Abstract
Fluorescence detection is a central component in biological research. In recent years there has been a growing interest in the interactions of fluorophores with metallic surfaces or particles. A single-stranded oligonucleotide was chemically bound to a single 50 nm diameter silver particle and a Cy5-labeled complementary single-stranded oligonucleotide was hybridized with the particle-bound oligonucleotide. The bound Cy5 molecules on the silver particles were spatially separated from the silver surface by the hybridized DNA duplex chains, which were about 8 nm in length, to reduce the competitive quenching. We use fluorescence lifetime correlation spectroscopy (FLCS) with picosecond time-resolved detection to separate the fluorescence correlation spectroscopy (FCS) contributions from fluorophores and metal-conjugated fluorophores. The single Cy5-labeled 50 nm silver particles displayed a factor of 15-fold increase in emission signal and 5-fold decrease in emission lifetimes in solution relative to the Cy5-DNA in the absence of metal. Lifetime measurements support the near-field interaction mechanism between the fluorophore and silver nanoparticle. In this study, FLCS is being applied to a system where the brightness and the fluorescent lifetime of the emitting species are significantly different. Our measurements suggest that FLCS is a powerful method for investigating the metal-fluorophore interaction at the single molecule level and to separate two different species from a mixture solution emitting at the same wavelength. Additionally, the highly bright Cy5-DNA-Ag molecules offer to be excellent probes in high background biological samples.
Keywords: fluorescence correlation spectroscopy, fluorescence lifetime correlation spectroscopy, silver nanoparticles, plasmonics, metal enhanced fluorescence, fluorescence
1. INTRODUCTION
Fluorescence detection is the basis of many measurements in biological research. Fluorescence provides numerous measurement opportunities including studies of biological macromolecules, cell imaging and DNA sequencing. Exotic phenomena such as multi-photon excitation and time-resolved microscopy are now routinely performed in many laboratories for cellular imaging. At present the use of fluorescence primarily depend on the spontaneous emission of photons occurring nearly isotropically in all directions. Information about the samples is obtained mostly from changes in the non-radiative decay rates, such as collisions of fluorophores with quenchers and fluorescence resonance energy transfer (FRET). The rates of spontaneous emission are not significantly changed in such experiments. In recent papers we began to consider methods to modify the radiative decay rates of fluorophores [1–2]. A review of existing literature revealed that radiative decay rates could be modified by proximity of fluorophores to metallic particles. We use the term metal to describe conducting metallic surfaces and particles, not metal ions. The effects of metals on fluorescence have been subject to prior theoretical reports [3–6], but the practical usefulness of these effects was not explicitly recognized. We observed a variety of favorable effects due to metal particles, such as increased fluorescence intensities, decrease lifetime, increased photostability and increased distances for fluorescence resonance energy transfer (FRET). We refer to these favorable effects as metal-enhanced fluorescence (MEF). MEF is increasingly finding applications in various fields including chemistry and biology. MEF is a complex phenomenon. Knowing more about MEF at the single molecule level will help implementing this phenomenon in versatile applications.
Fluorescence lifetime correlation spectroscopy (FLCS) [7–11] is a combination of fluorescence lifetime and fluorescence correlation spectroscopy (FCS) measurement. In general, FCS is used to measure freely diffusing molecules. Significant fluctuations in fluorescence intensity only occurs if the number of fluorophores observed at any given time are small, typically less than 10 molecules. The observed volume is usually diffraction-limited. In FLCS measurement, the fluorescence excitation is accomplished with a pulsed laser, and the fluorescence photons are detected on two different times scales so called time-tagged time-correlated photon counting: on a pico- to nanosecond time scale, where the distance between the exciting laser pulses and the photon detection events is time stamped (time-correlated single-photon counting or TCSPC); and on a much larger time-scale from 100 nanoseconds to seconds. The absolute arrival time of detected photons is recorded, which is subsequently used for calculating the auto-correlation function. A separate autocorrelation function is created for each emission lifetime component resulting from different fluorescent species rather than fitting complex autocorrelation function with a multi-parameter model. The resolution of the measured auto-correlation function with two or more separate functions is possible because of the additional intensity decay information. This resolution assumes that individual intensity decay is in fact associated with its own correlation function, that is, separate diffusing species with distinct intensity decays.
In this paper, we use FLCS with picosecond time-resolved detection to separate the FCS contributions from fluorophores and silver nanoparticle-conjugated fluorophores. For this study, a single-stranded oligonucleotide was chemically bound to a single 50 nm diameter silver particle and a Cy5-labeled complementary single-stranded oligonucleotide was hybridized with the particle-bound oligonucleotide. The bound Cy5 molecules on the silver particles were spatially separated from the silver surface by the hybridized DNA duplex chains, to reduce the competitive quenching. The DNA used in the current experiment contains 23 base pairs, so the separation between the fluorophore and metal core is about 8 nm. This distance is approximately considered to be an optimized value for the most efficient metal-enhanced fluorescence [1–2].
2. EXPERIMENTAL SECTION
Materials
All reagents and spectroscopic grade solvents were used as received from Fisher or Aldrich. RC dialysis membrane (MWCO 50,000) was obtained from Spectrum Laboratories, Inc. Water (18.2 MΩ-cm) purified using Millipore Milli-Q gradient system, was used in all experiments. (2-mercapto-propionylamino) acetic acid 2,5-dioxopyrrolidin-1-ylester was synthesized as previously report. Oligonucleotides were synthesized by the Biopolymer Laboratory at the University of Maryland at Baltimore, in which aminated oligonucleotide was also synthesized with one amino group substituted on the pyrimidine ring of a thymine base and the complementary oligonucleotide was labeled by Cy5.
Preparation of succinimidylated tiopronin-coated metal nanoparticles
Tiopronin-coated silver nanoparticles were prepared by chemical reduction of silver nitrate using ascorbic acid. [12–14] A 10 mg amount of silver nitrate and 30 mg of trisodium citrate were co-dissolved in 50 mL of water. A 100 μL aliquot of 0.1 N NaOH solution was added dropwise under stirring for 2 min, respectively. A 20 mg amount of ascorbic acid in 10 mL of water was then added dropwise for 5 min, and the solution was stirred for an additional 1 h. A 50 mg amount of tiopronin in 5 mL of water was added at pH 7.0, and the solution was stirred for 1 h. The solution was centrifuged at 8000 rpm to remove the suspension. After being washed with water, the residue solids were redispersed in 50 mL of water. These tiopronin-coated silver particles were succinimidylated by ligand exchange.[15] (2-Mercaptopropionylamino)-acetic acid 2,5-dioxopyrrolidin-1-yl ester and tiopronin-coated silver particles were co-dissolved in water at a molar ratio of 1/2 and stirred for 24 h. Unbound organic components were left in solution by centrifugation at 8000 rpm. The residual was washed with water and then dispersed in 50 mL of water.
Aminated oligonucleotides were chemically bound onto the succinimidylated silver particles by condensation between the amino moieties on the oligonucleotides and the terminal succinimidyl ester moieties on the silver particles. The oligonucleotides and succinimidylated metal particles were co-dissolved in water at a molar ratio of 1/1 with continuous stirring for 24 h. The metal particles were centrifuged at 8000 rpm to remove the unbound oligonucleotides in suspension. The residue was washed with water and dispersed in 50 mM phosphate-buffered saline (PBS) buffer solution at pH 7.2. The fluorophore-labeled complementary oligonucleotides were bound to the metal particles by hybridization with the bound oligonucleotides, which was performed at a molar ratio of particle/labeled oligonucleotide) 1/1 for 24 h. The suspension was removed by centrifugation at 8000 rpm. The residual particles were washed with the buffer and then dialyzed against the buffer solution (MWCO 50 000) to remove any unbound impurities for the spectral measurements.
Absorption and Fluorescence Spectroscopy
Absorption spectra were collected using a Hewlett-Packard 8453 spectrophotometer. Fluorescence spectra were recorded using a Varian Cary Eclipse Fluorescence Spectrophotometer using front face illumination geometry with excitation from a Xenon arc lamp.
Fluorescence lifetime correlation spectroscopy (FLCS) measurements
FLCS measurements were performed using a scanning confocal time-resolved microscope (Picoquant MicroTime 200). The excitation laser (λex ~ 635 nm) was reflected by a dichroic mirror to a high numerical aperture (NA) oil objective (100x, NA 1.3) and focused onto the solution sample. The fluorescence was collected by avalanche photodiodes through a dichroic beam splitter and a bandpass (650–720 nm, Chroma) filter thus eliminating the scattered excitation light and collecting the fluorescence from the Cy5 probes in the region of interest. We have used a PicoQuant TimeHarp 200 PC board operated in Time-Tagged Time-Resolved (TTTR) mode. For fluorophore conjugated silver nanoparticle, we have obtained two different lifetime components. Calculations of time-correlated single photon counting filtered auto-correlation of Cy5-DNA-Ag nanoparticles are performed with the PicoQuant Symphotime software (version 4.3).
A schematic of the experimental setup for the FLCS measurements is shown in Figure 1.
Figure 1.
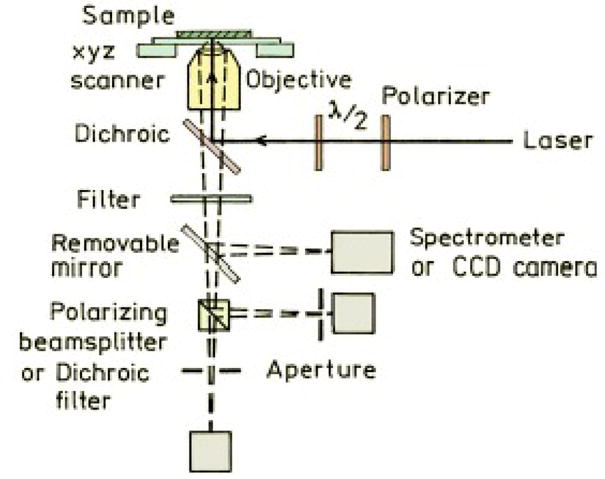
Schematic of the FLCS system.
The fluorescence intensity decays were analyzed in terms of the multi-exponential model as the sum of individual single exponential decays [16]:
| (1) |
In this expression τi are the decay times and αi are the amplitudes and . The fractional contribution of each component to the steady-state intensity is described by:
| (2) |
The average lifetime is represented by:
| (3) |
The values of αi and τi were determined using the PicoQuant symphotime software with nonlinear least squares fitting. The goodness-of-fit was determined by the χ2 value.
3. RESULTS AND DISCUSSION
Silver particles were synthesized by reduction of silver salt using ascorbic acid as the reduction agent. The silver particles were stabilized by the coated citrate molecules. The citrate molecules were further replaced by the tiopronin, and the tiopronin-coated silver particles were observed to display goodsolubility and chemical stability in water. Representative TEM image showed average diameters of the metal cores to be 50 nm (Figure 2). The size distributions of metal particles were analyzed with Scion Image Beta Release 2 counting multiple TEM images. It was shown from the histograms that the majority of the synthesized particles are in the 50 nm diameter range.
Figure 2.
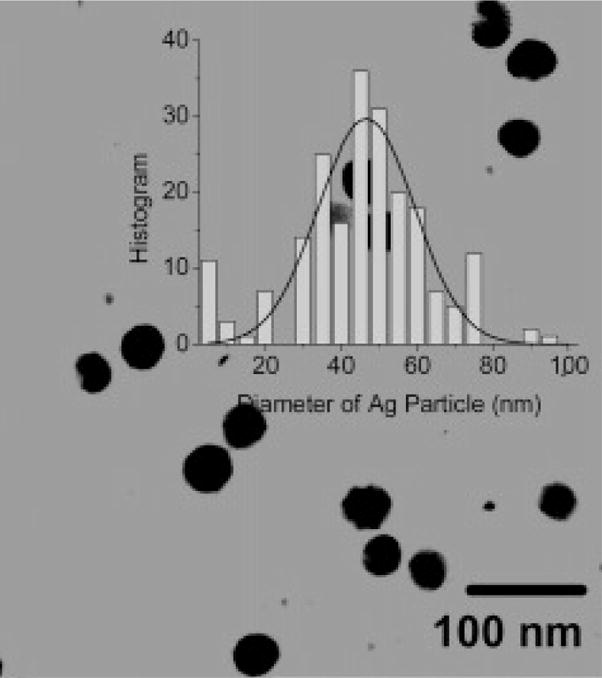
TEM image of silver particles.
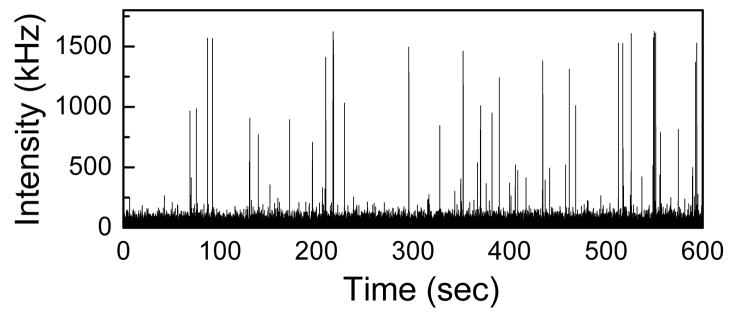
The fluorescence intensity fluctuation of a mixture of Cy5-DNA (concentration: 1nM) and Cy5-DNA-Ag (concentration: 100pM) is shown in Figure 3. The average intensity (pulse height) for Cy5-DNA is about 50 kHz where as for Cy5-DNA-Ag is about 1GHz. So there is a significant enhancement of signal when a Cy5-DNA is covalently attached to a 50 nm silver particle.
Figure 3.
Intensity fluctuations of a mixture of Cy5-DNA (1nM) and Cy5-DNA-Ag (100pM)
The Cy5-DNA-Ag-particle showed much faster intensity decay with ~5-fold decrease in average fluorescence lifetime (from ~1.8 ns for free Cy5-DNA to 0.35 ns for Cy5-DNA-Ag-particle). These unusual fluorescence properties i.e. increase in fluorescence intensity and decrease in lifetime of Cy5-DNA-Ag-particles are thought to be due to the presence of enhanced local fields around the 50 nm silver particle and the increased emission rate of the metal-fluorophore conjugates.
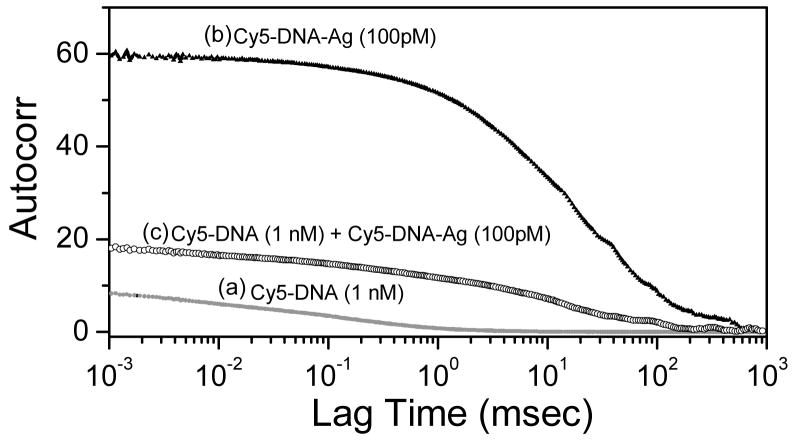
Figure 4 shows the measured correlation functions for Cy5-DNA free (1 nM) and Cy5-DNA-Ag-particle (100 pM) in solution. As expected the correlation function for the bound Cy5 is strongly shifted to longer times because of the slower diffusion of the Cy5-DNA-Ag-particle as compared to the free Cy5-DNA. The autocorrelation curve for a mixture of Cy5-DNA (1 nM) and Cy5-DNA-Ag (100 pM) is also shown in Figure 4. It is important to note that this curve is shifted more than half way towards the Ag-particle-only curve, even though the Cy5-DNA-Ag particle concentration is 10-fold less than Cy5-DNA. This dominant contribution of the Ag-particle-bound form, even with a 10-fold lower concentration of Cy5-DNA-Ag-particle, is due to the higher brightness of Ag-particle-bound Cy5-DNA as compared with free Cy5-DNA.
Figure 4.
Autocorrelation of Cy5-DNA, bound to a silver particle and the mixture.
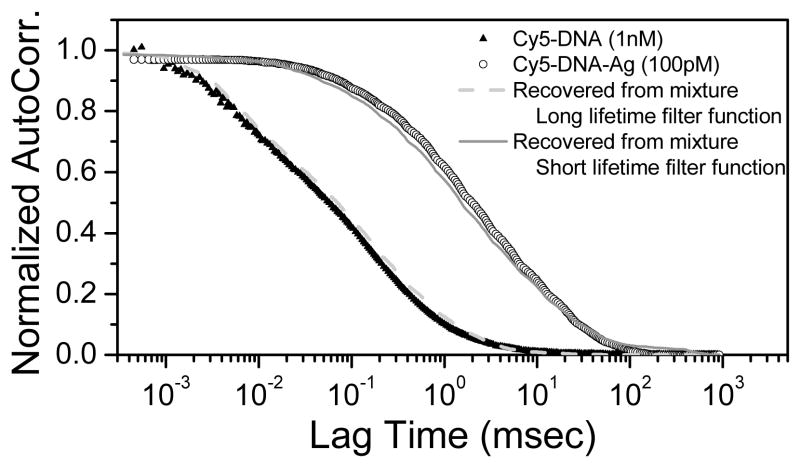
The intensity decays can be used with the data from a mixture to recover the separate correlation curves of each species in the mixture. Figure 5 shows the recovered autocorrelation from the mixture of free Cy5-DNA and of the Ag-particle-bound form using long lifetime (in this case: Cy5-DNA lifetime of 1.8 nsec) and short lifetime (corresponding to the Cy5-DNA-Ag lifetime of 0.35 nsec) functions [10].
Figure 5.
Recovered autocorrelation based on lifetime
4. CONCLUSION
In this article, we use fluorescence lifetime correlation spectroscopy (FLCS) to separate the fluorescence correlation spectroscopy (FCS) contributions from fluorophores and metal-conjugated fluorophores. The single Cy5-labeled 50 nm silver particles displayed a factor of 15-fold increase in emission signal and 5-fold decrease in emission lifetimes in solution relative to the Cy5-DNA. Our measurements suggest that FLCS is a powerful method for investigating the metal-fluorophore interaction at the single molecule level and to separate two different species from a mixture solution emitting at the same wavelength. Additionally, the highly bright Cy5-DNA-Ag molecules offer to be excellent probes in high background biological samples.
Acknowledgments
This work was supported by the National Institutes of Health (NIH): the National Institute of Biomedical Imaging and Bioengineering (Grant No. EB00682, EB-006521), and the National Human Genome Research Institute (Grant No. HG002655).
References
- 1.Lakowicz JR. Radiative decay engineering: Biophysical and biomedical applications. Anal Biochem. 2005;337:171. doi: 10.1006/abio.2001.5377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lakowicz JR, Ray K, Chowdhury M, Szmacinski H, Fu Y, Zhang J, Nowaczyk K. Plasmon controlled fluorescence: a new paradigm in fluorescence spectroscopy. Analyst. 2008;133:1308. doi: 10.1039/b802918k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chance RR, Prock A, Silbey R. Molecular fluorescence and energy transfer near interfaces. Adv Chem Phys. 1973;37:1. [Google Scholar]
- 4.Ford G, Weber W. Electromagnetic interactions of molecules with metal surfaces. Phys Reports. 1984;113:195. [Google Scholar]
- 5.Gersten J, Nitzan A. Spectroscopic properties of molecules interacting with small dielectric particles. J Chem Phys. 1981;75:1139. [Google Scholar]
- 6.Gersten JI. Theory of fluorophore-metallic surface interactions. In: Geddes CD, Lakowicz JR, editors. Topics in Fluorescence Spectroscopy, Vol. 8: Radiative Decay Engineering. Springer Science+Business Media, Inc; New York: 2005. pp. 197–221. [Google Scholar]
- 7.Gregor I, Enderlein J. Time-resolved methods in biophysics. 3 Fluorescence lifetime correlation spectroscopy. Photochem Photobiol Sci. 2007;6:13. doi: 10.1039/b610310c. [DOI] [PubMed] [Google Scholar]
- 8.Benda A, Fagulova V, Deyneka A, Enderlein J, Hof M. Fluorescence Lifetime Correlation Spectroscopy Combined with Lifetime Tuning: New Perspectives in Supported Phospholipid Bilayer Research. Langmuir. 2006;22:9580. doi: 10.1021/la061573d. [DOI] [PubMed] [Google Scholar]
- 9.Enderlein J, Gregor I. Using fluorescence lifetime for discriminating detector afterpulsing in fluorescence-correlation spectroscopy. Rev Sci Instr. 2005;76:033102. [Google Scholar]
- 10.Kapusta P, Wahl M, Benda A, Hof M, Enderlein J. Fluorescence Lifetime Correlation Spectroscopy. J Fluoresc. 2007;17:43. doi: 10.1007/s10895-006-0145-1. [DOI] [PubMed] [Google Scholar]
- 11.Bohmer M, Wahl M, Hans-Jurgen R, Erdmann R, Enderlein J. Time-resolved fluorescence correlation spectroscopy. Chem Phys Lett. 2002;353:439. [Google Scholar]
- 12.Chen S, Wang ZL, Ballato J, Foulger SH, Carroll DL. Monopod, Bipod, Tripod, and Tetrapod Gold Nanocrystals. J Am Chem Soc. 2003;125:16186. doi: 10.1021/ja038927x. [DOI] [PubMed] [Google Scholar]
- 13.Nikoobakht B, El-Sayed MA. Preparation and Growth Mechanism of Gold Nanorods (NRs) Using Seed-Mediated Growth Method. Chem Mater. 2003;15:1957. [Google Scholar]
- 14.Orendorff CJ, Murphy CJ. Quantitation of Metal Content in the Silver-Assisted Growth of Gold Nanorods. J Phys Chem B. 2006;110:3990. doi: 10.1021/jp0570972. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Fu Y, Lakowicz JR. Enhanced Foerster Resonance Energy Transfer (FRET) on a Single Metal Particle. J Phys Chem C. 2007;111:50. doi: 10.1021/jp062665e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lakowicz JR. Principles of Fluorescence Spectroscopy. 3. Kluwer Academic/Plenum Publishers; New York: 2006. [Google Scholar]