Abstract
Considering the biological abundance and importance of Mg2+, there is a surprising lack of information regarding the proteins that transport Mg2+, the mechanisms by which they do so, and their physiological roles within the cell. The best characterized Mg2+ channel to date is the bacterial protein CorA, present in a wide range of bacterial species. The CorA homolog Mrs2 forms the mitochondrial Mg2+ channel in all eukaryotes. Physiologically, CorA is involved in bacterial pathogenesis, and the Mrs2 eukaryotic homolog is essential for cell survival. A second Mg2+ channel widespread in bacteria is MgtE. Its eukaryotic homologs are the SLC41 family of carriers. Physiological roles for MgtE and its homologs have not been established. Recently, the crystal structures for the bacterial CorA and MgtE Mg2+ channels were solved, the first structures of any divalent cation channel. As befits the unique biological chemistry of Mg2+, both structures are unique, unlike that of any other channel or transporter. Although structurally quite different, both CorA and MgtE appear to be gated in a similar manner through multiple Mg2+ binding sites in the cytosolic domain of the channels. These sites essentially serve as Mg2+ “sensors” of cytosolic Mg2+ concentration. Many questions about these channels remain, however, including the molecular basis of Mg2+ selectivity and the physiological role(s) of their eukaryotic homologs.
Magnesium
Mg2+ is the most abundant divalent cation in living cells. It is present at a total cellular concentration of 15−25 mM in both prokaryotic and mammalian cells (39, 53, 55). In the cytosol, the majority of Mg2+ is bound to ATP and other phosphonucleotides and to multiple enzymes. In all cells, Mg2+ serves as an essential structural element for ribosomes and membranes and as a required cofactor for ATP in the catalytic pocket of a multitude of enzymes. In prokaryotes, Mg2+ has also been identified as an important regulatory signal essential for virulence (10, 70).
The chemistry of divalent magnesium is unique among the biologically important cations. The hydrated radius of Mg2+ is ∼400 times larger than the dehydrated radius, a much larger difference than that seen with Na+ and Ca2+ (∼25-fold) or K+ (fourfold). Of all biological cations, Mg2+ is the most charge dense, holding the waters within its hydration shell tighter by a factor of 103−104 than do Ca2+, K+, and Na2+ (39). In addition, the hydrated Mg2+ cation is more rigid than other cations, always hexacoordinate, and almost always prefers to coordinate with oxygen. Proteins that transport Mg2+ must be able to recognize the very large hydrated cation, strip the tightly bound hydration shell from the cation, and only then transport the dehydrated form. These chemical properties of Mg2+ thus predict that proteins that recognize and transport Mg2+ will be unique (19, 36).
Mg2+ Transport Proteins
The most thoroughly characterized Mg2+ transport proteins to date are from prokaryotic sources. Although several genes associated with Mg2+ transport in eukaryotic systems have been recently identified (Table 1), this review will focus on the only two divalent cation channels to be crystallized: CorA and MgtE. The first prokaryotic Mg2+ transport system identified and cloned was termed corA for the Co2+ resistance screen by which it was discovered in Escherichia coli and Salmonella enterica serovar Typhimurium (22, 23, 44, 49, 61). A locus termed mgt was also found to be associated with Mg2+ transport and eventually shown to encode a P-type ATPase that mediates Mg2+ influx with rather than against its electrochemical gradient (22, 66-69, 72, 73). Additional work uncovered another widespread Mg2+ influx system in prokaryotes encoded by mgtE (65, 75). Work from this laboratory has subsequently characterized Mg2+ flux mediated by each of these systems.
Table 1.
Prokaryotic and eukaryotic Mg2+ transporters
| Superfamily | Identified Members | Apparent Km for Mg2+ | Type of Transporter | References |
|---|---|---|---|---|
| CorA | CorA, ALR1/ALR2, Mrs2/AtMrs2, Lpe10 | ∼ 15 μM | Channel, Channels | 22, 23, 66, 67 |
| MgtE | MgtE, SLC41A1, SLC41A2 | 0.7 mM, 0.3 mM | Channel, Carriers | 65, 75, 13, 14, 54 |
| TRPM | TRPM6 TRPM7 (LTRPC7, TRP-PLIK) |
Channel/kinase (Chanzyme) | 1, 5, 57, 58, 76, 42, 43, 59 | |
| Claudins | Claudin-16 (Paracellin-1) Claudin-19 |
Claudins | 62 | |
| ACDP | ACDP2 (CNNM2) | 0.6 mM | Putative channel | 12 |
| MagT1 | MagT1 | 0.2 mM | Putative channel | 15 |
| Mgt | MgtA, MgtB | 10 μM | P-type ATPases | 64, 66, 69 |
| NIPA1 | NIPA1(SPG6) | 0.7 mM | Putative channel | 12 |
| MMgT | MMgT1, MMgT2 | 1.5 mM, 0.6 mM | Putative channels | 16 |
Text in bold denotes prokaryotic origin. Text in regular font denotes eukaryotic origin.
CorA is an ion channel present in approximately half of the microbial genomes currently sequenced. It mediates the influx of Mg2+, Co2+, and Ni2+; it does not transport Mn2+, Ca2+, Zn2+, or Fe2+ (45, 66). The eukaryotic homolog of CorA is Mrs2, the Mg2+ channel of the inner mitochondrial membrane (3, 56).
MgtE, like CorA, is the primary Mg2+ transport system in about half of bacterial genomes sequenced to date. A minority of organisms carry both CorA and MgtE. MgtE is able to mediate the flux of Mg2+ and Co2+. Ni2+ is an inhibitor of MgtE, but, unlike CorA, Ni2+ is not transported by MgtE (65, 75). The eukaryotic homologs of MgtE are the SLC41 family of solute carriers (77).
The CorA Mg2+ Channel
CorA structure
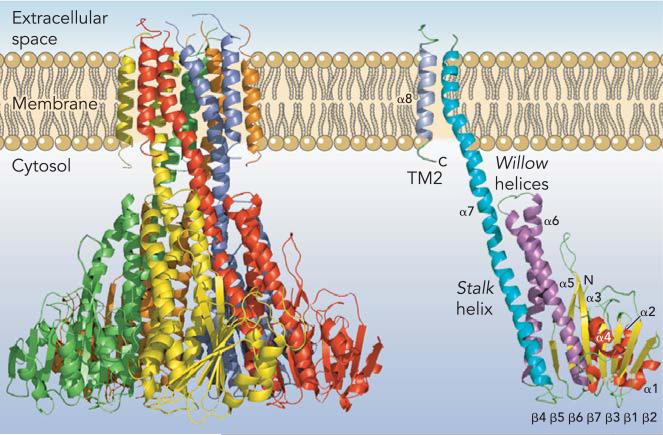
Three independent crystal structures of Thermotoga maritima CorA have recently been published with resolutions from 2.9 to 3.9 Å. All three structures give essentially the same picture of an apparent closed form of the channel (9, 33, 51). CorA is a homopentamer with two transmembrane (TM) segments per monomer (FIGURE 1). Both the amino and carboxytermini are positioned in the cytosol. The large NH2-terminal cytoplasmic domain structure is formed from a new protein fold, consisting of a seven stranded parallel/anti-parallel β-sheet sandwiched between two sets of α-helices (α1α2α3) and (α4α5α6). A stalk helix (α7) links this cytosolic domain to TM1. The NH2-terminal regions of the stalk helix with part of the α6 helix form a funnel-like structure opening into the cytosol. The stalk helix extends ∼100 Å from the cytoplasm into the membrane, forming the inner wall of the funnel and TM1. At the membrane-cytosol interface, the funnel is relatively narrow in diameter at 5 Å, whereas its cytoplasmic mouth is much wider at 20 Å. Many of the α6 and α7 stalk helix residues facing the interior of the funnel are negatively charged or bear hydroxyl groups (FIGURE 2). Since CorA is a homopentamer, this arrangement provides a succession of concentric, negatively charged or polar residues that putatively would interact with Mg2+ as it exits the membrane pore.
FIGURE 1. CorA Mg2+ channel.
A: CorA Mg2+ channel. The homopentamer is shown from the side with each of the five monomers in a different color. The extracellular space (periplasm) is at top. B: a single monomer is shown with TM2/α8 in gray, TM1/α7 in blue, the willow helices (α5 and α6) in purple, the β-sheet (β1-β7) in yellow, and the remaining helices (α1, α2, α3) in red.
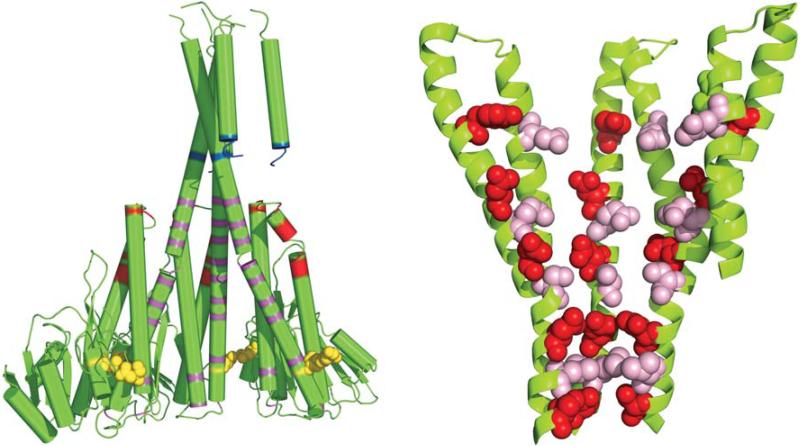
FIGURE 2. Cytosolic charged domains of CorA.
A: cartoon view of charged residues in the CorA cytosolic domain. The CorA Mg2+ channel of T. maritima is shown in cartoon form in green with two monomers removed for clarity. Lysine residues 284, 292, and 346−349 shown in blue comprise the “basic sphincter.” Aspartate and glutamate residues at the membrane proximal ends of the willow helices are shown in red (D189, D190, E191, D193, E196, E197, E198, E201, E204, and E206). Charged and hydroxyl-bearing residues that line the pore are shown in magenta (E230, S233, D238, E246, Y255, D263, E266, D270, S273, D277, S280, and S284). Aspartates 89 and 253, which bind a Mg2+ ion between adjacent monomers, are shown as yellow spheres. An alternative view is also shown in Figure 4. B: cartoon view of charged residues lining the interior face of the funnel of CorA. The cytosolic portions of most of the α6 and α7/stalk helices of the T. maritima CorA are shown in green. Two of the five monomers have been removed for clarity to allow better visualization. The charged residues that line the inner wall of the funnel formed by α6 and α7 are represented as spheres with alternate rings in red (from bottom of funnel: S284, D277, D270, D263, and Y255) and pink (from bottom of funnel: S280, S273, E266, and E230). Residues S233, D238, and E246 are not colored for clarity.
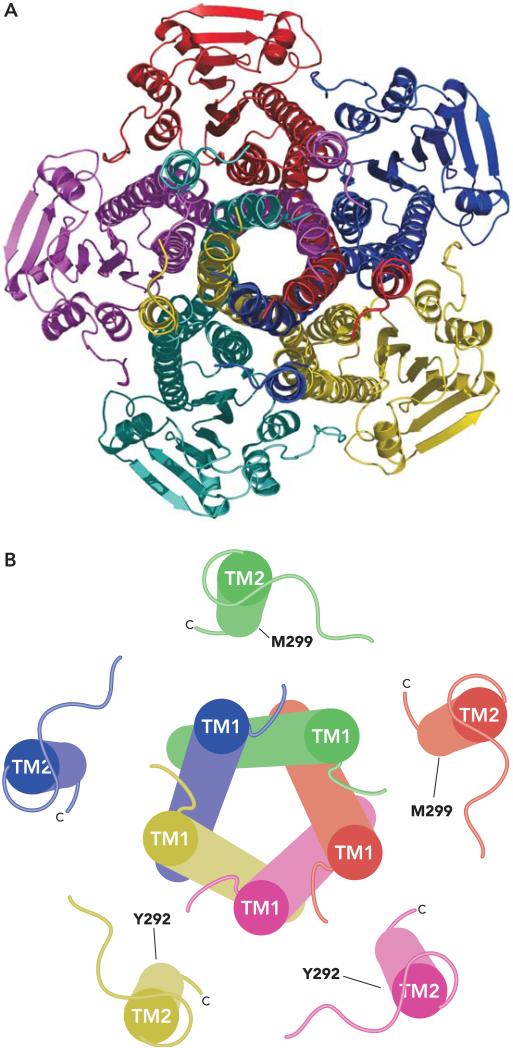
The membrane domain of CorA is formed by two TM segments per monomer, giving 10 TM segments in total. The helices of TM2 form a ring around the outside of the pore, which is composed solely of TM1 helices in the apparent closed form of the channel (FIGURE 3). A proline and a glycine cause TM1 to kink and taper the pore near the periplasmic surface. Distortion by proline and glycine residues in similar positions is a common property of ion channels (74). The distal portion of TM1 near the periplasm contains the signature sequence of all CorA proteins, YGMNF. A BLAST (2) search with this five-amino acid string returns no hits except CorA. This sequence is absolutely required for CorA function (71). In the homopentamer, the asparagine residues contributed by the YGMNF motif appear to block the pore at the periplasmic face. They are held in place by stacking interactions between the tyrosine of the YGMNF sequence from one monomer and the phenylalanine of the adjacent monomer's YGMNF sequence.
FIGURE 3. CorA membrane domain.
A: CorA as seen from the periplasm. The CorA pentamer is shown from outside the cell looking down the pore, which is formed by TM1 in this closed form. Each monomer is colored differently. The five TM2 helices lie outside this pore, approximately 15 Å from the adjacent TM1 and 23 Å from another TM2. B: CorA membrane domain as cylinders. TM1 and TM2 are shown as cylinders with each monomer colored differently. The view is from the periplasm. Each TM2 is approximately perpendicular to the membrane, whereas each TM1 is angled slightly. Y292 near the periplasmic end of TM2 and M299 near the cytosolic end of TM2 are indicated on two monomers each. Cysteine substitution at either of these residues results in a pentamer containing five cysteines within the membrane. It is of interest that Y292C can spontaneously cross-link to another Y292C residue. CorA carrying the M299C substitution does not spontaneously cross-link via a disulfide bond, but it can be induced to do so with copper-phenanthroline oxidation (71). Since each TM2 is 23 <ang from the adjacent TM2, these data strongly suggest that the TM2 helices must move in toward the apparent TM1 pore at some point during flux of Mg2+.
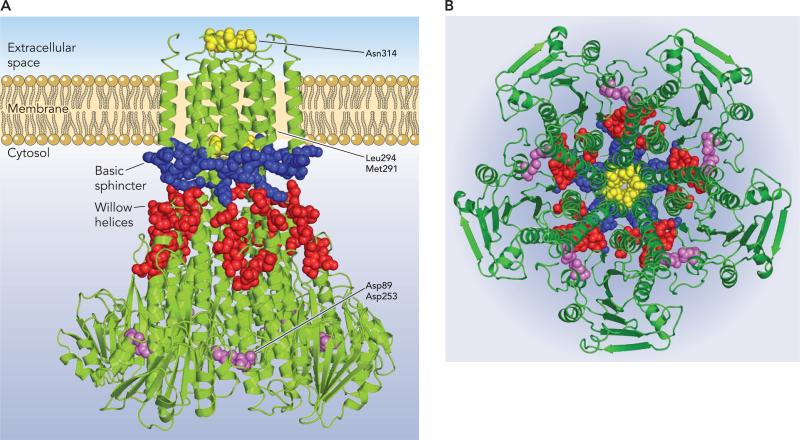
TM2 lies outside the pore formed by TM1 and returns the carboxy-terminus to the cytosol. The periplasmic loop connecting TM1 and TM2 as well as the last two carboxy-terminal amino acids were not resolved in any of the published crystal structures. A universally conserved feature of CorA is a short six-amino acid cytosolic domain of multiple arginines and/or lysines at the COOH terminus. For example, in S. typhimurium, this sequence is KRKNWL, wheras in T. maritima it is KKKKWL. Including two conserved lysines on the outside face of the α7 stalk helix, this places a ring of 25−30 lysines 50 Å in diameter external to the pore and adjacent to the membrane, termed the “basic sphincter” (FIGURE 4). This ring is putatively countered by 50 aspartate and glutamate residues from the membrane proximal halves of helices α5 and α6, which are positioned almost perpendicular to the membrane, providing a ring of negative charges just above the basic sphincter termed the willow helices.
FIGURE 4. Important residues of the CorA Mg2+ channel.
A: a view of the T. maritima Mg2+ channel from the side. Monomers are all in green. Potentially important residues are represented in space-filling form. The yellow residues are those within the pore formed by TM1 (N314, L294, M292) that appear to block the pore in its closed state. The blue residues are the lysines of the basic sphincter. The red residues are the aspartate and glutamate residues in the membrane proximal halves of the willow helices (α5 and α6). The lavender residues are the aspartate residues (D89 and D253) that bind Mg2+ between each monomer, apparently helping to hold the αβα domain of one monomer to the α7 stalk helix of an adjacent monomer. B: a view of the T. maritima Mg2+ channel from the cytosol. The CorA channel is viewed down the funnel toward the membrane. Residues are colored as in part A but without labels.
Interaction of CorA with Mg2+
The large size of a hydrated Mg2+ creates a unique problem for Mg2+ transporters in terms of selectivity and transport. The ability of cation hexa ammines to selectively inhibit CorA has suggested the hypothesis that CorA initially interacts with the fully hydrated Mg2+ ion (30) since the covalently bound ammine groups mimic the size and shape of a hydrated Mg2+ cation (6). In addition, the ability of Co(III) and Ru(III) hexaammines to inhibit CorA indicates that this initial interaction is not based strictly on charge since both divalent and trivalent cations bind to CorA (30).
An obvious candidate for site of initial interaction of the channel with a Mg2+ cation is the only part of CorA exposed on the periplasmic face of the membrane, the nine-amino acid loop connecting TM1 and TM2 (33). This loop always contains a significant negative charge as well as the highly conserved MPEL motif. This suggests that the periplasmic loop may act as a selectivity filter. In contrast, Payandeh and Pai's crystal structure (51) modeled this unresolved loop by lengthening TM2 and reducing the loop to seven amino acids. The results of their modeling suggested that the MPEL loop lies roughly parallel to the membrane and thus would be poorly positioned to interact with Mg2+ and act as a selectivity filter. Further experiments will be required to determine where Mg2+ initially binds to CorA.
The CorA pore is ∼40 Å in length. It is noteworthy that none of the almost 800 currently known CorA homologs contains a charged amino acid in either TM1 or TM2. Thus electrostatic interactions are not involved in movement of the most charge dense of all biological cations through the membrane. Indeed mutational analysis of the S. typhimurium CorA indicates that it is the backbone carbonyls within the pore that primarily interact with Mg2+ during its passage (71). In addition to the constriction formed by the ring of five asparagine residues from the YGMNF motif, two additional sites of pore constriction are apparent, creating a maximum width of 6 Å narrowing to 2.5 Å. The smallest of these constrictions is formed by bulky hydrophobic residues at the cytosol-membrane interface (Leu294 and Met291 in the T. maritima CorA). A large hydrophobic residue is conserved at the equivalent of position 294 in CorA homologs. Adjacent to this constriction but outside the pore is the basic sphincter, the lysine/arginine ring formed from the COOH terminus. The combination of positive potential from this ring and pore constriction would appear to provide a formidable barrier to passage of a positive cation.
How does Mg2+ pass through all of these barriers? The relative positioning of the positively charged basic sphincter and the negatively charged willow helices suggests an answer (9, 33, 51). The electrostatic attraction generated between the basic sphincter and the willow helices could induce movement of the basic sphincter outward and/or upward further into the cytosol, away from the pore and the block formed by Leu294 and Met291. This would necessitate movement of the α7 stalk helix since part of the lysine/arginine ring is formed from residues of α7 near the cytosolic end of the pore. The 100 Å length of the stalk helix provides a very long relatively rigid “lever” through which movements elsewhere in the homopentamer could be transmitted to the pore, thereby opening it. Recent mutational analysis of T. maritima CorA by Payandeh et al. (50) supports such a gating role for the willow helices.
Regulation of Mg2+ transport
How might movement of the willow helices and basic sphincter be controlled to regulate opening and closing of the channel? The structure from Lunin et al. (33) and that from Payandeh and Pai (51) were solved in the presence of Mg2+ and Ca2+, respectively. In both, electron density was detected near residues Asp89 of the α3 helix and Asp253 of the α7 stalk helix consistent with cation binding (FIGURE 4). Mg2+ bound to this site would stabilize a closed conformation of the protein by associating the NH2-terminal αβα domain of one monomer with the α7 stalk helix of the adjacent monomer. Mg2+ dissociation from these sites could elicit rotation of the αβα domain of one monomer away from the α7 helix of the funnel wall (9, 33, 51). The movement of the willow helices as part of the αβα domain would presumably exert an attraction on the positively charged basic sphincter residues, relieving the block at the membrane-cytosol interface and allowing the protein to open like an iris. Recent mutational data supports this hypothesis (50). It remains to be definitively demonstrated that the Mg2+ ions bound to the cytosolic domain have an actual function in gating. Nonetheless, available data indicate that these bound ions act essentially as sensors of cytosolic Mg2+ concentration, thus tightly regulating Mg2+ movement through CorA and consequently regulating Mg2+ homeostasis.
The CorA superfamily
The CorA protein superfamily is widespread in the Eubacteria with about 800 sequences currently known. Although phylogenetic analysis of the family suggests at least two additional subfamilies in addition to the CorA Mg2+ channels (26, 27), information on function is available only for the subfamily represented by ZntB, which mediates the efflux of Zn2+ (4, 80). Given its sequence similarity and identical secondary structure to CorA, it has been hypothesized to have a structure similar to that of CorA (38). This hypothesis has recently been confirmed with solution of the crystal structure of the soluble domain of ZntB from Vibrio parahaemolyticus (Tan et al., PDB code 3CK6) and S. typhimurium (Maguire et al., unpublished observations). These structures show that ZntB is a funnel-shaped homopentamer assembled almost identically to CorA. Efflux of Zn2+ via ZntB would move the cation against the electrochemical gradient. This obviously requires energy, most likely in the form of a counter- or co-transported ion, although this has not been formally shown. Thus the CorA superfamily appears to contain both ion channels and transporters, much like the ClC family of carriers (35, 41).
Eukaryotic homologs of CorA include the Mrs2 and Lpe10 inner mitochondrial membrane proteins, which were originally identified as being required for group II intron splicing (3, 18, 79). Mrs2 is most homologous to CorA in the membrane domain and exhibits the YGMNF motif. That Mrs2 is a true homolog of CorA has been confirmed by demonstration that Mrs2 can functionally substitute for CorA in prokaryotes and that a prokaryotic CorA can in turn functionally substitute for Mrs2 (3, 29, 81). The human homolog of Mrs2 has been shown to complement the yeast mrs2 phenotype (81). Arabidopsis thaliana also expresses an Mrs2 homolog that can complement both bacterial corA and yeast mrs2 mutants (31, 60).
Mrs2 directly transports Mg2+ into the mitochondrion as shown both with MagFura-2, a fluorescent Mg2+ indicator dye, (29) and from patch clamping of giant lipid vesicles (56). In the latter experiments, Mrs2 exhibits a conductance of 150 pS. The involvement of these proteins in group II intron splicing is apparently indirect, as the ribozyme structure involved requires adequate Mg2+ concentration for function. Mrs2 is apparently the only Mg2+ influx pathway in the mitochondrion. Recent studies involving conditional knock down of the human Mrs2 homolog (Mrs2L) show that Mrs2 is necessary for Mg2+ uptake in mitochondria as well as maintenance of respiratory complex I. Long-term knockdown of Mrs2 results in apoptosis (52).
The ALR1 and ALR2 eukaryotic homologs of CorA were first identified in a screen testing for resistance to aluminum. Overexpression of either protein confers resistance to Al3+ and Ga3+ ions (34). These proteins are localized in the yeast plasma membrane, and their expression and turnover via endocytosis is controlled by the Mg2+ concentration in the environment (17). Electrophysiological studies of ALR1 provide evidence that ALR1 mediates Mg2+ influx (32). Recent work has shown that ALR1 and ALR2 are able to form homo-oligomers and hetero-oligomers (78). ALR2 transports Mg2+ rather poorly, possibly because the glutamic acid of the highly conserved MPEL motif in the loop between TM1 and TM2 is replaced by a positively charged arginine. Mutation of this arginine to glutamic acid greatly increased the Mg2+ transport capacity of ALR2 (78). The ALR proteins are around 800 amino acids long, much larger than CorA, and have an NH2-terminal extension that presumably binds trivalent cations. Whether the ALR homologs transport trivalent cations is not known, although the phenotype of increased resistance with their overexpression suggests that they mediate either influx of trivalent cations into a vacuole or their efflux across the plasma membrane.
The MgtE Mg2+ Channel
MgtE structure
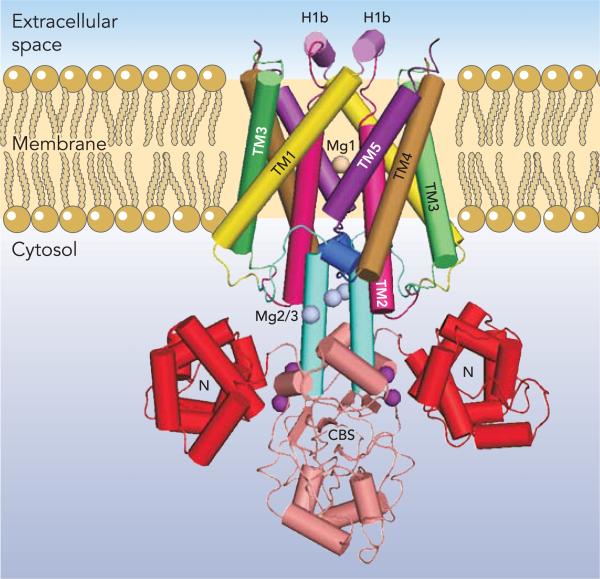
The crystal structure of the full-length bacterial Thermus thermophilus MgtE was recently determined by Hattori et al. (20, 21) to a resolution of 3.5 Å (FIGURE 5). In addition, the structure of the cytosolic domain was resolved to 2.3 Å in the presence of Mg2+ and to 3.9 Å in the absence of Mg2+. MgtE exists as a homodimer with five transmembrane segments per monomer giving, like CorA, 10 total TM segments. The cytosolic domain is highly acidic and composed of two subdomains. The first, the N domain, is located at the NH2-terminal region of the cytosolic domain and is a right-handed superhelix with 10 total α-helices in each domain. Pallen and Gophna noted that the N domain is structurally homologous to the soluble domain of FliG, a component motor of the bacterial flagellum (44a). Immediately following the N domain is a tandemly repeated cystathionine-β-synthase (CBS) domain, a known dimerization domain in several transporters (25).
FIGURE 5. The MgtE Mg2+ channel.
Structure of MgtE from T. thermophilus. MgtE is shown with each dimer colored identically. The N domain is shown in red, the CBS domains in beige, and the connecting helices in blue. Each of the TM segments of each monomer are shown in different colors and labeled. The H1b helices at the extracellular membrane face are shown in purple. Bound Mg2+ ions are labeled as follows: Mg1 within the pore is in wheat, Mg2 and Mg3 are in gray, and Mg4 and Mg5 are in purple. One of the Mg2 residues is obscured in this view.
Each monomer has five transmembrane helices. The helices are connected by five loops, designated L0–L4 with the L1 and L4 loops containing short α-helices termed H1b and H4b, respectively (FIGURE 5). The transmembrane domain of each monomer is linked with the cytosolic domain by a connecting helix, which interacts with the TM5, TM2, and H4b helices through both dipole moments and van der Waals forces. The connecting helix of each monomer interacts with and is parallel to the connecting helix of the other monomer. Hydrophobic interactions between the TM domains appear to drive dimerization, which is stabilized by hydrogen bond interactions between highly conserved charged and/or polar residues. As is a general feature of many ion channels, several MgtE TM helices are kinked at either a glycine or proline residue.
The ion-conduction pore of MgtE appears to be formed primarily by the TM2 and TM5 helices. The periplasmic entrance to the pore is formed from residues in the L1 loop. In this loop, the H1b helix is exposed to the periplasm and parallel to the membrane. Immediately following H1b, the continuation of L1 forms an initial nonhelical and highly conserved hydrophobic portion of TM2. A typical sequence here is VVILA. The entrance to the pore at the periplasmic side is ∼15 Å in diameter. The entrance on the cytoplasmic side is ∼6 Å in diameter. However, in contrast to the periplasmic end of the pore, the cytosolic end is lined with hydrophilic residues, including a conserved aspartate. This is also in contrast to CorA where not one of the almost 800 homologs now known carries a charged residue in either TM1 or TM2.
Interaction of MgtE with Mg2+
The full-length crystal structure of MgtE was solved in the presence of 40 mM Mg2+. The structure revealed five peaks of electron density on each monomer near conserved acidic residues. Bond angles and bond lengths support the interpretation of these densities as bound Mg2+ ions (FIGURE 5). The densities are referred to as Mg1–Mg5 (20). Mg1 is in the ion conducting pore and coordinated with the carboxyl group of the aspartate residue in TM5 and to the carbonyl group of an alanine one turn of the helix away. Mg1 coordination is clearly octahedral with short 2.0 Å bond angles near 90°, which is typical of Mg2+. Mg2 and Mg3 are located ∼6 Å apart at the interface of the cytosolic and transmembrane domains. Mg2 is coordinated with one residue of the CBS domain and two residues of the connecting helix. Mg3 coordinates with a residue of the CBS domain, a residue of the connecting helix, and a third residue between TM4 and TM5. These locations suggest that Mg2+ ions are involved in positioning the connecting helix for proper interaction with TM5, TM2, and H4b to lock the channel in a closed conformation. Mg4 and Mg5 are ∼8 Å apart and appear to connect the N and CBS domains. These Mg2+ ions are positioned to fix the connecting helix in place and stabilize the interactions between the N and CBS domains. Mg2-Mg3 and/or Mg4-Mg5 would thus appear to play a role similar to the Mg2+ ions bound between monomers in CorA.
Regulation of MgtE
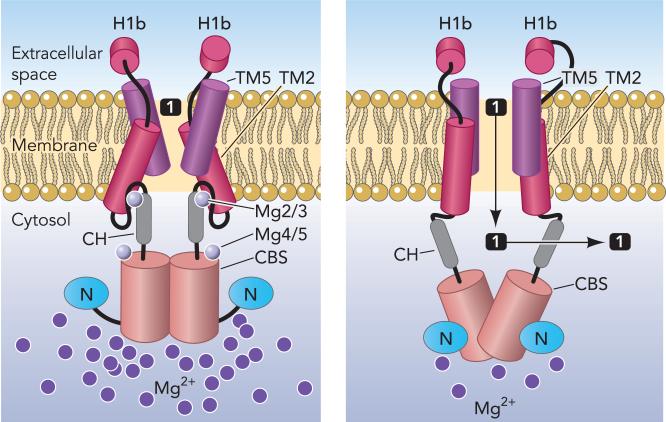
The structure of the N domain and the structure of the CBS domain in the presence and absence of Mg2+ are virtually identical. However, the absence of Mg2+ from the Mg2-Mg3 and Mg4-Mg5 sites appears to allow the N domain to rotate ∼120° away from the CBS domain. The dimerization of the two tandem CBS domains within each monomer is also altered in the absence of Mg2+. The connecting helices unlock and rotate ∼20° away from each other. Thus the absence of Mg2+ induces marked cytosolic domain flexibility. These movements would disrupt the interaction between the transmembrane domains and the connecting helices, allowing rearrangement of the helices that form the pore and presumably opening the pore (FIGURE 6).
FIGURE 6. Cartoon structure of MgtE showing proposed domain movements during Mg2+ flux.
This drawing is based on the model shown in Fig. 4, C and D, of Hattori et al. (20). The bound Mg2+ ions are shown as numbered light gray circles, whereas the additional light purple circles in the cytosol represent relative concentration of cytosolic free Mg2+. The various domains are labeled as in previous figures. At left, Mg2/3 and Mg4/5 hold the CH to the TM domain and the N to the CBS domain, respectively. However, as cytosolic Mg2+ decreases, these bound Mg2+ ions may dissociate from MgtE. Loss of Mg2/3 would free the connecting helices from the TM domain, putatively allowing TM2 to move outward, changing the angle of TM5 relative to the membrane (right). Concomitantly, the loss of Mg4/5 would allow the N domain to swing out markedly, pulling the CBS domains apart, which in turn separates the connecting helices opening the pore for entry of Mg1 represented by the black square.
These observations suggest that the cytosolic domain of MgtE acts essentially as a Mg2+ sensor, allowing the cytosolic domain to regulate gating of the ion-conducting pore. Similarly in CorA, the Mg2+ ions bound between each monomer suggest that the cytosolic domain of CorA acts as a Mg2+ sensor. Thus, despite their markedly different structures, CorA and MgtE permeability likely is regulated identically in response to changes in cytosolic Mg2+ concentration. Unlike CorA, MgtE exhibits an additional level of regulation by Mg2+. Recently, Mg2+ controlled riboswitches have been demonstrated in the promoters of two different classes of Mg2+ transporter, the Bacillus sub-tilis mgtE and S. Typhimurium mgtA genes (7, 8).
The MgtE superfamily
Phylogenetic analysis of bacterial MgtE proteins appears to indicate a single family (77), unlike CorA, which has two and likely three subfamilies (26, 27). MgtE homologs in eukaryotes are represented by the SLC41 family of solute carriers with three members to date (SLC41A1−3). The SLC41 family is homologous to MgtE within the transmembrane domain; however, SLC41 transporters have only a short NH2-terminal domain that lacks similarity to either the N or CBS domains (77). Furthermore, although they contain 10 TM segments like MgtE, these are the product of a single gene, indicating a tandem fusion during evolution. Both SLC41A1 and SLC41A2 have been expressed in Xenopus laevis oocytes and have been shown to generate Mg2+ currents (13, 14). More detailed characterization has been performed by Kolisek et al. (28) after expression of SLC41A1 in both HEK293 cells and the Mg2+ transport-deficient strain of S. Typhimurium (22). Interestingly these studies revealed that SLC41A1 functions as a Mg2+ efflux system in contrast to the Mg2+ influx activity of MgtE. SLC41A2 has been subsequently expressed in TRPM7-deficient DT40 cells by Sahni et al. (54) where it appears to mediate Mg2+ influx. It remains to be determined whether SLC41A3 mediates influx or efflux.
SLC41A1 mRNA is highly expressed in heart and testis tissues and to a lesser degree in skeletal muscle, kidney, colon, pancreas, prostate, ovaries, adrenal gland, and thyroid gland (77). Expression is regulated by dietary Mg2+. Mice fed a low magnesium diet for 5 days showed a two- to fourfold increase in SLC41A1 mRNA in kidney, colon, and heart (13). In contrast, expression of SLC41A2 mRNA was not significantly changed in either mouse distal convoluted tubule cells maintained in low magnesium or in kidney tissue isolated from mice kept on low magnesium diets (14). The molecular mechanism and physiological role of SLC41A1 regulation are not known.
Remaining Questions and Future Directions
Although the determination of the crystal structures of CorA and MgtE have provided valuable insight into the function of Mg2+ channels, there are still numerous aspects of protein function and physiology that have not been resolved. Some important outstanding questions are explored below.
First, how do CorA and MgtE recognize and differentiate between cations? Each channel is highly selective for Mg2+ (66). Other than Co2+ and Ni2+, neither transports other common divalent cations such as Fe2+ and Ca2+. Moreover, for the most part, no other divalent cations even inhibit Mg2+ flux (23, 37, 46, 63, 65, 75). CorA does not differentiate between divalent and trivalent cations as evidenced by its inhibition by trivalent cation hexaammines (30). The mechanism by which this selectivity occurs is unknown.
The structures of CorA and MgtE present an interesting question in terms of symmetry. How does a pentameric protein bind a cation that is always hexacoordinate without greatly distorting the octahedral configuration of the cation? Which residues does Mg2+ coordinate with? With CorA, electrostatic interactions can be ruled out since no CorA homolog has a charged residue in TM1 or TM2. Although some serine and threonine residues appear in TM1 or TM2 of the CorA channel, hydroxyl groups appear to have no role, at least from mutational analysis of the S. Typhimurium CorA (71). Thus, in CorA, Mg2+ appears to interact primarily if not solely with backbone carbonyls within the pore. Given the pentameric nature of the channel, this raises issues of coordination. Mg2+ strongly prefers interaction with oxygens in an octahedral conformation with all bond angles close to 90°. Hexacoordinate binding of Mg2+ with backbone carbonyls while maintaining anything close to a 90° bond angle is possible only if Mg2+ interacts with a single carbonyl atom at a time, the other sites being filled with water. This would require a very large pore diameter. Since such a large pore diameter would not be particularly selective for any cation over another cation, this consideration in turn suggests either that Mg2+ coordination within the CorA pore is distorted markedly from the preferred octahedral configuration or that movement of TM1 somehow allows residues within TM2 to also interact with Mg2+. The former scenario would require a great deal of energy to form and maintain the distorted configuration. It seems more likely that the open form of the channel will involve interaction of Mg2+ with residues within TM2, even though the currently available structures of CorA suggest that the pore is formed entirely by TM1.
Like CorA, the outer half of the MgtE pore also appears to be highly hydrophobic in virtually all homologs, again suggesting Mg2+ interaction with backbone carbonyls. In contrast to CorA however, the inner half of the pore contains a single negatively charged residue, usually an aspartate. In the homodimer, this results in two carboxyl groups that interact with Mg2+, as shown in the crystal structure. Nevertheless, sequence alignments of the hundreds of MgtE homologs currently known indicate that this conserved aspartate is likely the only charged or polar residue within the pore. In both CorA and MgtE, therefore, the primary interaction of Mg2+ within the pore is with backbone carbonyls. In MgtE, with its homodimeric structure, this would provide two cognate carbonyl groups spaced 180° apart all through the pore thus readily satisfying Mg2+'s preference for rather rigid 90° bond angles. This preference for 90° bond angles is also satisfied in the binding of Mg1 within the MgtE pore to the carboxyl groups of the two aspartate residues and the carbonyls of the adjacent alanine residues of the dimer, leaving two positions to be filled by waters.
Gating of the CorA and MgtE Mg2+ is also unknown. Both structures contain Mg2+ ions bound within the cytosolic domain of the channel with a potential to regulate conformation and thus activity. The cation-free crystal structures of the soluble domains, compared with their structures in the presence of cation, clearly suggest that association and dissociation of these bound Mg2+ ions has marked effects on the structure and positioning of the cytosolic domain relative to the membrane domain. In the intact channels, these changes presumably are carried to the membrane domain, largely by the α7 helices of CorA or the connecting helices of MgtE. Although it seems very likely that such regulation via the Mg2+ bound to the cytosolic domain of each channel would be physiologically relevant, this remains to be demonstrated.
Another obvious question is to what extent these Mg2+ channel structures can provide insight into the structures of Ca2+ or other divalent cation channels. We would argue that this is unlikely due to the differences in the chemistry of Mg2+ compared with Ca2+ and other divalent cations. Independent of structure, however, regulation of divalent cation channels by divalent cation bound to the cytosolic domain could well be a common feature.
Finally, the apparent function of the CorA paralog ZntB is to mediate efflux of Zn2+ rather than the influx of Mg2+ (80). The recent deposition of coordinates for the structure of the ZntB soluble domain and our own unpublished structural data on ZntB (see above) clearly show that CorA and ZntB share a quite similar architecture. However, the cation binding sites of Mg2+ on CorA and Zn2+ on ZntB must be different despite similar protein structures. Zn2+ has to initially bind to one or more sites within the cytosolic domain before passage out through the pore. Given the current CorA structures, Mg2+ must bind either to the external loop between TM1 and TM2 or to residues at the periplasmic end of TM1. In contrast, Zn2+ would not need to interact with similar residues at the external end of the pore since this would only impede flux. This is evident in the sequence of the short loop between TM1 and TM2. In CorA, this loop is always composed of large bulky residues, several of which are charged. This loop in ZntB homologs contains little or no charge and is composed largely of smaller residues including three or even four glycines. Moreover, since energy would be required for Zn2+ efflux, a counterion would be required, most likely Na+ or H+, with unknown stoichiometry. The CorA Mg2+ channel requires only a single ion permeation pathway. With ZntB, although H+ or Na+ passage could and likely does share some part of the Zn2+ pathway, there must be some difference in the routes for each ion. This same issue arises, for example, with the various chloride transporters, some of which are channels and some of which are Cl−/H+ symporters (35, 41).
The physiological roles of the CorA and MgtE families of Mg2+ channels/carriers in both prokaryotes and eukaryotes remain largely unexplored. In bacteria, mutation of CorA attenuates virulence of S. Typhimurium (47, 48), and loss of MgtE reduces biofilm formation and motility in another pathogen Aeromonas hydrophila (40). Thus these Mg2+ channels appear important for virulence. The molecular basis for these effects is not yet known. In mammals, diseases of Mg2+ homeostasis linked to Mrs2 (CorA) or SLC41A1 (MgtE) have not been identified to date. Nonetheless, regulation of SLC41 by Mg2+ and conditional knockdown of Mrs2 suggest that these Mg2+ transporters have significant roles in the homeostasis of Mg2+ in eukaryotes. Their regulation suggests the likelihood that mutations causing human disease will be identified, similar to Mg2+ deficiency syndromes (1, 24) elicited by mutations in the claudin-16 (paracellin-1) and claudin-19 (62), NIPA1 (11), and TRPM6 (58) Mg2+ transport systems.
Regarding the MgtE family, we do not yet know what cellular membranes contain the various SLC41 homologs. With regard to the direction of Mg2+ flux, SLC41A1 apparently mediates only Mg2+ efflux as demonstrated by Kolisek et al. (28). Although SLC41A2 mediates Mg2+ influx as demonstrated by Sahni et al. (54), it is not known whether it can mediate efflux. The direction of flux for SLC41A3 is completely unexplored. The SLC41 family members also appear likely to have accessory proteins in the cytosol since they have very short NH2 termini (28). Although more data exists concerning the mitochondrial homolog Mrs2, many questions regarding its function in the cell also remain. Recent data from Mrs2 knockdown experiments indicates involvement in apoptosis (52). Is this involvement due simply to loss of Mg2+ when the channel is not present, or is Mrs2 an active participant in the mitochondrial apoptosis pathway? Likewise, there is also nothing known about the role or regulation of the numerous Mrs2 and ALR proteins that are expressed by plants.
Determination of the structures of the CorA and MgtE Mg2+ channels has provided valuable insight into the mechanism by which Mg2+ moves across membranes. Befitting the unique chemistry of Mg2+, neither channel has similarity to other known channels or transporters. Nonetheless, although important, solution of their structures provides little information about their functional role(s). Data about the physiology relevant to specific Mg2+ transport systems is sorely lacking and must be obtained for a complete picture of Mg2+ homeostasis to be synthesized.
References
- 1.Alexander RT, Hoenderop JG, Bindels RJ. Molecular determinants of magnesium homeostasis: insights from human disease. J Am Soc Nephrol. 2008;19:1451–1458. doi: 10.1681/ASN.2008010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 3.Bui DM, Gregan J, Jarosch E, Ragnini A, Schweyen RJ. The bacterial magnesium transporter CorA can functionally substitute for its putative homologue Mrs2p in the yeast inner mitochondrial membrane. J Biol Chem. 1999;274:20438–20443. doi: 10.1074/jbc.274.29.20438. [DOI] [PubMed] [Google Scholar]
- 4.Caldwell AM, Smith RL. Membrane topology of the ZntB efflux system of Salmonella enterica serovar Typhimurium. J Bacteriol. 2003;185:374–376. doi: 10.1128/JB.185.1.374-376.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chubanov V, Gudermann T, Schlingmann KP. Essential role for TRPM6 in epithelial magnesium transport and body magnesium homeostasis. Pflügers Arch. 2005;451:228–234. doi: 10.1007/s00424-005-1470-y. [DOI] [PubMed] [Google Scholar]
- 6.Cowan JA. Metallobiochemistry of RNA. Co(NH3)63+ as a probe for Mg2+(aq) binding sites. J Inorg Biochem. 1993;49:171–175. doi: 10.1016/0162-0134(93)80002-q. [DOI] [PubMed] [Google Scholar]
- 7.Cromie MJ, Shi Y, Latifi T, Groisman EA. An RNA sensor for intracellular Mg2+. Cell. 2006;125:71–84. doi: 10.1016/j.cell.2006.01.043. [DOI] [PubMed] [Google Scholar]
- 8.Dann CE, III, Wakeman CA, Sieling CL, Baker SC, Irnov I, Winkler WC. Structure and mechanism of a metal-sensing regulatory RNA. Cell. 2007;130:878–892. doi: 10.1016/j.cell.2007.06.051. [DOI] [PubMed] [Google Scholar]
- 9.Eshaghi S, Niegowski D, Kohl A, Martinez MD, Lesley SA, Nordlund P. Crystal structure of a divalent metal ion transporter CorA at 2.9 angstrom resolution. Science. 2006;313:354–357. doi: 10.1126/science.1127121. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Vescovi E, Soncini FC, Groisman EA. Mg2+ as an extracellular signal: environmental regulation of Salmonella virulence. Cell. 1996;84:165–174. doi: 10.1016/s0092-8674(00)81003-x. [DOI] [PubMed] [Google Scholar]
- 11.Goytain A, Hines RM, El-Husseini A, Quamme GA. NIPA1(SPG6), the basis for autosomal dominant form of hereditary spastic paraplegia, encodes a functional Mg2+ transporter. J Biol Chem. 2007;282:8060–8068. doi: 10.1074/jbc.M610314200. [DOI] [PubMed] [Google Scholar]
- 12.Goytain A, Quamme GA. Functional characterization of ACDP2 (ancient conserved domain protein), a divalent metal transporter. Physiol Genomics. 2005;22:382–389. doi: 10.1152/physiolgenomics.00058.2005. [DOI] [PubMed] [Google Scholar]
- 13.Goytain A, Quamme GA. Functional characterization of human SLC41A1, a Mg2+ transporter with similarity to prokaryotic MgtE Mg2+ transporters. Physiol Genomics. 2005;21:337–342. doi: 10.1152/physiolgenomics.00261.2004. [DOI] [PubMed] [Google Scholar]
- 14.Goytain A, Quamme GA. Functional characterization of the human solute carrier, SLC41A2. Biochem Biophys Res Commun. 2005;330:701–705. doi: 10.1016/j.bbrc.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 15.Goytain A, Quamme GA. Identification and characterization of a novel mammalian Mg2+ transporter with channel-like properties. BMC Genomics. 2005;6:48. doi: 10.1186/1471-2164-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goytain A, Quamme GA. Identification and characterization of a novel family of membrane magnesium transporters, MMgT1 and MMgT2. Am J Physiol Cell Physiol. 2008;294:C495–C502. doi: 10.1152/ajpcell.00238.2007. [DOI] [PubMed] [Google Scholar]
- 17.Graschopf A, Stadler JA, Hoellerer MK, Eder S, Sieghardt M, Kohlwein SD, Schweyen RJ. The yeast plasma membrane protein Alr1 controls Mg2+ homeostasis and is subject to Mg2+-dependent control of its synthesis and degradation. J Biol Chem. 2001;276:16216–16222. doi: 10.1074/jbc.M101504200. [DOI] [PubMed] [Google Scholar]
- 18.Gregan J, Bui DM, Pillich R, Fink M, Zsurka G, Schweyen RJ. The mitochondrial inner membrane protein Lpe10p, a homologue of Mrs2p, is essential for magnesium homeostasis and group II intron splicing in yeast. Mol Gen Genet. 2001;264:773–781. doi: 10.1007/s004380000366. [DOI] [PubMed] [Google Scholar]
- 19.Grubbs RD, Maguire ME. Magnesium as a regulatory cation: criteria and evaluation. Magnesium. 1987;6:113–127. [PubMed] [Google Scholar]
- 20.Hattori M, Tanaka Y, Fukai S, Ishitani R, Nureki O. Crystal structure of the MgtE Mg2+ transporter. Nature. 2007;448:1072–1075. doi: 10.1038/nature06093. [DOI] [PubMed] [Google Scholar]
- 21.Hattori M, Tanaka Y, Fukai S, Ishitani R, Nureki O. Crystallization and preliminary X-ray diffraction analysis of the full-length Mg2+ transporter MgtE. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2007;63:682–684. doi: 10.1107/S1744309107032332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hmiel SP, Snavely MD, Florer JB, Maguire ME, Miller CG. Magnesium transport in Salmonella typhimurium: genetic characterization and cloning of three magnesium transport loci. J Bacteriol. 1989;171:4742–4751. doi: 10.1128/jb.171.9.4742-4751.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hmiel SP, Snavely MD, Miller CG, Maguire ME. Magnesium transport in Salmonella typhimurium: characterization of magnesium influx and cloning of a transport gene. J Bacteriol. 1986;168:1444–1450. doi: 10.1128/jb.168.3.1444-1450.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoenderop JG, Bindels RJ. Calciotropic and magnesiotropic TRP channels. Physiology. 2008;23:32–40. doi: 10.1152/physiol.00039.2007. [DOI] [PubMed] [Google Scholar]
- 25.Ignoul S, Eggermont J. CBS domains: structure, function, and pathology in human proteins. Am J Physiol Cell Physiol. 2005;289:C1369–C1378. doi: 10.1152/ajpcell.00282.2005. [DOI] [PubMed] [Google Scholar]
- 26.Kehres DG, Lawyer CH, Maguire ME. The CorA magnesium transporter gene family. Microbial Comp Genomics. 1998;43:151–169. doi: 10.1089/omi.1.1998.3.151. [DOI] [PubMed] [Google Scholar]
- 27.Knoop V, Groth-Malonek M, Gebert M, Eifler K, Weyand K. Transport of magnesium and other divalent cations: evolution of the 2-TM-GxN proteins in the MIT superfamily. Mol Genet Genomics. 2005;274:205–216. doi: 10.1007/s00438-005-0011-x. [DOI] [PubMed] [Google Scholar]
- 28.Kolisek M, Launay P, Beck A, Sponder G, Serafini N, Brenkus M, Froschauer EM, Martens H, Fleig A, Schweigel M. SLC41A1 is a novel mammalian Mg2+ carrier. J Biol Chem. 2008;283:16235–16247. doi: 10.1074/jbc.M707276200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kolisek M, Zsurka G, Samaj J, Weghuber J, Schweyen RJ, Schweigel M. Mrs2p is an essential component of the major electrophoretic Mg2+ influx system in mitochondria. EMBO J. 2003;22:1235–1244. doi: 10.1093/emboj/cdg122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kucharski LM, Lubbe WJ, Maguire ME. Cation hexaammines are selective and potent inhibitors of the CorA magnesium transport system. J Biol Chem. 2000;275:16767–16773. doi: 10.1074/jbc.M001507200. [DOI] [PubMed] [Google Scholar]
- 31.Li L, Tutone AF, Drummond RS, Gardner RC, Luan S. A novel family of magnesium transport genes in Arabidopsis. Plant Cell. 2001;13:2761–2775. doi: 10.1105/tpc.010352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu GJ, Martin DK, Gardner RC, Ryan PR. Large Mg2+-dependent currents are associated with the increased expression of ALR1 in Saccharomyces cerevisiae. FEMS Microbiol Lett. 2002;213:231–237. doi: 10.1111/j.1574-6968.2002.tb11311.x. [DOI] [PubMed] [Google Scholar]
- 33.Lunin VV, Dobrovetsky E, Khutoreskaya G, Zhang R, Joachimiak A, Doyle DA, Bochkarev A, Maguire ME, Edwards AM, Koth CM. Crystal structure of the CorA Mg2+ transporter. Nature. 2006;440:833–837. doi: 10.1038/nature04642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.MacDiarmid CW, Gardner RC. Overexpression of the Saccharomyces cerevisiae magnesium transport system confers resistance to aluminum ion. J Biol Chem. 1998;273:1727–1732. doi: 10.1074/jbc.273.3.1727. [DOI] [PubMed] [Google Scholar]
- 35.Maduke M, Miller C, Mindell JA. A decade of CLC chloride channels: structure, mechanism, and many unsettled questions. Annu Rev Biophys Biomol Struct. 2000;29:411–438. doi: 10.1146/annurev.biophys.29.1.411. [DOI] [PubMed] [Google Scholar]
- 36.Maguire ME. Magnesium: a regulated and regulatory cation. Met Ions Biol Syst. 1990;26:135–153. [Google Scholar]
- 37.Maguire ME. Magnesium transporters: properties, regulation and structure. Front Biosci. 2006;11:3149–3163. doi: 10.2741/2039. [DOI] [PubMed] [Google Scholar]
- 38.Maguire ME. The structure of the CorA magnesium transporter, a divalent cation channel. Curr Opin Struct Biol. 2006;4:432–438. doi: 10.1016/j.sbi.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 39.Maguire ME, Cowan JA. Mg2+ chemistry and biochemistry. Biometals. 2002;15:203–210. doi: 10.1023/a:1016058229972. [DOI] [PubMed] [Google Scholar]
- 40.Merino S, Gavin R, Altarriba M, Izquierdo L, Maguire ME, Tomas JM. The MgtE Mg2+ transport protein is involved in Aeromonas hydrophila adherence. FEMS Microbiol Lett. 2001;198:189–195. doi: 10.1111/j.1574-6968.2001.tb10641.x. [DOI] [PubMed] [Google Scholar]
- 41.Mindell JA, Maduke M. ClC chloride channels. Genome Biol. 2001;2:3003. doi: 10.1186/gb-2001-2-2-reviews3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Monteilh-Zoller MK, Hermosura MC, Nadler MJ, Scharenberg AM, Penner R, Fleig A. TRPM7 provides an ion channel mechanism for cellular entry of trace metal ions. J Gen Physiol. 2003;121:49–60. doi: 10.1085/jgp.20028740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nadler MJ, Hermosura MC, Inabe K, Perraud AL, Zhu Q, Stokes AJ, Kurosaki T, Kinet JP, Penner R, Scharenberg AM, Fleig A. LTRPC7 is a Mg ATP-regulated divalent cation channel required for cell viability. Nature. 2001;411:590–595. doi: 10.1038/35079092. [DOI] [PubMed] [Google Scholar]
- 44.Nelson DL, Kennedy EP. Magnesium transport in Escherichia coli. Inhibition by cobaltous ion. J Biol Chem. 1971;246:3042–3049. [PubMed] [Google Scholar]
- 44a.Pallen MF, Gophna U. Bacterial flagella and type III secretion: case studies in the evolution of complexity. In: Volff JN, editor. Gene and Protein Evolution. Genome Dyn. Vol. 3. Karger; Basel: 2007. pp. 30–47. [DOI] [PubMed] [Google Scholar]
- 45.Papp KM, Maguire ME. The CorA Mg2+ transporter does not transport Fe2+. J Bacteriol. 2004;186:7653–7658. doi: 10.1128/JB.186.22.7653-7658.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Papp KM, Maguire ME. The CorA Mg2+ transporter does not transport Fe2+. J Bacteriol. 2004;186:7653–7658. doi: 10.1128/JB.186.22.7653-7658.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Papp-Wallace KM, Maguire ME. Regulation of CorA Mg2+ channel function affects the virulence of Salmonella enterica serovar Typhimurium. J Bacteriology. 2008;190:6509–6516. doi: 10.1128/JB.00144-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Papp-Wallace KM, Nartea M, Kehres DG, Porwollik S, McClelland M, Libby SJ, Fang FC, Maguire ME. The CorA Mg2+ channel is required for the virulence of Salmonella enterica serovar Typhimurium. J Bacteriology. 2008;190:6517–6523. doi: 10.1128/JB.00772-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park MH, Wong BB, Lusk JE. Mutants in three genes affecting transport of magnesium in Escherichia coli: physiology and genetics. J Bacteriol. 1976;126:1096–1103. doi: 10.1128/jb.126.3.1096-1103.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Payandeh J, Li C, Ramjeesingh M, Poduch E, Bear CE, Pai EF. Probing structure-function relationships and gating mechanisms in the CorA Mg2+ transport system. J Biol Chem. 2008;283:11721–11733. doi: 10.1074/jbc.M707889200. [DOI] [PubMed] [Google Scholar]
- 51.Payandeh J, Pai EF. A structural basis for Mg2+ homeostasis and the CorA translocation cycle. EMBO J. 2006;25:3762–3773. doi: 10.1038/sj.emboj.7601269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Piskacek M, Zotova L, Zsurka G, Schweyen RJ. Conditional knock-down of hMRS2 results in loss of mitochondrial Mg2+ uptake and cell death. J Cell Mol Med. doi: 10.1111/j.1582-4934.2008.00328.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Romani A, Scarpa A. Regulation of cell magnesium. Arch Biochem Biophys. 1992;298:1–12. doi: 10.1016/0003-9861(92)90086-c. [DOI] [PubMed] [Google Scholar]
- 54.Sahni J, Nelson B, Scharenberg AM. SLC41A2 encodes a plasma-membrane Mg2+ transporter. Biochem J. 2007;401:505–513. doi: 10.1042/BJ20060673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scarpa A, Brinley FJ. In situ measurements of free cytosolic magnesium ions. Fed Proc. 1981;40:2646–2652. [PubMed] [Google Scholar]
- 56.Schindl R, Weghuber J, Romanin C, Schweyen RJ. Mrs2p forms a high conductance Mg2+ selective channel in mitochondria. Biophys J. 2007;93:3872–3883. doi: 10.1529/biophysj.107.112318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schlingmann KP, Gudermann T. A critical role of TRPM channel-kinase for human magnesium transport. J Physiol. 2005;566:301–308. doi: 10.1113/jphysiol.2004.080200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schlingmann KP, Weber S, Peters M, Niemann NL, Vitzthum H, Klingel K, Kratz M, Haddad E, Ristoff E, Dinour D, Syrrou M, Nielsen S, Sassen M, Waldegger S, Seyberth HW, Konrad M. Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat Genet. 2002;31:166–170. doi: 10.1038/ng889. [DOI] [PubMed] [Google Scholar]
- 59.Schmitz C, Perraud AL, Johnson CO, Inabe K, Smith MK, Penner R, Kurosaki T, Fleig A, Scharenberg AM. Regulation of vertebrate cellular Mg2+ homeostasis by TRPM7. Cell. 2003;114:191–200. doi: 10.1016/s0092-8674(03)00556-7. [DOI] [PubMed] [Google Scholar]
- 60.Schock I, Gregan J, Steinhauser S, Schweyen R, Brennicke A, Knoop V. A member of a novel Arabidopsis thaliana gene family of candidate Mg2+ ion transporters complements a yeast mitochondrial group II intron-splicing mutant. Plant J. 2000;24:489–501. doi: 10.1046/j.1365-313x.2000.00895.x. [DOI] [PubMed] [Google Scholar]
- 61.Silver S. Active transport of magnesium in Escherichia coli. Proc Natl Acad Sci USA. 1969;62:764–771. doi: 10.1073/pnas.62.3.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Simon DB, Lu Y, Choate KA, Velazquez H, Al-Sabban E, Praga M, Casari G, Bettinelli A, Colussi G, Rodriguez-Soriano J, McCredie D, Milford D, Sanjad S, Lifton RP. Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science. 1999;285:103–106. doi: 10.1126/science.285.5424.103. [DOI] [PubMed] [Google Scholar]
- 63.Smith RL, Banks JL, Snavely MD, Maguire ME. Sequence and topology of the CorA magnesium transport systems of Salmonella typhimurium and Escherichia coli. Identification of a new class of transport protein. J Biol Chem. 1993;268:14071–14080. [PubMed] [Google Scholar]
- 64.Smith RL, Kaczmarek ML, Kucharski LM, Maguire ME. Magnesium transport in Salmonella typhimurium: induction of mgtA and mgtCB expression during invasion of epithelial and macrophage cells. Microbiology. 1998;144:1835–1843. doi: 10.1099/00221287-144-7-1835. [DOI] [PubMed] [Google Scholar]
- 65.Smith RL, Thompson LJ, Maguire ME. Cloning and characterization of mgtE, a putative new class of Mg2+ transporter from Bacillus firmus OF4. J Bacteriol. 1995;177:1233–1238. doi: 10.1128/jb.177.5.1233-1238.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Snavely MD, Florer JB, Miller CG, Maguire ME. Magnesium transport in Salmonella typhimurium: 28Mg2+ transport by the CorA, MgtA, and MgtB systems. J Bacteriol. 1989;171:4761–4766. doi: 10.1128/jb.171.9.4761-4766.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Snavely MD, Florer JB, Miller CG, Maguire ME. Magnesium transport in Salmonella typhimurium: expression of cloned genes for three distinct Mg2+ transport systems. J Bacteriol. 1989;171:4752–4760. doi: 10.1128/jb.171.9.4752-4760.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Snavely MD, Gravina SA, Cheung TT, Miller CG, Maguire ME. Magnesium transport in Salmonella typhimurium: regulation of mgtA and mgtB expression. J Biol Chem. 1991;266:824–829. [PubMed] [Google Scholar]
- 69.Snavely MD, Miller CG, Maguire ME. The mgtB Mg2+ transport locus of Salmonella typhimurium encodes a P-type ATPase. J Biol Chem. 1991;266:815–823. [PubMed] [Google Scholar]
- 70.Soncini FC, Garcia VE, Solomon F, Groisman EA. Molecular basis of the magnesium deprivation response in Salmonella typhimurium: identification of PhoP-regulated genes. J Bacteriol. 1996;178:5092–5099. doi: 10.1128/jb.178.17.5092-5099.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Szegedy MA, Maguire ME. The CorA Mg2+ transport protein of Salmonella typhimurium. Mutagenesis of conserved residues in the second membrane domain. J Biol Chem. 1999;274:36973–36979. doi: 10.1074/jbc.274.52.36973. [DOI] [PubMed] [Google Scholar]
- 72.Tao T, Grulich PF, Kucharski LM, Smith RL, Maguire ME. Magnesium transport in Salmonella typhimurium: biphasic time and magnesium dependence of the transcription of the mgtA and mgtCB loci. Microbiology. 1998;144:655–664. doi: 10.1099/00221287-144-3-655. [DOI] [PubMed] [Google Scholar]
- 73.Tao T, Snavely MD, Farr SG, Maguire ME. Magnesium transport in Salmonella typhimurium: mgtA encodes a P-type ATPase and is regulated by Mg2+ in a manner similar to that of the mgtB P-type ATPase. J Bacteriol. 1995;177:2654–2662. doi: 10.1128/jb.177.10.2654-2662.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tieleman DP, Shrivastava IH, Ulmschneider MR, Sansom MS. Proline-induced hinges in transmembrane helices: possible roles in ion channel gating. Proteins. 2001;44:63–72. doi: 10.1002/prot.1073. [DOI] [PubMed] [Google Scholar]
- 75.Townsend DE, Esenwine AJ, George J, III, Bross D, Maguire ME, Smith RL. Cloning of the mgtE Mg2+ transporter from Providencia stuartii and the distribution of mgtE in the eubacteria. J Bacteriol. 1995;177:5350–5354. doi: 10.1128/jb.177.18.5350-5354.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Voets T, Nilius B, Hoefs S, van der Kemp AW, Droogmans G, Bindels RJ, Hoenderop JG. TRPM6 forms the Mg2+ influx channel involved in intestinal and renal Mg2+ absorption. J Biol Chem. 2004;279:19–25. doi: 10.1074/jbc.M311201200. [DOI] [PubMed] [Google Scholar]
- 77.Wabakken T, Rian E, Kveine M, Aasheim HC. The human solute carrier SLC41A1 belongs to a novel eukaryotic subfamily with homology to prokaryotic MgtE Mg2+ transporters. Biochem Biophys Res Commun. 2003;306:718–724. doi: 10.1016/s0006-291x(03)01030-1. [DOI] [PubMed] [Google Scholar]
- 78.Wachek M, Aichinger MC, Stadler JA, Schweyen RJ, Graschopf A. Oligomerization of the Mg2+-transport proteins Alr1p and Alr2p in yeast plasma membrane. FEBS J. 2006;273:4236–4249. doi: 10.1111/j.1742-4658.2006.05424.x. [DOI] [PubMed] [Google Scholar]
- 79.Wiesenberger G, Waldherr M, Schweyen RJ. The nuclear gene MRS2 is essential for the excision of group II introns from yeast mitochondrial transcripts in vivo. J Biol Chem. 1992;267:6963–6969. [PubMed] [Google Scholar]
- 80.Worlock AJ, Smith RL. ZntB Is a Novel Zn2+ Transporter in Salmonella enterica Serovar Typhimurium. J Bacteriol. 2002;184:4369–4373. doi: 10.1128/JB.184.16.4369-4373.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zsurka G, Gregan J, Schweyen RJ. The human mitochondrial Mrs2 protein functionally substitutes for its yeast homologue, a candidate magnesium transporter. Genomics. 2001;72:158–168. doi: 10.1006/geno.2000.6407. [DOI] [PubMed] [Google Scholar]