Abstract
Although vascular endothelial growth factor (VEGF), angiopoietin-1 (Ang-1) and Ang-2 have important roles in angiogenesis, very little is known about the regulation of these factors in the villous placenta during human pregnancy. In the present study, to investigate whether placental expression of Ang-1, Ang-2 and VEGF was altered in a cell-specific manner with advancing baboon gestation, the mRNA levels of these growth factors were determined by RT-PCR in cells isolated by Percoll gradient centrifugation from and protein localization assessed by immunocytochemistry in the villous placenta at early (day 60), mid (day 100) and late (day 170, term is 184 days) baboon gestation. Mean (± SE) Ang-1 mRNA levels, relative to 18S rRNA, in villous syncytiotrophoblast (3.92 ± 0.68) and cytotrophoblast (1.31 ± 0.31) cell fractions were highest on day 60 of gestation, then decreased by approximately 2.5-fold (P<0.05) to 1.39 ± 0.29 and 0.49 ±0.07, respectively, on day 170. Moreover, Ang-1 mRNA levels in the villous stromal cells and Ang-2 mRNA levels in all placental villous cell fractions were similar on days 60, 100, and 170 of gestation. In contrast to Ang-1 and Ang-2, placental villous cytotrophoblast VEGF mRNA levels were increased 2.94 fold (P<0.05) between mid (0.67 ± 0.15) and late (1.97 ± 0.49) gestation. A corresponding decrease in Ang-1, absence of change in Ang-2, and increase in VEGF protein immunocytochemical expression were exhibited in placental trophoblast with advancing baboon pregnancy. Ang-1/-2 and the angiopoietin Tie-2 receptor were expressed in vascular endothelial cells of the villous placenta, indicating that these blood vessel cells are a major site of ligand-receptor interaction for angiogenesis during primate pregnancy. We conclude that there is a cell-specific differential change in placental villous trophoblast expression of VEGF, Ang-1, and Ang-2 which we propose is important in regulating angiogenesis in the villous placenta during primate pregnancy.
Keywords: Placenta, Trophoblast, Angiopoietin, VEGF, Baboon, Development
Introduction
The vascular network within the villous placenta develops by de novo formation of vessels, i.e. vasculogenesis, and proliferation of existing vessels, i.e. angiogenesis, processes regulated by stimulatory and inhibitory factors. The most widely studied angiostimulatory factor is vascular endothelial growth factor (VEGF) [1]. Two other proteins, angiopoietin-1 (Ang-1) and Ang-2, both of which bind to the Tie-2 tyrosine kinase receptor, work in concert with VEGF to control vascular morphogenesis, remodeling, and maturation [2–4]. VEGF stimulates endothelial cell proliferation and capillary tube formation, while Ang-1 promotes association of endothelial cells with periendothelial cells to mature and stabilize newly-formed blood vessels. Ang-2 loosens the vessel wall rendering endothelial cells accessible to VEGF to further promote angiogenesis, although in elevated levels Ang-2 causes vessel disruption.
VEGF, Ang-1, Ang-2, and the Tie-2 receptor are expressed by cytotrophoblasts and syncytiotrophoblast as well as vascular and avascular cells within the human villous placenta [5–9], consistent with their proposed role upon placental vascular development. However, very little is known about the regulation of these respective angioregulatory factors within the villous placenta during human pregnancy. We have recently shown a developmental increase in the expression of placental villous cytotrophoblast VEGF levels and vascularization, coinciding with the rise in estrogen levels of advancing baboon pregnancy [10]. Moreover, estrogen administration increased placental villous cytotrophoblast VEGF expression and vessel density early in baboon gestation when estrogen levels are typically low [11, 12], indicating that estrogen promotes placental angiogenesis during early primate pregnancy. In the present study, to investigate whether placental expression of Ang-1 and Ang-2, as well as VEGF, is altered in a cell-specific manner with advancing gestation, the mRNA levels in isolated cell fractions of, and protein localization by immunocytochemistry within, the villous placenta of these growth factors were determined at early, mid and late baboon pregnancy.
Methods and Materials
Animals
Adult female baboons (Papio Anubis) weighing 12–15 kg were housed individually in aluminum/stainless-steel large primate cages and maintained in a controlled temperature (22°C) environment under a 12:12 h light: dark cycle. Baboons were fed high-protein monkey chow twice daily, fresh fruit and vitamins daily, and water ad libitum. Females were paired with male baboons for a period of 5 days at midcycle as determined by daily record of menstrual cycle history and perineal turgescence. Pregnancy was determined by palpation and ultrasonography.
Pregnant baboons were anesthetized with isoflurane and placentas obtained via caesarean section on days 59–61 (early), 100–102 (mid) or 166–180 (late) of gestation (term = 184 days). Throughout the study period, baboons were briefly sedated with ketamine (10 mg/kg body weight, im) and blood samples (4 ml) withdrawn from a maternal saphenous vein for the purpose of determining serum estradiol levels by RIA using an automated chemiluminescent immunoassay system (Immulite, Diagnostic Products Corp., Los Angeles, CA) as previously described [13].
Baboons were cared for and used strictly in accordance with USDA regulations and the NIH Guide for the Care and Use of Laboratory Animals (Publication No. 86–23, 1985). The experiments conducted were approved by the Institutional Animal Care and Use Committee of the University of Maryland School of Medicine.
Placental cell isolation
At least 8 randomly selected sections (approximately 5 mm3) of placental villous tissue were isolated from each placenta and fixed overnight in 10% formalin and embedded in paraffin for immunohistochemical analysis. Most of the remaining villous tissue was then subjected to enzymatic digests to obtain enriched fractions of villous cytotrophoblasts, syncytiotrophoblast and villous stromal cells as previously described by our laboratory [14] and Kliman et al [15]. Briefly, placental villous tissue was dispersed in HBSS (Invitrogen, Carlsbad, CA) containing either 0.1% collagenase (type H, Sigma Blend, Sigma Chemical Co., St. Louis, MO), 0.1% hyaluronidase (bovine testes, type I-S Sigma), 0.025% soybean trypsin inhibitor (type I-S, Sigma) and 0.02% DNase I (bovine pancreas type IV, Sigma) or 0.125% trypsin (bovine pancreas type I, Sigma) and 0.02% DNase I. Cell dispersions were then layered onto 5–70% Percoll (Amersham Biosciences, Piscataway, NJ) gradients and enriched cell fractions isolated by centrifugation at 1200 × g. Kliman et al [15] and we (unpublished data) have previously shown that enriched cytokeratin-positive cytotrophoblast, placental lactogen-positive syncytiotrophoblast, and α1-antichymotrypsin/vimentin-positive macrophage, endothelial and mesenchymal villous stromal cell fractions were obtained from the placenta using the latter cell isolation procedure.
RT-PCR
The mRNA levels for Ang-1, Ang-2 and VEGF were determined by RT-PCR as modified from our recent study [10]. Total RNA was extracted from villous cell fractions via guanidine isothiocyanate-cesium chloride gradient centrifugation and total RNA quantified by UV absorbance at 260 nm. Oligonucleotide primers were designed (Invitrogen) and derived from the human sequences for: Ang-1 [16], forward, 5'-GGGGGAGGTTGGACTGTAAT-3', and reverse, 5'-AGGGCACATTTGCACATACA-3'; Ang-2 [17], forward, 5'-GGATCTGGGGAGAGAGGAAC-3', and reverse, 5'-CTCTGCACCGAGTCATCGTA-3'; VEGF [18], forward 5'-CTGCTGTCTTGGGTGCATTGG-3' and reverse, 5'-GGTTTGATCCGCATAATCTGC-3', and 18S [19], forward, 5'-TCAAGAACGAAAGTCGGAGG-3', and reverse, 5'-GGACATCTAAGGGCATCACA-3'. Total RNA (75–300 ng) was reversed transcribed at 42°C for 60 min in a 20 µl mixture consisting of 200 U Moloney murine leukemia virus reverse transcriptase (RT, Invitrogen), 1 × RT buffer, 250 ng random primers (Invitrogen), 1 mM dithiothreitol (Invitrogen), 40 U RNAguard ribonuclease inhibitor (Amersham) and 1 mM dNTPs (Invitrogen). Negative controls, in which either the RT enzyme or RNA was not added to the RT reaction, were performed to test for genomic DNA contamination. After 60 min, the RT reactions were incubated at 70°C for 15 min, cooled to 4°C and 1 or 5 µl added to separate PCR reactions (final volume 50 µl) containing 1.25 U cloned Thermus aquaticus DNA polymerase (Amplitaq, Perkin-Elmer Corp/Cetus, Norwalk, CT), 1X PCR buffer, 0.2 mM dNTPs, and 10 pmol each of primer sets for Ang-1, Ang-2, VEGF, and 18S rRNA. PCR was performed in a programmable thermal cycler (MJ Research Inc., Cambridge, MA) and a preliminary study to assess optimal conditions for linearity of PCR amplification was performed for each primer pair and placental cell fraction. PCR conditions consisted of amplification for 33 (Ang-1), 28 (Ang-2), 24 (VEGF) and 14–16 (18S), cycles with denaturing at 94°C for 1 min, annealing at 60°C for 1 min, extension at 72°C for 2 min with a final extension at 72°C for 5 min. The PCR products were then agarose gel (2%) fractionated, visualized with ethidium bromide and gels scanned using Gel-Doc imaging system and Multi- Analyst software (Bio-Rad Laboratories, Hercules, CA). The intensity of amplified products was represented as the relative area under each sample band. The levels of Ang-1, Ang-2, and VEGF were normalized to 18S rRNA. No difference in 18S rRNA levels was observed with advancing gestation.
Immunocytochemistry
Placental villous tissue was embedded in paraffin, sectioned (5 µm), deparaffinized, rehydrated in graded concentrations of ethanol, and boiled in 0.01 M Na citrate for 5 min and allowed to cool for 20 min in buffer. Sections were then incubated in H2O2 for 10 min to block endogenous peroxidase and blocked with 5% normal horse serum. Sections were incubated overnight with polyclonal goat antibodies to Ang-1 (1:80 dilution, R & D Systems, Minneapolis, MN), Ang-2 (1:160 dilution, R & D), Tie-2 (1:50 dilution, R & D), and VEGF (1:400 dilution, Zymed, South San Francisco, CA). Sections were then incubated 1 h (room temperature) with biotinylated antigoat immunoglobulins (Vector Laboratories, Inc., Burlingame, CA), immersed for 1 h in avidin-biotin-peroxidase complex (ABC Elite, Vector), developed using diaminobenzidine as the chromagen, and counterstained with Harris hematoxylin. Negative controls included omission of the primary antibody.
Statistics
Data are presented as means ± SE and were analyzed by ANOVA with post hoc comparison of means by Newman Keuls multiple comparison statistic.
Results
Placental/fetal body weights and placental Ang-1, Ang -2, and VEGF mRNA
Placental and fetal body weights increased (P<0.001) progressively between days 60, 100, and 170 of gestation (Table 1).
Table 1.
Placental and fetal body weights and placental villous cytotrophoblast VEGF mRNA levels and blood vessel density during baboon pregnancy.
| Days of gestation | Placental weight (gm) | Fetal weight (gm) | Cytotrophoblast VEGF mRNA/18 S rRNA | Placental villous blood vessel density (number/mm2) |
|---|---|---|---|---|
| 60 | 34.5 ± 4.0a | 12.3 ± 1.3a | 1.10 ± 0.24a | 493 ± 34a |
| 100 | 79.6 ± 2.1b | 163.2 ± 3.9b | 0.67 ± 0.15a | 977 ± 101b |
| 170 | 183.5 ± 5.2c | 849.2 ± 17.2c | 1.97 ± 0.49b | 1,375 ± 71c |
VEGF mRNA levels in cytotrophoblasts isolated from villous placenta of baboons on days 60 (early, n = 7), 100 (mid, n = 7) and 170 (late, n = 9) of gestation. Data of placental villous density as reported in our previous study [10]. Values (means ± SE) with different letter superscripts differ (P<0.001 or P<0.05 for VEGF mRNA) from each other as analyzed by ANOVA and Newman Keul's multiple statistic.
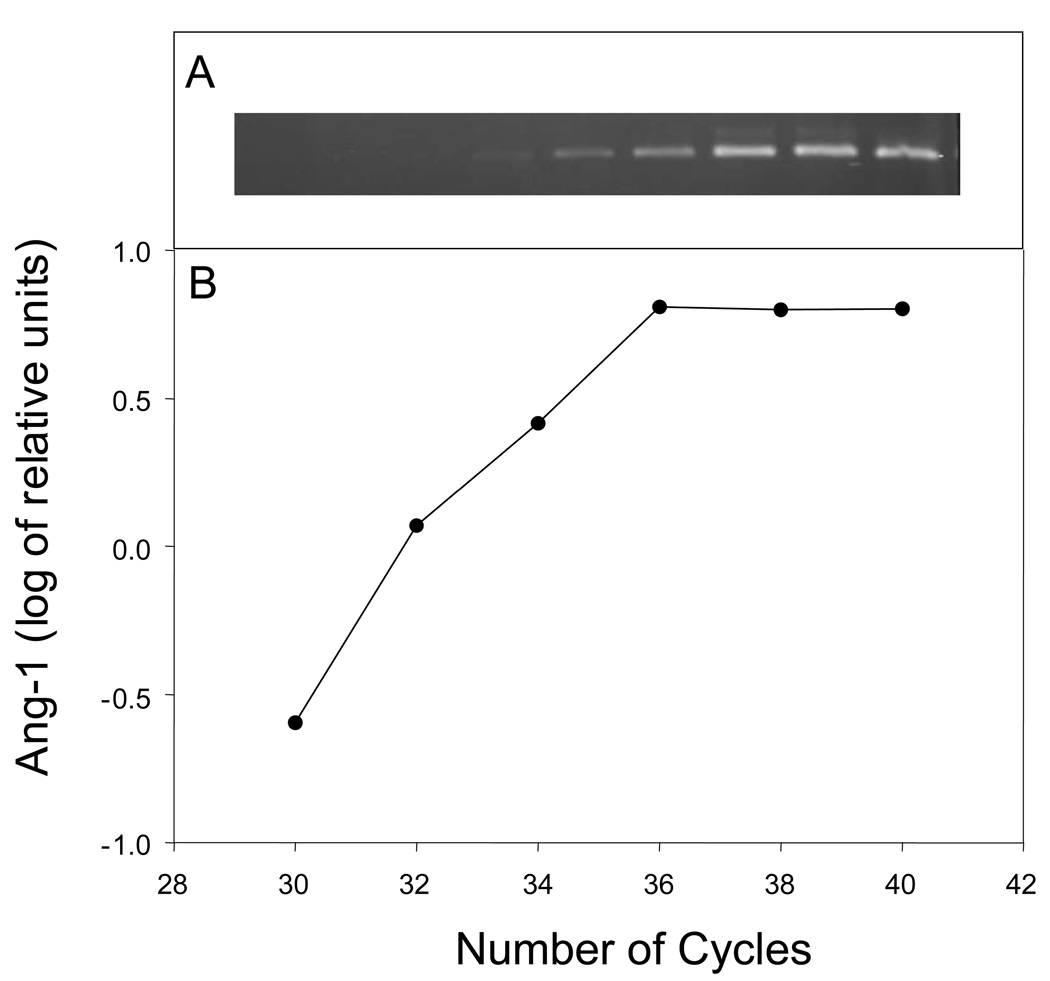
Fig. 1 illustrates validation of PCR for Ang-1 mRNA (362-bp) in cytotrophoblasts isolated from the baboon placenta on day 100 of gestation. Linearity of the PCR amplification occurred through 36 cycles and thus 33 cycles were chosen for subsequent Ang-1 PCR analyses. Similar validation experiments were conducted for Ang-2 mRNA (535-bp) in which 28 cycles was employed.
Fig. 1.
Ang-1 PCR amplification of RNA obtained from cytotrophoblasts isolated from the baboon villous placenta on day 100 of gestation. RNA (75 ng) was reverse transcribed and incubated with primers for Ang-1 at increasing number of PCR cycles. (A) PCR products isolated on agarose gel. (B) Log of Ang-1 relative units versus number of cycles.
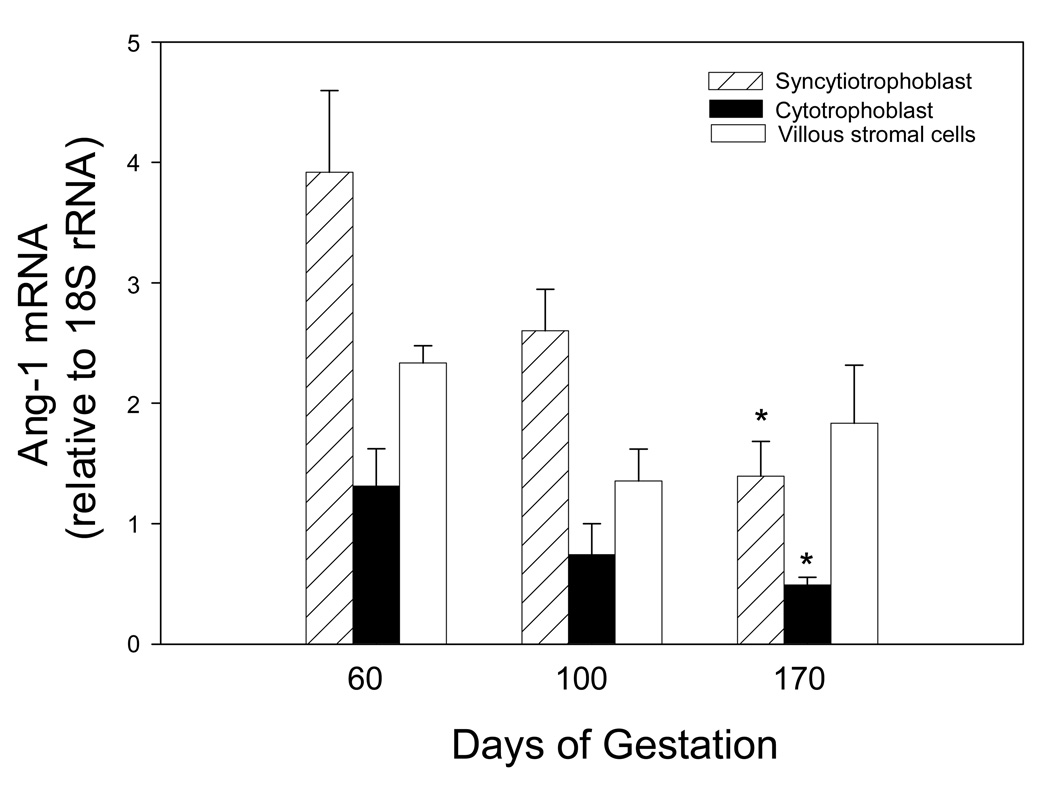
Fig. 2 shows mRNA levels of Ang-1 in cell fractions isolated from the villous placenta on days 60, 100, and 170 of baboon pregnancy. Mean ± SE Ang-1 mRNA levels, relative to 18S rRNA, in enriched placental villous syncytiotrophoblast (3.92 ± 0.68 relative units) and cytotrophoblast (1.31 ± 0.31) cell fractions were highest on day 60 of gestation, then decreased over 2.5-fold (P<0.05) to 1.39 ± 0.29 and 0.49 ± 0.07 relative units, respectively, on day 170 (Fig. 2). In contrast Ang-1 mRNA levels in the villous stromal cells were similar on days 60, 100, and 170 of gestation.
Fig. 2.
Ang-1 mRNA levels expressed as a ratio of 18S rRNA in placental villous cells isolated from baboons on day 60 (early), 100 (mid), and 170 (late) gestation (term is 184 days). Cell fractions were isolated via 5–70% Percoll gradient centrifugation and mRNA levels determined by RT-PCR. Values represent the means ± SE of 3 to 11 baboons for each cell fraction and time of gestation. *P<0.05 versus day 60 of gestation (ANOVA and Newman Keuls Multiple Comparison statistic).
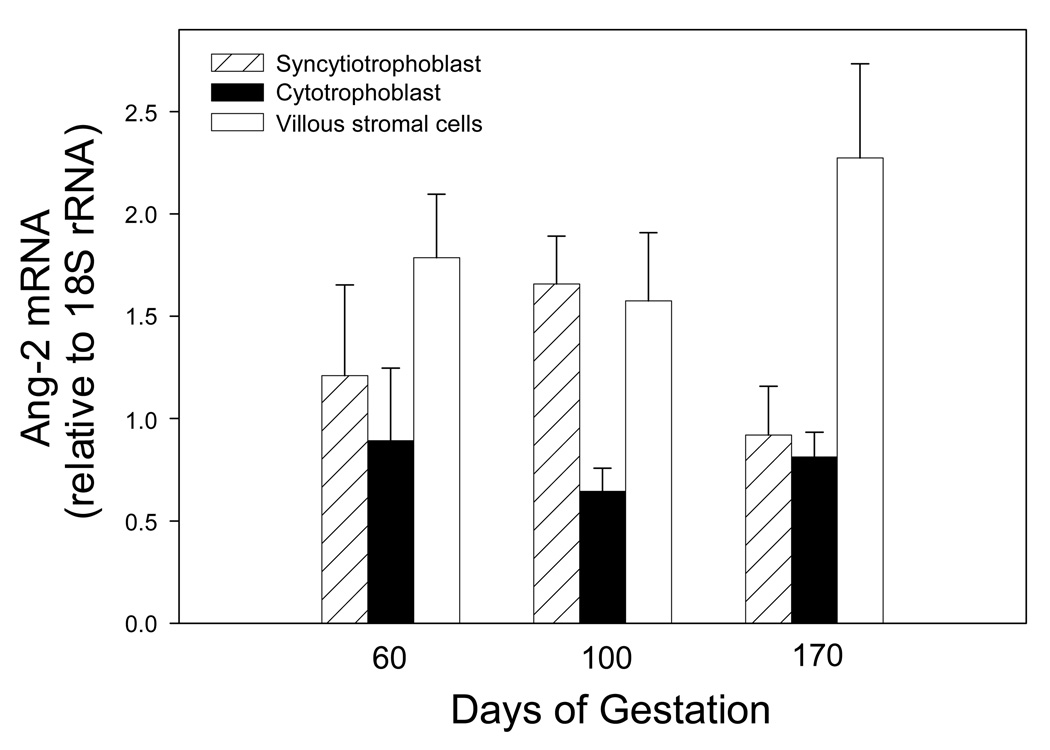
Unlike the decrease in Ang-1 mRNA expression with advancing baboon pregnancy, Ang-2 mRNA levels were similar on days 60, 100 and 170 of gestation in the cytotrophoblast, syncytiotrophoblast and villous stromal cells (Fig. 3).
Fig. 3.
Ang-2 mRNA levels expressed as a ratio of 18S rRNA in placental villous cells isolated from baboons on days 60, 100, and 170 of gestation. Values represent the means ± SE of 3 to 11 animals for each cell fraction and time of gestation.
In sharp contrast to the progressive decline in Ang-1 and unaltered Ang-2 mRNA levels within the villous placenta with advancing gestation, placental villous cytotrophoblast VEGF mRNA levels (corrected for 18 S rRNA) were similar on days 60 (1.10 ± 0.24) and 100 (0.67 ± 0.15) of gestation (Table 1) and then increased 2.94-fold (P<0.05) between mid and late (1.97 ± 0.49) gestation in baboons of the present study, as shown in our previous report [10]. The rise in placental villous trophoblast VEGF expression was associated with a corresponding developmental increase in blood vessel density within the villous placenta with advancing pregnancy (Table 1) shown previously [10].
Placental Ang-1, Ang-2, Tie-2, and VEGF immunocytochemistry
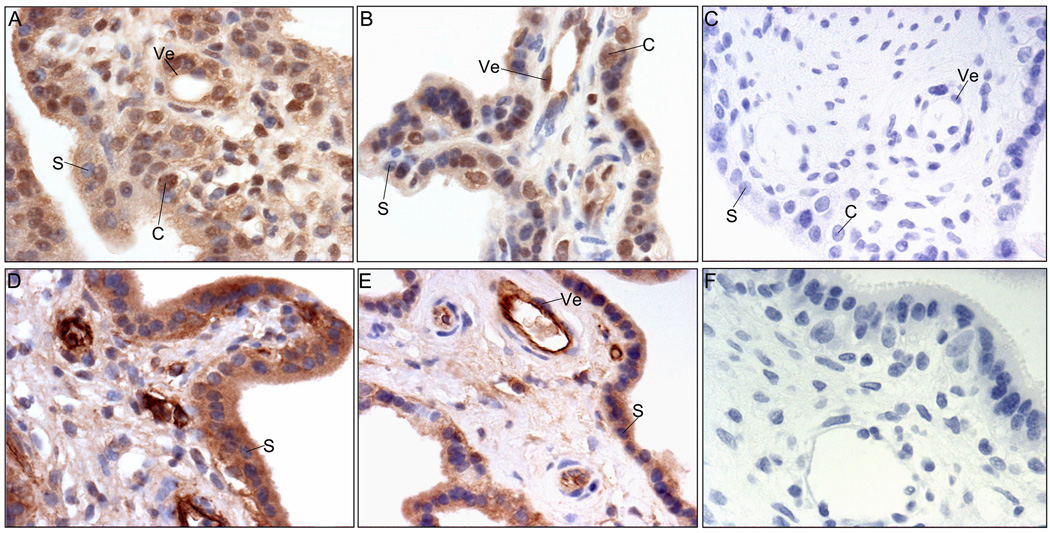
Ang-1 protein was localized by immunocytochemistry and abundantly expressed in the syncytiotrophoblast and cytotrophoblasts of the chorionic villi and in the vascular endothelial and nonvascular cells of the villous core, on days 60 and 170 of baboon pregnancy (Figs. 4A and B). Consistent with the decrease in Ang-1 mRNA levels with advancing baboon pregnancy, Ang-1 immunoreactivity in the syncytiotrophoblast appeared lighter on day 170 than on day 60 of gestation. However, Ang-1 staining in cytotrophoblasts and vascular cells within the villous stromal appeared similar at early, mid (not shown), and late gestation.
Fig. 4.
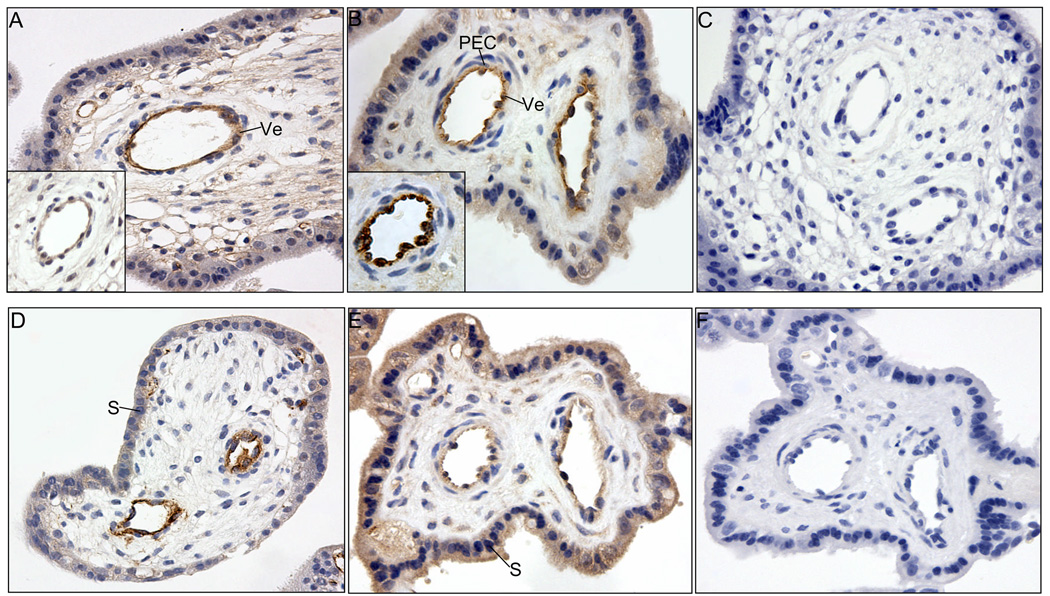
Representative photomicrographs of Ang-1 (A, B, C) and Ang-2 (D, E, F) immunocytochemistry in baboon placenta on days 60 (early, A, D) and 170 (late, B, E) of gestation. Omission of the primary Ang-1 (C) and Ang-2 (F) antibodies. C, cytotrophoblast nucleus; S, syncytiotrophoblast nucleus; Ve, vascular endothelium. Magnification is 200X.
Ang-2 protein was also localized in the syncytiotrophoblast, cytotrophoblast, and vasculature and nonvascular cells of the villous stroma (Figs. 4D and E). Moreover, consistent with Ang-2 mRNA levels, there was no apparent change in the pattern or level of Ang-2 protein immunostaining between early (Fig. 4D), mid (not shown) and late (Fig. 4E) gestation. Specificity for Ang-1 and Ang-2 immunocytochemistry was demonstrated by the absence of staining when primary antibodies were omitted from the reaction (Fig. 4C and F).
Tie-2 receptor (Fig. 5A) as well as Ang-1 ligand (Fig. 5A insert) were abundantly expressed in the same cells of the blood vessel walls of the villous placenta throughout early (Fig. 5A), mid (not shown) and late (Fig. 5B) baboon gestation, particularly within vascular endothelial cells (Fig. 5B) identified by von Willebrand Factor immunolocalization (Fig. 5B insert). In contrast, little or no immunostaining for Tie-2 occurred in periendothelial cells (i.e. vascular smooth muscle cells/pericytes, Fig. 5B). Relatively low expression of Tie-2 was exhibited in the syncytiotrophoblast and stromal cells not associated with blood vessels of the villous placenta (Figs. 5A and B).
Fig. 5.
Representative photomicrographs of Tie-2 (A, B, C) and VEGF (D, E, F) immunocytochemistry in baboon placenta on days 60 (A and D) and 170 (B and E) of gestation. Immunocytochemical localization of Ang-1 and von Willebrand Factor is shown in inserts of panels A and B, respectively. Omission of primary Tie-2 (C) and VEGF (F) antibodies. S, syncytiotrophoblast nucleus; Ve vascular endothelium; PEC, periendothelial cells (vascular smooth muscle cells/pericytes). Magnification is 200X.
VEGF was detected within the cytoplasm of the trophoblast where expression appeared to increase between early (Fig. 5D) and late (Fig. 5E) gestation. VEGF was also abundantly expressed within the vascular endothelium at early (Fig. 5D), mid (not shown) and late (Fig. 5E) gestation.
Discussion
The results of the present study show that baboon placental villous cytotrophoblasts, syncytiotrophoblast and vascular endothelial cells of vessels within the villous stroma expressed the angioregulatory factors VEGF, Ang-1 and Ang-2 and the Tie-2 receptor. The expression of both Ang-1/-2 and Tie-2 in the vascular endothelium indicates that these blood vessel cells are a major site of ligand-receptor interaction for angiogenesis within the villous placenta during primate pregnancy. The current study further shows that placental trophoblast Ang-1, Ang-2 and VEGF were differentially expressed at early, mid and late gestation. Thus, the mRNA levels for Ang-1 in the cytotrophoblast and syncytiotrophoblast of the villous placenta exhibited a progressive decrease between early, mid, and late baboon pregnancy. Coinciding with the decline in mRNA levels, Ang-1 protein expression within the syncytiotrophoblast also decreased with advancing gestation, although there are limitations to quantification of protein levels by immunocytochemistry. The reduction in Ang-1 appeared to be specific for the trophoblast cell fraction, because Ang-1 mRNA levels and protein localization were unaltered in the mesenchymal and vascular cells of the villous stroma. Moreover, only placental villous trophoblast Ang-1 expression was changed with advancing gestation, while Ang-2 mRNA levels and protein localization were not significantly different at early, mid, and late baboon pregnancy in the respective placental villous cell fractions. Furthermore, the marked decrease in placental trophoblast Ang-1 expression sharply contrasted with the increase in VEGF mRNA levels in villous cytotrophoblasts between mid and late gestation shown in the present and our previously published study [10]. Therefore, we propose that the expression of VEGF, Ang-1 and Ang-2 is differentially regulated in a cell-specific manner within the villous placenta with advancing primate pregnancy.
Placental VEGF, Ang-1 and Ang-2 expression has been studied by various approaches in the human and other primate species. As in baboons of the present study, Ang-1 assessed by in situ hybridization was expressed in high level within the cytotrophoblast and syncytiotrophoblast of the human placenta, where expression also was decreased in late gestation [20]. Also, as shown in baboons, Ang-2 assessed by laser densitometric analysis and immunocytochemistry was localized within villous trophoblast and perivascular tissue of the human placenta [20–23], where levels showed only a small increase with advancing gestation [20]. In contrast, Ang-2 mRNA levels determined by PCR and Northern blot analysis in whole villous tissue decreased with advancing human [22] and marmoset [24] gestation. Collectively, therefore, there is considerable agreement in the cellular localization and expression of Ang-1 and Ang-2 with advancing gestation in the human, baboon, and marmoset monkey villous placenta. Differences in results seem to occur when whole placental tissue is analyzed, pointing to the advantage as in the present study of quantifying angioregulatory or other molecules after cell isolation to selectively assess expression, particularly in heterogenous tissues such as the placenta.
The factor(s) that regulate the developmental change in placental cell-specific expression of Ang-1 remain to be identified. We have recently shown that estrogen, the levels of which increase with advancing human and baboon pregnancy [25], stimulated placental trophoblast VEGF expression in the baboon [11, 12]. In contrast, estrogen decreased the expression of Ang-1 in the lung, kidney and heart [26]. Moreover, estrogen may interact with other factors, including hypoxia which increased Ang-2 mRNA levels in human placental villous explants [23], in regulating the differential expression of placental VEGF, Ang-1 and Ang-2 with advancing stages of gestation. We suggest that the baboon in which estrogen levels can be manipulated provides a valuable nonhuman primate model to determine in future studies whether the differential expression of placental villous VEGF, Ang-1 and Ang-2 exhibited with advancing primate pregnancy is regulated by estrogen.
Although the physiological importance of the cell-specific change in placental expression of the angioregulatory factors during baboon pregnancy requires further study, it is likely that the alterations underlie and are important in regulating the pattern of vascular remodeling of the villous placenta that occurs during advancing primate pregnancy. Thus, during the first trimester of human pregnancy, a dense network of capillaries is formed via vasculogenesis and branching angiogenesis subjacent to the trophoblast layer within the mesenchymal and immature intermediate villi [27–30]. With advancing gestation, as immature intermediate villi are transformed into stem villi, the peripheral capillary network regresses and vessels differentiate and mature into arteries and veins within the villous core. It is possible, therefore, that cytotrophoblast VEGF in concert with the elevated expression of Ang-1 by the trophoblast and villous stromal cells promotes branching angiogenesis and maturation of blood vessels within stem villi as they are formed early in gestation. During the second half of gestation, mature intermediate villi comprised of slender fetal capillaries and terminal villi dominated by sinusoidally dilated, highly-coiled fetal capillaries develop [27, 31]. Consequently, unlike stem and immature intermediate villi where vessel maturation leads to formation of arterioles/arteries and venules/veins, capillaries predominate in the terminal villi which comprise 50% of total villous volume [29]. Thus, the progressive increase in VEGF, coinciding with a decrease in Ang-1 and maintenance of Ang-2 and Tie-2 receptor expression within the villous trophoblast with advancing baboon gestation, as shown in the present study, may promote capillarization and restrain vessel maturation, particularly within the terminal villi where most of the maternal-fetal exchange occurs.
In summary, the present study shows that villous placental trophoblast expression of VEGF increased, Ang-1 decreased, and Ang-2 was unaltered in a cell-specific manner with advancing baboon pregnancy. Therefore, a cell-specific differential change in the expression of these angioregulatory molecules was exhibited, which we propose is important in regulating angiogenesis in the villous placenta during primate pregnancy.
Acknowledgements
This work was supported by National Institutes of Health Research Grant RO1 HD 13294.
The secretarial assistance of Mrs. Wanda James with the manuscript is great appreciated.
References
- 1.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 2.Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D. Signaling vascular morphogenesis and maintenance. Science. 1997;277:48–50. doi: 10.1126/science.277.5322.48. [DOI] [PubMed] [Google Scholar]
- 4.Gale NW, Yancopoulos GD. Growth factors acting via endothelial cell-specific receptor tyrosine kinases: VEGFs, Angiopoietins, and ephrins in vascular development. Genes Dev. 1999;13:1055–1066. doi: 10.1101/gad.13.9.1055. [DOI] [PubMed] [Google Scholar]
- 5.Sharkey AM, Charnock-Jones DS, Boocock CA, Brown KD, Smith SK. Expression of mRNA for vascular endothelial growth factor in human placenta. J Reprod Fertil. 1993;99:609–615. doi: 10.1530/jrf.0.0990609. [DOI] [PubMed] [Google Scholar]
- 6.Clark DE, Smith SK, Sharkey AM, Charnock-Jones DS. Localization of VEGF and expression of its receptors flt and KDR in human placenta throughout pregnancy. Human Reprod. 1996;11:1090–1098. doi: 10.1093/oxfordjournals.humrep.a019303. [DOI] [PubMed] [Google Scholar]
- 7.Goldman-Wohl DS, Ariel I, Greenfield C, Lavy Y, Yagel S. Tie-2 and angiopoietin-2 expression at the fetal-maternal interface: a receptor ligand model for vascular remodelling. Mol Human Reprod. 2000;6:81–87. doi: 10.1093/molehr/6.1.81. [DOI] [PubMed] [Google Scholar]
- 8.Wulff C, Wiegand M, Kreienberg R, Fraser HM. Angiogenesis during primate placentation in health and disease. Reproduction. 2003;126:569–577. doi: 10.1530/rep.0.1260569. [DOI] [PubMed] [Google Scholar]
- 9.Bussolati B, Perkins J, Shams M, Rhaman M, Nijjar S, Qui Y, Kniss D, Dunk C, Yancopoulos G, Ahmed A. Angiopoietin-1 and angiopoietin-2 are differentially expressed during placental development and stimulate trophoblast proliferation, migration and release of nitric oxide. J Soc Gynecol Invest. 2000;7(Suppl) 158A. [Google Scholar]
- 10.Hildebrandt VA, Babischkin JS, Koos RD, Pepe GJ, Albrecht ED. Developmental regulation of vascular endothelial growth/permeability factor messenger ribonucleic acid levels in and vascularization of the villous placenta during baboon pregnancy. Endocrinology. 2001;142:2050–2057. doi: 10.1210/endo.142.5.8174. [DOI] [PubMed] [Google Scholar]
- 11.Albrecht ED, Robb VA, Pepe GJ. Regulation of placental vascular endothelial growth/permeability factor expression and angiogenesis by estrogen during early baboon pregnancy. J Clin Endocrinol Metab. 2004;89:5803–5809. doi: 10.1210/jc.2004-0479. [DOI] [PubMed] [Google Scholar]
- 12.Robb VA, Pepe GJ, Albrecht ED. Acute temporal regulation of placental vascular endothelial growth/permeability factor expression in baboons by estrogen. Biol Reprod. 2004;71:1694–1698. doi: 10.1095/biolreprod.104.030882. [DOI] [PubMed] [Google Scholar]
- 13.Albrecht ED, Aberdeen GW, Pepe GJ. The role of estrogen in the maintenance of primate pregnancy. Am J Obstet Gynecol. 2000;182:432–438. doi: 10.1016/s0002-9378(00)70235-3. [DOI] [PubMed] [Google Scholar]
- 14.Henson MC, Babischkin JS, Pepe GJ, Albrecht ED. Effect of the antiestrogen ethamoxytriphetol (MER-25) on placental low density liproprotein uptake and degradation in baboons. Endocrinology. 1988;122:2019–2026. doi: 10.1210/endo-122-5-2019. [DOI] [PubMed] [Google Scholar]
- 15.Kliman HJ, Nestler JE, Sermasi E, Sanger JM, Strauss JF., III Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae. Endocrinology. 1986;118:1567–1582. doi: 10.1210/endo-118-4-1567. [DOI] [PubMed] [Google Scholar]
- 16.Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V, Ryan TE, Bruno J, Radziejewski C, Maisonpierre PC, Yancopoulos GD. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996;87:1161–1169. doi: 10.1016/s0092-8674(00)81812-7. [DOI] [PubMed] [Google Scholar]
- 17.Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 18.Tischer E, Mitchell R, Hartman T, Silva M, Gospodarowicz D, Fiddes JC, Abraham JA. The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J Biol Chem. 1991;266:11947–11954. [PubMed] [Google Scholar]
- 19.Torczynski RM, Fuke M, Bollon AP. Cloning and sequencing of a human 18S ribosomal RNA gene. DNA. 1985;4:283–291. doi: 10.1089/dna.1985.4.283. [DOI] [PubMed] [Google Scholar]
- 20.Dunk C, Shams M, Nijjar S, Rhaman M, Qiu Y, Bussolati B, Ahmed A. Angiopoietin-1 and angiopoietin-2 activate trophoblast Tie-2 to promote growth and migration during placental development. Am J Pathol. 2000;156(6):2185–2199. doi: 10.1016/S0002-9440(10)65089-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leach L, Babawale MO, Anderson M, Lammiman M. Vasculogenesis, angiogenesis and the molecular organization of endothelial junctions in the early human placenta. J Vas Res. 2002;39:246–259. doi: 10.1159/000063690. [DOI] [PubMed] [Google Scholar]
- 22.Geva E, Ginzinger DG, Zaloudek CJ, Moore DH, Byrne A, Jaffe RB. Human placental vascular development: vasculogenic and angiogenic (branching and nonbranching) transformation is regulated by vascular endothelial growth factor-A, angiopoietin-1, and angiopoietin-2. J Clin Endocrinol Metab. 2002;87:4213–4224. doi: 10.1210/jc.2002-020195. [DOI] [PubMed] [Google Scholar]
- 23.Zhang EG, Smith SK, Baker PN, Charnock-Jones DS. The regulation and localization of angiopoietin-1,-2, and their receptor Tie2 in normal and pathologic human placentae. Molecular Med. 2001;7:624–635. [PMC free article] [PubMed] [Google Scholar]
- 24.Wulff C, Wilson H, Dickson SE, Wiegand SJ, Fraser HM. Hemochorial placentation in the primate: expression of vascular endothelial growth factor, angiopoietins, and their receptors throughout pregnancy. Biol Reprod. 2002;66:802–812. doi: 10.1095/biolreprod66.3.802. [DOI] [PubMed] [Google Scholar]
- 25.Albrecht ED, Pepe GJ. Placental steroid hormone biosynthesis in primate pregnancy. Endocrine Rev. 1990;11:124–150. doi: 10.1210/edrv-11-1-124. [DOI] [PubMed] [Google Scholar]
- 26.Ye F, Florian M, Magder SA, Hussain SN. Regulation of angiopoietin and Tie-2 receptor in non-reproductive tissues by estrogen. Steroids. 2002;67:305–310. doi: 10.1016/s0039-128x(01)00163-5. [DOI] [PubMed] [Google Scholar]
- 27.Kaufmann P, Mayhew TM, Charnock-Jones DS. Aspects of human fetoplacental vasculogenesis and angiogenesis. II. Changes during normal pregnancy. Placenta. 2004;25:114–126. doi: 10.1016/j.placenta.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 28.Charnock-Jones DS, Kaufmann P, Mayhew TM. Aspects of human fetoplacental vasculogenesis and angiogenesis. I. Molecular Regulation. Placenta. 2004;25:103–113. doi: 10.1016/j.placenta.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 29.Mayhew TM, Charnock-Jones DS, Kaufmann P. Aspects of human fetoplacental vasculogenesis and angiogenesis. III. Changes in complicated pregnancies. Placenta. 2004;25:127–139. doi: 10.1016/j.placenta.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 30.Demir R, Kayisli UA, Seval Y, Celik-Ozenci C, Korgun ET, Demir-Weusten AY, Huppertz B. Sequential expression of VEGF and its receptors in human placental villi during very early pregnancy: differences between vasculogenesis and angiogenesis. Placenta. 2004;25:560–572. doi: 10.1016/j.placenta.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 31.Bernischke K, Kaufman P. Pathology of the human placenta. 4th ed. New York: Springer Verlag; 2000. p. 947. [Google Scholar]