g. for the example in Fig. 1, τ1 was 9 min 48 s and τ2 was 1 h 2 min). It should be noted that it was not possible to model the data well when a short period of contraction occurred and so this was omitted from the data when it occurred. However, as is explained below, it seems likely that this transient length decrease was due to a log-time element causing shortening when cell wall water content was increased.
When the wall water potential was reduced, the best match to the data was provided by eqn (3) (r2 was generally >0·99):
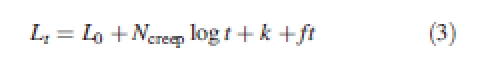 |
3 |
In this case, k only corrects for the log-time element with no instantaneous elastic element. Ncreep was always positive and the so the log-time element describes the transient increase in creep rate after exchange. ft is a viscous flow element equivalent to that in eqn (1), but f after control buffer was replaced with buffer containing 1·0 MPa PEG was substantially less than f3 (for the data shown in Fig. 1, the decrease is approximately by a factor of 12).
It seems plausible that the rheological components in eqns (2) and (3) correspond to the equivalent terms in eqn (1) and this interpretation is reinforced by observation of equivalent exchanges using boiled tissue. Figure 2 compares the effects of altering cell wall water content in hypocotyl segments that were boiled and those that were frozen and thawed. Figure 2A illustrates creep of segments where cell wall water content was increased and Fig. 2B shows the opposite exchange. It is clear that in both treatments boiling caused a reduction in extension rate over longer time-scales. However, the effects of increasing and decreasing cell wall spacing appeared substantially different. When water content was reduced the pattern of response was virtually identical in boiled and frozen and thawed tissue except that the extension rate after the initial period of rapid extension was lower in the boiled segments (although proportionally comparable to that before the exchange). However, when the water potential of boiled cell walls was increased, instead of a rapid increase in length (with or without a slight shortening) there was a long and pronounced contraction before extension resumed so that segments only regained their length prior to the exchange after approx. 1 h. Thereafter, although extension rates remained low, they were still greater than they had been before the exchange.
Fig. 2.
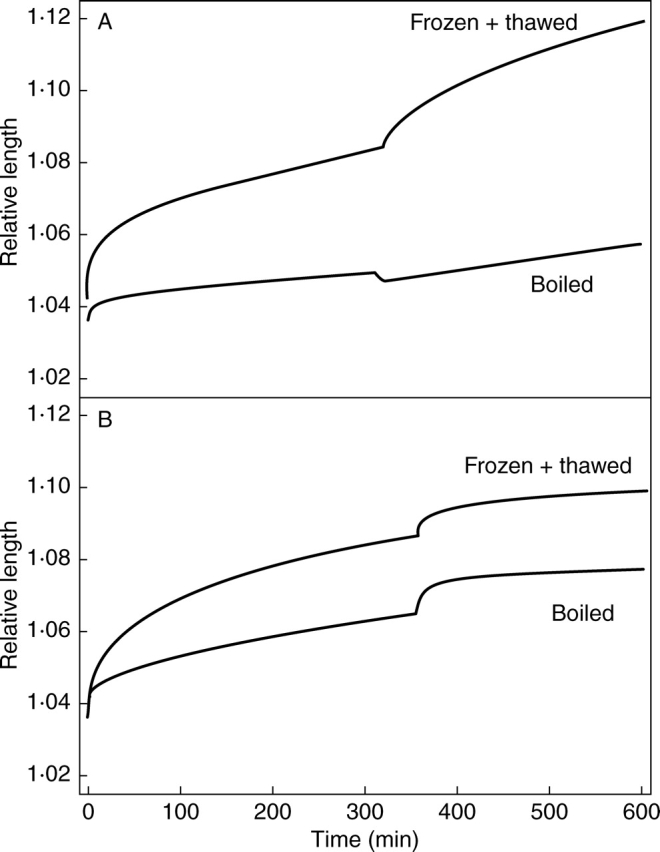
For the experimental data in graph (A) cell wall water content was increased by replacing buffer containing PEG to give π = 1·0 MPa with control buffer and, in (B), cell wall water content was reduced by exchanging control buffer for buffer containing PEG. In both graphs representative examples of creep of frozen and thawed and boiled segments of hypocotyls of etiolated 5-d-old sunflower seedlings are plotted.
These observations are readily explicable if it is assumed that the log-time element is insensitive to boiling and extends as cell wall spacing is reduced and contracts as cell wall spacing increases. If an increase in cell wall water content caused contraction of the log-time element and extension of Kelvin element 1, Kelvin element 2 and the viscous flow element but only the three extending elements were reduced by boiling, then the pattern observed in Fig. 2A might be expected. Likewise extension of the log-time element when cell wall spacing was reduced would be unaffected by boiling, but overall extensibility would be decreased, explaining the behaviour shown in Fig. 2B. Therefore, the effects of boiling are the same as for the equivalent components of eqn (1).
Summary of rheological elements
This analysis demonstrates that it is possible to define a number of separate biomechanical processes operating over different time-scales during creep of growing tissues, some of which are inhibited by boiling while others are not and some of which are limited by increases in cell wall spacing and others by decreases. Figure 3 shows the four elements of eqn (1) also found in eqns (2) and (3) and shows extension due to each after a step increase in stress. These are:
(1) Log-time element. This is most important over reasonably short time-scales (decreasing to <2·5 % of its initial rate within 30 min) and is not substantially affected by heat treatment (and therefore probably not enzymically mediated) or pH. It seems to extend when stress is applied or when cell wall spacing is decreased and to contract when cell wall spacing is increased. It seems possible that it relates to microfibril realignment as the cell wall thickness changes during changes in stress or water content. Reshaping of the cell spaces may also be involved but is unlikely to account for this element completely as a log-time element was also required for modelling creep of Acetobacter cellulose pellicles (Thompson, 2005).
(2 and 3) Kelvin element 1 and Kelvin element 2. These are both reduced at higher pH and by inactivation of cell wall enzymes (Thompson, 2001). Kelvin element 1 is relatively minor in this system and is short term (decreasing to <2 % of its initial rate within 30 min) but Kelvin element 2 continues to extend over longer periods (extension was still approx. 20 % of its initial rate after 3 h). Both are increased (or restarted) after an increase in cell wall spacing but are not apparent (at least as separate elements) after a decrease. The observation (not presented) that creep after decreasing cell wall water potential from the control value by 0·25 MPa conforms to eqn (3) but with a greater flow rate than at the higher water potential suggest that for Kelvin element 2 this may be because f2 remains but whatever is causing it to decrease has been removed so that it behaves as additional viscous flow. Kelvin elements are often visualized as a flow element and spring in parallel in which flow reduces as the spring extends and accepts a progressively greater proportion of the total stress (Scott Blair, 1969); in such a thought experiment the effect of decreasing wall water content might be equivalent to removing the spring. Therefore, for Kelvin element 2 (and perhaps for Kelvin element 1 as well) the flow and retarding components may be separate processes affected by water potential in different ways.
(4) Viscous flow element. This element is most analogous to growth and while it is of least importance over the initial 30 min of extension, it comes to predominate over periods of several hours. It is inhibited by increased pH, inactivation of cell wall enzymes and reduced cell wall spacing.
Fig. 3.
A plot illustrating extension of the rheological elements utilized in eqns (1) and (2) or (3) after a step increase in applied force from 0·098 N to 0·196 N (the parameters used are the average for creep of nine segments of hypocotyls of etiolated 5-d-old sunflower seedlings in pH 5·0 MES buffer with no PEG). The elements for which modelled values are shown are the log-time element, Kelvin element 1, Kelvin element 2 and the viscous flow element.
Additionally, near instantaneous and probably elastic extension takes place when a step increase in force is applied. This seems to be unaffected by cell wall spacing, pH or boiling.
PHYSIOLOGICAL IMPLICATIONS OF A MULTIPLICITY OF CELL WALL BIOMECHANICAL PROCESSES
It would be surprising if all cell and tissue types necessarily exhibit the same type of behaviour and (as will be discussed below), there may be particular reasons that growing tissues have the properties described above. In most mature cell types, the requirements are likely to be reasonably simple with the main requirements being that cell walls are strong enough to contain the cells' own turgor pressure (and any tissue pressure exerted on them by adjacent cells) and that there is no long-term flow or relaxation so that any deformations are reversible and capable of storing elastic energy in the walls to maintain the interplay between turgor pressure and elastic stretching of the cell walls that supports the structure of many tissues. However, in other cases particular cell types may require cell walls that exhibit quite specific properties. The extensive lignification during differentiation of xylem vessels, modifying the wall properties so that cells do not collapse as a result of internal hydraulic tension is a particularly extreme example. Additionally many cell types need to be able to regulate the biomechanical properties of their cell walls and this may require modulation of some rheological elements with limited effect on others.
A number of situations where particular wall behaviour is needed for physiological function, including examples where controlled modification of specific cell wall properties is important will now be considered.
Growing tissues
Growth of plant tissues provides a good illustration of the importance of differential regulation of cell wall behaviour over short- and long-term time-scales. As has already been noted, growing tissues must exhibit solid behaviour over short time-scales (with extension at high Deborah numbers mainly due to the log-time element) but in a more fluid fashion over longer time-scales, with extension of Kelvin element 2 and the viscous flow element becoming increasingly important for lower Deborah numbers.
Additionally, there are numerous examples of changing growth rates in response to hormonal signals (e.g. Malloch and Osborne, 1976; Zhang and Davies, 1990), environmental factors (e.g. Palmer et al., 1996) or developmental effects (e.g. Palmer and Davies, 1996; Thompson et al., 1998) and as growth takes place over periods of hours, days or even weeks, regulation of growth must involve alteration of long-term behaviour. However, growth rates can increase or decrease several-fold and severe effects on tissue dimensions, water relations or both would result if cell wall properties manifesting over short time-scales were altered to a similar degree. For example, if growth promotion was associated with a decrease in the elastic modulus, a combination two effects would be observed:
A rapid increase in cell size until the balance between cell wall stress and the new modulus is restored. This would involve some dilution of cell solutes so that there would also be a reduction in turgor.
Cell wall relaxation with a consequent decrease in turgor and tissue water potential.
Exact effects would depend on the balance of cell wall extensibility, solute dilution and hydraulic conductivity but could include a rapid increase in tissue size and a collapse in tissue water potential, and, if there was a decrease in turgor due to dilution of cell contents or a reduction in tissue water potential, growth rate might actually reduce even though the cell walls were ‘loosened’. Therefore, it is necessary for long-term processes to be controlled with limited effect on those acting over shorter periods.
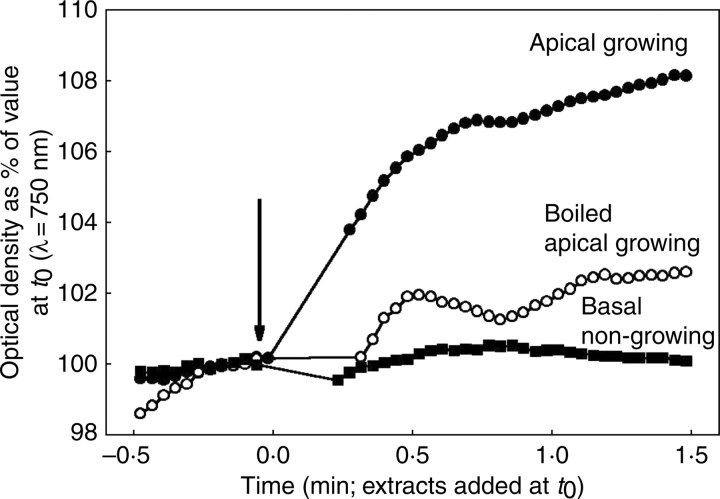
An interesting possibility, suggested by the observation that f2 and f3 are increased by high cell wall water content and that this is reduced by boiling is that a component of control of longer-term properties affecting growth is by regulation of cell wall spacing, and that this might be enzymically mediated. This was investigated by examining the effects of extracts from growing tissue on particle size in suspensions of cell wall fragments from boiled growing hypocotyls (measured by the effect on turbidity). Suspensions were prepared by boiling etiolated hypocotyls for 2 min and then homogenizing them in 10 mL distilled water containing 0·025 % Tween using a laboratory mixer/emulsifier at full speed for 5 min (Silverson Machines Ltd, Waterside, UK). The homogenate was then centrifuged for 5 min at 650 g to remove unfragmented tissue pieces and the supernatant centrifuged for 10 min at 1450 g. The pellet was resuspended in 10 mL of pH 5·0 MES buffer containing PEG to give an osmotic pressure of 1·0 MPa, 5 mM KCl and 1 mm CaCl2. The suspension was then again centrifuged at 1450 g for 10 min, after which the supernatant was discarded and the pellet resuspended in 10 mL of pH 5·0 MES buffer with 5 mm KCl and 1 mm CaCl2, but no PEG giving a cloudy, grey suspension of cell wall fragments. The optical density of the suspension was recorded for 30 s to measure initial optical density before addition of extract to the cuvette (extract from 17 mg f. wt for each ml suspension), inverted to mix the solutions and returned to the spectrophotometer. Extinction was then recorded for a further 90 s. Spectrophotometer readings used a wavelength of 750 nm but effects have been found to be independent of light wavelength across the range tested (400–750 nm). The effect of extracts of unboiled growing tissue, boiled growing tissue and non-growing tissue from the base of the hypocotyl are shown in Figure 4 (extracts were prepared by homogenizing tissue in 10 µL mg–1 f. wt of 1 m NaCl in a hand-held homogenizer with the same weight of acid-washed sand as fresh tissue). A rapid increase in turbidity was observed after addition of extract from unboiled growing tissue with an increase in optical density of approx. 8 % within 90 s, but there was little effect after addition of extracts from boiled tissue or tissue that had ceased to expand. Therefore it does seem that one or more enzymes present in growing tissues can cause cell wall swelling in vitro and it is possible that they facilitate growth by reducing spatial constraint in extending cell walls in vivo. The recent observation by Yennawar et al. (2006) that pellets of centrifuged maize cell walls treated with expansins expanded over the following 48 h suggests that expansins may cause some or all of the swelling and supports the proposal in Thompson (2005) that this may contribute to the effect of expansins on creep of plant tissue.
Fig. 4.
Graph showing the effects of adding enzyme extracts from apical growing, boiled apical growing or basal non-growing segments of hypocotyls of etiolated 5-d-old sunflower seedlings on turbidity of suspensions of cell wall fragments.
It is interesting to compare these properties of plant growth with use of temperature in shaping some plastics. An established factor in rheology of synthetic polymers is ‘time-temperature correspondence’, which describes the observation that analogous behaviour is observed over long time-scales at low temperatures and short time-scales at high temperatures (Aklonis et al., 1972). It could be argued that plants' use of long time-scale flow in growth is to some degree analogous to high temperature moulding of plastics, when flow at high temperatures is used to shape objects which are solid at lower temperatures. Indeed, microthermal analysis of plant cell walls has shown a melt-type transition occurs at much lower temperatures in cell walls of growing tissues at low pH (Lin et al., 1991).
At this point it might be useful to consider the phenomenon of tissue pressure and the associated hypothesis that epidermal tissues are particularly involved in regulating growth where tissue pressure is observed (e.g. Kutschera, 1992, 1995). This model has been disputed (Peters and Tomos, 1996) and it is clearly possible to consider a tissue where there are two or more cell types, none of which are growing but where their cell walls have different elastic moduli. Under these circumstances, stress will be distributed between the cell walls according to their respective elasticities but with no regulation of growth. If one of the cell types is still capable of expanding (i.e. the cell walls are capable of long-term flow) but another is not, then relaxation of the growing cell walls will occur with progressive transfer of stress to the non-flowing walls until either the inextensible walls fail, or stress in the extensible cell walls drops to the minimum required for flow. This is the situation proposed to exist in mature green tomato fruit (Thompson et al., 1998) and under these circumstances the non-extending tissue would be responsible for terminating growth at a point determined by temporal, environmental or hormonal cues (provided that it did not break or split under the increased stress). However, the third hypothetical possibility is a tissue that is still growing in which the cell walls of different tissues exhibit different viscosities. In this case, stress will be transmitted from the cells with more extensible walls (generally cortical cells) to those with less extensible walls (often epidermal cells). Kutschera (1995) has presented evidence suggesting that this is the case in growing hypocotyls of sunflower and Cucurbita pepo (zucchini) hypocotyls, growing epicotyls in Pisum sativum (pea) and growing coleoptiles in Avena sativa (oat) and Zea mays (maize). Alteration of the viscosity of either wall type could alter growth rate and so there is no physical reason why both cortical and epidermal cell walls should not be involved in regulating growth, but there may be biological advantages for control primarily residing in the cells with the ‘strongest’ walls. In these situations, altering wall properties would lead to a redistribution of stress between cell types unless changes were tightly co-ordinated. Such fluctuations in stress might well hinder precise regulation of growth and perhaps make the epidermis prone to failure (a risk illustrated by splitting of grape and tomato fruit after sudden watering or when heavy rain follows a dry spell). These difficulties would be minimized if cell wall viscosity of epidermal cell walls exceeded that of cortical walls by a large margin as cortical cell wall stresses would then remain at a near constant level close to the minimum required for extension of those cells and growth rates could be altered without substantial redistributions of stress between cell types. Under this arrangement, epidermal cells would act as target cells in growth regulation as has been hypothesized (although this argument remains speculative and may not be reflected in vivo).
Stomatal guard cells
Regulation of stomatal aperture is another instance where responses to hormonal and other regulatory signals require specific cell wall biomechanical properties. In many respects, the properties that guard cell walls must exhibit are opposite to those of growing cells in that it is estimated that turgor pressures can increase to >4 MPa during opening (Franks et al., 1998) which must cause an elastic change in cell shape taking place over a short period of time, but despite the extremely high stresses involved it is important that the cell walls do not permanently deform at their open dimensions as the walls must be able to ‘store’ a substantial proportion of the work done in opening the stomata so that it can be utilized during closure. If the energy was dissipated some closure might be driven by epidermal back pressure but the original cell shape and correct modulus for further opening and closing cycles would not be restored. Additionally, if stored elasticity is to be utilized effectively, frictional losses during closure must be minimized. Therefore, proper functioning of guard cells depends on short-term flexible and reversible extension but it is essential that the long-term irreversible flows of primary importance in growing tissue are completely suppressed.
Jones et al. (2003) have examined the effect of selective digestion of cell wall components on stomatal opening and closing in Commelina communis. They found that both opening (by fusicoccin) and closure (by ABA or 0·5 m mannitol) were inhibited completely by treatment with arabinanase and partially by feruloyl esterase, but that inhibition of closing was reversed when a combination of pectin methyl esterase and endopolygalacturonase were used to digest homogalacturonan. Stomatal ‘locking’ by arabinanase was plausibly attributed to removal of side branches of rhamnogalacturonan I so that the only the backbone (which includes stretches of homoglacturonan) remained and calcium ions could form cross-links between the charged galacturonic acid monomers of adjacent stretches. This then made the wall too rigid for the stomata to open or close. This is usually prevented because the homogalacturonan stretches are held apart by arabinan branches (which can be linked to one another via feruloyl esters cross-links, explaining the effect of feruloyl esterase). Additionally digestion of homogalacturonan with pectin methyl esterase and endopolygalacturonase caused the stomata to ‘gape’ wider than normal when open. These components of the guard cell wall and their function seem to be highly conserved with similar composition and behaviour also observed in maize and broad bean (Jones et al., 2005). It therefore seems that rhamnogalacturonan I is involved in maintaining the correct degree of polymer mobility in guard cell walls, and that this is at least partially due to spatial organization within the wall.
There have been reports of expression of the AtExp1 expansin genes in guard cells of arabidopsis (Cosgrove, 2000) and of increased stomatal opening and closure in tobacco overexpressing AtExp1 (Zhao et al., 2006). It seems odd (given the requirements for guard cell wall properties noted above) that a gene for a type of enzyme generally associated with growth and stress relaxation should be expressed in guard cells. However, expansins have also been found to increase in fruit ripening (Rose and Bennett, 1999) and both dehydration and rehydration of resurrection plants (Jones and McQueen-Mason, 2004), neither of which involve growth per se, but which do require cell wall flexibility. Although, expansins generally modify long-term properties with limited effect on the short-term reversible extension required here, the correct properties would result if the cell wall extension and contraction involved in stomatal opening and closure were substantially due to an equivalent of Kelvin element 1 in eqns (1) and (2) (the behaviour of which is consistent with promotion by expansins), provided that Kelvin element 2 and the viscous flow element were absent or suppressed. Given the posited role of expansins in growth regulation, an intriguing possibility is that they might also be involved in regulating stomatal function. Controlling cell wall flexibility could not determine the direction of change of aperture (which must be determined by guard cell and companion cell turgors), but there might advantages to modulating fluidity of the guard cell wall. Freeing polymer movement during closure could compensate for the inevitable loss of some elastic energy and facilitate closure, while ‘fixing’ the cell walls once stomata had opened could help prevent permanent deformation or dissipation of elastic energy. Also, suppression of loosening might be consistent with the occasional instances of stomatal lock-open observed in vivo (e.g. Allègre et al., 2007).
Abscission cells
Abscission of plant organs involves separation of functionally differentiated abscission cells (Wright and Osborne, 1974) and appears to include at least two mechanical events:
Reduced adherence between cells caused by dissolution of the pectin-rich middle lamellae between abscission cells (e.g. Valdovinos and Jensen, 1968).
Growth and rounding of the abscission cells (e.g. Osborne and Sargent, 1976; Sexton and Redshaw, 1981).
Dissolution of the middle lamella would be expected to be associated with increased levels of polygalacturonases and related enzymes and this is the case (e.g. Taylor et al., 1993) although it is worth noting that expression of abscission-associated polygalacturonase genes is not confined to the abscission zone (Hong et al., 2000) suggesting their presence alone is not sufficient for cell separation to occur.
It seems probable that cell rounding and growth are the ultimate consequences of cell wall loosening processes that initially generate increasing tissue pressures culminating in fracture of cells and tissues that cannot extend, such as the vascular traces. This is actually analogous to the situations described in the earlier consideration of tissue pressure where one cell type has walls that are more extensible than another so that stress is progressively transferred to the more rigid walls until (in this case) they fail. Sexton and Redshaw (1981) have noted that sufficient pressure was generated for abscission of leaves of Impatiens sultani by the abscission cells losing their initial flattened polygonal shape and becoming more spherical so that their volume increased with little increase in surface area, although in other cases the abscission cells seem to expand too much for this to be the case (e.g. Wright and Osborne, 1974). Adoption of a round shape is clearly mechanically favourable for any pressurized structure and may have additional advantages for abscission, as adjacent cells will push one another apart across most of their common faces but will pull away from one another at the edges, peeling the middle lamellae apart.
Abscission cell growth and reshaping could be due to removal of growth suppression in cells where the walls have retained the capacity for extension or to a restored capacity for growth in secondary cell walls from which it has been lost. The latter seems more likely because rounding of abscission cells involves extension in directions in which growth was limited during initial expansion and because mature cells can transdifferentiate into functional abscission cells (McManus et al., 1998).
Cell rounding could be due to enzymes capable of breaking down cell wall components reinforcing the cell corners, but there have also been a number of observations of cell wall swelling during abscission (e.g. Valdovinos and Jensen, 1968; Sexton et al., 1977) and in the framework suggested earlier, it might be suggested that increased spacing allowing greater polysaccharide mobility might actually be a key component of the abscission process and not just a consequence of other wall loosening events. Increased separation between cellulose microfibrils could release the tight entanglement in mature cell walls and allow them to pull through the matrix and to realign. It appears that the cell walls of abscission cells can swell to a very substantial degree [Sexton et al. (1977) report that abscission cell walls expand to 5–10 µm during foliar abscission in Coleus blumei] and stresses on the cell wall would tend to cause realignment of microfibrils to give a more spherical cell shape once they were released from other constraints.
The period over which abscission occurs (typically 24–72 h) would tend to suggest that expansion and rounding result from reasonably long-term cell wall processes but, in this case, the interpretation is not completely clear cut. The reason is that abscission requires a high pI endo-1,4-β-glucanhydrolase (Sexton et al., 1980; Clements and Atkins, 2001) and perhaps other enzymes and so it is not possible to separate the time required for de novo protein synthesis and for the enzymes to have their effect from the time period over which rheological processes occur. However, if cell wall swelling is important, then the process might be analogous to the effect of increasing cell wall water content on creep of growing material as described above, and which primarily promoted long-term processes.
SUMMARY
This paper has argued that plant cell walls are complex systems in which multiple rheological processes occur, some reversible and others irreversible and that each of these processes takes place over a different and distinctive time-scale. Additionally, evidence has been presented showing that separation between cell wall components, perhaps particularly the cellulose microfibrils, affects these rheological elements and modulates cell wall biomechanical properties.
In the light of these propositions, it was noted that different cell types require cell walls that exhibit specific and often different biomechanical behaviour. Physiological control may then depend upon regulation of individual rheological processes in the wall, depending upon whether changes need to be reversible or not and the time-scales over which they must occur. Finally, controlled spatial organization within the cell wall often seems to be fundamental in ensuring correct cell wall and cell function.
ACKNOWLEDGEMENTS
Bhavita Majevadia helped with much of the preliminary work. Support for the research has been provided by grants from the Nuffield Foundation and the University of Westminster. The extensiometer was constructed by Mr Philip Smith and is on loan from Lancaster University.
NOTE ADDED IN PROOF
The osmotic pressures in this manuscript were determined by freezing point osmometry. Subsequent measurements using vapour pressure osmometry have been found to differ from data obtained by freezing point for PEG solutions [a phenomenon noted in Kiyosawa, (2003)]. Measurements of PEG solutions determined to have osmotic pressures of 1.0 MPa and 0.25 MPa by freezing point osmometry gave osmotic pressures of 0.62 MPa and 0.11 MPa respectively by vapour pressure osmometry.
LITERATURE CITED
- Aklonis JJ, MacKnight WJ, Shen M. Introduction to polymer viscoelasticity. New York: John Wiley and Sons; 1972. [Google Scholar]
- Allègre M, Daire X, Héloir M-C, Trouvelot S, Mercier L, Adrian M, et al. Stomatal deregulation in Plasmopara viticola-infected grapevine leaves. New Phytologist. 2007;173:832–840. doi: 10.1111/j.1469-8137.2006.01959.x. [DOI] [PubMed] [Google Scholar]
- Büntemeyer K, Lüthen H, Böttger M. Auxin-induced changes in cell wall extensibility of maize roots. Planta. 1998;204:515–519. [Google Scholar]
- Carpita N, Sabularse D, Montezinos D, Delmer DP. Determination of the pore size of cell walls of living plant cells. Science (New Series) 1979;205:1144–1147. doi: 10.1126/science.205.4411.1144. [DOI] [PubMed] [Google Scholar]
- Chanliaud E, Gidley MJ. In vitro synthesis and properties of pectin/Acetobacter xylinus cellulose composites. The Plant Journal. 1999;20:25–35. doi: 10.1046/j.1365-313x.1999.00571.x. [DOI] [PubMed] [Google Scholar]
- Clements JC, Atkins CA. Characterization of a non-abscission mutant in Lupinus angustifolius L.: physiological aspects. Annals of Botany. 2001;88:629–635. [PubMed] [Google Scholar]
- Cosgrove DJ. New genes and new biological roles for expansins. Current Opinion in Plant Biology. 2000;3:73–78. doi: 10.1016/s1369-5266(99)00039-4. [DOI] [PubMed] [Google Scholar]
- Edelmann HG. Water potential modulates extensibility of rye coleoptile cell walls. Botanica Acta. 1995;108:374–380. [Google Scholar]
- Franks PJ, Cowan IR, Farquhar GD. A study of stomatal mechanics using the cell pressure probe. Plant, Cell and Environment. 1998;21:94–100. [Google Scholar]
- Hong S-B, Sexton R, Tucker ML. Analysis of gene promoters for two tomato polygalacturonases expressed in abscission zones and the stigma. Plant Physiology. 2000;123:869–881. doi: 10.1104/pp.123.3.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L, McQueen-Mason S. A role for expansins in dehydration and rehydration of the resurrection plant Craterostigma plantagineum. Federation of European Biochemical Societies Letters. 2004;559:61–65. doi: 10.1016/S0014-5793(04)00023-7. [DOI] [PubMed] [Google Scholar]
- Jones L, Milne JL, Ashford D, McCann MC, McQueen-Mason SJ. A conserved functional role of pectic polymers in stomatal guard cells from a range of plant species. Planta. 2005;221:255–264. doi: 10.1007/s00425-004-1432-1. [DOI] [PubMed] [Google Scholar]
- Jones L, Milne JL, Ashford D, McQueen-Mason SJ. Cell wall arabinan is essential for guard cell function. Proceedings of the National Academy of Sciences of the USA. 2003;100:11783–11788. doi: 10.1073/pnas.1832434100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutschera U. The role of the epidermis in the control of elongation growth in stems and coleoptiles. Botanica Acta. 1992;105:246–252. [Google Scholar]
- Kutschera U. Tissue pressure and cell turgor in axial plant organs: implications for the organismal theory of multicellularity. Journal of Plant Physiology. 1995;146:126–132. [Google Scholar]
- Lin L-S, Yuen HK, Varner JE. Differential scanning calorimetry of plant cell walls. Proceedings of the National Academy of Sciences of the USA. 1991;88:2214–2243. doi: 10.1073/pnas.88.6.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus MT, Thompson DS, Merriman C, Lyne L, Osborne DJ. Transdifferentiation of mature cortical cells to functional abscission cells in bean. Plant Physiology. 1998;116:891–899. doi: 10.1104/pp.116.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malloch KR, Osborne DJ. Auxin and ethylene control of growth in seedlings of Zea mays L. and Avena sativa L. Journal of Experimental Botany. 1976;27:992–1103. [Google Scholar]
- Osborne DJ, Sargent JA. The positional differentiation of ethylene-responsive cells in rachis abscission-zones in leaves of Sambucus nigra and their growth and ultrastructural changes at senescence and separation. Planta. 1976;130:203–210. doi: 10.1007/BF00384421. [DOI] [PubMed] [Google Scholar]
- Palmer SJ, Davies WJ. An analysis of relative elemental growth rate, epidermal cell size and xyloglucan endotransglycosylase activity through the growing zone of ageing maize leaves. Journal of Experimental Botany. 1996;47:339–347. [Google Scholar]
- Palmer SJ, Berridge DM, McDonald AJS, Davies WJ. Control of leaf expansion in sunflower (Helianthus annuus L.) by nitrogen nutrition. Journal of Experimental Botany. 1996;47:359–368. [Google Scholar]
- Peters WS, Tomos AD. The history of tissue tension. Annals of Botany. 1996;77:657–665. doi: 10.1006/anbo.1996.0082. [DOI] [PubMed] [Google Scholar]
- Rose JKC, Bennett AB. Cooperative disassembly of the cellulose-xyloglucan network of plant cell walls: parallels between cell expansion and fruit ripening. Trends in Plant Science. 1999;4:176–183. doi: 10.1016/s1360-1385(99)01405-3. [DOI] [PubMed] [Google Scholar]
- Scott Blair GW. Elementary rheology. London: Academic Press; 1969. [Google Scholar]
- Sexton R, Redshaw AJ. The role of cell expansion in the abscission of Impatiens leaves. Annals of Botany. 1981;48:745–757. [Google Scholar]
- Sexton R, Durbin ML, Lewis LN, Thomson WW. Use of cellulase antibodies to study leaf abscission. Nature. 1980;283:873–874. [Google Scholar]
- Sexton R, Jamieson GGC, Allan MHIL. An ultrastructural study of abscission zone cells with special reference to the mechanism of enzyme secretion. Protoplasma. 1977;91:369–387. [Google Scholar]
- Taylor JE, Webb STJ, Coupe SA, Tucker GA, Roberts JA. Changes in polygalacturonase activity and solubility of polyuronides during ethylene-stimulated abscission in Sambucus nigra. Journal of Experimental Botany. 1993;44:93–98. [Google Scholar]
- Thompson DS. Extensiometric determination of the rheological properties of the epidermis of growing tomato fruit. Journal of Experimental Botany. 2001;52:1291–1301. [PubMed] [Google Scholar]
- Thompson DS. How do cell walls regulate plant growth? Journal of Experimental Botany. 2005;56:2275–2285. doi: 10.1093/jxb/eri247. [DOI] [PubMed] [Google Scholar]
- Thompson DS, Davies WJ, Ho LC. Regulation of tomato fruit growth by epidermal cell wall enzymes. Plant, Cell and Environment. 1998;21:589–599. [Google Scholar]
- Valdovinos JG, Jensen TE. Fine structure of abscission zones. II. Cell wall changes in abscising pedicels of tobacco and tomato flowers. Planta. 1968;83:295–302. doi: 10.1007/BF00385339. [DOI] [PubMed] [Google Scholar]
- Ward IM, Hadley DW. An introduction to the mechanical properties of solid polymers. Chichester: John Wiley & Sons; 1993. [Google Scholar]
- Whitney SEC, Gothard GE, Mitchell JT, Gidley MJ. Roles of cellulose and xyloglucan in determining the mechanical properties of primary cell walls. Plant Physiology. 1999;121:657–663. doi: 10.1104/pp.121.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright M, Osborne DJ. Abscission in Phaseolus vulgaris: the positional differentiation and ethylene-induced expansion growth of specialised cells. Planta. 1974;120:163–170. doi: 10.1007/BF00384926. [DOI] [PubMed] [Google Scholar]
- Yennawar NH, Li L-C, Dudzinski DM, Tabuchi A, Daniel J., Cosgrove DJ. Crystal structure and activities of EXPB1 (Zea m 1), a β-expansin and group-1 pollen allergen from maize. Proceedings of the National Academy of Sciences of the USA. 2006;103:14664–14671. doi: 10.1073/pnas.0605979103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Davies WJ. Does ABA in the xylem control the rate of leaf growth in soil-dried maize and sunflower plants? Journal of Experimental Botany. 1990;41:1125–1132. [Google Scholar]
- Zhao P, Chen S, Wang X-C. Arabidopsis expansin AtEXP1 involved in the regulation of stomatal movement. Acta Agronomica Sinica. 2006;32:562–567. [Google Scholar]
References
- Kiyosawa K. Theoretical and experimental studies on freezing point depression and vapor pressure deficit as methods to measure osmotic pressure of aqueous polyethylene glycol and bovine serum albumin solutions. Biophysical Chemistry. 2003;104:171–188. doi: 10.1016/s0301-4622(02)00365-4. [DOI] [PubMed] [Google Scholar]