Abstract
Objectives
To describe a large multicenter cohort of pediatric cardiac arrest (CA) with return of circulation (ROC) from either the in-hospital (IH) or out-of-hospital (OH) setting in order to determine if significant differences related to pre-event, arrest event, early post-arrest event characteristics and outcomes exist that would be critical in planning a clinical trial of therapeutic hypothermia (TH).
Design
Retrospective cohort study
Setting
Fifteen Pediatric Emergency Care Applied Research Network (PECARN) sites.
Patients
Patients from 24 hours (h) to 18 years with either IH or OH CA who had a history of at least 1 minute of chest compressions and ROC for at least 20 minutes were eligible.
Interventions
None
Measurements and Main Results
A total of 491 patients met study entry criteria with 353 IH cases and 138 OH cases. Major differences between the IH and OH cohorts were observed for patient pre-arrest characteristics; arrest event initial rhythm described; and arrest medication use. Several post-arrest interventions were utilized differently, however, the use of TH was similar (<5%) in both cohorts. During the 0–12 h interval following ROC, OH cases had lower minimum temperature and pH, and higher maximum serum glucose recorded. Mortality was greater in the OH cohort (62% vs. 51%, p=0.04) with the cause attributed to a neurological indication much more frequent in the OH than IH cohort (69% vs. 20%; p < 0.01).
Conclusions
For pediatric CA with ROC, several major differences exist between IH and OH cohorts. The finding that the etiology of death was attributed to neurological indications much more frequently in OH arrests has important implications for future research. Investigators planning to evaluate the efficacy of new interventions such as TH should be aware that the IH and OH populations differ greatly and require independent clinical trials.
Keywords: cardiac arrest, children, pediatric, cohort study, out of hospital, in hospital, return of spontaneous circulation, mortality, outcome, therapeutic hypothermia, randomized controlled trial
INTRODUCTION
Cardiac arrest (CA) in childhood is often a catastrophic event that is associated with mortality or poor neurological outcome in the in-hospital (IH) or out-of hospital (OH) setting (1–19). Until recently, most studies of pediatric OH and IH CA with return of circulation (ROC) have been relatively small retrospective reports from single sites or geographical areas (5–19). Two contemporary reviews of the literature about OH pediatric CA have summarized available information and concluded that non uniform data collection and inadequate outcome information exist for this setting (1,2).
For cardiac arrest in the IH setting, the American Heart Association’s National Registry of Cardiopulmonary Resuscitation (NRCPR) database has addressed many of the limitations of small case series reports (3). It has been utilized extensively for quality improvement and research. The data collection for adults and pediatric cases has been standardized and includes consistent data field definitions and data abstractor training for review of primary data sources at each site. At this time, however, there is no comparable data set for OH CA in children.
Because of the dismal neurological outcomes associated with CA, especially in the OH setting, interventions to ameliorate hypoxic-ischemic encephalopathy following CA have been sought for decades. Until recently, no therapy had been demonstrated to improve outcome in any human population. In 2002, two randomized clinical trials (RCTs) of therapeutic hypothermia (TH) for adults with OH ventricular fibrillation or pulseless ventricular tachycardia reported improved survival with good neurological outcome (20, 21). In 2005, three trials of TH in newborns with hypoxic-ischemic encephalopathy reported improved survival with good neurological outcome (22–24). Clinical trials of TH in adult and pediatric populations with traumatic brain injury (TBI) have not demonstrated benefit at this time (25, 26). A recently completed pediatric trial of TBI observed a trend for lower survival in patients receiving TH (25). Clinical trials of TH for pediatric CA have not been conducted to date, although expert surveys have ranked the efficacy of TH for pediatric CA as an urgent research priority (27).
The Pediatric Emergency Care Applied Research Network (PECARN) is a federally-funded multi-institutional emergency medicine network that conducts research on prevention and management of acute illness and injuries in children (28). The scope of both the Emergency Medical Services for Children (EMSC) and the PECARN research agenda includes the continuum of care from prehospital, emergency department (ED), operating room, pediatric intensive care unit (PICU), ward, through rehabilitation and discharge. This represents an ideal setting in which to study pediatric CA interventions and evaluate long-term outcomes. As part of the planning for a clinical trial to investigate the efficacy of TH and potentially other interventions to improve outcomes in children after CA, we conducted a NIH-sponsored pre-clinical trial cohort study of IH and OH cardiac arrest at 15 PECARN associated hospitals. The current report is the first large multicenter cohort study of both IH and OH pediatric CA in the U.S. This report 1) describes pre-arrest, arrest, early post-arrest variables and outcomes at hospital discharge for IH and OH cohorts of pediatric patients with CA and ROC and 2) compares and contrasts the IH and OH cohorts of cases that might be eligible for an interventional trial of TH. We hypothesized that we would identify significant differences between children with CA and ROC in the IH and OH setting that would require either a priori planning to adjust for differences or performance of separate clinical trials for each setting.
METHODS
Our retrospective cohort study of IH and OH CA was conducted between July 1, 2003 and December 31, 2004 at 15 sites associated with the PECARN. Patients from one day [24 hours (h)] to 18 years of age (inclusive) who experienced CA requiring at least one minute(min) of chest compressions and who had a ROC for a minimum of 20 min, were eligible for inclusion. Case classification as OH was assigned if chest compressions were initiated prior to hospital arrival. IH classification was assigned when chest compressions were initiated in the emergency department or other hospital setting. Patients cared for in a neonatal ICU or who had planned CA in the operating room as part of congenital heart disease surgical repair were excluded. These criteria were selected to identify a cohort of pediatric patients similar to those who would be potentially eligible for a future TH trial.
Patients were identified by medical record ICD-9 codes (427.5 cardiac arrest, and 437.4 ventricular fibrillation/flutter), procedure codes (99.60 cardiopulmonary resuscitation not otherwise specified, 99.63 closed chest cardiac massage, and 99.62 other electric counter shock of heart), institutional arrest logs (e.g. CPR committee or Quality Assurance Committee), morbidity and mortality reviews, emergency department records, trauma records, Pediatric Risk of Mortality (PRISM) scores (29), and other site specific mechanisms. If a patient experienced more than one CA during the study time period, only the first arrest meeting eligibility criteria was included. The study was approved with a waiver of informed consent granted by the Institutional Review Board at all 15 clinical sites and the data coordinating center.
The PECARN Central Data Management and Coordinating Center (CDMCC) at the University of Utah trained investigators and data abstractors at each site to review patient records and collect data. Training included review of a manual of operations, teleconferences, and comparative coding of hypothetical patient records. During data collection, a sample of nearly 20% of records coded by data abstractors was reviewed by the site investigators for 27 key data fields. Overall accuracy was > 96%. Data fields reviewed by the site investigator that did not match with those of the abstractor were flagged for site investigator review and resolution. All data were double-entered into a secure, encrypted Internet site and electronically submitted to the CDMCC. The CDMCC performed a secondary review to ensure data quality, and site abstractors were queried to resolve data discrepancies.
Data collected included 1) patient characteristics such as age, weight, gender, race, ethnicity, insurance type, and chronic pre-existing conditions; 2) event characteristics including location and timing of CA, first and subsequent monitored cardiac rhythms, presence and types of vascular access, endotracheal tube, monitoring devices and other interventions prior to arrest, use of defibrillation, and drugs administered during the arrest; 3) etiology of CA; 4) hospital course including use of extracorporeal membrane oxygenation (ECMO), TH, other intensive care monitoring devices and interventions, drug therapies, and subsequent arrests and seizures; 5) physiologic and laboratory data such as pupillary reflexes, body temperature, blood pH, glucose and lactate concentrations in the first 12 h post-arrest; 6) Pediatric Cerebral Performance Category (PCPC) scores prior to CA and at hospital discharge; and 7) survival to hospital discharge. Dates and times of important clinical events were recorded and related time intervals determined. Utstein-style definitions were used for most variables where such definitions exist (30, 31). PCPC scores (from 1–6) estimate cognitive function (1 = normal, 2 = mild disability, 3 = moderate disability, 4 = severe disability, 5 = coma or vegetative state, and 6 = death) (32, 33). Good neurological outcome was defined as PCPC score of 1 or 2 at hospital discharge, or no change in score from pre-arrest to hospital discharge. Two approaches were used to classify CA as day or night and weekday or weekend. Events were coded as day-time if they occurred from 7:00 AM–6:59 PM and as night-time from 7 PM–6:59AM (Method 1). Weekday arrests were classified as Monday 12:00 AM to Friday 11:59 PM and weekend was defined as Saturday 12:00 AM to Sunday 11:59 PM. We also described night and weekend time periods with night defined as interval from 11:00 PM–6:59 AM and weekend classified from Friday 11:00 PM–Monday 6:59 AM (Method 2); Method 2 was described in a recent adult IH CA report (34).
Several steps were taken to prepare the data for analysis. We reviewed time intervals for invalid or extreme values. If a value was considered impossible (e.g., negative values) or extremely unlikely based on a valid range for that variable, it was set to missing for the analysis. Physiologic and laboratory data were collected as minimum and maximum values obtained from 0–6 h and >6–12 h. If there was only one value provided for a time interval, it was assigned to both the minimum and maximum. To obtain minimum and maximum values for the first 12 h, data from both time intervals were included; a missing value was assigned only if it was missing across both 0–6 h and >6–12 h. For drugs administered during CA in the OH cohort, data were based on any documentation of drugs received either prior to hospital arrival or in the hospital.
Statistical Analyses
For the descriptive data analyses, medians with interquartile ranges (IQR) are described for continuous variables and proportions depicted as percents for categorical variables. A non-parametric Wilcoxon rank-sum test was used for continuous univariate comparisons between groups, while a Chi-square or Fisher’s exact test was used for categorical variable comparisons between groups. The Cochran-Armitage test for trend was used for ordered categorical variables. A significance level of 0.05 was used for all analyses. Since this was an exploratory study to define factors that may differ in the OH and IH cohort settings, statistical adjustment for multiple comparisons was not performed. We graphically summarized cumulative survival and discharged alive for the first 60 days post arrest in each cohort. Patients discharged alive prior to day 60 were assumed alive through day 60. All analyses were performed utilizing SAS 9.1 for Windows (Cary, NC).
RESULTS
Four hundred and ninety-three (493) cases were included in this report of pediatric CA with ROC; 138 were OH and 353 were IH events. Table 1 describes demographic and pre-existing conditions for both cohorts. Median age was older (2.9 vs. 0.9 years; p<0.01) and gender more often male in the OH cohort (69% vs. 57%; p=0.02). The frequency of a chronic pre-existing condition was much less common for the OH cohort (49% vs. 88%; p<0.01).
Table 1.
Comparison of patient demographic and pre-existing conditions for In-hospital and Out-of-hospital cardiac arrest cohortsa
| IH (N = 353) | OH (N = 138) | P-Valueb | |
|---|---|---|---|
| Median (IQR) | Median (IQR) | ||
| Age (years) | 0.9 (0.2, 6.1) | 2.9 (0.6, 11.4) | < 0.01 |
| Weight (kg) | 7.4 (3.7, 20.0) | 15.0 (7.5, 35.0) | < 0.01 |
| n (percent) | n (percent) | ||
| Gender (male) | 202 (57) | 94 (69) | 0.02 |
| Race | 0.67 | ||
| White | 168 (48) | 63 (46) | |
| Black | 91 (26) | 41 (30) | |
| Other/Unknown | 94 (27) | 34 (25) | |
| Ethnicity | 0.07 | ||
| Hispanic | 26 (7) | 5 (4) | |
| Not Hispanic | 117 (33) | 59 (43) | |
| Unknown | 210 (60) | 74 (54) | |
| Insurance type | |||
| Commercial | 188 (56) | 61 (47) | 0.13 |
| Medicaid | 127 (38) | 55 (42) | |
| Other insurance | 22 (7) | 14 (11) | |
| Any chronic pre-existing condition | 310 (88) | 68 (49) | < 0.01 |
| Specific chronic pre-existing conditionsc | |||
| Prenatal conditions or complications | 46 (13) | 17 (12) | 0.83 |
| Lung or airway disease | 94 (27) | 29 (21) | 0.20 |
| Congenital heart disease | 176 (50) | 14 (10) | < 0.01 |
| Acquired heart disease | 43 (12) | 6 (4) | 0.01 |
| Hematologic, oncologic or immunologic | 56 (16) | 2 (1) | < 0.01 |
| Gastrointestinal | 75 (21) | 13 (9) | < 0.01 |
| Genetic/metabolic | 54 (15) | 6 (4) | < 0.01 |
| Endocrine | 12 (3) | 2 (1) | 0.37 |
| Renal | 45 (13) | 0 (0.0) | < 0.01 |
| Neurological | 82 (23) | 30 (22) | 0.72 |
Unavailable (missing) values were excluded from calculations of percentages and summary statistics for the following variables: age (1), weight (9), gender (3), insurance type (24).
For comparison between IH and OH arrests, Chi-square or Fisher’s exact was used for categorical variables and Wilcoxon rank-sum test was used for continuous variables.
For chronic pre-existing conditions, a condition was assumed to be not present unless specifically noted otherwise.
Table 2 describes CA event characteristics. The cohorts were similar with respect to proportion of weekend or night occurrence of the study eligible events using Method 1 definitions; with Method 2 definitions, there was a trend for more daytime events in the OH cohort (p=0.05). The initial cardiac rhythms differed (p<0.01); asystole was more commonly reported as the initial OH rhythm (46% vs. 16%), while bradycardia was the more frequent initial IH rhythm (49% vs. 10%). Asystole occurring anytime during an arrest event occurred more commonly in OH than IH arrests (51% vs. 29%; p<0.01), while ventricular fibrillation or ventricular tachycardia at any time during an arrest occurred at a similar frequency of about 1 in 5 IH or OH arrests. The administration of Pediatric Advanced Life Support (PALS) drugs during CA differed; atropine was more commonly administered during OH arrests, while sodium bicarbonate and calcium were administered much more frequently during IH events. Median number of epinephrine doses administered was similar for IH and OH arrests, while the proportion receiving any dose of epinephrine was higher in the IH cohort. The duration of CPR was shorter in the IH compared to OH cohort (median 9.0 (4.0, 25.0) vs. 31 (18.0, 50.0) min; p<0.01); however, for the OH cohort, data were available for only 69 events.
Table 2.
Comparison of cardiac arrest event characteristics for In-hospital and Out-of-hospital cohortsa
| IH Overall (N = 353) | OH Overall (N = 138) | P-Valueb | |
|---|---|---|---|
| n (percent) | n (percent) | ||
| Day of arrest (if unavailable, using CPR, ROC, or arrival at hospital) | 0.71 | ||
| Weekday (Mon 12:00 am–Fri 11:59 pm) | 255 (72) | 102 (74) | |
| Weekend (Sat 12:00 am–Sun 11:59 pm) | 98 (28) | 36 (26) | |
| Time of arrest (if unavailable, using CPR, ROC, or arrival at hospital) | 0.28 | ||
| Day (7:00 am–6:59 pm) | 190 (54) | 80 (60) | |
| Night (7:00 pm–6:59 am) | 160 (46) | 54 (40) | |
| Day of arrest (if unavailable, using CPR, ROC, or arrival at hospital) | 0.95 | ||
| Weekday (Mon 7:00 am–Fri 10:59 pm) | 244 (70) | 93 (69) | |
| Weekend (Fri 11:00 pm–Mon 6:59 am) | 106 (30) | 41 (31) | |
| Time of arrest (if unavailable, using CPR, ROC, or arrival at hospital) | 0.05 | ||
| Day (7:00 am–10:59 pm) | 252 (72) | 108 (81) | |
| Night (11:00 pm–6:59 am) | 98 (28) | 26 (19) | |
| First monitored rhythm | < 0.01 | ||
| Asystole | 55 (16) | 64 (46) | |
| Bradycardia | 173 (49) | 14 (10) | |
| Pulseless electrical activity | 31 (9) | 14 (10) | |
| Ventricular fibrillation/tachycardia | 35 (10) | 9 (7) | |
| Other/Unknown | 59 (17) | 37 (27) | |
| Asystole rhythm documented (any time) | 101 (29) | 71 (51) | < 0.01 |
| VF/VT rhythm documented (any time) | 67 (19) | 30 (22) | 0.49 |
| Drugs administered during arrest | |||
| Fluid bolus | 139 (42) | 58 (43) | 0.77 |
| Atropine | 124 (37) | 67 (50) | 0.01 |
| Sodium Bicarbonate | 203 (61) | 56 (41) | < 0.01 |
| Calcium | 174 (52) | 10 (7) | < 0.01 |
| Vasopressin | 18 (5) | 2 (1) | 0.06 |
| Lidocaine | 33 (10) | 12 (9) | 0.75 |
| Procainamide | 0 (0) | 0 (0) | NA |
| Amiodarone | 19 (6) | 2 (1) | 0.05 |
| Epinephrine (any received) | 293 (86) | 96 (76) | 0.01 |
| Median (IQR) | Median (IQR) | ||
| Epinephrine doses administered | 2.0 (1.0, 4.0) | 3.0 (1.0, 5.0) | 0.28 |
Unavailable (missing) values were excluded from calculations of percentages and summary statistics for the following variables: day of arrest (definition 2 only) (7), time of arrest (7), drugs administered (except epinephrine) (21), epinephrine administered (24).
For comparison between IH and OH arrests, Chi-square or Fisher’s exact was used for categorical variables and Wilcoxon rank-sum test was used for continuous variables.
Table 3 describes the etiology of the arrest events. There were important differences between the two cohorts; cardiac etiologies were much more frequently reported in IH arrests [congenital heart disease (CHD) 37% vs. 4% and non-CHD 36% vs. 15%; both p<0.01], while respiratory etiologies were more common in OH arrests (72% vs. 42%; p<0.01).
Table 3.
Comparison of the etiology of cardiac arrest for In-hospital and Out-of-hospital cohortsa
| IH Overall (N=353) | OH Overall (N = 138) | P-Valueb | |
|---|---|---|---|
| n (percent) | n (percent) | ||
| Cardiac (not congenital heart disease) | 124 (36) | 20 (15) | < 0.01 |
| Arrhythmia | 42 (12) | 13 (9) | |
| Hypovolemic shock | 19 (5) | 2 (1) | |
| Septic shock | 28 (8) | 2 (1) | |
| Cardiomyopathy | 8 (2) | 1 (1) | |
| Other | 41 (12) | 4 (3) | |
| Congenital heart disease | 130 (37) | 6 (4) | < 0.01 |
| Arrhythmia | 69 (20) | 4 (3) | |
| Low cardiac output | 38 (11) | 1 (1) | |
| Hypoxemia | 15 (4) | 2 (1) | |
| During post-op course | 52 (15) | 1 (1) | |
| Tamponade | 4 (1) | 0 (0) | |
| Other | 9 (3) | 2 (2) | |
| Respiratory | 145 (42) | 98 (72) | < 0.01 |
| Acute life threatening event | 3 (1) | 22 (16) | |
| Endotracheal tube displacement | 19 (5) | 2 (1) | |
| Respiratory failure | 112 (32) | 37 (27) | |
| Airway obstruction | 8 (2) | 5 (4) | |
| Drowning/Asphyxia | 0 (0) | 43 (31) | |
| Other | 8 (2) | 4 (3) | |
| Neurological | 8 (2) | 5 (4) | 0.53 |
| Drug overdose/Ingestion | 3 (1) | 4 (3) | 0.10 |
| Trauma | 22 (6) | 15 (11) | 0.09 |
| Electrolyte imbalance | 30 (9) | 4 (3) | 0.03 |
| Terminal condition | 12 (3) | 1 (1) | 0.12 |
Patients could have multiple categories identified for etiology of arrest. There was one OH and six IH arrests with no information documented for etiology of arrest. These records were excluded from percentage calculations.
Chi-square or Fisher’s exact used for comparison between IH and OH arrests.
Table 4 describes monitoring and interventions in the 12 h post-arrest period. Pulse oximetry and cardiac monitoring were nearly universal and the use of mechanical ventilation was >90% in both groups. Intraosseous catheters were used more commonly in OH cases, while central venous catheters (includes all central access catheters) and arterial lines were more common in the IH cases. TH was infrequently utilized in either group (<5%). ECMO and dialysis were utilized more commonly in IH cases. Anticonvulsant agents were administered more commonly in the OH group, while administration of one or more inotrope or vasopressor agents was more common in the IH group. An epinephrine infusion was the most frequently used inotrope/vasopressor agent; its use was similar in the cohorts. Dopamine, milrinone, vasopressin, steroids and antimicrobial use were more common in IH cases, while mannitol was employed more often following OH events.
Table 4.
Post-arrest hospital course (0–12 Hours) for In-hospital and Out-of-hospital cohortsa
| IH Overall (N=353) | OH Overall (N = 138) | P-Valueb | |
|---|---|---|---|
| n (percent) | n (percent) | ||
| ICU interventions and monitoring devices | |||
| Cardiac monitor | 350 (100) | 130 (98) | 0.07 |
| Pulse oximeter | 350 (100) | 130 (98) | 0.07 |
| Peripheral intravenous catheter | 256 (73) | 116 (87) | < 0.01 |
| Intraosseous access | 20 (6) | 31 (23) | < 0.01 |
| Central venous catheter/CVP | 307 (87) | 100 (75) | < 0.01 |
| Arterial catheter | 281 (80) | 90 (68) | < 0.01 |
| Mechanical ventilation | 335 (95) | 122 (92) | 0.11 |
| ECMO | 58 (17) | 3 (2) | < 0.01 |
| Dialysis | 19 (5) | 0 (0) | 0.01 |
| Intracranial pressure monitor | 9 (3) | 1 (1) | 0.30 |
| Therapeutic hypothermia (TH) | 13 (4) | 3 (2) | 0.57 |
| Drug therapies | |||
| Antiarrhythmics | 66 (19) | 33 (25) | 0.14 |
| Anticonvulsants | 54 (15) | 35 (26) | 0.01 |
| Any inotrope or vasopressor | 293 (83) | 93 (70) | < 0.01 |
| Dopamine | 180 (51) | 48 (36) | < 0.01 |
| Dobutamine | 50 (14) | 17 (13) | 0.68 |
| Epinephrine | 216 (62) | 76 (57) | 0.38 |
| Norepinephrine | 27 (8) | 5 (4) | 0.12 |
| Milrinone or amrinone | 117 (33) | 6 (5) | < 0.01 |
| Vasopressin | 54 (15) | 10 (8) | 0.02 |
| Other inotrope or vasopressor | 49 (14) | 18 (14) | 0.90 |
| Antimicrobials | 257 (73) | 73 (55) | < 0.01 |
| Steroids | 87 (25) | 7 (5) | < 0.01 |
| Mannitol | 8 (2) | 11 (8) | < 0.01 |
| Hypertonic saline | 3 (1) | 1 (1) | 1.00 |
Unavailable (missing) values were excluded from calculations of percentages and summary statistics for the following variables: ICU intervention and monitoring devices (7) [except therapeutic hypothermia (0)], drug therapies (7).
Table 5 describes important physiologic and laboratory measurements in the 0–12 h time period post-arrest. In the OH cohort, the minimum body temperature recorded was lower than for IH cases (median 34.1 vs. IH 35.3° C; p<0.01). The maximum body temperature was also higher in the OH cohort. The minimum recorded pH was lower in OH arrests (median 7.03 vs. 7.20; p<0.01), while maximum lactate measurements were similar (8.0 and 7.8 mmol/L). The maximum glucose was higher in the OH cohort, while the minimum glucose was lower in the IH cohort. Bilaterally reactive pupils were documented more often during the 12 h following ROC in IH cohort. Seizures occurred after CA and prior to hospital discharge nearly twice as often in the OH cases. Subsequent CA within 24 h occurred in about 1/4 cases in each group.
Table 5.
Comparison of physiologic and laboratory values (0–12 hours after ROC) for in-hospital versus out-of-hospital cohortsa
| IH Overall (N = 353) | OH Overall (N = 138) | P-Valueb | |||
|---|---|---|---|---|---|
| N | Median (IQR) | N | Median (IQR) | ||
| Minimum body temperature, °C | 338 | 35.3 (34.2, 36.3) | 133 | 34.1 (32.5, 35.6) | < 0.01 |
| Maximum body temperature, °C | 338 | 37.1 (36.5, 38.0) | 133 | 37.8 (36.7, 38.8) | < 0.01 |
| Minimum pH | 328 | 7.20 (7.04, 7.33) | 123 | 7.03 (6.80, 7.20) | < 0.01 |
| Maximum pH | 328 | 7.46 (7.36, 7.53) | 123 | 7.37 (7.31, 7.46) | < 0.01 |
| Maximum lactate, mmol/L | 230 | 7.8 (3.1, 14.0) | 76 | 8.0 (4.1, 13.8) | 0.74 |
| Minimum glucose, mg/dL | 313 | 113 (82, 172) | 126 | 135 (97, 203) | < 0.01 |
| Maximum glucose, mg/dL | 313 | 195 (120, 291) | 126 | 291 (188, 346) | < 0.01 |
| N | n (percent) | N | n (percent) | ||
| Two responsive pupils | 313 | 235 (75) | 131 | 42 (32) | < 0.01 |
| Seizures post-arrest (prior to hospital discharge) | 346 | 50 (14) | 133 | 35 (26) | < 0.01 |
| Subsequent arrests w/in 24 hrs of initial CA | 353 | 98 (28) | 138 | 31 (22) | 0.23 |
Unavailable (missing) values were excluded from calculations of summary statistics.
For comparison between IH and OH arrests, Chi-square test was used for categorical variables and Wilcoxon rank-sum test was used for continuous variables.
Table 6 depicts PCPC score changes in the two cohorts. Baseline PCPC was normal in 83% of OH cases and 66% of IH cases (p<0.01). At hospital discharge, PCPC was unchanged in only 24% of OH cases compared with 44% of IH cases (p<0.01). Good neurological outcome (defined as PCPC equal to 1 or 2, or no change in baseline PCPC) occurred in 24% of the OH cohort compared to 47% in the IH cohort (p<0.01). Mortality rate was higher in the OH cohort (62% vs. 51%, p=0.04). The primary cause of death differed markedly (p<0.01); death was attributed to neurological futility or brain death much more frequently in the OH cohort (69% vs. 20%), while cardiovascular causes occurred more commonly in the IH cohort (50% vs. 22%).
Table 6.
Pre-arrest PCPC and hospital outcomes for children with in-hospital versus out-of-hospital arresta
| IH Overall (N = 353) | OH Overall (N = 138) | P valueb | |
|---|---|---|---|
| n (percent) | n (percent) | ||
| Baseline PCPC | < 0.01 | ||
| Normal | 187 (66) | 105 (83) | |
| Mild disability | 42 (15) | 9 (7) | |
| Moderate disability | 35 (12) | 7 (6) | |
| Severe disability | 16 (6) | 5 (4) | |
| Vegetative | 4 (1) | 1 (1) | |
| Hospital d/c PCPC | 0.01 | ||
| Normal | 89 (27) | 23 (17) | |
| Mild disability | 27 (8) | 6 (4) | |
| Moderate disability | 19 (6) | 5 (4) | |
| Severe disability | 11 (3) | 12 (9) | |
| Vegetative | 0 (0) | 6 (4) | |
| Dead | 181 (55) | 85 (62) | |
| No change in PCPC | 124 (44) | 30 (24) | < 0.01 |
| Good neurologic outcomec | 132 (47) | 31 (24) | <0.01 |
| Death (includes cases with missing PCPC data) | 181(51) | 85 (62) | 0.04 |
| Cause of Death | < 0.01 | ||
| Neurologic | 36 (20) | 59 (69) | |
| Cardiovascular | 90 (50) | 19 (22) | |
| Other | 54 (30) | 7 (8) |
Unavailable (missing) values were excluded from percentage calculations for the following variables: baseline PCPC (69 IH, 11 OH), PCPC at hospital discharge (26 IH, 1 OH), change in PCPC and neurologic outcome (74 IH, 11 OH cases), cause of death (1 IH).
Cochran-Armitage trend test used for comparison between IH and OH arrests for ordered categorical variables (ie, baseline and hospital d/c PCPC). Chi-square test used for all other variables.
Good neurologic outcome defined as either no change in PCPC from baseline to hospital discharge or a PCPC of Normal or Mild Disability at hospital discharge.
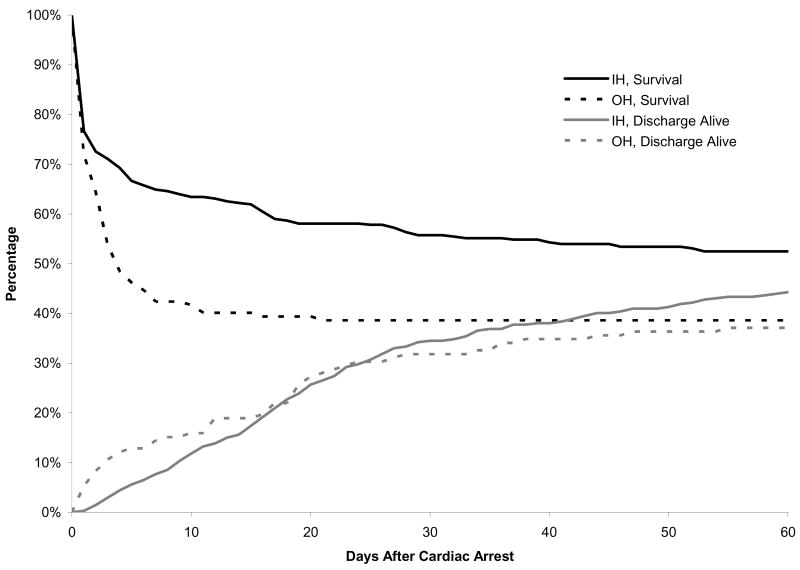
Figure 1 highlights differences in the percentage surviving and discharged alive for the first 60 days following the CA. Trends differed remarkably between cohorts with survival in the OH cohort falling below 50% within the first five days post-arrest. Survival at 10 days post-arrest was 42% in the OH cohort compared to 63% in the IH arrest group. Discharge alive was similar between the two groups at approximately day 15; the durations of PICU and hospital stay post-arrest were longer for the IH compared to the OH cohort (median 7.0 (1.0, 20.0) vs. 3.0 (1.0, 7.0) and median 12.0 (2.0, 27.0) vs. 3.0 (1.0, 11.0); p<0.01 in both cases).
Figure 1. Overall survivala and discharge alive proportion among cases with In-hospital and Out-of-hospital cardiac arrest and return of circulation.
a Patients discharged alive prior to day 60 were assumed alive through day 60.
DISCUSSION
This is the first multicenter cohort study conducted in a US population of children with CA and ROC that compares and contrasts major clinical features of IH and OH CA. This study was performed to provide information needed to plan an interventional RCT of TH after pediatric CA. An essential prerequisite for evaluation of new therapies in these patients is information about whether there are differences that preclude combining IH and OH cases in clinical trials. Our analyses demonstrate that there are important differences between the IH and OH CA patients who would be eligible for an interventional clinical trial of TH to improve neurobehavioral outcome.
There were five major differences between the OH and IH cohorts: 1) pre-arrest baseline neurological status (based on PCPC) was more frequently normal in OH cases [83% vs. 66%]; 2) chronic pre-existing conditions were nearly twice as common in the IH cohort [88% vs. 49%]; 3) etiology was predominately respiratory for the large majority of the OH cohort [72% vs. 42%] and cardiac for the IH cohort; 4) neurological status at hospital discharge was unchanged from baseline nearly twice as often in the IH cohort [44% vs. 24%]; and 5) most important for an interventional trial of TH, the attributed cause for death was neurological injury in the vast majority in the OH cohort while uncommon in the IH cohort [69% vs. 20%]. These findings strongly suggest that combining OH and IH cases in a single clinical trial would be ill advised especially for interventions designed to improve neurobehavioral outcomes after pediatric CA.
Several other cohort differences merit comment. Baseline characteristics of the IH and OH cases differed with respect to age and gender. OH cases were older and more likely to be male. Specific arrest event associated factors also were dissimilar between IH and OH cases. For example, the initial cardiac rhythm documented after start of chest compressions was bradycardia for IH arrests and asystole for OH arrests. This is not surprising given that most IH CA occur in monitored settings (i.e. PICU) where early rhythm and heart rate changes would be optimally identified, while OH arrests most likely documented the onset of the arrest and initial rhythm at a much later time when monitoring equipment was available (3). The use of PALS medications during resuscitations differed. Epinephrine use was reported in a somewhat higher proportion of IH cases, but this could reflect poor documentation or unavailable records from the pre-hospital setting in the OH group. The use of calcium in more than 50% of IH cases but only 7% of OH cases is quite notable. Calcium administration is not generally recommended for children in the IH setting, and its use may be associated with poorer outcomes (35).
Supportive therapies and monitoring utilized during the first twelve hours following ROC for OH and IH cohorts were dissimilar in some respects. Central venous and arterial catheters were documented more commonly in IH cases; in some cases, they may have been placed prior to the arrest. The higher use of IO catheters in OH cases is not surprising since this route is recommended when IV access can not be established rapidly. ECMO was used more commonly in IH cases where rapid response to an arrest event would be possible compared to OH arrests. It is noteworthy that the use of TH was very low in both groups (<5%), even though the study period followed publication of the adult hypothermia RCTs (20, 21). The low use is likely due to absence of pediatric RCTs and protocols to guide use; this suggests equipoise exists for TH at our PECARN sites. Equipoise was also described in a recent survey report (27).
Figure 1 depicts the large difference in the time courses of survival and live discharge between OH and IH cohorts. Mortality peaked rapidly (survival declined most) in the first 5 days in the OH cohort, which may reflect greater multi-organ hypoxic-ischemic injury compared to the IH cohort. This interpretation is supported by the lower minimum pH measured within 12 h of ROC in the OH cohort. Seizures were reported and treated nearly twice as often in the OH as the IH cohort, while bilateral reactive pupils were present less than half as often in the OH cohort. Both observations are congruent with greater neurological injury sustained in the OH survivors, and the much higher proportion of deaths due to neurological injury in the OH group.
For the planning of a TH RCT it was important to determine the range of temperatures observed in the early post-ROC period. For the 12 hours immediately following OH arrest, the median for lowest temperature reported was 34.1° C and median maximum temperature was 37.8° C. This lowest temperature is very close to the range of hypothermia utilized in adult RCTs (32–34° C) and neonatal clinical trials (20, 21, 23, 24). This suggests a goal temperature in the 32–34° C range could be achieved quickly and easily in this group. The mildly elevated maximum temperatures observed in the OH group were an unexpected finding that could have been the result of over aggressive warming. Thus, rigorous monitoring will be necessary to avoid hyperthermia in the control groups of future hypothermia trials (36, 37). For the IH group, median recorded lowest temperature was higher (35.3° C); this likely reflects preexisting or early placement of temperature monitoring and warming equipment in the PICU or monitored setting.
Comparison of findings from our study to existing information in the literature may be limited. Our study population had unique characteristics related to our goal to delineate explicit inclusion and exclusion criteria for future pediatric TH trials. For example, a minimum requirement for inclusion was duration of chest compressions for at least one minute followed by ROC for at least 20 minutes; this time interval was selected in order to capture a population at some risk for adverse neurological outcome. Patients receiving shorter durations of chest compression, whether or not they received defibrillation, epinephrine or other PALS medications, were excluded. Also, we excluded cases that did not survive the initial resuscitation event (no ROC for at least 20 minutes) since our long-term goal was to plan an interventional clinical trial of hypothermia. This limits direct comparison of our results with studies that included cases without ROC. A recent review reported that about 70% of OH arrests did not have ROC and the NRCPR reported that approximately 50% of IH arrests do not have ROC (3,4). Additionally, we did not use the exact NRCPR definition of IH CA, which emphasizes documentation in the medical record of the absence of a palpable pulse or a rhythm not associated with a pulse. Pulse detection is an extremely unreliable and problematic physical finding to accurately measure in adults under optimal conditions; trained pediatric caregivers perform poorly as well (38–40). An AHA-affiliated expert group recently proposed a different ‘pragmatic definition’ for OH CA to include ‘receives chest compressions by EMS personnel’ (41). A criterion of chest compressions for at least a minute (or other duration) could be applicable for clinical trials in both the OH and IH settings. We suspect that few cases with a minute or more of chest compressions would actually be associated with a clinically detectable pulse and cases classified as bradycardia in our study would likely have been classified as pulseless electrical activity (PEA) using the NRCPR algorithm. This is indirectly supported by mortality rates observed in our cohorts. For example, our IH cohort mortality was very similar to a recent NRCPR report containing pediatric cases with ROC (49%) (3), and our OH cohort mortality was similar to the largest report to date that used Utstein criteria (66%) (4).
A limitation of our report common to other reports on pediatric CA is missing data for some variables. For example, we observed missing initial rhythm data in 20% for IH and OH events, which is similar to a NRCPR report with 22% missing data for this variable. Another limitation, which is shared with most other reports on pediatric CA survivors, is the classification of neurological outcome, both with respect to early timing at hospital discharge and reliance on relatively crude measures like PCPC. Although a few reports indicate that status of children at hospital discharge is largely unchanged at one year follow-up (42, 43), detailed neurobehavioral testing at one year or more after the CA event is needed to confirm these initial reports and to delineate the impact of the event on more subtle areas of neurobehavioral function (44, 45).
A major strength of our report is that the 15 sites were supported by a federally funded research network (PECARN) with a data coordinating center (CDMCC). A standard data collection tool and training were used by all sites and the CDMCC systematically verified data. The entire spectrum of pediatric cardiac arrest cases, including IH, OH and transfers from another facility were captured. The participating clinical sites had at least 12 PICU beds and were distributed across most large geographical areas of the U.S. except southern states. In contrast, most prior U.S. reports were from single sites or geographical areas. The notable exception is the NRCPR registry, which is currently limited to IH CA.
In summary, this study demonstrates global differences between pediatric CA in the OH and IH settings. The most important differences in the context of planning a clinical trial of TH to improve neurobehavioral outcome relate to neurological issues. Patients from the OH setting are much more likely than IH cases to have normal baseline PCPC scores, while IH survivors are much more likely than OH survivors to have good PCPC outcomes at hospital discharge. Our most important new finding is that mortality was attributed to neurological factors in nearly 70% of OH cases, in significant contrast to only 20% of IH cases. Other important differences were that etiology of CA was respiratory in the majority of the OH cohort and pre-existing conditions were much more common in IH cohort. These findings provide a compelling rationale for implementation of separate clinical trials of TH for pediatric cardiac arrest in the two groups.
Acknowledgments
Supported by the following federal grants: NIH NICHD R21 HD044955 and R34 HD 050531 (FWM). The Pediatric Emergency Care Applied Research Network (PECARN) is supported by cooperative agreements U03MC00001, U03MC00003, U03MC00006, U03MC00007, and U03MC00008 from the Emergency Medical Services for Children (EMSC) program of the Maternal and Child Health Bureau, Health Resources and Services Administration, US Department of Health and Human Services.
We acknowledge the efforts of the following individuals participating in PECARN at the time this study was initiated.
PECARN Steering Committee: N. Kuppermann, Chair; E. Alpern, J. Chamberlain, J. M. Dean, M. Gerardi, J. Goepp, M. Gorelick, J. Hoyle, D. Jaffe, C. Johns, N. Levick, P. Mahajan, R. Maio, K. Melville, S. Miller*, D. Monroe, R. Ruddy, R. Stanley, D. Treloar, M. Tunik, A. Walker. MCHB/EMSC liaisons: D. Kavanaugh, H. Park.
Central Data Management and Coordinating Center (CDMCC): M. Dean, R. Holubkov, S. Knight, A. Donaldson.
Data Analysis and Management Subcommittee (DAMS): J. Chamberlain, Chair; M. Brown, H. Corneli, J. Goepp, R. Holubkov, P. Mahajan, K. Melville, E. Stremski, M. Tunik
Grants and Publications Subcommittee (GAPS): M. Gorelick, Chair; E. Alpern, J. M. Dean, G. Foltin, J. Joseph, S. Miller*, F. Moler, R. Stanley, S. Teach
Protocol Concept Review and Development Subcommittee (PCRADS): D. Jaffe, Chair; K. Brown, A. Cooper, J. M. Dean, C. Johns, R. Maio, N. C. Mann, D. Monroe, K. Shaw, D. Teitelbaum, D. Treloar
Quality Assurance Subcommittee (QAS): R. Stanley, Chair; D. Alexander, J. Brown, M. Gerardi, M. Gregor, R. Holubkov, K. Lillis, B. Nordberg, R. Ruddy, M. Shults, A. Walker
Safety and Regulatory Affairs Subcommittee (SRAS): N. Levick, Chair; J. Brennan, J. Brown, J. M. Dean, J. Hoyle, R. Maio, R. Ruddy, W. Schalick, T. Singh, J. Wright
* deceased
Footnotes
Presented in part at Society of Critical Care Medicine’s 36th Critical Care Congress, Orlando, FL. Crit Care Med 2006; 34 [Suppl] A421.
Participating children’s hospital, university affiliation and site investigators are listed below in alphabetical order:
Children’s Hospital Medical Center, University of Cincinnati, Cincinnati, Ohio (R. Brilli)
Children’s Hospital of Buffalo, SUNY-Buffalo, Buffalo, NY (L. Hernan)
Children’s Hospital of Michigan, Wayne State University, Detroit, MI (K. Meert)
Children’s Hospital of New York, Columbia University, New York, NY (C. Schleien)
Children’s Hospital of Philadelphia, University of Pennsylvania, Philadelphia, PA (V. Nadkarni)
Children’s Hospital of Pittsburgh, University of Pittsburgh, Pittsburgh, PA (R. Clark)
Children’s Hospital of Wisconsin, Medical Collage of Wisconsin, Milwaukee, WI (K. Tieves)
Children’s National Medical Center, George Washington University, Washington D.C. (H. Dalton)
C.S. Mott Children’s Hospital, University of Michigan, Ann Arbor, MI (F. Moler)
Golisano Children’s Hospital, University of Rochester, Rochester, NY (E. van der Jagt)
Helen DeVos Children’s Hospital, Michigan State University, Grand Rapids, MI (R. Hackbarth)
Primary Children’s Medical Center, University of Utah, Salt Lake City, UT (K. Statler)
St. Louis Children’s Hospital, Washington University St. Louis, MO (F. Levy)
The Johns Hopkins Hospital, Johns Hopkins University, Baltimore, MD (H. Shaffner)
University of California at Davis, Sacramento, CA (R. Pretzlaff)
References
- 1.Young KD, Seidel JS. Pediatric cardiopulmonary resuscitation: a collective review. Ann Emer Med. 1999;33:195–205. doi: 10.1016/s0196-0644(99)70394-x. [DOI] [PubMed] [Google Scholar]
- 2.Donoghue AG, Nadkarni V, Berg RA, et al. Out-of-hospital pediatric cardiac arrest: an epidemiologic review and assessment of current knowledge. Ann Emerg Med. 2005;46:512–22. doi: 10.1016/j.annemergmed.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 3.Nadkarni VM, Larkin GL, Peberdy MA, et al. for the National Registry of Cardiopulmonary Resuscitation Investigators. First Documented Rhythm and Clinical Outcome from In-Hospital Cardiac Arrest Among Children and Adults. JAMA. 2006;295:50–57. doi: 10.1001/jama.295.1.50. [DOI] [PubMed] [Google Scholar]
- 4.Young KD, Gausche-Hill M, McClung CD, Lewis RJ. A prospective, population-based study of the epidemiology and outcome of out-of-hospital pediatric cardiopulmonary arrest. Pediatrics. 2004;114:157–164. doi: 10.1542/peds.114.1.157. [DOI] [PubMed] [Google Scholar]
- 5.Sirbaugh PE, Pepe PE, Shook JE, et al. A prospective, population-based study of the demographics, epidemiology, management, and outcome of out-of-hospital pediatric cardiopulmonary arrest. Ann Emerg Med. 1999;33:174–184. doi: 10.1016/s0196-0644(99)70391-4. [DOI] [PubMed] [Google Scholar]
- 6.Schindler MB, Bohn DB, Cox PN, et al. Outcome of out-of-hospital cardiac or respiratory arrest in children. N Engl J Med. 1996;335:1473–1479. doi: 10.1056/NEJM199611143352001. [DOI] [PubMed] [Google Scholar]
- 7.Ronco R, King W, Donley DK, et al. Outcome and cost at a children’s hospital following resuscitation for out-of-hospital cardiopulmonary arrest. Arch Pediatr Adolesc Med. 1995;149:210–214. doi: 10.1001/archpedi.1995.02170140092017. [DOI] [PubMed] [Google Scholar]
- 8.O’Rourke PP. Outcome of children who are apneic and pulseless in the emergency room. Crit Care Med. 1986;14:466–468. [PubMed] [Google Scholar]
- 9.Torphy DE, Minter MG, Thompson BM. Cardiorespiratory arrest and resuscitation of children. Am J Dis Child. 1984;138:1099–1102. doi: 10.1001/archpedi.1984.02140500005003. [DOI] [PubMed] [Google Scholar]
- 10.Eisenberg M, Bergner L, Hallstrom A. Epidemiology of cardiac arrest and resuscitation in children. Ann Emerg Med. 1983;12:672–674. doi: 10.1016/s0196-0644(83)80413-2. [DOI] [PubMed] [Google Scholar]
- 11.Thompson JE, Bonner B, Lower GM. Pediatric cardiopulmonary arrests in rural populations. Pediatrics. 1990;86:302–306. [PubMed] [Google Scholar]
- 12.Hickey RW, Cohen DM, Strausbaugh S, et al. Pediatric patients requiring CPR in the prehospital setting. Ann Emerg Med. 1995;25:495–501. doi: 10.1016/s0196-0644(95)70265-2. [DOI] [PubMed] [Google Scholar]
- 13.Nichols DG, Kettrick RG, Swedlow DB, et al. Factors influencing outcome of cardiopulmonary resuscitation in children. Pediatr Emerg Care. 1986;2:1–5. doi: 10.1097/00006565-198603000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Fiser DH, Wrape V. Outcome of cardiopulmonary resuscitation in children. Ped Emerg Care. 1987;3:235–238. doi: 10.1097/00006565-198712000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Torres A, Pichert CB, Firestone J, et al. Long-term functional outcome of inpatient pediatric cardiopulmonary resuscitation. Pediatr Emerg Care. 1997;13:369–373. doi: 10.1097/00006565-199712000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Reis AG, Nadkarni V, Perondi MB, et al. A prospective investigation into the epidemiology of in-hospital pediatric cardiopulmonary resuscitation using the internation Utstein reporting style. Pediatrics. 2002;109:200–209. doi: 10.1542/peds.109.2.200. [DOI] [PubMed] [Google Scholar]
- 17.Gillis J, Dickson D, Rieder M, et al. Results of inpatient pediatric resuscitation. Crit Care Med. 1986;14:469–471. doi: 10.1097/00003246-198605000-00007. [DOI] [PubMed] [Google Scholar]
- 18.de Mos N, van Litsenburg RR, McCrindle B, et al. Pediatric in-intensive-care-unit cardiac arrest: Incidence, survival, and predictive factors. Crit Care Med. 2006;34:1209–1215. doi: 10.1097/01.CCM.0000208440.66756.C2. [DOI] [PubMed] [Google Scholar]
- 19.Ehrlich R, Emmett SM, Rodriquez-Torres Pediatric cardiac resuscitation team: A 6 year study. J Pediatr. 1974;84:152–155. doi: 10.1016/s0022-3476(74)80580-9. [DOI] [PubMed] [Google Scholar]
- 20.The Hypothermia After Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurological outcome after cardiac arrest. N Engl J Med. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 21.Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 22.Gluckman PG, Wyatt JS, Azzopardi D, et al. on behalf of the CoolCap Study Group. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomized trial. Lancet. 2005;365:663–670. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- 23.Shankaran S, Laptook AR, Ehrenkranz RA, et al. for the National Institute of Health and Human Development Neonatal Research Network. Whole-Body Hypothermia for Neonates with Hypoxic-Ischemic Encephalopathy. N Engl J Med. 2005;353:1574–84. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 24.Eicher DJ, Wagner CL, Katikaneni LP, et al. Moderate Hypothermia in Neonatal Encephalopathy: Efficacy Outcomes. Pediatr Neurol. 2005;32:11–17. doi: 10.1016/j.pediatrneurol.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 25.Hutchison JS, Ward RE, Lacrois J, et al. Hypothermia Therapy after Traumatic Brain Injury in Chilren. N Engl J Med. 2008;358:2447–56. doi: 10.1056/NEJMoa0706930. [DOI] [PubMed] [Google Scholar]
- 26.Clifton GL, Miller ER, Choi SC, et al. Lack of effect of induction of hypothermia after acute brain injury. N Engl J Med. 2001;344:556–63. doi: 10.1056/NEJM200102223440803. [DOI] [PubMed] [Google Scholar]
- 27.Haque IU, LaTour MC, Zaritsky AL. Pediatric critical care community survey of knowledge and attitudes toward therapeutic hypothermia in comatose children after cardiac arrest. Pediatr Crit Care Med. 2006;7:7–14. doi: 10.1097/01.pcc.0000192322.45123.80. [DOI] [PubMed] [Google Scholar]
- 28.The Pediatric Emergency Care Applied Research Network. The Pediatric Emergency Care Applied Research Network (PECARN): Rational, Development, and First Steps. Acad Emerg Med. 2003;10:661–668. [PubMed] [Google Scholar]
- 29.Pollack MM, Patel KM, Ruttimann UE. The Pediatric Risk of Mortality III--Acute Physiology Score (PRISM III-APS): a method of assessing physiologic instability for pediatric intensive care unit patients. J Pediatr. 1997;131:575–81. doi: 10.1016/s0022-3476(97)70065-9. [DOI] [PubMed] [Google Scholar]
- 30.Zaritsky A, Nadkarni V, Hazinski MF, et al. Recommended guidelines for uniform reporting of pediatric advanced life support: the pediatric Utstein style. American Academy of Pediatrics, American Heart Association and the European Resuscitation Council. Ann Emerg Med. 1995;26:487–503. doi: 10.1016/s0196-0644(95)70119-2. [DOI] [PubMed] [Google Scholar]
- 31.Jacobs I, Nadkarni V, The ILCOR Task Force on Cardiac Arrest and Cardiopulmonary Resuscitation Outcomes, et al: Cardiac arrest and cardiopulmonary resuscitation outcomes reports: Update and simplification of the Utstein templates for resuscitation registries: A statement for healthcare professionals from a task force of the international liaison committee on resuscitation (American Heart Association, European Resuscitation Council, Australian Resuscitation Council, New Zealand Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Councils of Southern Africa. Circulation 2004; 110:3385–3397
- 32.Fiser DH. Assessing the outcome of pediatric intensive care. J Pediatr. 1992;121(1):68–74. doi: 10.1016/s0022-3476(05)82544-2. [DOI] [PubMed] [Google Scholar]
- 33.Fiser DH, Long N, Roberson PK, et al. Relationship of pediatric overall performance category and pediatric cerebral performance category scores at pediatric intensive care unit discharge with outcome measures collected at hospital discharge and 1- and 6-month follow-up assessments. Crit Care Med. 2000;28:2616–20. doi: 10.1097/00003246-200007000-00072. [DOI] [PubMed] [Google Scholar]
- 34.Peberdy MA, Ornato JP, Larkin GL, et al. Survival from in-hospital cardiac arrest during nights and weekends. JAMA. 2008;299:785–792. doi: 10.1001/jama.299.7.785. [DOI] [PubMed] [Google Scholar]
- 35.Srinivasan V, Morris MC, Helfaer MA, et al. American Heart Association National Registry of CPR Investigators. Calcium use during in-hospital pediatric cardiopulmonary resuscitation: A report from the National Registry of Cardiopulmonary Resuscitation. Pediatrics. 2008;121:e1144–e1151. doi: 10.1542/peds.2007-1555. [DOI] [PubMed] [Google Scholar]
- 36.Hickey RW, Kochanek PM, Ferimer H, et al. Hypothermia and hyperthermia in children after resuscitation from cardiac arrest. Pediatrics. 2000;106:118–122. doi: 10.1542/peds.106.1.118. [DOI] [PubMed] [Google Scholar]
- 37.Takino M, Okada Y. Hyperthermia following cardiopulmonary resuscitation. Intensive Care Med. 1991;17:419–420. doi: 10.1007/BF01720680. [DOI] [PubMed] [Google Scholar]
- 38.Eberle B, Dick WF, Schneider T, et al. Checking the carotid pulse check: diagnostic accuracy of first responders in patients with and without a pulse. Resuscitation. 1996;33:107–116. doi: 10.1016/s0300-9572(96)01016-7. [DOI] [PubMed] [Google Scholar]
- 39.The 2005 AHA Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Part 11: Pediatric Basic Life Support. Circulation. 2005;112(Suppl IV):IV-160–IV166. doi: 10.1161/CIRCULATIONAHA.110.971085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gazmuri RJ, Nadkarni VM, Nolan JP, et al. Scientific Knowledge Gaps and Clinical Research Priorities for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Identified During the 2005 International Consensus Conference on ECC and CPR Science With Treatment Recommendations. Circulation. 2007;116:2501–2512. doi: 10.1161/CIRCULATIONAHA.107.186228. [DOI] [PubMed] [Google Scholar]
- 41.Nichol G, Runsfeld J, Eigel B, et al. Essential Features of Designating Out-of-Hospital Cardiac Arrest as a Reportable Event. [AHA Scientific Statement] Circulation. 2008;117:2299–2308. doi: 10.1161/CIRCULATIONAHA.107.189472. [DOI] [PubMed] [Google Scholar]
- 42.Horisberger T, Fischer E, Fanconi S. One-year survival and neurological outcome after pediatric cardiopulmonary resuscitation. Intensive Care Med. 2002;28:365–8. doi: 10.1007/s00134-001-1188-z. [DOI] [PubMed] [Google Scholar]
- 43.Lopez-Herce J, Garcia C, Rodriguez-Nunez A, et al. Long-term outcome of paediatric cardiorespiratory arrest in Spain. Resuscitation. 2005;64:79–85. doi: 10.1016/j.resuscitation.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 44.Morris RD, Krawiecki NS, Wright JA, et al. Neuropsychological, academic, and adaptive functioning in children who survive in-hospital cardiac arrest and resuscitation. J Learn Disabil. 1993;26:46–51. doi: 10.1177/002221949302600105. [DOI] [PubMed] [Google Scholar]
- 45.Bloom AA, Wright JA, Morris RD, et al. Additive impact of in-hospital cardiac arrest on the functioning of children with heart disease. Pediatrics. 1997;99:390–398. doi: 10.1542/peds.99.3.390. [DOI] [PubMed] [Google Scholar]