Abstract
Objective
The hypothalamic control of energy balance is regulated by a complex network of neuropeptide-releasing neurons. Whilst the effect of these neuropeptides on individual aspects of energy homeostasis has been studied, the coordinated response of these effects has not been comprehensively investigated. We have simultaneously monitored a number of metabolic parameters following ICV administration of 1nmol and 3nmol of neuropeptides with established roles in the regulation of feeding, activity and metabolism. Ad libitum fed rats received the orexigenic neuropeptides neuropeptide Y (NPY), agouti-related protein (AgRP), melanin-concentrating hormone (MCH) or orexin-A. Overnight food deprived rats received an ICV injection of the anorectic peptides α-MSH, corticotrophin releasing factor (CRF) or neuromedin U (NMU).
Results
Our results reveal the temporal sequence of the effects of these neuropeptides on both energy intake and expenditure, highlighting key differences in their function as mediators of energy balance. NPY and AgRP increased feeding and decreased oxygen consumption, with the effects of AgRP being more prolonged. In contrast, orexin-A increased both feeding and oxygen consumption, consistent with an observed increase in activity. The potent anorexigenic effects of CRF were accompanied by a prolonged increase in activity whilst NMU injection resulted in significant but short-lasting inhibition of food intake, ambulatory activity and oxygen consumption. Alpha-MSH injection resulted in significant increases in both ambulatory activity and oxygen consumption, and reduced food intake following administration of 3nmol of the peptide.
Conclusion
We have for the first time, simultaneously measured several metabolic parameters following hypothalamic administration of a number of neuropeptides within the same experimental system. This work has demonstrated the interrelated effects of these neuropeotides on activity, energy expenditure and food intake thus facilitating comparison between the different hypothalamic systems.
Keywords: Hypothalamus, neuropeptide, food intake, energy expenditure
Introduction
Energy balance in mammals is a highly regulated physiological process and is maintained by a homeostatic system involving both the central nervous system (CNS) and the periphery. The hypothalamus and the brainstem are CNS sites critical in the regulation of energy homeostasis. Reciprocal neuronal connections exist between these two regions which, in response to peripheral signals of nutritional status, regulate both short and long-term energy homeostasis.
The hypothalamus contains an extensive neuronal network which releases neuropeptides in a coordinated manner and contributes to the physiological responses to changes in nutritional status. The regulation of energy homeostasis integrates energy intake - i.e. food intake - with energy outputs such as resting metabolic rate, activity and thermogenesis. The neuropeptides within the hypothalamus that regulate energy homeostasis specifically influence energy intake and energy output to exert their overall effect on energy balance. Thus, determining the coordinated effects of specific neuropeptides on energy intake, behavior and energy expenditure is essential to understanding their physiological role.
The hypothalamic circuits controlling energy homeostasis are still to be precisely determined. The best characterized neuronal populations involved in the regulation of appetite and energy expenditure are the orexigenic neuropeptide Y (NPY)/ agouti-related protein (AgRP) neuron and the anorexigenic pro-opiomelanocortin (POMC) neuron located in the arcuate nucleus (Arc). The Arc is located at the base of the hypothalamus and is incompletely isolated by the blood brain barrier. It is thus able to be directly influenced by peripherally circulating signals of acute food intake and nutritional status. Neuronal populations in the Arc respond to these circulating factors and signal to extra-hypothalamic regions such as the brainstem, as well as other hypothalamic nuclei, including the paraventricular nucleus (PVH) and lateral hypothalamic area (LHA), where they influence second order neurons (1). These include melanin-concentrating hormone (MCH) and orexin-A neurons, located primarily in the LHA (2;3), corticotrophin releasing factor (CRF) neurons, found predominantly in the PVH (4) and neurons releasing neuromedin U (NMU), which in turn interact with the NMU2R located in the PVH (5).
Whilst the effects of these neuropeptides on food intake and energy expenditure have been studied, they have not been comprehensively and simultaneously measured in the same experimental system. The Comprehensive Laboratory Animal Monitoring System (CLAMS) (Columbus Instruments) enables 24 hour profiles of food intake, activity and energy expenditure to be determined. Simultaneous measurements of food intake, activity and energy expenditure allow the comprehensive metabolic effects of specific neuropeptides to be determined.
To further understand the complex responses involved in appetite regulation, we studied the role of the Arc neuropeptides NPY, AgRP, and α-MSH (a product of the POMC gene), the predominantly PVH neuropeptides CRF and NMU, and the LHA neuropeptides MCH and orexin-A in the regulation of energy balance. The coordinated effects of ICV administration of these neuropeptides on food intake, oxygen consumption, respiratory exchange ratio (RER) and activity were determined using the CLAMS. These comprehensive measurements provide further insight into the roles of these peptides in the regulation of the specific components of energy balance.
Materials and Methods
Animals
Male Wistar rats (specific pathogen free; Charles River, Margate, UK), weighing 300-350 g, were maintained in individual cages under controlled temperature (21 - 23 °C) and light (12:12 light-dark cycle; lights on at 0700 h) with ad libitum access to food (RM1 diet, SDS Ltd., Witham, UK) and water. Animal procedures were approved under the British Home Office Animals (Scientific Procedures) Act 1986 (Project Licence 70/6402).
Materials
Cannulation materials were purchased from Plastics One, Inc. (Roanoke, VA, USA). Rat CRF, orexin-A and α-MSH were synthesized by Bachem (St Helen’s, UK). NMU-23 was synthesized by IAF Biochem International (Quebec, ON, Canada). MCH and AgRP (83-132) were synthesized by the Peptide Institute (Osaka, Japan). NPY was synthesized as previously described (6).
ICV cannulation and injection
Animals were implanted with a permanent 22 gauge stainless steel cannula projecting to the third ventricle, as previously described (7). Animals were allowed seven days recovery after surgery and were then accustomed to handling and being weighed on a daily basis. All compounds were injected using a 28-gauge stainless steel injector placed in and projecting 1mm below the tip of the cannula. Cannula placement was confirmed by a positive dipsogenic response to angiotensin II (50ng/rat). Only those animals with a positive dipsogenic response were included in the data analysis. Three days after the angiotensin II injection, all animals were habituated to the injection process by receiving a single injection of 5μl saline injected over 1 minute as previously described (7).
Study design
ICV cannulated animals were monitored using a 24 chamber open-circuit Oxymax Comprehensive Laboratory Animal Monitoring System (CLAMS; Columbus instruments, Columbus, OH, USA). Individually housed rats were maintained at 21-23°C under a 12:12h light-dark cycle (light period 0700-1900h). Powdered RM1 diet (SDS Ltd.) and water were available ad libitum unless otherwise stated. Animals were individually housed in plexiglass cages, through which air was passed at a flow rate of 2.5L/min. Prior to all metabolic cage studies, rats were acclimatized to their cages for 48h in order to generate a stable metabolic background against which to test the effects of the neuropeptides.
Baseline studies
In order to generate reference data for subsequent neuropeptide studies, baseline data was recorded in ad libitum fed and food deprived animals. Ad libitum fed rats (n = 24) were placed in the CLAMS metabolic cages for 48h and then food deprived for an additional 24h. Metabolic parameters (VO2 and VCO2) were measured by indirect calorimetry. Exhaust air from each chamber was sampled at 30min intervals for a period of 1min. Sample air was sequentially passed through O2 and CO2 sensors (Columbus Instruments) for determination of O2 and CO2 content. Oxygen consumption and CO2 production values were normalised with respect to body weight and O2 consumption was corrected to metabolic body size (W0.75) (8). RER was calculated by dividing VCO2 by VO2. Ambulatory activity of each animal was measured simultaneously using the optical beam technique (Opto M3, Columbus Instruments). Consecutive photo-beam breaks were scored as an ambulatory movement. Activity counts in x and z axes were recorded every minute for 24h and were used to determine horizontal (XAMB) and vertical (ZTOT) movement respectively. Food intake was measured every minute.
ICV injection studies
A dose of 3nmol has been previously demonstrated to cause a robust effect on food intake for all neuropeptides examined in this study (5;6;9-12). Based on this data, we have administered 1nmol and 3nmol of each peptide studied in order to facilitate direct comparison of their effects. VCO2, VO2, activity (horizontal and vertical) and cumulative food intake were monitored as described above.
Satiated studies
Ad libitum fed rats received a single ICV injection of either saline or peptide (NPY, AgRP, MCH or orexin-A) (n = 8 per group) at doses of either 1nmol or 3nmol in the early light phase (0900-1000h).
Food deprived studies
Rats were food deprived for 20h prior to injection. All rats received a single injection of either saline or peptide (α-MSH, CRF or NMU) (n = 8 per group) at doses of 1nmol or 3nmol in the early light phase (0900-1000h). Animals were returned to their home cage with ad libitum access to food.
Statistics
Statistical advice was provided by J. Eliahoo at the Statistical Advisory Service, Imperial College London. For all parameters, differences between the treatment groups were determined using the Mann-Whitney U test (Stata 9, Statacorp, College Station, TX, USA). All significant effects of neuropeptides were recorded in the initial 12h post injection and are presented as such, with the exception of AgRP which is shown as a 24h profile. In all cases P < 0.05 was considered to be statistically significant. All feeding data are presented as percentage of saline to facilitate comparison between different neuropeptide treatments.
Results
Twenty four hour baseline readings of food intake, activity and energy expenditure in ad libitum fed and food deprived rats
Increased levels of feeding and locomotor activity occurred during the dark phase compared to the light phase. These activities exhibited a bimodal pattern, with levels peaking at the beginning and end of the dark phase (Figure 1). Corresponding with these diurnal variations, VO2 and RER were also elevated during the dark phase whilst fasting resulted in a reduction in VO2 and VCO2 during the dark phase (Figure 1).
Figure 1.
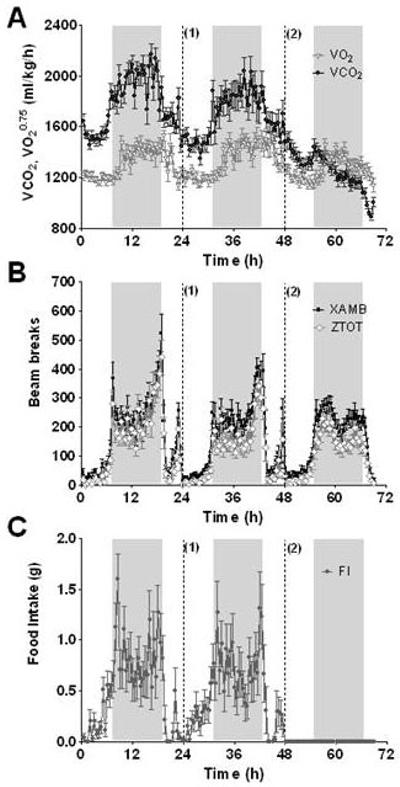
3 day baseline profiles of A) VO2 and VCO2, B) activity (XAMB and ZTOT) and C) food intake. Food dispensers were refilled at 24h (1) and animals were food deprived at 48h (2). Food intake was measured every minute and is presented as cumulative food intake at 30min intervals. Horizontal activity was measured every 30min for 24h and is presented as total number of beams broken during a 30min period. Metabolic parameters for each individually housed animal were measured every 30min for 24h. Shaded bars represent the dark phase. n = 21-24 per group, results are mean ± SEM.
NPY
ICV injection of either 1nmol or 3nmol NPY resulted in an immediate increase in food intake, 5min (3nmol) and 10min (1nmol) post injection (Table 1, Figure 2). A significant increase in XAMB was also observed by 1h post injection, with the 3nmol dose of NPY causing a more sustained effect (Table 1, Figure 3). A similar pattern was also seen for ZTOT (data not shown). Despite the increase in activity, ICV administration of either dose of NPY caused a significant decrease in VO2 (Table 1, Figure 4), and a significant increase in RER (Figure 5).
Table 1.
Effect of ICV hypothalamic neuropeptide administration (1 and 3nmol dose) on food intake, activity (XAMB) and VO2. Data are presented as mean onset and duration of significant effect vs. saline controls P<0.05, N.S. - not significant, n = 6-8 per group
| Food intake | XAMB | O2 consumption | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Peptide | Dose (nmol) | Onset | Duration | ↑/↓ | Onset | Duration | ↑/↓ | Onset | Duration | ↑/↓ |
| NPY | 1 | 10min | 17h | ↑ | 1h | 30min | ↑ | 30min | 1h 30min | ↓ |
| 3 | 5min | 14h 55min. | ↑ | 1h | 1h | ↑ | 30min | 2h | ↓ | |
| AgRP | 1 | 3h 10min | >24h | ↑ | 12h 17h | 30min 2h | ↓ | N.S. | N.S. | ↓ |
| 3 | 3h 50min | >24h | ↑ | 12h 16h 30min | 30min 3h | ↓ | N.S. | N.S. | ↓ | |
| Orexin-A | 1 | N.S. | N.S. | ↑ | 1h | N.S. | ↑ | 1h | - | ↑ |
| 3 | N.S. | N.S. | ↑ | 30min | 1h | ↑ | 30min | 1h | ↑ | |
| MCH | 1 | N.S. | N.S. | ↑ | N.S. | N.S. | - | N.S. | N.S. | - |
| 3 | N.S. | N.S. | ↑ | N.S. | N.S. | - | N.S. | N.S. | - | |
| α-MSH | 1 | N.S. | N.S. | ↓ | 30min | - | ↑ | 30min | - | ↑ |
| 3 | 30min | 6h 50min | ↓ | 30min | 30min | ↑ | 30min | 1h | ↑ | |
| CRF | 1 | 10mins | >24h | ↓ | 10min | 4h 30min | ↑ | 30min | 30min | ↑ |
| 3 | 15min | >24h | ↓ | 15min | 8h 30min | ↑ | 30min | 30min | ↑ | |
| NMU | 1 | 10min | 45min | ↓ | 30min | 1h 30min | ↑ | 30min | - | ↑ |
| 3 | 15min | 1h 10min | ↓ | 30min | 1h 30min | ↑ | 30min | 30min | ↑ | |
Figure 2.
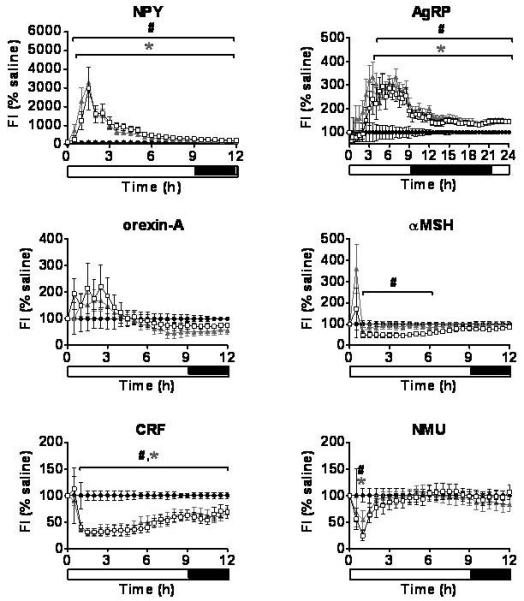
Food Intake. The effect of a single ICV injection of hypothalamic neuropeptides at doses of 1nmol and 3nmol on food intake. Food intake was measured every minute for 24h and is presented as a percentage of saline at 30 minute intervals. *P < 0.05 1nmol treatment group vs. saline, #P < 0.05 3nmol treatment group vs. saline (n = 6-8/group). Saline - ●, 1nmol dose - ▲, 3nmol dose - □. Results are mean ± SEM.
Figure 3.
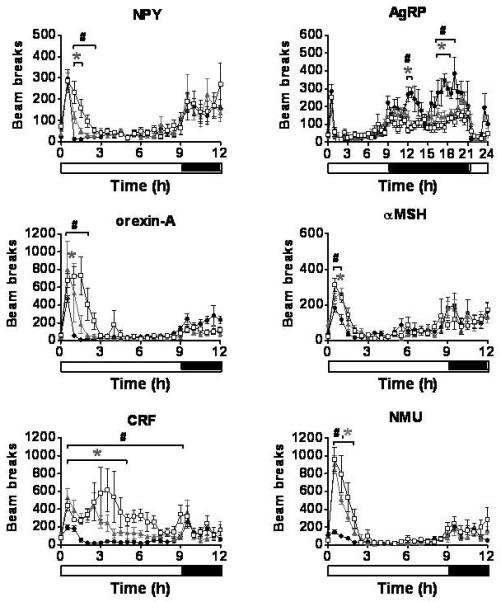
Ambulatory activity (XAMB). The effect of a single ICV injection of hypothalamic neuropeptides at doses of 1nmol and 3nmol on XAMB. Activity was measured every 30min for 24h and is presented as total number of horizontal beams broken during a 30min period. *P < 0.05 1nmol treatment group vs. saline, #P < 0.05 3nmol treatment group vs. saline (n = 6-8/group). Saline - ●, 1nmol - ▲, 3nmol - □. Results are mean ± SEM.
Figure 4.
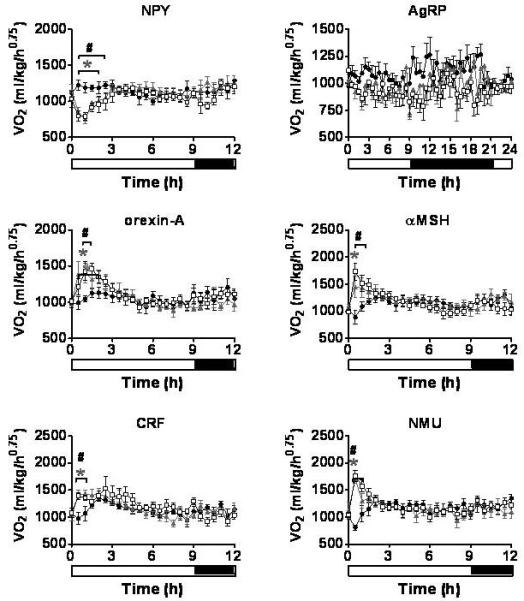
Oxygen Consumption. The effect of a single ICV injection of hypothalamic neuropeptides at doses of 1nmol and 3nmol on VO2. VO2 was measured every 30min for 24h. *P < 0.05 1nmol treatment group vs. saline, #P < 0.05 3nmol treatment group vs. saline (n = 6-8/group). Saline - ●, 1nmol - ▲, 3nmol - □. Results are mean ± SEM.
Figure 5.
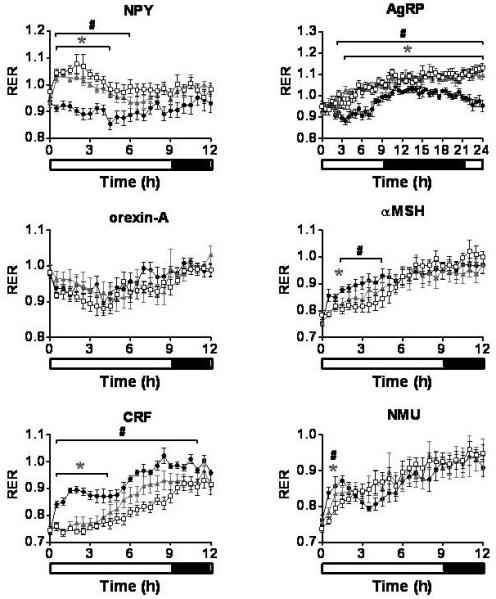
Respiratory exchange ratio (RER). The effect of a single ICV injection of hypothalamic neuropeptides at doses of 1nmol and 3nmol on RER. RER was measured every 30min for 24h. *P < 0.05 1nmol treatment group vs. saline, #P < 0.05 3nmol treatment group vs. saline (n = 6-8/group). Saline - ●, 1nmol - ▲, 3nmol - □. Results are mean ± SEM.
AgRP
A single ICV injection of either 1nmol or 3nmol AgRP produced a significant increase in food intake with the effect lasting throughout the remaining 24h measurement period (Table 1, Figure 2). During the dark phase, significant reductions in XAMB were observed in AgRP injected animals (Table 1, Figure 3). These reductions in XAMB coincided with the peaks in activity at the beginning and the end of the dark phase observed in saline injected controls (Figure 3). A similar pattern was also seen in ZTOT (data not shown). There was a trend towards a decrease in VO2 in AgRP treated animals compared to saline controls; however, this did not achieve statistical significance (Figure 4). A significant increase in RER was observed following administration of 1 or 3nmol AgRP (Figure 5).
Orexin-A
A trend towards an increase in food intake was observed for 3h following a single injection of either 1nmol or 3nmol orexin-A. This effect, however, did not reach statistical significance (Figure 2). In contrast to the non-significant effects on feeding, a rapid and potent increase in locomotion was observed at both doses (Table 1, Figure 3). A similar pattern was observed with ZTOT (data not shown). Consistent with the effects on activity, an increase in VO2 was also observed at 1nmol and 3nmol doses. (Table 1, Figure 4). No effect was seen on RER (Figure 5).
MCH
A trend towards a stimulation in feeding was observed at 30 minutes following a single ICV injection of either dose of MCH; however, this effect did not reach statistical significance (Supplementary Figure 1). A small decrease in VO2 at 30min post injection and a small rise in RER lasting up to 4h post injection, neither of which reached statistical significance, were also recorded (Supplementary Figure 1).
α-MSH
A single ICV injection of 3nmol α-MSH rapidly decreased food intake in food deprived rats (Table 1, Figure 2). No statistically significant effects on food intake were observed with 1nmol α-MSH. There was a small but significant increase in XAMB at both 1nmol and 3nmol doses (Table 1, Figure 3). A similar pattern was observed for ZTOT (data not shown). A rapid and dramatic increase in VO2 was observed by 30min post injection in animals receiving either 1nmol or 3nmol α-MSH (Table 1, Figure 4). Injection of both 1nmol and 3nmol of α-MSH also resulted in a significantly reduced RER (Figure 5).
CRF
A rapid and potent reduction in food intake was seen following a single ICV injection of either 1nmol or 3nmol CRF. Food intake was significantly lower than saline controls by 10min and 15min post injection for 1nmol and 3nmol respectively, and remained significantly lower for the duration of the 24h measurement period (Table 1, Figure 2). The decrease in food intake was accompanied by a prolonged stimulation of XAMB (Table 1, Figure 3). A similar pattern was also observed for ZTOT (data not shown). In addition, an increase in VO2 was also observed. This, however, was not as prolonged as the effect seen on food intake and activity and was only significant from 30min to 1h post injection for both doses (Table 1, Figure 4). RER was significantly reduced following administration of both doses of CRF (Figure 5).
NMU
A single ICV injection of either 1nmol or 3nmol NMU significantly reduced food intake (Table 1, Figure 2). A rapid and significant increase in XAMB was also observed (Table 1, Figure 3). A similar pattern was also observed for ZTOT (data not shown). In addition, 1nmol and 3nmol NMU significantly increased VO2 by 30min post injection (Table 1, Figure 4). A small decrease in RER was also seen at 1h post injection, in accordance with the reduction in food intake (Figure 5).
Discussion
Understanding the coordinated effects of neuropeptides involved in energy intake, behavior and energy expenditure is necessary to determine their physiological role in the regulation of energy homeostasis. The changes observed in energy expenditure in our studies are influenced by alterations in physical activity, thermogenesis and food intake in rats. In a food deprived state, energy expenditure and activity are reduced as a means of conserving energy, whereas during feeding the opposite effect is observed. This change is reflected in the altered RER values, indicating the metabolism of readily available carbohydrate in the fed state compared to the utilization of fat stores in the food deprived state.
Under ad libitum fed conditions, rats on a 12 hour light:dark cycle consume most of their daily food intake during the dark phase with peaks at the beginning and end of this period (13;14). The increase in food intake at the beginning of the dark phase is thought to be due to an energy deficiency from the preceding light phase during which food intake is relatively low (15). The increase in food intake at the end of the dark phase may exist to ensure sufficient energy availability throughout the light phase (16).
In the present study, all animals were injected in the early light phase. Orexigenic neuropeptides were administered to ad libitum fed rats whilst anorectic neuropeptides were injected in food deprived rats. These models were used in accord with work previously published by ourselves and others (17-19). It has been shown, however, that the time of day that a peptide is administered can have an effect on its potency and duration of action (20). Had the rats been injected at different times during the light/dark cycle, for example, at the beginning of the dark phase, different food intake, activity and energy expenditure profiles may have been observed. Similarly administering the orexigenic neuropeptides to food deprived rats and the anorectic neuropeptides to ad libitum fed rats would also likely have resulted in different profiles. Thus while our experiments demonstrate the effects of the neuropeptides in the same experimental system, their results cannot be assumed to be applicable to rats injected under different experimental conditions.
Physiologically, neuropeptides are released in coordinated fashion in response to changes in energy balance. It is possible to study the effects of administering more than one neuropeptide on energy balance. For example, co-administration of NPY and AgRP has been shown to additively increase food intake compared with each peptide alone (20). Unfortunately, little is known regarding the concentrations of neuropeptides released or their specific sites of action within the hypothalamus under different physiological conditions. It is therefore currently impossible to accurately mimic physiology by administering combinations of neuropeptides. We administered neuropeptides individually, which can provide useful data regarding the role of these signals, but does not mimic physiological conditions.
Continuous measurements revealed the unique food intake profiles of each neuropeptide. A marked stimulation of food intake was seen following ICV administration of NPY and AgRP in line with previously published data (21;22), whereas MCH and orexin-A were less potent, resulting in a non-significant (P = 0.09) increase in food intake. Previous studies have demonstrated a significant increase in food intake following ICV injection of MCH and orexin at equivalent doses (2;12;23). The differing food intake profiles from our studies highlight the characteristics of specific neuropeptide signaling pathways. For example, ICV administration of NPY caused an immediate and potent increase in food intake which was relatively short-lived compared to the orexigenic effects of AgRP which only became statistically significant 3h after injection and lasted the duration of the study. Interestingly, AgRP caused a significant reduction in XAMB during the dark phase, 12 hours post injection. It is possible that AgRP has similar suppressive effects on activity, as measured using XAMB, in the light phase, but that these effects are difficult to discern against the low activity of the control animals during this period. The mechanism by which AgRP reduces activity is unclear and requires further investigation. The increased RER values of AgRP injected animals likely reflect their increased food intake leading to increased use of carbohydrate as fuel source. Aside from a slight decrease in dark phase ambulatory activity, no discernible differences between the 1nmol and 3nmol doses of AgRP were detected. If the experiment had continued beyond 24h it is possible that a distinction between high and low doses may have been observed. Despite NPY and AgRP largely being secreted from the same neurons in the Arc (24), the different food intake, activity and energy expenditure profiles exhibited following ICV administration into rats in the present studies highlight their distinct effects.
In rats, fasting or a reduction in food intake will usually result in a reduction in energy expenditure as the body acts to conserve energy stores. Interestingly, following the administration of specific neuropeptides, changes in energy expenditure were contrary to those predicted from the observed changes in food intake alone. For example, NPY and orexin-A both stimulated food intake, but orexin-A increased energy expenditure whilst NPY reduced it. This coordinated metabolic response to NPY is in line with previous work that has shown that NPY stimulates food intake, promotes white fat lipid storage and decreases brown adipose tissue (BAT) thermogenesis (25). It is unclear how NPY decreases energy expenditure and it is likely that multiple mechanisms are involved. NPY has been shown to suppress sympathetic nervous system (SNS) activity to BAT (26). Furthermore, NPY is an important regulator of the hypothalamic-pituitary-thyroid (HPT) axis which is critical for the regulation of energy expenditure. Previous work has demonstrated the projection of NPY neurons from the Arc to the PVH and the presence of the Y1R on TRH neurons in the PVH (1). ICV administration of NPY has also been shown to suppress PVH proTRH mRNA and circulating thyroid hormone levels (27). Acute changes in TRH have been shown to influence oxygen consumption within a similar timeframe to the changes in energy expenditure observed in the current study (28) and ICV administration of TRH stimulates oxygen consumption, perhaps due to increased sympathetic outflow to brown adipose tissue (BAT) (29). By contrast, the increase in VO2 following ICV administration of orexin-A was associated with a marked increase in activity, consistent with its role in arousal. Orexin-A neurons project to a number of extrahypothalamic regions including the locus coeruleus, an area important in the control of arousal and anxiety-like behaviours and c-fos activiation is seen in this nucleus following ICV administration of orexin-A. (30). In addition to effects on arousal, orexin-A also directly increases energy expenditure by increasing the firing rate of sympathetic nerves to BAT (31).
Unique profiles of food intake, activity and energy expenditure were also observed following injection of α-MSH, CRF and NMU. For example, NMU demonstrated an inhibition of food intake at 5 minutes post injection, with the effect lasting for just over an hour. This is a rather short lived effect compared to previously published data demonstrating a sustained reduction in food intake for 12h (5). The ICV injections in this previous study, however, were administered at the start of the dark phase, compared to the early light phase injections performed in the current studies, which may explain the different durations of the anorectic effects observed. In contrast, CRF produced a more rapid and potent inhibition of food intake lasting for at least 24 hours. Interestingly, the rapid and potent reduction in food intake seen following ICV administration of 3nmol CRF was accompanied by a prolonged increase in activity lasting for up to 9 hours. The ability of CRF to alter grooming and exploratory behavior has been well established (32;33). In addition, de Groote et al (34) have shown ICV injection of low dose CRF (0.2nmol) stimulated behavioral changes lasting for 4 hours. The contrasting temporal effects of CRF on food intake and activity suggest that these responses may be regulated by different hypothalamic circuits. As opposed to the feeding effects of CRF, which are mediated by the PVH, there is evidence to suggest that the locomotor effects of CRF may be mediated via activation of brainstem serotonergic systems (35). ICV administration of CRF also increased VO2, in line with previous data demonstrating CRF stimulates sympathetic outflow to BAT (36).
The inhibitory effects of NMU on food intake have been suggested to be secondary to its effects on behavior and the stress response. In addition to its anorexigenic effects (5), ICV administration of NMU has also been shown to increase locomotor activity, face washing and grooming behavior in rodents (37). These effects are thought to be mediated via the CRF system (38) as they are absent in the CRF knockout mouse (39), and NMU has been shown to stimulate CRF secretion from hypothalamic explants (40). It is therefore difficult to ascertain NMU-specific effects, given the wide range of metabolic effects induced by CRF itself. In the present study, NMU and CRF were shown to produce effects on activity of differing durations. NMU reduced food intake for up to 1 hour 10 minutes whereas the effects of CRF were much more prolonged, lasting for at least 24 hours. It is possible; however, that peptide half-life may also contribute towards these differences. The mechanisms through which NMU and CRF regulate food intake, activity and energy expenditure require further study.
ICV administration of the α-MSH caused a marked decrease in food intake in line with previously published data (41). This was associated with a rapid and potent increase in VO2. Although decreased food intake would be expected to reduce energy expenditure per se, the observed increase in VO2 is consistent with the known effects of the melanocortin system on the HPT axis. As with NPY/AgRP neurons, POMC neurons from the Arc project onto TRH neurons in the PVH (42) and ICV administration of α-MSH to food deprived rats potently stimulates plasma TSH (43). Activation of the melanocortin 4 receptor (MC4R) by α-MSH is also thought to stimulate TRH gene transcription (44). The increase in VO2 following ICV administration of α-MSH is therefore likely to be due, at least in part, to its stimulatory effects on TRH neurons. An additional mechanism may be via direct activation of sympathetic outflow to brown adipose tissue (BAT); the α-MSH synthetic analogue melanotan II (MTII) has been shown to directly stimulate sympathetic outflow to BAT and the MC4R is expressed on BAT SNS outflow neurons (45). Interestingly, the 1nmol dose of α-MSH significantly increased both oxygen consumption and ambulatory activity, but, unlike the 3nmol dose, had no effect on food intake. These data suggest a dose-dependent hierarchy of metabolic effects for α-MSH, and that the pathways controlling energy expenditure may be more sensitive to the effects of α-MSH than those regulating food intake. Alpha MSH binds to the MC3R and MC4R with similar affinities (46). Whilst the role of the MC4R in the control of both food intake and energy expenditure has been extensively studied in rodents and humans (47;48), the role of the MC3R, whilst important (10;49), is less well established and requires further study. It is possible, however, that the effects of α-MSH on food intake and energy expenditure are mediated by the different receptors. Further work is required to determine the exact role of the MC3 and MC4R in mediating the effects of αMSH on energy balance.
In summary, these data demonstrate the important quantitative and temporal differences in the response of metabolic parameters following the administration of key hypothalamic peptides. These studies all performed in the same experimental system, facilitate comparison between these neuropeptide systems and provide useful information on the time course and latencies of specific neuropeptides. It must be considered, however, that within the hypothalamus, neuropeptides are released in a coordinated fashion to mount an appropriate physiological response to nutritional status. Further studies are required to elucidate the precise neuronal pathways that allow the hypothalamus to sense changes in nutritional status and how these are coordinated to mediate an appropriate physiological response by altering energy intake and expenditure.
Supplementary Material
Acknowledgements
This research is funded by programme grants from the MRC (G7811974) and Wellcome Trust (072643/Z/03/Z) and by an EU FP6 Integrated Project Grant LSHM-CT-2003-503041. We are also grateful for support from the NIHR Biomedical Research Centre funding scheme and an IMB Capacity building award. The CLAMS were purchased with joint funding from the Imperial College Strategic Infrastructure Fund and the MRC. KLS and KGM both hold BBSRC New Investigator Awards. The authors would also like to thank Linton Instrumentation (Norfolk, UK) for their assistance in the installation and maintenance of the CLAMS.
Reference List
- 1.Broberger C, Visser TJ, Kuhar MJ, Hokfelt T. Neuropeptide Y innervation and neuropeptide-Y-Y1-receptor-expressing neurons in the paraventricular hypothalamic nucleus of the mouse. Neuroendocrinology. 1999;70(5):295–305. doi: 10.1159/000054490. [DOI] [PubMed] [Google Scholar]
- 2.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richarson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92(5):1. doi: 10.1016/s0092-8674(02)09256-5. [DOI] [PubMed] [Google Scholar]
- 3.Vaughan JM, Fischer WH, Hoeger C, Rivier J, Vale W. Characterization of melanin-concentrating hormone from rat hypothalamus. Endocrinology. 1989;125(3):1660–1665. doi: 10.1210/endo-125-3-1660. [DOI] [PubMed] [Google Scholar]
- 4.Hashimoto K, Ohno N, Aoki Y, Kageyama J, Takahara J, Ofuji T. Distribution and characterization of corticotropin-releasing factor and arginine vasopressin in rat hypothalamic nuclei. Neuroendocrinology. 1982;34(1):32–37. doi: 10.1159/000123274. [DOI] [PubMed] [Google Scholar]
- 5.Howard AD, Wang R, Pong SS, Mellin TN, Strack A, Guan XM, Zeng Z, Williams DL, Jr., Feighner SD, Nunes CN, Murphy B, Stair JN, Yu H, Jiang Q, Clements MK, Tan CP, McKee KK, Hreniuk DL, McDonald TP, Lynch KR, Evans JF, Austin CP, Caskey CT, Van der Ploeg LH, Liu Q. Identification of receptors for neuromedin U and its role in feeding. Nature. 2000;406(6791):70–74. doi: 10.1038/35017610. [DOI] [PubMed] [Google Scholar]
- 6.Edwards CM, Abusnana S, Sunter D, Murphy KG, Ghatei MA, Bloom SR. The effect of the orexins on food intake: comparison with neuropeptide Y, melanin-concentrating hormone and galanin. J Endocrinol. 1999;160(3):R7–12. doi: 10.1677/joe.0.160r007. [DOI] [PubMed] [Google Scholar]
- 7.Smith KL, Patterson M, Dhillo WS, Patel SR, Semjonous NM, Gardiner JV, Ghatei MA, Bloom SR. Neuropeptide S stimulates the hypothalamo-pituitary-adrenal axis and inhibits food intake. Endocrinology. 2006;147(7):3510–3518. doi: 10.1210/en.2005-1280. [DOI] [PubMed] [Google Scholar]
- 8.Blaxter KL. Energy metabolism in Animals and Man. CUP Archive; 1989. [Google Scholar]
- 9.Rossi M, Kim MS, Morgan DG, Small CJ, Edwards CM, Sunter D, Abusnana S, Goldstone AP, Russell SH, Stanley SA, Smith DM, Yagaloff K, Ghatei MA, Bloom SR. A C-terminal fragment of Agouti-related protein increases feeding and antagonizes the effect of alpha-melanocyte stimulating hormone in vivo. Endocrinology. 1998;139(10):4428–4431. doi: 10.1210/endo.139.10.6332. [DOI] [PubMed] [Google Scholar]
- 10.Abbott CR, Rossi M, Kim M, AlAhmed SH, Taylor GM, Ghatei MA, Smith DM, Bloom SR. Investigation of the melanocyte stimulating hormones on food intake. Lack Of evidence to support a role for the melanocortin-3-receptor. Brain Res. 2000;869(1–2):203–210. doi: 10.1016/s0006-8993(00)02386-6. [DOI] [PubMed] [Google Scholar]
- 11.Negri L, Noviello L, Noviello V. Effects of sauvagine, urotensin I and CRF on food intake in rats. Peptides. 1985;6(Suppl 3):53–57. doi: 10.1016/0196-9781(85)90350-x. [DOI] [PubMed] [Google Scholar]
- 12.Rossi M, Choi SJ, O’Shea D, Miyoshi T, Ghatei MA, Bloom SR. Melanin-concentrating hormone acutely stimulates feeding, but chronic administration has no effect on body weight. Endocrinology. 1997;138(1):351–355. doi: 10.1210/endo.138.1.4887. [DOI] [PubMed] [Google Scholar]
- 13.le MJ, Tallon S. The spontaneous periodicity of ad libitum food intake in white rats. J Physiol (Paris) 1966;58(3):323–349. [PubMed] [Google Scholar]
- 14.le MJ. Peripheral and systemic actions of food in the caloric regulation of intake. Ann N Y Acad Sci. 1969;157(2):1126–1157. doi: 10.1111/j.1749-6632.1969.tb12940.x. [DOI] [PubMed] [Google Scholar]
- 15.Strubbe JH, Spiteri NJ, ingh Prins AJ. Effect of skeleton photoperiod and food availability on the circadian pattern of feeding and drinking in rats. Physiol Behav. 1986;36(4):647–651. doi: 10.1016/0031-9384(86)90348-3. [DOI] [PubMed] [Google Scholar]
- 16.Spiteri NJ, Prins AA, Keyser J, Strubbe JH. Circadian pacemaker control of feeding in the rat, at dawn. Physiol Behav. 1982;29(6):1141–1145. doi: 10.1016/0031-9384(82)90311-0. [DOI] [PubMed] [Google Scholar]
- 17.Kim MS, Rossi M, Abusnana S, Sunter D, Morgan DG, Small CJ, Edwards CM, Heath MM, Stanley SA, Seal LJ, Bhatti JR, Smith DM, Ghatei MA, Bloom SR. Hypothalamic localization of the feeding effect of agouti-related peptide and alpha-melanocyte-stimulating hormone. Diabetes. 2000;49(2):177–182. doi: 10.2337/diabetes.49.2.177. [DOI] [PubMed] [Google Scholar]
- 18.Abbott CR, Kennedy AR, Wren AM, Rossi M, Murphy KG, Seal LJ, Todd JF, Ghatei MA, Small CJ, Bloom SR. Identification of hypothalamic nuclei involved in the orexigenic effect of melanin-concentrating hormone. Endocrinology. 2003;144(9):3943–3949. doi: 10.1210/en.2003-0149. [DOI] [PubMed] [Google Scholar]
- 19.Lawrence CB, Liu YL, Stock MJ, Luckman SM. Anorectic actions of prolactin-releasing peptide are mediated by corticotropin-releasing hormone receptors. Am J Physiol Regul Integr Comp Physiol. 2004;286(1):R101–R107. doi: 10.1152/ajpregu.00402.2003. [DOI] [PubMed] [Google Scholar]
- 20.Wirth MM, Giraudo SQ. Agouti-related protein in the hypothalamic paraventricular nucleus: effect on feeding. Peptides. 2000;21(9):1369–1375. doi: 10.1016/s0196-9781(00)00280-1. [DOI] [PubMed] [Google Scholar]
- 21.Clark JT, Kalra PS, Crowley WR, Kalra SP. Neuropeptide Y and human pancreatic polypeptide stimulate feeding behavior in rats. Endocrinology. 1984;115(1):427–429. doi: 10.1210/endo-115-1-427. [DOI] [PubMed] [Google Scholar]
- 22.Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, Barsh GS. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278(5335):135–138. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- 23.Qu D, Ludwig DS, Gammeltoft S, Piper M, Pelleymounter MA, Cullen MJ, Mathes WF, Przypek R, Kanarek R, Maratos-Flier E. A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature. 1996;380(6571):243–247. doi: 10.1038/380243a0. [DOI] [PubMed] [Google Scholar]
- 24.Goldstone AP, Unmehopa UA, Bloom SR, Swaab DF. Hypothalamic NPY and agouti-related protein are increased in human illness but not in Prader-Willi syndrome and other obese subjects. J Clin Endocrinol Metab. 2002;87(2):927–937. doi: 10.1210/jcem.87.2.8230. [DOI] [PubMed] [Google Scholar]
- 25.Billington CJ, Briggs JE, Grace M, Levine AS. Effects of intracerebroventricular injection of neuropeptide Y on energy metabolism. Am J Physiol. 1991;260(2 Pt 2):R321–R327. doi: 10.1152/ajpregu.1991.260.2.R321. [DOI] [PubMed] [Google Scholar]
- 26.Egawa M, Yoshimatsu H, Bray GA. Neuropeptide Y suppresses sympathetic activity to interscapular brown adipose tissue in rats. Am J Physiol. 1991;260(2 Pt 2):R328–R334. doi: 10.1152/ajpregu.1991.260.2.R328. [DOI] [PubMed] [Google Scholar]
- 27.Fekete C, Kelly J, Mihaly E, Sarkar S, Rand WM, Legradi G, Emerson CH, Lechan RM. Neuropeptide Y has a central inhibitory action on the hypothalamic-pituitary-thyroid axis. Endocrinology. 2001;142(6):2606–2613. doi: 10.1210/endo.142.6.8207. [DOI] [PubMed] [Google Scholar]
- 28.Schuhler S, Warner A, Finney N, Bennett GW, Ebling FJ, Brameld JM. Thyrotrophin-releasing hormone decreases feeding and increases body temperature, activity and oxygen consumption in Siberian hamsters. J Neuroendocrinol. 2007;19(4):239–249. doi: 10.1111/j.1365-2826.2006.01524.x. [DOI] [PubMed] [Google Scholar]
- 29.Griffiths EC, Rothwell NJ, Stock MJ. Thermogenic effects of thyrotrophin-releasing hormone and its analogues in the rat. Experientia. 1988;44(1):40–42. doi: 10.1007/BF01960238. [DOI] [PubMed] [Google Scholar]
- 30.Hagan JJ, Leslie RA, Patel S, Evans ML, Wattam TA, Holmes S, Benham CD, Taylor SG, Routledge C, Hemmati P, Munton RP, Ashmeade TE, Shah AS, Hatcher JP, Hatcher PD, Jones DN, Smith MI, Piper DC, Hunter AJ, Porter RA, Upton N. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci U S A. 1999;96(19):10911–10916. doi: 10.1073/pnas.96.19.10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monda M, Viggiano A, Viggiano A, Viggiano E, Messina G, Tafuri D, De L V. Sympathetic and hyperthermic reactions by orexin A: role of cerebral catecholaminergic neurons. Regul Pept. 2007;139(1–3):39–44. doi: 10.1016/j.regpep.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 32.Morley JE, Levine AS. Corticotrophin releasing factor, grooming and ingestive behavior. Life Sci. 1982;31(14):1459–1464. doi: 10.1016/0024-3205(82)90007-8. [DOI] [PubMed] [Google Scholar]
- 33.Dunn AJ, Berridge CW, Lai YI, Yachabach TL. CRF-induced excessive grooming behavior in rats and mice. Peptides. 1987;8(5):841–844. doi: 10.1016/0196-9781(87)90069-6. [DOI] [PubMed] [Google Scholar]
- 34.de GL, Penalva RG, Flachskamm C, Reul JM, Linthorst AC. Differential monoaminergic, neuroendocrine and behavioural responses after central administration of corticotropin-releasing factor receptor type 1 and type 2 agonists. J Neurochem. 2005;94(1):45–56. doi: 10.1111/j.1471-4159.2005.03164.x. [DOI] [PubMed] [Google Scholar]
- 35.Linthorst AC, Penalva RG, Flachskamm C, Holsboer F, Reul JM. Forced swim stress activates rat hippocampal serotonergic neurotransmission involving a corticotropin-releasing hormone receptor-dependent mechanism. Eur J Neurosci. 2002;16(12):2441–2452. doi: 10.1046/j.1460-9568.2002.02400.x. [DOI] [PubMed] [Google Scholar]
- 36.Egawa M, Yoshimatsu H, Bray GA. Effect of corticotropin releasing hormone and neuropeptide Y on electrophysiological activity of sympathetic nerves to interscapular brown adipose tissue. Neuroscience. 1990;34(3):771–775. doi: 10.1016/0306-4522(90)90181-3. [DOI] [PubMed] [Google Scholar]
- 37.Hanada R, Nakazato M, Murakami N, Sakihara S, Yoshimatsu H, Toshinai K, Hanada T, Suda T, Kangawa K, Matsukura S, Sakata T. A role for neuromedin U in stress response. Biochem Biophys Res Commun. 2001;289(1):225–228. doi: 10.1006/bbrc.2001.5945. [DOI] [PubMed] [Google Scholar]
- 38.Yokota M, Ozaki Y, Sakamoto F, Yamada S, Saito J, Fujihara H, Ueta Y. Fos expression in CRF-containing neurons in the rat paraventricular nucleus after central administration of neuromedin U. Stress. 2004;7(2):109–112. doi: 10.1080/10253890410001727370. [DOI] [PubMed] [Google Scholar]
- 39.Hanada T, Date Y, Shimbara T, Sakihara S, Murakami N, Hayashi Y, Kanai Y, Suda T, Kangawa K, Nakazato M. Central actions of neuromedin U via corticotropin-releasing hormone. Biochem Biophys Res Commun. 2003;311(4):954–958. doi: 10.1016/j.bbrc.2003.10.098. [DOI] [PubMed] [Google Scholar]
- 40.Wren AM, Small CJ, Abbott CR, Jethwa PH, Kennedy AR, Murphy KG, Stanley SA, Zollner AN, Ghatei MA, Bloom SR. Hypothalamic actions of neuromedin U. Endocrinology. 2002;143(11):4227–4234. doi: 10.1210/en.2002-220308. [DOI] [PubMed] [Google Scholar]
- 41.Poggioli R, Vergoni AV, Bertolini A. ACTH-(1-24) and alpha-MSH antagonize feeding behavior stimulated by kappa opiate agonists. Peptides. 1986;7(5):843–848. doi: 10.1016/0196-9781(86)90104-x. [DOI] [PubMed] [Google Scholar]
- 42.Fekete C, Legradi G, Mihaly E, Huang QH, Tatro JB, Rand WM, Emerson CH, Lechan RM. alpha-Melanocyte-stimulating hormone is contained in nerve terminals innervating thyrotropin-releasing hormone-synthesizing neurons in the hypothalamic paraventricular nucleus and prevents fasting-induced suppression of prothyrotropin-releasing hormone gene expression.PG- J Neurosci. 2000;20(4) doi: 10.1523/JNEUROSCI.20-04-01550.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim MS, Small CJ, Stanley SA, Morgan DG, Seal LJ, Kong WM, Edwards CM, Abusnana S, Sunter D, Ghatei MA, Bloom SR. The central melanocortin system affects the hypothalamo-pituitary thyroid axis and may mediate the effect of leptin. J Clin Invest. 2000;105(7):1005–1011. doi: 10.1172/JCI8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harris M, Aschkenasi C, Elias CF, Chandrankunnel A, Nillni EA, Bjoorbaek C, Elmquist JK, Flier JS, Hollenberg AN. Transcriptional regulation of the thyrotropin-releasing hormone gene by leptin and melanocortin signaling. J Clin Invest. 2001;107(1):111–120. doi: 10.1172/JCI10741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Song CK, Vaughan CH, Keen-Rhinehart E, Harris RB, Richard D, Bartness TJ. Melanocortin-4 receptor mRNA expressed in sympathetic outflow neurons to brown adipose tissue: neuroanatomical and functional evidence. Am J Physiol Regul Integr Comp Physiol. 2008;295(2):R417–R428. doi: 10.1152/ajpregu.00174.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mountjoy KG, Robbins LS, Mortrud MT, Cone RD. The cloning of a family of genes that encode the melanocortin receptors. Science. 1992;257(5074):1248–1251. doi: 10.1126/science.1325670. [DOI] [PubMed] [Google Scholar]
- 47.Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, Smith FJ, Campfield LA, Burn P, Lee F. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88(1):131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 48.Yeo GS, Farooqi IS, Aminian S, Halsall DJ, Stanhope RG, O’Rahilly S. A frameshift mutation in MC4R associated with dominantly inherited human obesity. Nat Genet. 1998;20(2):111–112. doi: 10.1038/2404. [DOI] [PubMed] [Google Scholar]
- 49.Chen AS, Marsh DJ, Trumbauer ME, Frazier EG, Guan XM, Yu H, Rosenblum CI, Vongs A, Feng Y, Cao L, Metzger JM, Strack AM, Camacho RE, Mellin TN, Nunes CN, Min W, Fisher J, Gopal-Truter S, MacIntyre DE, Chen HY, Van der Ploeg LH. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat Genet. 2000;26(1):97–102. doi: 10.1038/79254. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


