Abstract
Background and Aims
The plants that have remained in the contaminated areas around Chernobyl since 1986 encapsulate the effects of radiation. Such plants are chronically exposed to radionuclides that they have accumulated internally as well as to α-, β- and γ-emitting radionuclides from external sources and from the soil. This radiation leads to genetic damage that can be countered by DNA repair systems. The objective of this study is to follow DNA repair and adaptation in haploid cells (birch pollen) and diploid cells (seed embryos of the evening primrose) from plants that have been growing in situ in different radionuclide fall-out sites in monitored regions surrounding the Chernobyl explosion of 1986.
Methods
Radionuclide levels in soil were detected using gamma-spectroscopy and radiochemistry. DNA repair assays included measurement of unscheduled DNA synthesis, electrophoretic determination of single-strand DNA breaks and image analysis of rDNA repeats after repair intervals. Nucleosome levels were established using an ELISA kit.
Key Results
Birch pollen collected in 1987 failed to perform unscheduled DNA synthesis, but pollen at γ/β-emitter sites has now recovered this ability. At a site with high levels of combined α- and γ/β-emitters, pollen still exhibits hidden damage, as shown by reduced unscheduled DNA synthesis and failure to repair lesions in rDNA repeats properly. Evening primrose seed embryos generated on plants at the same γ/β-emitter sites now show an improved DNA repair capacity and ability to germinate under abiotic stresses (salinity and accelerated ageing). Again those from combined α- and γ/β-contaminated site do not show this improvement.
Conclusions
Chronic irradiation at γ/β-emitter sites has provided opportunities for plant cells (both pollen and embryo cells) to adapt to ionizing irradiation and other environmental stresses. This may be explained by facilitation of DNA repair function.
Key words: DNA repair, adaptation, pollen, seed, Chernobyl, radionuclides, Betula verrucosa, Oenothera biennis
INTRODUCTION
After the accident at Chernobyl, large areas of Europe were contaminated by a spectrum of radionuclides causing major concerns about environmental pollution (Hohenemser et al., 1986). The wide dispersal and non-uniformity of this contamination added special problems (Levi, 1991; Lindner, 2000). These include the presence of numerous ‘hot spots’ originating from particular types of fall-out, different solubility and toxicity of the generated radionuclides and their subsequent interaction with other chemical compounds in the environment (Salbu et al., 1994; Bondarkov et al., 1999; Chesser et al., 2004). As a result, prediction of the long-term impact of Chernobyl contamination on biological subjects remains uncertain.
The plants that have remained in the contaminated areas since 1986 encapsulate all the effects of radiation. Such plants are exposed to α-, β- and γ-emitting radionuclides from external sources as well as to radionuclides that are absorbed from the soil and accumulated internally. This combined irradiation leads to an accumulation of DNA damage within each cell. Pollen cells, because of their haploid status, are particularly susceptible to loss of genetic integrity in a radio-contaminated environment. DNA damage arising from accumulated radiation doses can cause genetic deficiencies in future offspring. Thus birch pollen, with its long formation period (10 months), spent mostly in the haploid state, is a most suitable biological material for the study of irradiation effects in a radio-contaminated area such as Chernobyl (Boubriak and Grodzinsky, 1989). This pollen model was also selected because birch trees are widespread in the Chernobyl area and so the same species is available at different contamination levels where radiation monitoring can be set up. Finally it is possible to collect significant amounts of pollen (grams) from the same trees, which is crucial for the biochemical assays used.
Genetic responses to irradiation mainly depend upon the organization, size and numbers of chromosomes, and on the efficiency of DNA repair systems in restoring the integrity of heritable information. Although a link between the extent of radiation damage (radiosensitivity) and the size of the chromosome target is well established (Osborne and Bacon, 1960; Sparrow et al., 1967) the involvement of different plant DNA repair systems is still not fully resolved.
Essential DNA maintenance in plants is carried out by base excision repair, which can eliminate single-base damage including oxidation and base loss (Morales-Ruiz et al., 2003; Mori et al., 2005), which are lesions commonly caused by ionizing radiation. The most versatile system for dealing with the DNA damage accumulated in consequence of irradiation is nucleotide excision repair (NER). This system can repair different types of damage because it recognizes conformational changes in the DNA duplex rather than a specific type of DNA damage. Constitutive repair of γ-radiation-induced DNA damage was first demonstrated in protoplasts isolated from cultured wild carrot cells (Howland, 1975). A number of enzymes involved in repair of radiation-induced DNA damage have been shown to affect mutation rate, chromosome aberration frequencies and viability in seeds and seedlings (Cheah and Osborne, 1978; Elder et al., 1987; Boubriak et al., 1997). Viability under irradiation also depends, to great extent, on the repair of double-strand DNA breaks which, in plants, is achieved both by homologous recombination and non-homologous end joining (Pacher et al., 2007), although non-homologous end joining seems to be more important (West et al., 2004; Puchta, 2005). Radiation-sensitive plant mutants have now been isolated (Jiang et al., 1997; Britt, 1999; Liu et al., 2001; Bray and West, 2005) and many plant genes from different repair pathways involved in repair of radiation-induced damage have been cloned (Bleuyard et al., 2005; Morgante et al., 2005; Liang et al., 2006; Vonarx et al., 2006). However, to date, the efficiency of NER and double-strand break repair and the stability/modification of these systems under chronic irradiation conditions have not been reported.
The efficiency of plant DNA excision repair is modified by continuous exposure to certain environmental pressures. For example, Corylus avellana pollen from trees grown at high altitude (under high levels of UV radiation) shows more efficient repair of both UV and γ-induced DNA damage than pollen of the same species grown near to sea level (Boubriak and Grodzinsky, 1985). The efficiency of DNA repair seems also to be sensitive to the type and duration of radiation encountered; chronic β-irradiation of parental plants gives an enhanced repair capacity in γ-irradiated seeds at germination. In contrast, no repair enhancement was found in similar experiments with chronic α-irradiation (Abramov et al., 1992b; Semov et al., 1997). The influence that combined chronic irradiation conditions have had on DNA repair in indigenous plants is therefore of special relevance to Chernobyl.
In early experiments with birch pollen from Chernobyl sites, no evidence of radiation stimulation of DNA repair activity or of any alteration in the expression of DNA repair enzymes was found (Boubriak et al., 1990) However, it was shown that UDS, which in binucleate pollen reflects the DNA repair synthesis step of NER (Jackson and Linskens, 1980; Boubriak and Grodzinsky, 1986), was strongly inhibited in the first (1987) generation of pollen formed on birch trees after the Chernobyl accident (Boubriak and Grodzinsky, 1989). In the years that followed (1988–1990) subsequent pollen generations have regained much of their capacity to perform UDS after radiation stress except in pollen from the highest levels of contamination from combined α- and γ/β-irradiation (Boubriak et al., 1991). We show evidence here that pollen formed at intermediate levels of contamination, with only γ/β-emitters present, are now showing an enhanced DNA repair capability and an increased tolerance to stress conditions.
The germination capacity under stress of seeds of a biennial species, Oenothera biennis, that grows wild in the areas around Chernobyl, was chosen as the second model plant system in which to assess whether adaptive survival mechanisms had arisen in the multiple generations of progeny (in contrast to pollen generations from the same trees) that have thrived there, undisturbed, in situ. Unfortunately no viable birch seeds were available for comparison but, where possible, Oenothera seeds were collected from the same contaminated ‘birch sites’. In fact this was the only suitable species growing at two out of the three selected ‘birch sites’ and Oenothera was also available at highly contaminated places with a significant α-emitter contribution. The choice of a biennial plant rather than a short-lived annual was intentional, because of the long-term radionuclide exposure to the parent before gamete formation.
The stresses imposed upon the harvested or germinating seed were either (a) an additional γ-irradiation dose; (b) the environmental pressures of enhanced temperature and increased humidity (accelerated ageing) given while the seed was in the ‘dry’ state; or (c) the inclusion of saline conditions in the medium in which the untreated seeds were germinated.
Previously, in an unpublished Royal Society Internal Report, it had been reported by the authors that whereas seeds from the high γ/β-emitter sites showed an improved capacity to survive ageing or additional stresses, those from high combined α-emitter and γ/β-emitter sites remain impaired in their responses.
In this paper, the long-term legacy of different types of radiation insult upon the inherent stability and adaptation of plants to chronic irradiation from radionuclide contamination is described.
MATERIALS AND METHODS
Plant material and radioactivity measurements
Pollen from birch trees (Betula verrucosa L.) has been collected every year since 1987 from three selected contamination sites inside the 30-km zone around the destroyed Chernobyl reactor. Radioactivity in soil was measured using a γ-spectrometer with DGDK-100 detector and an AM-A-02F1 analyser and by radiochemistry (Kashparov et al., 2001; Yoschenko et al., 2006). The contamination levels and radionuclide composition of soils at different locations in 1987 were: level I (Chernobyl control), total activity 5·2 × 1010 Bq km−2; level II (Kopachy), total activity 4·9 × 1013 Bq km−2; level III (Novo-Tchepelichy), total activity 1·3 × 1014 Bq km−2. The radionuclide composition for soils at these sites is presented in Table 1 (section A). Only level-III contamination had a significant amount of α-emitters in the soil (3·6 × 102 Bq kg−1). In the final year of pollen collection (1998) the total activity in soils had fallen to the following amounts: level I (Chernobyl control), 3·3 × 109 Bq km−2; level II (Kopachy), 1·1 × 1012 Bq km−2; level III (Novo-Tchepelichy), 1·8 × 1013 Bq km−2. Pollen was collected each year from the same trees by cutting branches with mature, but unopened, catkins. After shedding, dry pollen was either stored at −20 °C in glass tubes or quickly frozen and stored in liquid nitrogen. Under these conditions pollen maintained a high germination capability (70–95 %) for up to 4 (freezer) or 10 (liquid nitrogen) years. For germination, 50 mg of pollen (in triplicate) was first hydrated in moist air (45 min) then held in aerated 15 % sucrose/0·01 % boric acid solution.
Table 1.
Radionuclide composition at the different contaminated sites in the Chernobyl zone used for pollen and seed collections
| Activity of radionuclide (103 Bq kg−1) |
||||||
|---|---|---|---|---|---|---|
| Year of collection | Collection site | 137Cs | 134Cs | 144Ce | 106Ru | 90Sr |
| (A) Pollen collection | ||||||
| 1987 | Chernobyl, control | 1·8 | 0·6 | – | – | 1·6 |
| Kopachy, level I | 9·3 | 2·9 | 1·0 | 0·6 | 4·7 | |
| Novo‐Tchepelichy, level II | 18 | 6·5 | 3·8 | 2·7 | 8·7 | |
| (B) Seed collections | ||||||
| 1998 | Chernobyl, control | 1·3 | – | – | – | 1·1 |
| Kopachy, level I | 6·8 | – | – | – | 2·7 | |
| Yanov, level II | 28 | 1·2 | – | – | 12 | |
Seed from Oenothera biennis was collected first in 1998 from the three contaminated sites. Level I and level II contamination is at the same sites as for the pollen (Chernobyl and Kopachy), but level III (the highest) contamination was from a new Yanov site (no Oenothera seeds were found at the Novo-Tchepelichy site). The total activity in soils for these sites in the first year of collection in 1998 was as follows: level I (Chernobyl control), 3·1 × 109 Bq km−2; level II (Kopachy), 7·2 × 1012 Bq km−2; level III (Yanov), 1·4 × 1014 Bq km−2. The radionuclide composition of soils at these sites is shown in Table 1 (section B). Only the Yanov site contained significant levels of α-emitters: 9·4 × 102 Bq kg−1. In the final year of seed collection (2001), the total activity in soils had fallen to the following amounts: level I (Chernobyl control), 2·8 × 109 Bq km−2, level II (Kopachy), 6·4 × 1012 Bq km−2; level III (Yanov), 8·6 × 1013 Bq km−2.
Irradiation and incorporation studies
Dry pollen or seeds (four repeats for each sample) were γ-irradiated (500–2000 Gy, dose rate 893 cGy min−1) from a 137Cs source (RX 30/55 M Irradiator, Gravatom Industries, UK) and used immediately in experiments. UDS was measured by the incorporation of [3H]methylthymidine (Amersham, UK) into TCA-insoluble material as described earlier (Boubriak and Grodzinsky, 1985; Elder and Osborne, 1993). Briefly, for pollen: 50 mg of pollen were germinated in aerated 15 % sucrose with 0·01 % boric acid in the presence of 40 µCi of methyl-[3H]thymidine (specific activity 2·0 TBq mmol−1, 74 Ci mmol−1; Amersham International, UK). After repair intervals pollen was fixed and washed six times with unlabelled cold thymidine solution followed by TCA washes. DNA was extracted with hot HClO4 and half of the sample was used for radioactivity measurement in a liquid scintillation counter (Rackbeta 1209, LKB Wallac, Finland) and another half for measuring DNA concentration.
Absence of replication in the first 24 h of Oenothera seed imbibition was confirmed using hydroxyurea (10 mm solution), an inhibitor of replication.
DNA isolation and Southern blot hybridization to rDNA
For DNA isolation (in triplicates) from pollen, a commercial Genomic DNA kit (InViSorb™, Bioline, UK) was used. The DNA content for each isolated sample was quantified using either absorption at 260/280 nm or fluorescence at 550 nm using the DNA-specific dye PicoGreen™ (Molecular Probes Europe, The Netherlands). After restriction of DNA with MboI (or DpnII), which cuts at both sides of the sequence GATC, followed by electrophoresis of pooled DNA samples, DNA was transferred to Hybond C+ (Amersham, UK) membrane and probed with the 9-kb rDNA repeat unit from wheat described by Gerlach and Bedbrook (1979). This probe, cloned into pTA71 plasmid, was successfully used to probe rDNA composition in birch DNA (Bousquet et al., 1989) and was kindly provided by Dr Leonid Sitailo (Layola University, USA). The DNA probe was labelled with 32P using a random-prime labelling kit (Amersham, UK), hybridized to the membrane (at 65 °C) and, after stringency washing, radioactivity was detected on Hyperfilm (Amersham, UK) and analysed on a UVP Image Analyser (UVP, UK) (Boubriak et al., 2002)
Nucleosome analyses
The nucleosome content of pollen was measured using a Nucleosome ELISA kit (Oncogene Research Products, Calbiochem, USA). For this, 100 mg of pollen (in triplicate) was germinated for 3 h, ground in dry ice and lysed in 750 µL of the buffer supplied. Samples were diluted 1:8 and 1:16 for immunological analysis against the kit Daudi cell standards. The data represent units of nucleosomes per 1 ml of original pollen lysis extract.
Seed germination experiments
Sets of seeds (six repeats for each sample) were assessed for their germination success either by planting onto 1·5 % agar at 27 °C for 3 d in the dark followed by transfer to the light or on filter paper at 24 °C in the dark and supplied with water or a solution of gibberellin A3 (500 µ m) to overcome any dormancy that might be inherent in the seed lots.
(a) Accelerated ageing treatments
Weighed sets of dry seed (four repeats for each sample), enclosed in muslin bags, were suspended over saturated solutions of NaCl in closed Kilner jars and held at 40 °C, providing conditions of 74 % relative humidity. Seeds were re-weighed immediately at the end of the treatment, air dried to the original weight and used at once. The original moisture content of all dry seeds was 9·1–9·4 %. Germination tests on filter paper (no gibberellin) were then carried out at 24 °C in the dark.
(b) Salinity stress treatments
Sets of 25-mg samples (six repeats per sample) of dry seed were held on filter paper moistened with an aqueous solution of NaCl (150 mm) at 24 °C in the dark (no gibberellin).
(c) Seedling survival after γ-irradiation stress
The ability to survive and continue to grow was assessed in 10-d-old seedlings (25 seedlings per repeat) after they were exposed to chronic γ-irradiation ranging from 0 to 150 Gy.
Analysis of repair of single-strand DNA breaks
Measurements of repair of DNA single-strand breaks (SSB) in germinating seed were carried out using an electrophoretic method (Yamamoto et al., 1982; Syomov et al., 1992). To allow repair, the seeds (in triplicates) were first halved longitudinally to expose the embryo and then soaked in water in the presence of chloramphenicol (10 µg ml−1) (6–24 h). Seeds irradiated with 750 Gy were mixed, after various repair intervals, with dry ice and homogenized. DNA was extracted using a Nucleon Phyto-Pure Plant DNA extraction kit (Amersham, UK) according to the manufacturer's specifications. The combined DNA extracts were purified further by ethanol precipitation, quantified and diluted to 100 µg ml−1. DNA was denatured with an equal volume of 10 m urea and heated at 95 °C and electrophoresis was carried out in TAE buffer. After staining with ethidium bromide, gels were analysed on a Specord M40 spectrometer (Carl Zeiss, Germany). A maximum was determined, as was the mean numerical DNA weight and the number of SSBs (Yamamoto et al., 1982).
Statistical analysis
Data in all tables are presented as mean value of all repeats (from three to six repeats in the different experiments) ± standard error (SEM). Statistical analysis was based on a two-tailed t-test using programme GraphPad Instat.
RESULTS
Pollen
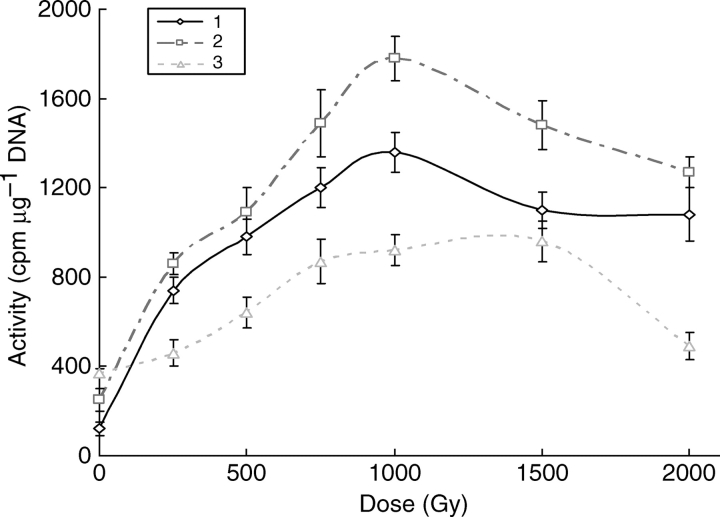
Unscheduled DNA synthesis during the first 3 h of pollen germination was followed in material collected from specific, identified birch trees at monitored radionuclide-contaminated sites in the 30-km zone around Chernobyl in successive years from 1987 to 1998. In the absence of replication and endoreduplication in birch pollen UDS represents repair synthesis in NER and can be used as a measure of dark repair efficiency (Boubriak and Grodzinsky, 1986; Jackson, 1987). Over the period of 11 years the ability of the pollen to carry out UDS on germination and, hence, to repair DNA lesions following an applied γ-radiation dose has improved annually and is now similar to that of unirradiated controls (Table 2). The results for 1998 for overall unscheduled DNA synthesis, i.e. repair of lesions developed during pollen formation, are now apparently little different from the capability of uncontaminated control trees. However, the ability to up-regulate this synthesis in response to a further radiation dose administered to the dry pollen after collection, shows a difference for pollen formed in a predominantly γ/β-emitter site and pollen from the combined α- and γ/β-irradiation sites (Fig. 1). The intermediate levels of chronic γ/β-irradiation result in an enhancement of the response to acute γ-irradiation, whilst a repair malfunction still exists in pollen from the trees that are highly contaminated with both α- and γ/β-emitters (Novo-Tchepelichy). The latter pollen is less able than the Chernobyl control to achieve full DNA repair capability following an additional radiation stress above 250 Gy.
Table 2.
Unscheduled DNA synthesis in birch pollen collected during 1987–1997 from Chernobyl sites with different radionuclide contamination
| Collection site | 1987 | 1989 | 1991 | 1993 | 1995 | 1997 |
|---|---|---|---|---|---|---|
| Chernobyl, control | 1050 ± 90 | 1280 ± 160 | 1070 ± 170 | 1160 ± 90 | 1320 ± 240 | 1160 ± 180 |
| Kopachy, level I | 70 ± 20* | 620 ± 310** | 880 ± 260 | 1010 ± 80 | 1430 ± 130 | 1580 ± 230* |
| Novo-Tchepelichy, level II | n/a | n/a | 450 ± 170* | 820 ± 90** | 710 ± 240* | 910 ± 160 |
After 500 Gy irradiation all pollen samples were allowed to repair on germination with 40 µCi of methyl-[3H]thymidine (specific activity varied in different years from 70 to 78 Ci mmol−1). Radioactivity data presented here in cpm−1 μg−1 DNA were corrected for the percentage of pollen germination in each year.
*,** Significantly different from the pollen from other sites at P ≤ 0·01 and P ≤ 0·05, respectively.
Fig. 1.
Dose dependence of unscheduled DNA synthesis in birch pollen from Chernobyl sites (1998) with different radionuclide contamination. 1, Chernobyl, control; 2, Kopachy, γ/β-emitter site (level II contamination); 3, Novo-Tchepelichy, combined α- and γ/β-irradiation (level III contamination). Radioactivity data presented here in cpm μg−1 DNA was corrected for the percentage of pollen germination. SEM presented for each point.
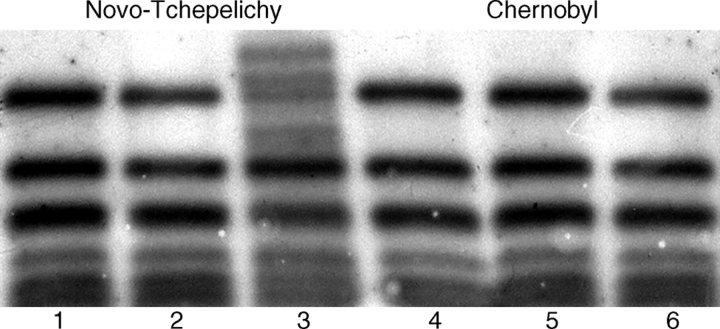
Further evidence that pollen collected from the Novo-Tchepelichy site in 1998 still exhibits limitations in DNA repair function is shown in the failure to properly repair newly imposed damage to the rDNA gene cluster. Gamma-irradiation of dry pollen (750 Gy) from a control site and from the Novo-Tchepelichy site, followed by 3 h germination, extraction and fractionation of DNA and Southern hybridization to a rDNA repeat (Fig. 2) shows quite clearly that the new radiation damage to Novo-Tchepelichy pollen (lane 3) has not been properly repaired on germination compared with the control sample (lane 6). An additional indication of nuclear instability to stress is provided by the presence of nucleosome multimers in the pollen collected from the radionuclide-contaminated sites. In eukaryotic systems, nucleosome accumulation represents a crisis state in the progression to programmed cell death (Coupe et al., 2004) and programmed cell death can be induced by irradiation or oxidative stress (Tiwari et al., 2002; Danon et al., 2004; Overmyer et al., 2005). Pollen collected from both Kopachy and Novo-Tchepelichy in 1995 possesses an increased level of free nucleosomes which is not attributable to differences in percentage viability compared with the control (Table 3), but by 1998 only Novo-Tchepelichy pollen shows this increase. It is not know at what stage of pollen formation, pollen desiccation, pollen dispersal or pollen germination, nucleosome accumulation occurs, but it represents further evidence for the continuing nuclear DNA abnormalities in pollen from birch trees that are still stressed by this chronic irradiation.
Fig. 2.
In vivo repair of rDNA in γ-irradiated pollen from differently radionuclide contaminated Chernobyl sites. Lanes 1–3, Novo-Tchepelichy level III contamination; lanes 4–6, Chernobyl control; lanes 1 and 4, MboI-digested DNA from pollen germinated for 3 h; lanes 2 and 5, MboI-digested DNA from dry pollen irradiated with 750 Gy; lanes 3 and 6, MboI-digested DNA from pollen irradiated with 750 Gy, germinated (repaired) for 3 h.
Table 3.
Nucleosome contents in germinated pollen samples from Chernobyl sites with different radionuclide contamination
| Collection sites/levels of pollution | 1995 relative units | Germination (%) | 1997 relative units | Germination (%) | 1998 relative units | Germination (%) |
|---|---|---|---|---|---|---|
| Chernobyl control | 0·14 ± 0·05 | 92·3 ± 3·3 | 0·11 ± 0·02 | 86·4 ± 6·3 | 0·17 ± 0·05 | 88·4 ± 7·7 |
| Kopachy level I | 0·47 ± 0·13* | 74·2 ± 2·2 | – | – | 0·19 ± 0·07 | 84·4 ± 6·6 |
| Novo-Tchepelichy level II | 0·51 ± 0·08* | 84·1 ± 5·7 | 0·92 ± 0·15* | 72·8 ± 2·9 | 0·84 ± 0·12* | 76·4 ± 8·3 |
Data are presented as nucleosome units per 1 ml of original lysate. One nucleosome unit per millilitre is defined as the amount of nucleosomes from 444 UV-treated Daudi cells ml−1.
The threshold for significant nucleosome detection is 0·1 U. ml−1
* Significantly different from controls at P ≤ 0·01.
Seeds
Ability of seeds to germinate and accelerated ageing
Sets of seeds collected from the Chernobyl control, Kopachy and Yanov sites (Table 4) were assessed for their final germination success with or without the addition of gibberellin.
Table 4.
Germination percentages of Oenothera biennis seeds collected in two years from differently radionuclide-contaminated sites and effect of 16-d accelerated ageing on these seeds
| 1998 |
2001 |
|||||
|---|---|---|---|---|---|---|
| Collection sites/levels of pollution | Germination (%) (–GA3) | Germination (%) (+GA3) | Accelerated ageing (%) (–GA3) | Germination (%) (–GA3) | Germination (%) (+GA3) | Accelerated ageing (%) (–GA3) |
| Chernobyl, control | 86·4 ± 6·2 | 90·1 ± 4·3 | 17·5 ± 2·2 | 88·3 ± 4·7 | 91·1 ± 3·8 | 19·5 ± 3·6 |
| Kopachy, level I | 82·2 ± 4·7 | 84·2 ± 4·2 | 55·5 ± 3·8* | 80·1 ± 3·8 | 82·6 ± 5·1 | 47·5 ± 4·4* |
| Yanov, level II | 75·9 ± 7·1 | 80·1 ± 5·6 | 22·5 ± 1·8 | 77·7 ± 6·1 | 83·1 ± 4·4 | 18·5 ± 2·6 |
Accelerated ageing carried out at 40 °C and 74 % humidity led to an increase in seed moisture from 9·1 ± 0·2 % to 14·0 ± 0·4 % in 1998 samples and from 9·4 ± 0·3 % to 14·5 ± 0·5 % in 2001 samples.
* Significantly different from the seed from other sites at P ≤ 0·01.
Seeds from each of the sites had a high germination potential at 11 d with no significant evidence of dormancy inherent in the seed lots. These results confirm earlier findings that, under non-stress conditions, the Chernobyl control seeds may have a slight germination advantage over those collected from either Kopachy or Yanov (Micheev et al., 2000).
When seeds were subjected to stress in the form of accelerated ageing, the highest germination was of seed from the intermediate radionuclide contaminated region (Kopachy), the lowest in the Chernobyl controls. In other species (wheat, rye, sugar beet) it has been shown that these accelerated ageing conditions cause enhanced DNA fragmentation and premature death of the enclosed embryo (Boubriak et al., 2000).
Ability of seed to germinate in saline conditions
The overall germination percentages of seeds held under an 150 mm salt stress (Table 5) suggested some improved ability of the Oenothera seeds from the contaminated Kopachy sites to survive saline-stress conditions, and also suggested that the older seed (1998 collection) expressed this capability more readily.
Table 5.
Germination percentages (%) under osmotic stress of Oenothera biennis seeds collected in two years from the differently radionuclide-contaminated Chernobyl sites
| 1998 |
2001 |
||||
|---|---|---|---|---|---|
| Collection sites/levels of pollution | 7 d | 12 d | 20 d | 7 d | 12 d |
| Chernobyl, control | 1·1 ± 0·7 | 0 | 3·7 ± 1·4 | 1·0 ± 0·6 | 1·6 ± 1·1 |
| Kopachy, level I | 6·6 ± 2·4* | 7·5 ± 1·2* | 11·5 ± 3·1* | 2·5 ± 1·3** | 3·0 ± 1·2** |
| Yanov, level II | 0 | 0 | 1·3 ± 0·8 | 1·7 ± 1·1 | 1·8 ± 0·9 |
Sets of 25-mg samples of dry seed were held in Petri dishes on filter paper moistened with 150 mm NaCl at 24 °C in the dark (no gibberellin).
*,** Significantly different from other treatments in same experiment at P ≤ 0·01 and P ≤ 0·05, respectively.
Ability of seedlings and seeds to survive additional radiation stress
Chernobyl control, Kopachy and Yanov seedlings of the 1998–2000 collections were compared for evidence of adaptation to chronic irradiation conditions by exposing them to a further quantified radiation dose. For this, the ability to survive successfully and continue to grow was assessed after seedlings of each lot had been exposed to a range of γ-irradiation doses. In the Chernobyl controls, growth impairment had already been observed at the lowest (20 Gy) dose when the overall root length was 15 % less than that of Kopachy seeds, though percentage germination was similar in each of the lots. These data (not shown here) are set out in a detailed report published elsewhere (Micheev et al., 2000).
Results for the Oenothera seed repair experiments show that at 24 h there is already some difference in the level of [3H]methylthymidine incorporation into the DNA of the imbibed unirradiated samples (0 Gy) in Kopachy or Yanov seeds compared with the Chernobyl control samples (Table 6). This may reflect differences in starting levels of DNA damage in the dry embryos of seeds formed under the different conditions of chronic irradiation because of the absence of replication in embryos at this time point. The later was confirmed in the experiments in which hydroxyurea was added to the incubation medium. This addition of a replication inhibitor had no significant effect on the level of incorporation of unirradiated samples up to 24 h (data not shown). After 750 Gy, all samples showed increased incorporation (reflecting DNA repair synthesis) with the highest values for Kopachy seed. At 1500 Gy, the Kopachy seeds show a significantly greater capacity for repair incorporation than the other samples. The Yanov seed failed to repair this higher level of imposed radiation damage as effectively as the control seed.
Table 6.
Dose dependence of unscheduled DNA synthesis in Oenothera seeds collected in two years from Chernobyl sites with different radionuclide contamination
| 1998 |
2001 |
|||||
|---|---|---|---|---|---|---|
| Collection sites/irradiation dose | 0 Gy | 750 Gy | 1500 Gy | 0 Gy | 750 Gy | 1500 Gy |
| Chernobyl, control | 320 ± 80 | 1280 ± 260 | 830 ± 210 | 260 ± 90 | 1110 ± 320 | 740 ± 170 |
| Kopachy, level I | 570 ± 140** | 1820 ± 360* | 1580 ± 290* | 460 ± 90 | 1430 ± 230** | 1200 ± 230* |
| Yanov, level II | 660 ± 110* | 1120 ± 170 | 580 ± 190** | 610 ± 90* | 960 ± 240 | 470 ± 160** |
After irradiation in the dry state, 100 mg of seeds per sample were halved longitudinally to expose embryos, imbibed in water in the presence of 40 µCi of methyl-[3H]thymidine for 12 h and fixed in ethanol/acetic acid (3:1). DNA was extracted as described in Materials and methods and radioactivity was measured and is presented here in cpm−1 μg−1 DNA.
*,** Significantly different from control in the same experiment: at P ≤ 0·01 and P ≤ 0·05, respectively.
Further evidence for an increased efficiency of NER in the Kopachy samples (both 1998 and 2001) was seen when SSB DNA repair in γ-irradiated seed was assessed. Two time points were analysed, one at 6 h and the other at 24 h after imbibition. At these time points, rapid repair of SSBs by simple ligation, which happens in the first minutes after irradiation, was not measured (Grodzinskii, 1989; Grodzinskii and Gudkov, 2006), but the remaining SSBs which are dealt with NER were monitored. Although repair is seen in all seeds at 6 h (Table 6), it seems that the first hour of imbibition may not provide a long enough period of hydration through the seed coat to permit full excision repair in either the unirradiated or the γ-irradiated samples.
After irradiation followed by 24-h imbibition, however, clear differences become apparent in the efficiency of SSB DNA repair. Although both the Chernobyl control and Kopachy seed from the 2001 samples show repair, after imbibition for 24 h, more SSBs are seen to be repaired in the Kopachy sample from 1998 (Table 7). This recovery at 24 h could accord with the significantly higher germination capacity found in the additionally γ-irradiated 1998 Kopachy samples compared with the 1998 samples from the Chernobyl control site, and with the greater ability of Kopachy seed to withstand salinity stress.
Table 7.
Efficiency of repair of DNA single-strand breaks formed in Oenothera seeds collected in two years atγ/β-contaminated site in Chernobyl after 750 Gy γ-irradiation
| 1998 |
2001 |
|||||
|---|---|---|---|---|---|---|
| Collection sites | DNA SSB(10−10 Gy Da −1) | Repair efficiency (%), 6 h | Repair efficiency (%), 24 h | DNA SSB(10−10 Gy Da −1) | Repair efficiency (%), 6 h | Repair efficiency (%), 24 h |
| Chernobyl, control | 8·3 ± 0·7 | 56 ± 4 | 87 ± 6 | 9·4 ± 0·4 | 47 ± 6 | 91 ± 9 |
| Kopachy, level I | 6·7 ± 0·8 | 68 ± 7* | 100 ± 5* | 7·8 ± 0·6 | 63 ± 7* | 98 ± 11 |
Number of SSB immediately after irradiation and following repair intervals was calculated from scans of denaturing DNA gels according to Yamamoto et al. (1982).
* Significantly different from control at the same time point at P ≤ 0·05.
DISCUSSION
Adaptation is a complex process by which populations of organisms respond to long-term environmental stresses through permanent genetic change (Kovalchuk et al., 2004) and, to date, our knowledge of the adaptation of plant populations to chronic irradiation is limited. A renewed interest in this topic was prompted by the Chernobyl disaster, which had major effects on plants growing in the vicinity of the reactor. These effects included the death of radiosensitive plants such as pine trees (Arkhipov et al., 1994), an increased frequency of mutations (Bubryak et al., 1992; Kovalchuk et al., 2000) and chromosome aberrations (Shkvarnikov, 1990) as well as embryonic lethal mutations (Abramov et al., 1992a). Despite the presence of genetic damage in chronically irradiated plants, ecological surveys in the Chernobyl area have revealed specific plant communities that have become adapted for survival in the radiocontaminated soils (Abramov et al., 1992a).
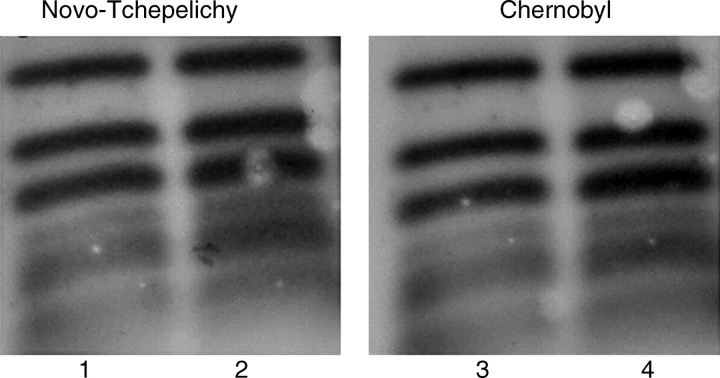
Adaptation is a complex process and this study has concentrated on only one possible mechanism of adaptation – up-regulation of DNA repair functions. Another study on the possible mechanisms of adaptation in the Chernobyl area showed extremely low recombination levels in chronically irradiated plants that may prevent extensive genome rearrangements (Kovalchuk et al., 2004). This is despite that fact that homologous recombination might be expected to be dealing with an increased incidence of double-strand breaks in such plants (although it is possible that plants grown in contaminated areas may shift their DSB repair mechanisms towards non-homologous end joining). In any case, more efficient dark repair systems in adapted plants should be able to deal with increased levels of radiation damage from the radionuclide contamination. The present data for increased excision repair capacity in Kopachy pollen strongly support this hypothesis. From the pollen repair studies it is clear that, in contrast to Kopachy, excision repair is impaired in Novo-Tchepelichy pollen. This pollen has a reduced level of DNA repair synthesis after gamma irradiation and the hybridization pattern for rDNA repeats following a repair interval showed significant rearrangements. These re-arrangements cannot be explained by non-homogeneous biological material, because the pollen from the same trees is used in all cases, nor by different DNA methylation patterns for control and chronically irradiated pollen samples. Despite being methylation sensitive, MboI, the restriction enzyme used to digest pollen DNA, provides the same restriction pattern for Chernobyl and Novo-Tchepelichy samples in all cases except when pollen samples are allowed to repair. To confirm further that DNA methylation is not sufficiently different to affect fragmentation patterns for pollen DNA from different Chernobyl sites, the restriction pattern produced by the isoschizomers MboI and DpnII, which have different sensitivities to eukaryotic CpG methylation, were compared (Fig. 3). The similar restriction pattern obtained by the use of these two enzymes confirms the limited effect of epigenetic changes in birch pollen at selected Chernobyl sites. This is why we suggest that the accumulation of unrepaired lesions in the rDNA cluster, due to the failure to properly repair remaining SSBs by NER, changes the MboI digestion pattern, thus confirming problems with DNA repair in the Novo-Tchepelichy sample.
Fig. 3.
Restriction pattern of pollen DNA from different Chernobyl sites. Lanes 1 and 2, Novo-Tchepelichy level III contamination; lanes 3 and 4, Chernobyl control; lanes 1 and 3, MboI-digested DNA from dry pollen; lanes 2 and 4, DpnII-digested DNA from dry pollen.
The most likely explanation of the opposite effects of combined irradiation on DNA repair functions in Kopachy and Novo-Tchepelychy pollen samples is the different composition of radionuclides at these Chernobyl sites.
The mechanisms responsible for the adaptive upgrading of DNA repair in the γ/β-emitter-contaminated pollen and seed, the repair of the lesions remaining in the pollen and the seed-repair mechanism at combined α- and γ/β-emitting sites have not been identified. A number of possibilities might operate in both pollen and seeds. Although immunological detection of α- and β-polymerases (involved in DNA repair in plants) indicates no differences between the levels of these enzymes in dry seeds from Chernobyl and pollen from Kopachy, the levels are significantly reduced in the pollen with impaired repair function from the most highly contaminated site at Novo-Tchepelichy (Boubriak et al., 2002). Up- and down-regulation of other enzymes involved in dark repair is the most obvious, but not the only mechanism explaining the differences in DNA repair efficiencies at different contamination levels. DNA repair enzymes are very labile and lose activity in stored seed (Elder et al., 1987) and this process can be facilitated by irradiation stress (Boubriak et al., 2002). It is not impossible that a chaperone component (perhaps of a LEA-type protein) might protect the relatively labile repair enzymes in adapted plants and account for the sustained repair activity in the Kopachy collection.
The present results on increased stress tolerance of seeds are interpreted as evidence for an adaptive selection for improved DNA repair capacity in the seeds of Oenothera plants growing in the highly γ/β-radionuclide-contaminated site at Kopachy. The failure of the Yanov seeds to accommodate damage from the highest (1500 Gy) imposed dose may be due, we suggest, to the proportionally higher extent of α-emitters present in the soil of the parent plants as shown in the direct radionuclide measurements. Because α-particles have a very high linear energy transfer, they are much more destructive than other types of radiation and repair of the damage caused by this irradiation is limited. This would be in agreement with the data obtained on birch pollen and with an earlier report of the successful adaptation of Vicia cracca plants to soils most highly contaminated by β-emitters, but not by α-emitters (Syomov et al., 1992; Semov et al., 1997).
In the present paper, the long-term legacy of different types of radiation insult upon the inherent stability of two genetically distinct plant genomes using haploid and diploid cells are described. The results show the capacity of plants to adapt to and survive chronic levels ofγ/β-emitters in the environment but an inability, so far, to make such an adaptation to high levels of α-emissions. The results alert us to a potential long-term threat to the successful survival of plant species under α-emitter contamination.
In this field of exploration there remains much to be elucidated in our understanding of long-term chronic irradiation damage from radioactive fall-out, not least in understanding the indicators that could lead to future, more successful, use of crops on contaminated land.
ACKNOWLEDGEMENTS
We thank The Royal Society of London and The Ukrainian Academy of Sciences, for supporting Joint Research Project UK-Ukraine Ref IJP 13727. We also wish to pay tribute to the late Professor Daphne J. Osborne, without whom much of the Chernobyl work described here would not have been done.
LITERATURE CITED
- Abramov VI, Fedorenko O, Schevchenko V. Genetic consequences of radioactive contaminations for populations of Arabidopsis. Science of the Total Environment. 1992;a 112:19–28. doi: 10.1016/0048-9697(92)90234-j. [DOI] [PubMed] [Google Scholar]
- Abramov VI, Sergeyeva SA, Ptitsyna SN, Semov AB, Shevchenko VA. Genetic-effects and repair of single-stranded-DNA breaks in populations of Arabidopsis-thaliana growing in the region of the Chernobyl-nuclear-power-station. Genetika. 1992;b 28:69–73. [Google Scholar]
- Arkhipov NP, Kuchma ND, Askbrant S, Pasternak PS, Musica VV. Acute and long-term effects of irradiation on pine (Pinus-Silvestris) stands post-Chernobyl. Science of the Total Environment. 1994;157:383–386. [PubMed] [Google Scholar]
- Bleuyard JY, Gallego ME, Savigny F, White CI. Differing requirements for the Arabidopsis Rad51 paralogs in meiosis and DNA repair. The Plant Journal. 2005;41:533–545. doi: 10.1111/j.1365-313X.2004.02318.x. [DOI] [PubMed] [Google Scholar]
- Bondarkov MD, Donetz NP, Zheltonozsky VA, Muck K, Sadovnikov LV, Stukin ED, et al. Investigation of gamma- and X-radiation of ‘hot particles’ from the zones of the Chernobyl accident and atomic explosions. Instruments and Experimental Techniques. 1999;42:409–412. [Google Scholar]
- Boubriak , II, Grodzinsky DM. Functioning of DNA-repair systems in pollen of plants growing under different ecological conditions. Fiziologiya I Biokhimiya Kulturnykh Rastenii. 1985;17:335–343. [Google Scholar]
- Boubriak I, Grodzinsky DM. Regulation of the physiological functions in plants. Kiev: Naukova dumka; 1986. Inhibition of UV-induced unscheduled DNA synthesis by aphidicolin in nut-grove pollen from a different altitude; pp. 141–145. [Google Scholar]
- Boubriak , II, Grodzinsky DM. DNA repair in pollen of birch plants grown in conditions of radioactive contamination. Radiobiology. 1989;29:589–594. [Google Scholar]
- Boubriak I, Naumenko V, Grodzinsky DM. Molecular and cellular mechanisms for the effects of chronic irradiation. Puschino: AN USSR; 1990. Cycloheximide and ethidium bromide modification of unscheduled DNA synthesis in birch pollen; pp. 40–42. [Google Scholar]
- Boubriak , II, Naymenko VD, Grodzinsky DM. Genetic damages occurred in birch pollen in conditions of radionuclide contamination. Radiobiology. 1991;31:564–567. [Google Scholar]
- Boubriak I, Kargiolaki H, Lyne L, Osborne DJ. The requirement for DNA repair in desiccation tolerance of germinating embryos. Seed Science Research. 1997;7:97–105. [Google Scholar]
- Boubriak I, Naumenko V, Lyne L, Osborne DJ. Loss of viability in rye embryos at different levels of hydration: senescence with apoptotic nucleosome cleavage or death with random DNA fragmentation. In: Black M, Bradford KJ, Vasquez-Ramos J, editors. Seed Biology: Advances and Applications. Cambridge: CAB International; 2000. pp. 205–214. [Google Scholar]
- Boubriak I, Naumenko V, Prokhnevsky A, Osborne DJ, Grodzinsky D. Long term impairment of DNA repair function in pollen of Betula verrucosa from heavily radiocontaminated sites of Chernobyl. In: Schevchenko V, editor. Genetic consequences of emergency radiation situation. Moscow: Publishing House of Russian Peoples Friendship; 2002. pp. 135–141. [Google Scholar]
- Bousquet J, Girouard E, Strobeck C, Dancik BP, Lalonde M. Restriction fragment polymorphisms in the rDNA region among 7 species of Alnus and Betula-Papyrifera. Plant and Soil. 1989;118:231–240. [Google Scholar]
- Bray CM, West CE. DNA repair mechanisms in plants: crucial sensors and effectors for the maintenance of genome integrity. New Phytologist. 2005;168:511–528. doi: 10.1111/j.1469-8137.2005.01548.x. [DOI] [PubMed] [Google Scholar]
- Britt AB. Molecular genetics of DNA repair in higher plants. Trends in Plant Sciences. 1999;4:20–25. doi: 10.1016/s1360-1385(98)01355-7. [DOI] [PubMed] [Google Scholar]
- Bubryak I, Vilensky E, Naumenko V, Grodzinsky D. Influence of combined alpha-, beta- and gamma-radionuclide contamination on the frequency of waxy-reversions in barley pollen. Science of the Total Environment. 1992;112:29–36. doi: 10.1016/0048-9697(92)90235-k. [DOI] [PubMed] [Google Scholar]
- Cheah KSE, Osborne DJ. DNA lesions occur with loss of viability in embryos of aging rye seed. Nature. 1978;272:593–599. doi: 10.1038/272593a0. [DOI] [PubMed] [Google Scholar]
- Chesser RK, Bondarkov M, Baker RJ, Wickliffe JK, Rodgers BE. Reconstruction of radioactive plume characteristics along Chernobyl's Western Trace. Journal of Environmental Radioactivity. 2004;71:147–157. doi: 10.1016/S0265-931X(03)00165-6. [DOI] [PubMed] [Google Scholar]
- Coupe SA, Watson LM, Ryan DJ, Pinkney TT, Eason JR. Molecular analysis of programmed cell death during senescence in Arabidopsis thaliana and Brassica oleracea: cloning broccoli LSD1, Bax inhibitor and serine palmitoyltransferase homologues. Journal of Experimental Botany. 2004;55:59–68. doi: 10.1093/jxb/erh018. [DOI] [PubMed] [Google Scholar]
- Danon A, Rotari VI, Gordon A, Mailhac N, Gallois P. Ultraviolet-C overexposure induces programmed cell death in Arabidopsis, which is mediated by caspase-like activities and which can be suppressed by caspase inhibitors, p35 and Defender against Apoptotic Death. Journal of Biological Chemistry. 2004;279:779–787. doi: 10.1074/jbc.M304468200. [DOI] [PubMed] [Google Scholar]
- Elder RH, Osborne DJ. Function of DNA synthesis and DNA repair in the survival of embryos during early germination and dormancy. Seed Science Research. 1993;3:45–53. [Google Scholar]
- Elder RH, Dellaquila A, Mezzina M, Sarasin A, Osborne DJ. DNA-ligase in repair and replication in the embryos of rye. Secale cereale. Mutation Research. 1987;181:61–71. [Google Scholar]
- Gerlach WL, Bedbrook JR. Cloning and characterization of ribosomal RNA genes from wheat and barley. Nucleic Acids Research. 1979;7:1869–1885. doi: 10.1093/nar/7.7.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodzinskii DM. Plant radiobiology. Kiev: Naykova Dymka; 1989. [Google Scholar]
- Grodzinskii DM, Gudkov IN. Radiation injury of the plants in the zone of influence of the accident on Chernobyl Nuclear Power Station (NPS) Radiatsionnia Biologiia, Radioecologiia. 2006;46:189–199. [PubMed] [Google Scholar]
- Hohenemser C, Deicher M, Ernst A, Hofsass H, Lindner G, Recknagel E. Chernobyl – an early report. Environment. 1986;28:6–13. [Google Scholar]
- Howland GP. Dark-repair of ultraviolet-induced pyrimidine dimers in the DNA of wild carrot protoplasts. Nature. 1975;254:160–161. doi: 10.1038/254160a0. [DOI] [PubMed] [Google Scholar]
- Jackson JF. DNA-repair in pollen – a review. Mutation Research. 1987;181:17–29. [Google Scholar]
- Jackson JF, Linskens HF. DNA-repair in pollen – range of mutagens inducing repair, effect of replication inhibitors and changes in thymidine nucleotide-metabolism during repair. Molecular & General Genetics. 1980;180:517–522. [Google Scholar]
- Jiang CZ, Yen CN, Cronin K, Mitchell D, Britt AB. UV- and gamma-radiation sensitive mutants of Arabidopsis thaliana. Genetics. 1997;147:1401–1409. doi: 10.1093/genetics/147.3.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashparov VA, Lundin SM, Khomutinin YV, Kaminsky SP, Levchuk SE, Protsak VP, et al. Soil contamination with 90Sr in the near zone of the Chernobyl accident. Journal of Environmental Radioactivity. 2001;56:285–298. doi: 10.1016/s0265-931x(00)00207-1. [DOI] [PubMed] [Google Scholar]
- Kovalchuk I, Abramov V, Pogribny I, Kovalchuk O. Molecular aspects of plant adaptation to life in the Chernobyl zone. Plant Physiology. 2004;135:357–363. doi: 10.1104/pp.104.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalchuk O, Dubrova YE, Arkhipov A, Hohn B, Kovalchuk I. Germline DNA – wheat mutation rate after Chernobyl. Nature. 2000;407:583–584. doi: 10.1038/35036692. [DOI] [PubMed] [Google Scholar]
- Levi HW. Radioactive deposition in Europe after the Chernobyl accident and its long-term consequences. Ecological Research. 1991;6:201–216. [Google Scholar]
- Liang L, Flury S, Kalck V, Hohn B, Molinier J. CENTRIN2 interacts with the Arabidopsis homolog of the human XPC protein (AtRAD4) and contributes to efficient synthesis-dependent repair of bulky DNA lesions. Plant Molecular Biology. 2006;61:345–356. doi: 10.1007/s11103-006-0016-9. [DOI] [PubMed] [Google Scholar]
- Lindner L. Chernobyl today and compared to other disasters. Atw-Internationale Zeitschrift Fur Kernenergie. 2000;45:282–292. [Google Scholar]
- Liu Z, Hall JD, Mount DW. Arabidopsis UVH3 gene is a homolog of the Saccharomyces cerevisiae RAD2 and human XPG DNA repair genes. The Plant Journal. 2001;26:329–338. doi: 10.1046/j.1365-313x.2001.01031.x. [DOI] [PubMed] [Google Scholar]
- Micheev AN, Gushcha NP, Shilina YV. Investigation of gipo- and giper-compensatory plant phases to the gamma-irradiation with the aim to correlate these to radioadaptation. Bulletin of Kiev National University. 2000;32:47–48. [Google Scholar]
- Morales-Ruiz T, Birincioglu M, Jaruga P, Rodriguez H, Roldan-Arjona T, Dizdaroglu M. Arabidopsis thaliana Ogg1 protein excises 8-hydroxyguanine and 2,6-diamino-4-hydroxy-5-formamidopyrimidine from oxidatively damaged DNA containing multiple lesions. Biochemistry. 2003;42:3089–3095. doi: 10.1021/bi027226u. [DOI] [PubMed] [Google Scholar]
- Morgante PG, Berra CM, Nakabashi M, Costa RM, Menck CF, Van Sluys MA. Functional XPB/RAD25 redundancy in Arabidopsis genome: characterization of AtXPB2 and expression analysis. Gene. 2005;344:93–103. doi: 10.1016/j.gene.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Mori Y, Kimura S, Saotome A, Kasai N, Sakaguchi N, Uchiyama Y, et al. Plastid DNA polymerases from higher plants, Arabidopsis thaliana. Biochemical and Biophysical Research Communications. 2005;334:43–50. doi: 10.1016/j.bbrc.2005.06.052. [DOI] [PubMed] [Google Scholar]
- Osborne TS, Bacon JA. Radiosensitivity of seeds. I. Reduction or stimulation of seeding growth as a function of gamma-ray dose. Radiation Research. 1960;13:686–690. [PubMed] [Google Scholar]
- Overmyer K, Brosche M, Pellinen R, Kuittinen T, Tuominen H, Ahlfors R, et al. Ozone-induced programmed cell death in the Arabidopsis radical-induced cell death1 mutant. Plant Physiology. 2005;137:1092–1104. doi: 10.1104/pp.104.055681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher M, Schmidt-Puchta W, Puchta H. Two unlinked double-strand breaks can induce reciprocal exchanges in plant genomes via homologous recombination and non-homologous end-joining. Genetics. 2007;175:21–29. doi: 10.1534/genetics.106.065185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puchta H. The repair of double-strand breaks in plants: mechanisms and consequences for genome evolution. Journal of Experimental Botany. 2005;56:1–14. doi: 10.1093/jxb/eri025. [DOI] [PubMed] [Google Scholar]
- Salbu B, Oughton DH, Ratnikov AV, Zhigareva TL, Kruglov SV, Petrov KV, et al. The mobility of 137Cs and 90Sr in agricultural soils in the Ukraine, Belarus, and Russia, 1991. Health Physics. 1994;67:518–528. doi: 10.1097/00004032-199411000-00007. [DOI] [PubMed] [Google Scholar]
- Semov AB, Ptitsina SN, Semova N. Features of DNA repair during chronic exposure to mutagenic factors. Radiatsionnija Biologiia Radioecoliia. 1997;37:565–568. [PubMed] [Google Scholar]
- Shkvarnikov PK. A cytological study of plants growing under exposure to different radiation levels. Tsitologia i Genetika. 1990;24:33–37. [PubMed] [Google Scholar]
- Sparrow AH, Underbrink AG, Sparrow RC. Chromosomes and cellular radiosensitivity. I. The relationship of D0 to chromosome volume and complexity in seventy-nine different organisms. Radiation Research. 1967;32:915–945. [PubMed] [Google Scholar]
- Syomov AB, Ptitsyna SN, Sergeeva SA. Analysis of DNA strand break induction and repair in plants from the vicinity of Chernobyl. Science of the Total Environment. 1992;112:1–8. doi: 10.1016/0048-9697(92)90232-h. [DOI] [PubMed] [Google Scholar]
- Tiwari BS, Belenghi B, Levine A. Oxidative stress increased respiration and generation of reactive oxygen species, resulting in ATP depletion, opening of mitochondrial permeability transition, and programmed cell death. Plant Physiology. 2002;128:1271–1281. doi: 10.1104/pp.010999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonarx EJ, Tabone EK, Osmond MJ, Anderson HJ, Kunz BA. Arabidopsis homologue of human transcription factor IIH/nucleotide excision repair factor p44 can function in transcription and DNA repair and interacts with AtXPD. The Plant Journal. 2006;46:512–521. doi: 10.1111/j.1365-313X.2006.02705.x. [DOI] [PubMed] [Google Scholar]
- West CE, Waterworth WM, Sunderland PA, Bray CM. Arabidopsis DNA double-strand break repair pathways. Biochemical Society Transactions. 2004;32:964–966. doi: 10.1042/BST0320964. [DOI] [PubMed] [Google Scholar]
- Yamamoto O, Ogama M, Koshi M. Comparison of the electrophoretic method with the sedimentation method for the analysis of DNA strand breaks. Journal of Radiation Research. 1982;23:385–398. doi: 10.1269/jrr.23.385. [DOI] [PubMed] [Google Scholar]
- Yoschenko VI, Kashparov VA, Protsak VP, Lundin SM, Levchuk SE, Kadygrib AM, et al. Resuspension and redistribution of radionuclides during grassland and forest fires in the Chernobyl exclusion zone: part I. Fire experiments. Journal of Environmental Radioactivity. 2006;86:143–163. doi: 10.1016/j.jenvrad.2005.08.003. [DOI] [PubMed] [Google Scholar]