Abstract
The p53-inducible BH3-only protein PUMA is a key mediator of p53-dependent apoptosis, and PUMA has been shown to function by activating Bax and mitochondrial outer membrane permeabilization. In this study we describe an ability of PUMA to induce autophagy that leads to the selective removal of mitochondria. This function of PUMA depends on Bax/Bak and can be reproduced by overexpression of Bax. The induction of autophagy coincides with cytochrome c release, and taken together the results suggest that PUMA functions through Bax to induce mitochondrial autophagy in response to mitochondrial perturbations. Surprisingly, inhibition of PUMA or Bax-induced autophagy dampens the apoptotic response, suggesting that under some circumstances the selective targeting of mitochondria for autophagy can enhance apoptosis.
Keywords: PUMA, Bax, autophagy
INTRODUCTION
Apoptotic cell death plays an important role in normal development and deregulation of apoptotic pathways can contribute to the genesis of various diseases, including cancer. The activation of apoptosis by oncogenic stress signals is thought to represent a key tumor suppressive mechanism that prevents the outgrowth of nascent tumor cells (1). Accordingly, inhibition or loss of at least some aspects of the apoptotic response pathways is a frequent characteristic of cancers in humans and model systems. One of the key pathways through which apoptosis is regulated depends on the control of mitochondrial outer membrane permeability (MOMP), which in turn regulates the release of apoptogenic factors from the mitochondria that can trigger a cascade of caspase activition (2). MOMP can be influenced by a family of proteins that consists of both pro- and anti-apoptotic members and show some similarity to the first of these proteins to be described, Bcl2 (3). At the core of the pathway lie Bax and Bak - two pro-apoptotic family members that function directly at the mitochondria to regulate MOMP (4). Bcl2, Bcl-xL and other anti-apoptotic family members act to inhibit Bax and Bak, and a large number of so-called BH3-only proteins function to promote apoptosis by either inhibiting Bcl2/Bcl-xL or by activating Bax and Bak (5). Of particular interest when considering a role of apoptosis in the suppression of cancer development is a BH3-only protein named PUMA (6). Expression of PUMA is regulated at the transcriptional level by p53, a tumor suppressor protein that is either directly or indirectly inactivated in most cancers. p53 functions to prevent tumor development through a number of different mechanisms, including the ability to reduce and repair genotoxic damage, and through an important contribution to the activation of oncogene induced senescence (7). p53 can also activate apoptosis in several cell types, and in some tissues the induction of p53-dependent apoptosis is critical for tumor suppression (8). Although p53 can activate the expression of a number of BH3-only proteins, studies in genetically modified mice have suggested that PUMA is the central mediator of p53’s apoptotic response in most cell types (6).
Autophagy refers to the highly regulated and evolutionarily conserved process of turnover and maintenance of cellular components that is required for cellular homeostasis (9). It is a multi-step process where portions of cytoplasm and organelles are engulfed in double-membrane vesicles called autophagososomes. These structures then fuse with lysosomes, resulting in the destruction of their contents by the acid hydrolases provided by the lysosome. Although autophagy is generally considered non-specific, in certain instances there appears to be selection for specific organelles such as ribosomes, the endoplasmic reticulum, peroxisomes or mitochondria. Selective loss of mitochondria has been termed mitophagy (10). While autophagy occurs constitutively in most cells, it can also be induced under conditions of stress such as starvation, hypoxia or drug treatment (11). In response to nutrient deprivation, autophagy clearly functions to promote survival - at least in the short term - by providing a source of energy through catabolism of the cell’s own components (12). Mitophagy has also been shown to contribute to survival by targeting the removal of damaged mitochondria and so eliminating the source of apoptogenic signals or reducing ROS levels (13, 14). However, despite these contributions to cell survival under stress (usually associated with tumor promotion), components of the autophagic pathways have been shown to function as tumor suppressors, suggesting that the ability to carry out autophagy somehow protects from tumor development (11, 15). This is further supported by the general observation that many oncogenes inhibit autophagy while tumor suppressor proteins can activate autophagy (16). This may reflect a role for autophagy in maintaining genome stability, or could reflect a recently described function for autophagy in supporting apoptosis in some systems (17, 18).
A number of recent studies have revealed interesting cross-talk between the apoptotic and autophagic pathways, with the identification of several proteins that can play a role in both responses (19). Bcl2 and Bcl-xL, for example, have been shown to inhibit autophagy as well as apoptosis by binding to Beclin-1, an autophagy-inducing protein that contains a BH3 domain (20). Interestingly, pro-apoptotic BH3-only proteins such as BNIP3 and Bad, as well as pharmacological BH3 mimetics, can function to induce autophagy by competitively disrupting the interaction between Beclin-1 and Bcl2 or Bcl-xL (14, 21). Conversely, Atg5 - a protein required for the formation of autophagosomes - can be rendered pro-apoptotic following cleavage by calpain. In this case the Atg5 cleavage product acquires the ability to move to the mitochondria, interact with Bcl-xL and drive cytochome c release and caspase activation (22). In light of these increasing links between the two responses, we investigated whether the p53-inducible pro-apoptotic protein PUMA might also have a function in regulating autophagy.
RESULTS
Our previous studies have shown that PUMA can localize to the mitochondria through a C-terminal hydrophobic domain, and by virtue of the BH3 domain-dependent interaction with Bcl2 (23). While studying the subcellular localization of Flag tagged PUMA we noted that the expression of PUMA reproducibly resulted in a characteristic clustering of the mitochondria around the nucleus. Co-staining of cells for Tom20, a mitochondrial outer membrane protein, revealed a filamentous network of mitochondria in untransfected cells, while cells expressing PUMA (which colocalizes with Tom 20) showed an aggregate clustering of the mitochondria around the nucleus - an effect that was seen within 12 hours of transfection of PUMA into a number of different cell lines (Figure 1A and B and supplementary Fig 1A). In time course experiments we noted that the mitochondrial clustering was observed as soon as PUMA expression could be detected, indicating that the changes in mitochondria occurs concurrently with or very rapidly after PUMA localization. In a number of cell, at later time points, the initial clustering of mitochondria in PUMA expressing cells appeared to be followed by a loss of mitochondria. Identical results were seen after staining for different mitochondrial proteins such as COX I and COX IV (data not shown). To confirm that this mitochondria loss was due to PUMA expression, we co-expressed GFP-C1 with PUMA to give an independent marker of which cells had been transfected with PUMA. This approach showed clearly that by 24 hours post-transfection, the depletion of mitochondria was evident only in the GFP positive cells (Figure 1C and supplementary Fig 1B). Our previous studies have shown that expression of extremely low levels of PUMA (below the level of detection by western blot) were sufficient to induce apoptosis, and we found that the clustering and loss of mitochondria were also induced by similarly low levels of PUMA expression (data not shown). Although the mitochondrial clustering and loss could be seen most clearly when the experiments were carried out in the presence of the pan-caspase inhibitor z-VAD-fmk to inhibit the loss of cells through PUMA-induced apoptosis, these effects of PUMA expression were also observed in the absence of z-VAD-fmk (Figure 1D), indicating that the loss was not a consequence of caspase inhibition. Although the activation of apoptosis in the absence of z-VAD-fmk made it difficult to quantify how many PUMA expressing cells showed mitochondrial loss, at earlier time points (8 hours) it was clear that the mitochondrial clustering occurred at a similar rate in the presence or absence of z-VAD-fmk (Supplementary Figure 1C).
Figure 1.
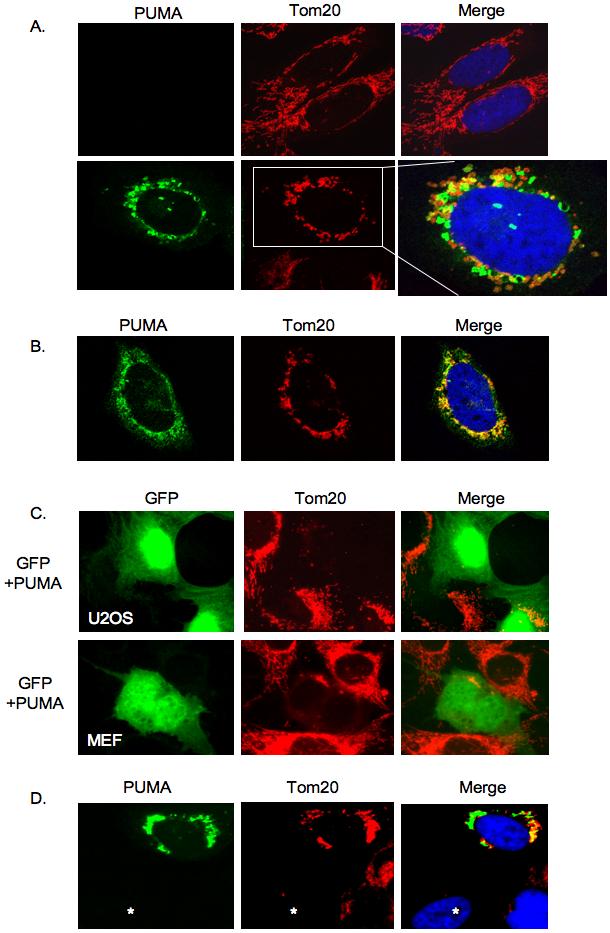
PUMA induces perinuclear accumulation and loss of mitochondria in different cell types.
A) U2OS cells were transfected with GFP-tagged PUMA expression plasmid and maintained in the presence of z-VAD-fmk to inhibit apoptosis. 24 hours later cells were fixed and subject to immunofluorescence analysis. Mitochondria were stained using anti-Tom20 antibody. The top panels show normal mitochondrial morphology in control transfected U2OS cells.
B) Saos-2 TetOn PUMA cells were treated with doxycycline to induce PUMA. 24 hours later PUMA was detected using an anti-Flag antibody while mitochondria were stained as above.
C) U2OS or MEF cells were co-transfected with a combination of pEGFP plasmid and PUMA expression plasmid and maintained in z-VAD-fmk. 24 hours later, cells were fixed and immunostained with anti-Tom20 antibody. GFP positivity indicated cells that co-expressed PUMA and showed loss of mitochondria (detected with anti-Tom20).
D) U2OS cells were transfected with GFP-PUMA and maintained without z-VAD-fmk. After 12 hours, mitochondrial clustering was evident in GFP-PUMA positive cells, with some cells (an example is indicated by *) showing loss of the mitochondrial marker.
It is well established that bulk degradation of cytoplasmic components is usually due to autophagy. Recent studies have illustrated the role of BH3 domain proteins in the regulation of autophagy, as well as apoptosis. We therefore considered the possibility that PUMA - in common with some other BH3-only proteins - may be stimulating autophagy and the consequent degradation of organelles including the mitochondria. A common method for detecting autophagy is by monitoring the conversion of GFP-tagged microtubule-associated protein 1 light chain 3 (MAP-LC3), from a diffuse cytosolic form (LC3-I) to a lipidated form (LC3-II) that forms part of the autophagic membrane and can be visualised as puncta accumulation by fluorescence microscopy (24). Using GFP-tagged LC3 to monitor the formation of autophagosomes, we found that PUMA strongly induced the formation of GFP-LC3 puncta, along with mitochondrial clustering (Figure 2A and Supplementary Figure 2A). An examination of the localization of the LC3 puncta showed that there was a generally closer localization with mitochondria (stained by Tom20) than with other organelles such as the golgi (stained with GM130) (Figure 2B). Although there was clearly not complete concurrence of the LC3 and Tom20 staining, the degree of overlap was similar to that seen previously under conditions where selective autophagic degradation of mitochondria - or mitophagy - had been identified (21). We therefore looked more directly at the PUMA-induced loss of various organelle-specific proteins by western blotting (Figure 2C), which showed that while PUMA expression clearly led to the loss of mitochondrial proteins (COXII and COXIV), there was no change in the levels of proteins marking other organelles, including Golgi (GM130) and ER (Calnexin). In agreement with these observations, PUMA expressing cells showed no reduction in ER or golgi staining by immunofluorescence (data not shown). These results therefore suggested that PUMA expression leads to an increased rate of autophagy that results in the selective loss of mitochondria.
Figure 2.
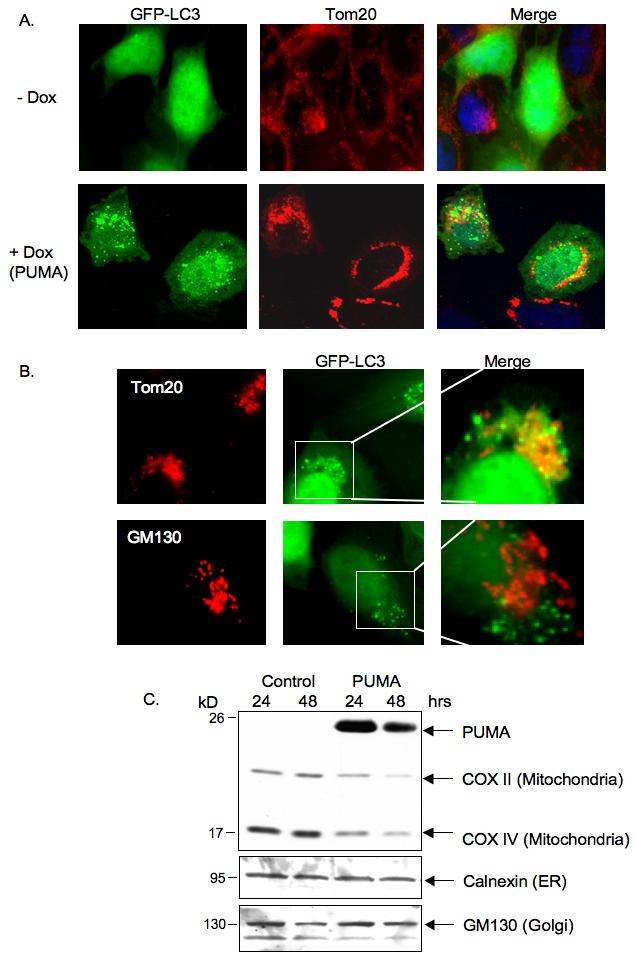
PUMA induces accumulation of GFP-LC3 puncta, which preferentially colocalize with mitochondria.
A) Saos-2 TetOn PUMA cells were infected with GFP-LC3 expressing Adenovirus, left for 16 hours and then treated with doxycycline. Cells were maintained in the presence of z-VAD-fmk throughout the experiment. 24 hours later, cells were fixed and immunostained with anti-Tom20 antibody to visualize the mitochondria.
B) U2OS cells were infected with GFP-LC3 expressing Adenovirus, left for 16 hours and then transfected with PUMA expression plasmid. Cells were maintained in the presence of z-VAD-fmk throughout the experiment. 24 hours later, cells were fixed and immunostained with anti-Tom20 (upper panel) or anti-GM130 (lower panel). Cells exhibiting GFP-LC3 puncta were then observed under a fluorescence microscope to compare the co-localization of GFP-LC3 puncta with mitchondria (Tom20) or Golgi (GM130).
C) U2OS cells were transfected with vector control or PUMA expression plasmid and maintained in the presence of z-VAD-fmk throughout the experiment. Cells were harvested 24 or 48 hours after transfection and cell lysates were immunoblotted with the indicated antibodies to detect levels of mitochondria, Golgi and ER proteins.
A number of recent reports have shown that BH3 domain proteins like BNIP3 and Bad can induce autophagy by releasing Beclin-1 - an essential component of the autophagic pathway - from an inhibitory interaction with Bcl2 or Bcl-xL. This function of BNIP3 would depend on its BH3 domain for interaction with Bcl2/BclXL, but would not be expected to require other mediators of the apoptotic response such as Bax or Bak. To test whether PUMA may be functioning in a similar manner we first examined the contribution of the PUMA BH3 domain. Deletion of 3 amino acids within the BH3 domain resulted in a failure of PUMA to induce the mitochondrial clustering seen following expression of the wild type protein (Figure 3A) and this mutant was also defective in the induction of LC3 puncta (Figure 3B). Interestingly, a PUMA mutant lacking the C-terminal domain - but retaining the BH3 domain and the ability to both localise to the mitochondria and induce apoptosis (23) - functioned like wild type PUMA in the induction of mitochondrial clustering, mitochondrial loss and LC3 puncta formation (Figure 3B and data not shown).
Figure 3.
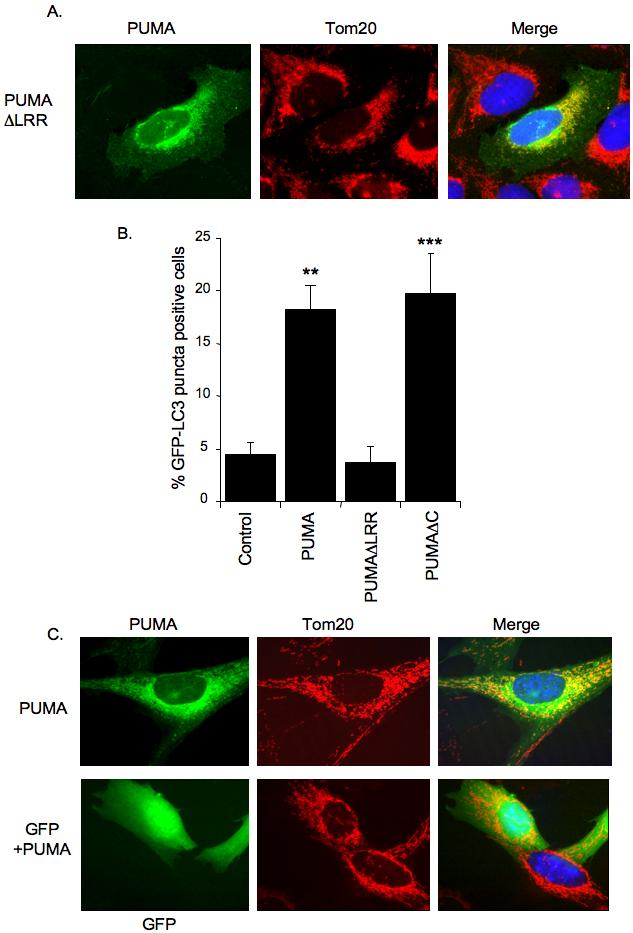
The ability of PUMA to affect mitochondria and LC3-punctae depends on Bax/Bak
A) U2OS cells were transfected with PUMAΔLRR expression plasmid and subjected to immunofluorescence analysis 24 hours later. PUMAΔLRR was detected using anti-FLAG antibody whilst mitochondria were stained with anti-Tom20 antibody.
B) Quantitation of GFP-LC3 puncta formation. U2OS cells were infected with GFP-LC3 expressing Adenovirus and then transfected with expression plasmids for the indicated proteins. Cells were maintained in the presence of z-VAD-fmk throughout the experiment. 24 hours later, cells were fixed and observed under a fluorescence microscope. Results are presented as mean percentage of GFP-LC3 positive cells displaying puncta. Data are mean and SD of three independent experiments. (**P<0.001, ***P<0.005 compared to control transfected cells).
C) Bax/Bak DKO MEFs were transfected with plasmids expressing the indicated proteins and subjected to immunofluorescence analysis after 24 hours. In the upper panel, PUMA was detected using an anti-Flag antibody whilst mitochondria were stained using anti-Tom20 antibody. In the lower panel, GFP positivity indicated cells that co-expressed PUMA and showed maintenance of mitochondria (detected with anti-Tom20).
If the induction of autophagy by PUMA is due to release of Beclin-1 from Bcl2/Bcl-xL, then it would not be predicted to be influenced by Bax or Bak expression. However, we have shown previously that induction of apoptosis by PUMA is absolutely dependent on the presence of Bax or Bak (25). Surprisingly, expression of PUMA in Bax/Bak double null cells had no effect at all on mitochondrial morphology or maintenance (Figure 3C). PUMA protein expressed in these cells colocalized with Tom20 staining in a broadly spread reticulate network (upper panel), and GFP/PUMA co-transfected cells retained their mitochondria (lower panel).
The requirement of Bax/Bak for PUMA to induce the mitochondrial changes led us to test whether expression of Bax alone would be able to mediate similar effects. Subsequent experiments showed that expression of Bax could induce the mitochondrial clustering seen following PUMA expression (Figure 4A). As expected, Bax also induced these mitochondrial changes in the Bax/Bak null cells (data not shown). It should be noted that in these experiments transfection of Bax leads to the expression of activated Bax that localizes to the mitochondria and is competent to induce apoptosis. As seen following PUMA expression, perinuclear clustering of mitochondria in response to Bax occurred in the presence or absence of z-VAD-fmk (Supplementary Figure 1C), and the loss of mitochondrial markers caused by the induction of Bax was not accompanied by a clear decrease in ER or golgi markers (Figure 4B). The ability of Bax to induce mitochondrial clustering and loss suggested that Bax may also be inducing autophagy. This was confirmed by showing that, like PUMA, expression of Bax induces LC3 puncta formation (Figure 4C and D, and Supplementary Figure 2B).
Figure 4.
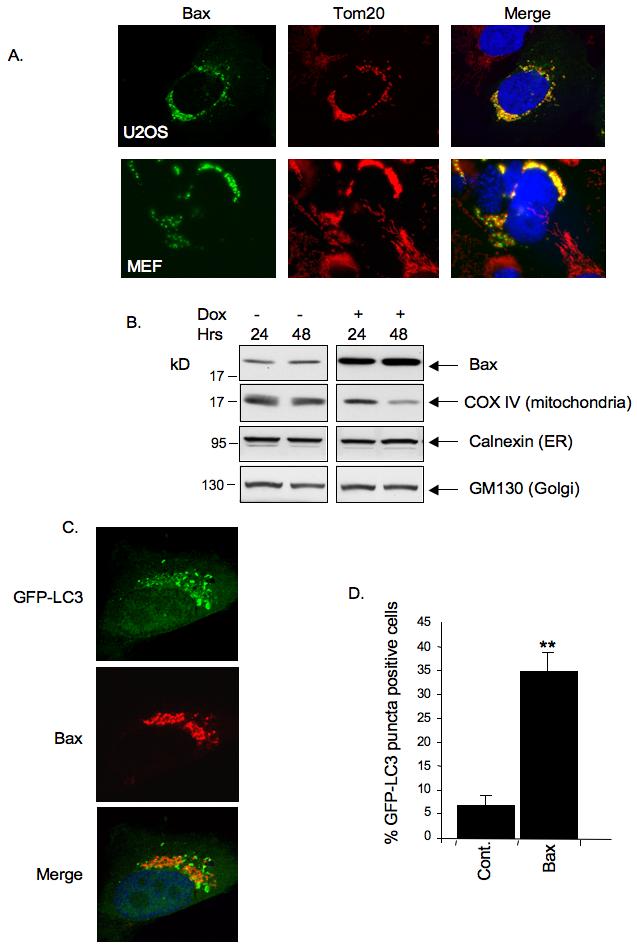
Bax functions like PUMA to induce mitochondrial loss and accumulation of LC3 punctae.
A) Bax induces mitochondrial perinuclear accumulation and loss. U2OS and WT MEFs were transfected with Bax expression plasmid and subjected to immunofluorescence analysis 24 hours later. Cells were maintained in the presence of z-VAD-fmk throughout the experiment. Bax was detected using anti-Bax antibody whilst mitochondria were stained using anti-Tom20 antibody.
B) Saos-2 TetOn Bax cells were treated with doxycycline to induce Bax expression and maintained in the presence of z-VAD-fmk throughout the experiment. Cell were harvested 24 or 48 hours after doxycycline treatment and cell lysates were immunoblotted with the indicated antibodies to detect levels of mitochondria, Golgi and ER proteins.
C) U2OS cells were infected with GFP-LC3 expressing Adenovirus and then transfected with Bax expression plasmid. Cells were maintained in the presence of z-VAD-fmk throughout the experiment. 24 hours later, cells were fixed, stained for Bax and observed under a fluorescence microscope.
D) Quantitation of GFP-LC3 puncta. Saos-2 TetOn Bax cells were infected with GFP-LC3 expressing Adenovirus and then treated with doxycycline for 24 hours. Cells were maintained in the presence of z-VAD-fmk throughout the experiment. Cells were fixed and analysed using a fluorescence microscope. Results are presented as mean percentage of GFP-LC3 positive cells displaying puncta. Data are mean and SD of three independent experiments (**P<0.001 compared to control cells).
To further establish the effect of PUMA and Bax on autophagy, we examined the conversion from LC3-I to LC3-II by Western blot, where LC3-II is detected as a faster migrating band. In agreement with the immunofluorescence studies, both PUMA and Bax expression enhanced the conversion of LC3, but this was not seen following expression of a BH3 domain mutant of PUMA (Figure 5A). Analysis by electron microscopy also showed the presence of vesicles consistent with the formation of autophagosomes following PUMA or Bax induction (Supplementary Figure 2C).
Figure 5.
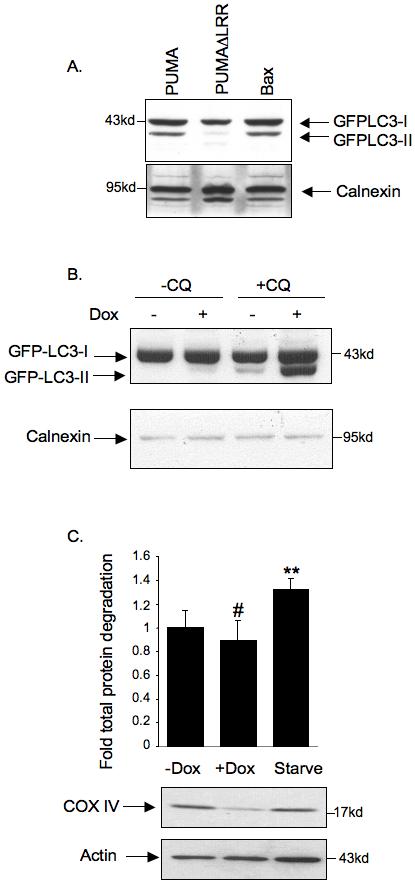
PUMA promotes autophagosome formation and loss of mitochondrial markers, but not general degradation of long-lived proteins
A) HeLa cells stably expressing GFP-LC3 were transfected with plasmids expressing the indicated proteins, maintained in the presence of z-VAD-fmk and harvested after 24 hours. Cell lysates were then subjected to Western blotting using anti-GFP antibodies to detect GFP-LC3-I and the faster migrating GFP-LC3-II. Calnexin was used as a loading control.
B) Inhibition of autophagosome degradation enhances levels of PUMA-induced GFP-LC3-II. Saos2 TetOn PUMA cells were infected with GFP-LC3 expressing Adenovirus and then treated with doxycycline for 24 hours. 30μM chloroquine was then added and cells were harvested after 9 hour. Cells were maintained in the presence of z-VAD-fmk throughout the experiment. Cell lysates were subjected to Western blotting using anti-GFP antibodies to detect GFP-LC3-I and the faster migrating GFP-LC3-II. Calnexin was used as a loading control.
C) Protein stability was assessed in Saos2 TetOn PUMA cells following doxycycline treatment to induce PUMA or starvation (# No significant difference; **P-value<0.001 compared to untreated cells). Western blot for COXIV protein levels in these samples is shown below. Actin was used as a loading control
The accumulation of LC3 punctae could result from either an enhanced rate of autophagy, or a block in the later stages of autophagy to prevent degradation of the autophagosomes. We therefore examined the effect of chloroquine (which inhibits the lysosome/autophagosome fusion) on the levels of LC3-II. As expected if PUMA is contributing to the enhancement of autophagy, the LC3-II levels were higher in PUMA/chloroquine cells compared to chloroquine only cell (Figure 5B). Taken together, our results suggest that PUMA enhances autophagy, that this effect requires Bax and/or Bak and that the autophagy induced by PUMA shows some selectivity in the removal of mitochondria. General autophagy can be assessed by an increased degradation of long-lived proteins, an effect that we observed in nutrient starved cells, as expected (Figure 5C). Interestingly, however, we did not detect a change in the degradation of long-lived proteins following activation of PUMA, despite a clear loss of the mitochondrial marker COX IV (Figure 5C). These results support the suggestion that PUMA can lead to specific loss of mitochondria, through mitophagy, without inducing a general and non-specific autophagic response. It is also interesting to note that we did not see non-apoptotic cell death following PUMA or Bax induction in the presence of z-VAD-fmk after 3 days treatment (as assessed by PI exclusion). This is in contrast to cells undergoing general autophagy in response to ER stress (26), where necrotic death is detected. This may therefore also reflect the difference between general autophagy and the more selective mitophagy seen here.
Having found that Bax and PUMA can induce autophagy and the removal of mitochondria, we were interested in understanding whether this would have any effect on their apoptotic activity. The removal of mitochondria through mitophagy has been reported previously and is thought to be triggered by mitochondrial membrane permeability transition caused by mitochondrial damage (27). Such a response would be entirely consistent with our observations that the autophagic effects of Bax and PUMA expression correlate with the ability to activate apoptosis. Most studies have indicated that the mitophagic response to mitochondrial damage is a pro-survival mechanism, removing damaged mitochondria and their associated apoptogenic signals. We therefore examined the contribution of autophagy to the sensitivity of cells to Bax-induced apoptosis by reducing the expression of components of the autophagic pathway. siRNA targeted to Atg5 lowered the expression of the Atg5 protein and partially rescued the mitochondrial loss induced by Bax expression (Figure 6A). Surprisingly, knock down of Atg5 also significantly reduced Bax-induced apoptosis (Figure 6B), suggesting that the autophagic response contributes to the apoptotic response. However, we were concerned that this result may be complicated by the recent description of a direct role for Atg5 in apoptosis following cleavage by calpain (22) - although we were unable to detect any increase in calpain activity following activation of either PUMA or Bax (data not shown). We therefore used siRNA to reduce the expression of other proteins required for autophagy and found that reduction of the expression of Atg7 or Atg10 also lowered the apoptotic response to Bax (Fig 6B). PUMA induced apoptosis was also inhibited by the reduction of autophagic proteins Atg5, Atg7 or Atg10 (Figure 6C).
Figure 6.
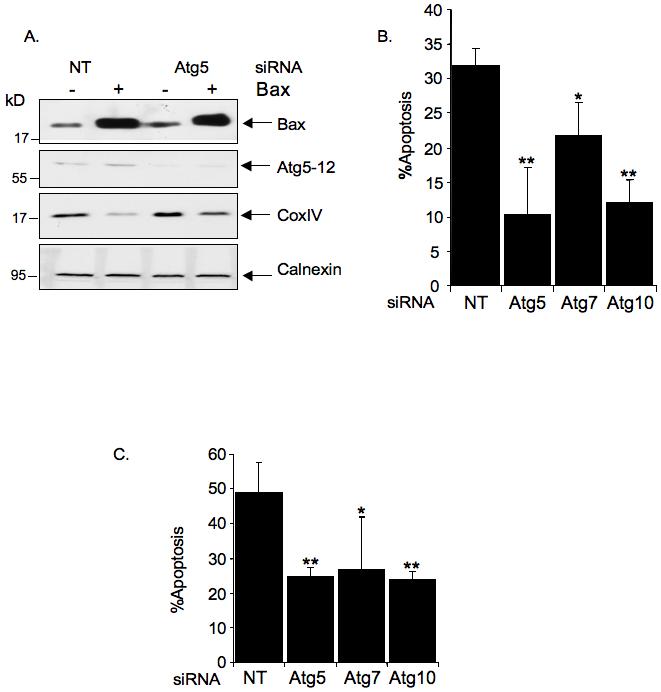
Inhibition of autophagy partially rescues the mitochondria loss and apoptosis induced by Bax and PUMA.
A) Saos-2 TetOn Bax cells were transfected with non-targeting siRNA or siRNA against Atg5. 72 hours later, doxycycline was added to induce Bax expression in the presence of z-VAD-fmk. 24 hours after induction, cells were harvested for Western blot analysis. Cell lysates were immunoblotted and probed with antibodies against the indicated proteins. Anti-Atg12 was used to detect Atg5-12 complex to confirm the decrease in Atg5 expression. Anti-COXIV antibody was used to detect mitochondria protein levels.
B) Saos-2 TetOn Bax cells were transfected with non-targeting siRNA as well as siRNA against Atg5, Atg7 and Atg10. 72 hours later, doxycycline was added to induce Bax expression. After 24 hours, cells were harvested and apoptosis was evaluated by flow cytometry. Results are the mean and standard deviation for at least three independent experiments. (* P-value<0.05, **P-value<0.001 compared with non-targeted siRNA transfected cells)
C) Saos-2 TetOn PUMA cells were transfected with non-targeted siRNA or siRNA against Atg5, Atg7 and Atg 10. 72 hours later, doxycycline was added to induce PUMA expression. After 24 hours, cells were harvested and apoptosis was evaluated by flow cytometry. Results are the mean and standard deviation for at least three independent experiments (* P-value<0.05, **P-value<0.001 compared with non-targeted siRNA transfected cells)
To try and elucidate the stage of apoptosis that was affected by reducing the expression of Atg5, we carried out cell fractionation to evaluate the effect on cytochrome c release. As expected, expression of Bax induced the release of cytochrome c from the mitochondria (Fig 7A), a response that was inhibited by reduction of Atg5 expression. Our studies suggest that, rather unexpectedly, autophagy may be contributing to the efficient release of cytochrome c and so the induction of apoptosis. However, it is also likely that mitochondrial membrane permeabilization induced by Bax is a trigger to induce autophagy - particularly the selective removal of these damaged mitochondria. We therefore tried to determine whether the formation of autophagosomes occurred before or after cytochrome c release. However, despite several attempts in different systems we were unable to clearly dissociate the appearance of LC3 puncta from the release of cytochrome c following either Bax or PUMA expression (Supplementary Figure 3).
Figure 7.
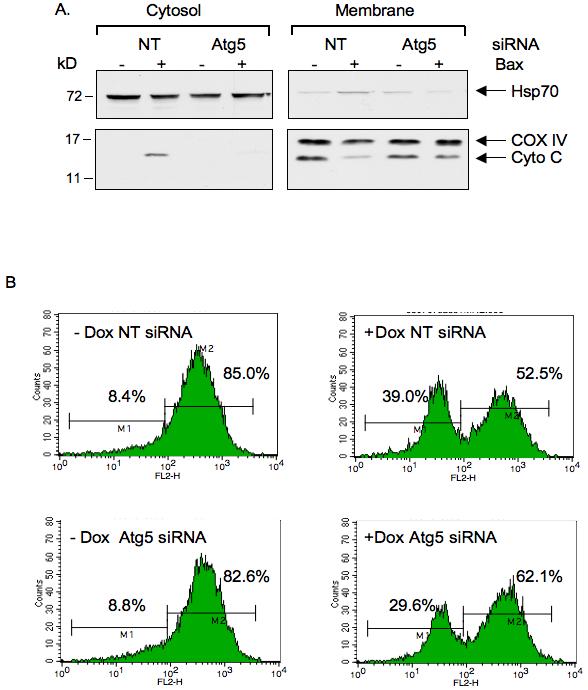
Inhibition of autophagy inhibits cytochrome c release and decrease of mitochondrial membrane potential induced by Bax.
Saos-2 TetOn Bax cells were transfected with non-targeting siRNA or siRNA against Atg5. 72 hours later, doxycycline was added to induce Bax expression in the presence of z-VAD-fmk.
A) 24 hours after Bax induction, cells were harvested and subjected to subcellular fractionation. Equivalent amounts of each fraction were subjected to Western blot analysis to detect cytochrome c levels and marker proteins; COXIV for membrane fraction and Hsp70 for cytosolic fraction.
B) 24 hours after Bax induction, cells were stained with TMRE to determine alterations in mitochondrial membrane potential (Δψm). A decrease in Δψm is demonstrated by the appearance of a peak with lower fluorescence intensity. The experiment was carried out at least three times and gave similar results.
Another feature frequently associated with apoptosis is the loss of mitochondrial inner membrane potential. Using the TMRE dye, which binds to mitochondria in a membrane potential dependent manner, we confirmed that expression of Bax resulted in the appearance of a population of cells with decreased potential. Interestingly, this population of cells was reduced following siRNA depletion of Atg5 (Figure 7B), suggesting that mitophagy contributes to the Bax-induced apopotosis by enhancing both cytochrome c release and loss of mitochondrial membrane potential.
DISCUSSION
We have shown that the BH3-only protein PUMA can induce autophagy concurrently with apoptosis, and that this appears to result in the selective removal of mitochondria. The induction of autophagy by PUMA requires its BH3 domain and is dependent on the presence of Bax or Bak. Bax alone can also induce mitochondrial-selective autophagy in the absence of PUMA activation, and we find a close correlation between the induction of apoptosis and autophagy in these systems.
A growing body of evidence supports a distinction between general autophagy and the specific removal of mitochondria through autophagic mechanisms, also termed mitophagy (28). General or non-selective autophagy is an important response to maintain metabolism and allow cells to survive limited periods of hypoxia, starvation or nutrient deprivation. This is a short-term survival solution, however, and extended or over-activated autophagy can result in the death of cells following extensive self-digestion of organelles and cytoplasm. This autophagic response is dependent on Beclin-1 and can be triggered by BH3-only proteins such as BNIP3 and Bad, which disrupt the inhibitory interaction between Bcl2/Bcl-xL and Beclin-1. More selective forms of autophagy that result in the engulfment and degradation of only mitochondria have been suggested to play an important role in the removal of damaged mitochondria, although the mechanism underlying the selectivity are not clear. Our results suggest that PUMA functions through Bax to induce mitochondrial selective autophagy that is triggered simultaneously with cytochrome c release. It would appear that induction of autophagy (as assessed by autophagosome appearance) does not induce nor precede the release of cytochrome c, but rather the same stimulus (such as changes in mitochondrial membrane potential) concurrently induces both these events.
The autophagic signal induced by PUMA is clearly different from that activated by starvation or ER stress, which are independent of Bax/Bak (12, 26). The dependence of PUMA-induced autophagy on Bax/Bak indicates that PUMA does not function directly to release Beclin-1 from Bcl2/Bcl-xL - at least in these model systems. This is further supported by the observation that Bax - which does not bind Beclin-1 and is unable to disrupt the interaction between Bcl2/Xl and Beclin-1 (29) - functions to induce mitochondrial autophagy in a manner very similar to PUMA. This activity of Bax would seem to be linked with the ability to induce apoptosis, since a recent study has shown that expression of cytoplasmic Bax (which is inactive) cannot induce autophagy in Bax/Bak null cells (26). Interestingly, we also found that knockdown of Beclin1 expression by siRNA did not prevent apoptosis in response to PUMA or Bax, even though reduction of Beclin-1 expression was able to inhibit the formation of autophagosomes in response to starvation (data not shown). It therefore seems possible that PUMA/Bax induced autophagy is Atg5, Atg7 and Atg10-dependent but Beclin-1-independent, a situation very similar to that described recently for neuronal autophagy induced by the parkinsonian neurotoxin 1-methyl-4-phenylpyridinium (MPP(+)) (30). Like PUMA and Bax, MPP(+) induces mitochondrial injury, autophagy and mitochondrial degradation that is inhibited by siRNA knockdown of Atg5, Atg7 and Atg8 but occurs independently of Beclin-1.
It seems likely that alterations in mitochondrial membrane potential-induced Bax/Bak can signal to both apoptosis and mitochondrial autophagy. Indeed, previous studies have shown that mitochondria that have sustained stress-induced damage such as MPT or MOMP can be removed by selective mitochondrial autophagy (13, 27, 30, 31). Furthermore, in yeast mitochondrial dysfunction (as measured by impaired mitochondrial membrane potential) also induces mitophagy, which is followed by cell death (32). What the precise signal from the altered mitochondrial membranes to autophagy is remains to be determined. Intriguingly, some BH3 only proteins which would be expected to activate Bax and induce the pathways described here - such as BNIP3 - also induce mitophagy through the activation of Beclin-1 (14). It remains possible that in different cell systems, or in response to different signals, PUMA may also engage this pathway.
The role of PUMA in p53-induced autophagy remains to be resolved. p53 transcriptionally activates the expression of DRAM (33), which can mediate the autophagic response. In cells where p53 drives DRAM expression, we were unable to identify an essential role for PUMA in the activation of autophagy (data not shown), consistent with DRAM being sufficient for this response. However, the response to p53 is complicated, with evidence that p53 can also function to repress autophagy (34). The ultimate response to p53 activation and the contribution of various p53 activities is therefore likely to be strongly stimulus and cell type dependent.
Despite descriptions of autophagic cell death, there is a consensus that autophagy itself is more usually a survival mechanism and although dying cells can show characteristics of autophagy, this is generally not the mechanism through which the cells die (35). However, it is possible that autophagy can contribute to the induction of other death responses, such as apoptosis. In light of the coordinate activation of both autophagic and apoptotic signals by PUMA and Bax, we examined whether autophagy could impact the apoptotic response. The impact of autophagy on apoptosis is highly context dependent, with compelling evidence that autophagy can both inhibit or promote apoptosis, depending on the system. As discussed above, general autophagy in response to starvation clearly plays a survival role, although a number of studies have also shown that general autophagy can play a role in promoting or initiating apoptosis (17, 19). There are many examples of systems in which a reduction or inhibition of autophagy leads to a reduction in apoptosis or cell death (18, 30, 31, 33, 36-38). The role for mitochondrial specific autophagy in the regulation of apoptosis is similarly complex. While selective removal of damaged mitochondria by autophagy has been proposed to protect or reduce the apoptotic signal (39), there are situations where mitophagy appears to contribute to apoptosis. Most interestingly, inhibition of the mitophagy associated with MPP(+) induced mitochondrial damage confers protection from MPP(+)-induced cell death in a manner reminiscent of the results described here with PUMA and Bax (30, 38). Bax has also been shown to induce both mitophagy and general autophagy, as well as death, in yeast. Interestingly, loss of expression of Uth1P - a protein that is specifically required for mitophagy - delays the rapid and regulated cell death induced by Bax, although these cells then die through necrosis (40).
Taken together, our data support a role for PUMA or Bax-induced mitochondrial autophagy in the promotion of apoptosis. Importantly, however, while this mitophagy can enhance apoptosis, it does not appear to induce cell death by itself in the short term, although loss of mitochondria is likely to be detrimental to the cell in the long term. The mechanism for this pro-apoptotic activity of mitochondrial autophagy remains unclear. It is possible that the process of autophagy enhances the breakdown of the mitochondria and so facilitates the release of cytochrome c and/or SMAC/DIABLO. Alternatively, the removal of mitochondria might promote cell death by reducing the cell’s capacity for energy production and survival (28).
MATERIALS AND METHODS
Reagents and antibodies
All reagents used in this study were from Sigma unless otherwise indicated. For Western blot and immunofluorescence experiments, cells were treated with 50 μM z-VAD-fmk (Bachem) to inhibit caspase activity and cell death.
The following antibodies were used: Flag M2 (F3165), Sigma; calnexin H-70( sc-11397), Tom20 FL-145(sc-11415), and Bax clone 2D2 (sc-20067) from Santa Cruz Biotechnology; OxPhos Complex IV subunit IV 10G8 (COXIV)(A21347), and OxPhos Complex IV subunit II 12C4 (COXII)(A6404) from Molecular Probes; cytochrome c (556433) and GM 130(610832) from BD Biosciences; HSP 70 from Transduction Labs; Apg12L (AP1816a ) from Abgent and GFP (7.1/13.1) from Roche.
Cell lines and plasmids
U2OS (osteosarcoma), hTERT-RPE (telomerase- immortalized human retinal pigment epithelial cells, Clontech), WT MEF (wild type mouse embryonic fibroblast) and Bax-/- Bak-/- double knockout (DKO) MEF (kind gift of Craig Thompson, Abramson Family Cancer Research Institute, University of Pennsylvania, Philadelphia, USA), Saos-2 TetOn PUMA-inducible (41) and Saos-2 TetOn Bax-inducible cell lines (42) have been described previously. HeLa cell line stably expressing GFP-LC3 was generated by hygromycin selection of cells infected with pBabe-Hygro retrovirus expressing BamHI-EcoRI subcloned EGFP-LC3 (24). All cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum, 2 mM glutamine, and antibiotics. Flag tagged PUMA, PUMAΔLRR, PUMAΔC (N164) and DO1-tagged Bax have been described previously (23, 42). GFP-PUMA and DSRed-PUMA were constructed by cloning PUMA into pEGFP-N1 (Clontech) and DSRed-N1 vectors respectively.
DNA and siRNA Transfections
Cells were transfected with indicated plasmids (generally 4 μg per 60mm dish) or induced with doxycycline (0.5μg/ml and 1μg/ml doxycycline for Saos-2 TetOn PUMA and Bax, respectively). DNA transfections were carried out using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. Small interfering RNA (siRNA) transfections were utilized to knock down protein levels of autophagic genes. Sequences of siRNA used are as follows - ATG5 (CAUCUGAGCUACCCGGAUAUU) (33); ATG7 (GGAGUCACAGCUCUUCCUU) (30); ATG10 (GGAGUUCAUGAGUGCUAUA) (43). Nontargeted siRNA purchased from Dharmacon was used as control. siRNA transfections were carried out using HiPerfect reagent (Qiagen) according to the manufacturers instructions. Cells were transfected at a concentration of 25nM siRNA twice on sequential days to enhance knock down. The cells were replated for various experiments 48 hours after siRNA transfection, left overnight and then treated with doxycycline. Induced cells were harvested at indicated times for various assays.
Western blotting
For Western blot analysis, transfected or induced cells were maintained in the presence of 50uM z-VAD-fmk and harvested and lysed in 1% CHAPS buffer (150mM NaCl, 10mM HEPES [pH 7.4], 1% CHAPS) after the indicated time. The protein content of the clarified cell lysates were determined and equal amounts were subjected to SDS-PAGE and Western blotting. For the chloroquine experiments, Saos2 TetOn PUMA cells were infected with Adenovirus expressing GFP-LC3, left overnight and then treated with doxycycline to induce PUMA. 24h later, 30uM chloroquine was added to the cells and left for 9h. Cells were then harvested and processed for Western blotting as described above. Calnexin protein levels were detected to confirm equal loading of protein content.
Subcellular fractionation
Saos-2 TetOn cells in 100 mm tissue culture dishes were transfected with the indicated siRNA, treated with doxycycline and then subjected to subcellular fractionation using the ProteoExtract Subcellular Proteome Extraction kit (Calbiochem) according to the manufacturer’s instructions. Equal amounts of membrane/organelle and cytosol fraction was separated using SDS-PAGE followed by Western blotting to detect cytochrome c release and various marker proteins; Hsp70 for cytosol fractions and COXIV for membrane/organelle fractions.
Immunofluorescence
Cells were plated on sterile glass coverslips (in 12-well tissue culture dishes) and transfected with 1 μg of the indicated plasmids or treated with doxycycline to induce PUMA or Bax. All experiments were carried out in the presence of z-VAD-fmk unless otherwise indicated. 24 h later, the cells were washed with PBS and then fixed in ice-cold 4% paraformaldehyde in PBS for 10 min. at room temperature. After fixation, the cells were permeabilized in PBS containing 0.2% Triton-X100 for 5 min. The cells were blocked in PBS containing 0.5% bovine serum albumin (blocking solution) at room temperature for 30 min. and then incubated overnight at 4°C with a combination of the indicated antibodies in blocking solution. The cells were washed three times with PBS and incubated for 1 h at room temperature with a mixture of donkey anti-mouse FITC-conjugated antibody and donkey anti-rabbit Cy3-conjugated antibody (both from Jackson ImmunoResearch Laboratories) in blocking solution containing 1 μg of DAPI (4′,6′-diamidino-2-phenylindole) (Sigma)/ml. The cells were washed three times with PBS, and slides were mounted with Hard-Set Vectashield (Vector). Cells were observed and images obtained with either a Zeiss Axiovert fluorescent microscope or a Bio-Rad Radiance 2000 confocal microscope. In the merged images DAPI staining was also shown to indicate the nucleus.
Apoptosis assay
Saos-2 TetOn cells were transfected with the indicated siRNAs and then treated with doxycycline to induce PUMA or Bax expression. Cells were harvested 24 hours later and analyzed by flow cytometry using a FACSCalibur flow cytometer (488-nm argon laser excitation source) (BD Biosciences). Apoptosis was assessed by evaluating caspase activation using the CaspACE FITC-VAD-fmk in situ marker from Promega.
Quantitation of GFP-LC3 puncta
Saos-2 TetOn cells were infected with Adenovirus expressing GFP-LC3 and then treated with doxycycline to induce PUMA or Bax expression. At the indicated times, cells were fixed with 4% PFA and then observed under a fluorescence microscope. Cells were classified as having a predominantly diffuse GFP stain or having numerous punctate structures representing autophagosomes. At least 100 cells were scored in each of 3 or more independent experiments. In experiments comparing the appearance of GFP-LC3 puncta to release of cytochrome c, cell were subjected to immunofluorescence staining with cytochrome c antibody before being evaluated under the microscope.
Measurement of mitochondrial membrane potential
TMRE (tetramethylrhodamine ethyl ester, Molecular Probes) was used to evaluate changes in mitochondria membrane potential. Doxycycline treated cells were harvested and resuspended in PBS containing 50nM TMRE. After incubation at 37 degrees for 20 min., samples were analyzed by flow cytometry. Cells with decreased mitochondrial membrane potential exhibited lower TMRE uptake (peak with lower fluorescence intensity).
Statistical Analysis
Statistical significance of experiments was determined using Student’s t test, with a significance level of p < 0.05.
Calpain activation assay
Calpain activity was measured using the Calpain-Glo kit (Promega). Saos2 TetOn cells were induced with doxycycline for 24 hours. Cells were then harvested and resuspended in Hepes lysis buffer (10mM HEPES pH 7.5, 10mM DTT, 1mM EDTA and 1mM EGTA). Cells were then centrifuged at full speed and the supernatant was used for the assay (according to the manufacturers instructions) as well as protein quantification.
Electron Microscopy
Saos2 TetOn PUMA and Bax cells were plated in 100 mm tissue culture dishes at near confluency and induced with doxycycline. After 24h, media was replaced with fixative (4% paraformaldehyde/2.5% glutaraldehde in 0.2M PIPES) and the cells were fixed for 1h. The fixative was then removed and the cells scraped into 1 ml of fixative, transferred to a microfuge tube and spun down. Pellets were treated with 1% osmium tetroxide (Agar Scientific), dehydrated in ethanol and propylene oxide before embedding in Durcupan resin Sigma). 60-70nm sections were cut on a Leica Ultracut UCT ultramicrotome and mounted on hexagonal, 100-mesh copper grids. Sections were stained with uranyl acetate and lead citrate and examined in a Jeol 1200 EX electron microsccope. Images were recorded on imaging plates and processed in a Ditabis plate scanner.
Measurement of degradation of long-lived proteins
Cells were labelled for 8 h with L-[35S]Met/Cys (5 μCi/ml) (Amersham), washed three times in PBS, then incubated overnight in serum-free DMEM with 2 mM unlabeled L-Met/Cys (plus z-VAD-fmk, plus/minus doxycycline). Subsequently, cells were washed and incubated in fresh DMEM (plus z-VAD-fmk plus/minus doxycycline) or EBSS plus z-VAD-fmk for 8h, whereupon the levels of degraded protein were calculated as described previously (43).
Supplementary Material
Supplementary Figure 1: PUMA induces perinuclear accumulation and loss of mitochondria in different cell types.
A) hTERT-RPE or wild type MEF cells were transfected with Flag-tagged PUMA expression plasmid and maintained in the presence of z-VAD-fmk to inhibit apoptosis. 24 hours later cells were fixed and subject to immunofluorescence analysis. PUMA was detected using an anti-Flag antibody whilst mitochondria were stained using anti-Tom20 antibody.
B) hTERT-RPE cells were co-transfected with a combination of pEGFP plasmid and PUMA expression plasmid and maintained in z-VAD-fmk. 24 hours later, cells were fixed and immunostained with anti-Tom20 antibody. GFP positivity indicated cells that co-expressed PUMA and showed loss of mitochondria (detected with anti-Tom20).
C) Perinuclear accumulation of mitochondria induced by PUMA or Bax is independent of z-VAD-fmk. U2OS cells were transfected with GFP-PUMA or Bax expression plasmid and maintained either in the absence of presence of z-VAD-fmk. 8 hours later, cells were fixed and subject to immunofluorescence analysis. Mitochondria were stained using anti-Tom20 antibody. Results are presented as mean percentage of cells displaying perinuclear accumulation of mitochondria. DAPI staining was used to confirm that these cells have not yet started to display nuclear condensation. There was no mitochondrial clustering in cells not expressing PUMA or Bax.
Supplementary Figure 2: PUMA and Bax expression leads to an accumulation of autophagosomes
A) U2OS cells were infected with GFP-LC3 expressing Adenovirus and then mock transfected or transfected with PUMA expression plasmid. Cells were maintained in the presence of z-VAD-fmk throughout the experiment. 24 hours later, cells were fixed, stained for PUMA and observed under a fluorescence microscope.
B) Saos-2 TetOn Bax cells were infected with GFP-LC3 expressing Adenovirus, left for 16 hours and then treated with doxycycline. Cells were maintained in the presence of z-VAD-fmk throughout the experiment. 24 hours later, cells were fixed and immunostained with anti-Tom20 antibody to visualize the mitochondria. Cells were then observed under a fluorescence microscope to compare the colocalization of GFP-LC3 puncta with mitochondria.
C) Electron micrographs of Saos-2 TetOn PUMA and Bax cells induced with doxycycline for 24 hours in the presence of z-VAD-fmk.
Supplementary Figure 3: GFP-LC3 puncta formation and cytochrome c release occur coincidentally in cells over-expressing Bax and PUMA.
A) Saos-2 TetOn Bax cells were infected with GFP-LC3 expressing Adenovirus, left for 16 hours and then treated with doxycycline in the presence of z-VAD-fmk. At the indicated time points, cells were fixed and immunostained with anti-cytochrome c antibody. The results were quantified as percentage of GFP positive cells exhibiting puncta compared to the percentage of GFP positive cells expressing diffuse cytochrome c.
B) As above using Saos-2 TetOn PUMA cells.
ACKNOWLEDGEMENTS
We thank Dr Aviva Tolkovsky (University of Cambridge) for the kind gift of GFP-LC3 expressing Adenovirus. This work was funded by Cancer Research UK and the West of Scotland Women’s Bowling Association.
REFERENCES
- 1.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004 Jul 30;305(5684):626–629. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- 3.Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 4.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:624–626. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chipuk JE, Green DR. How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends in cell biology. 2008 Apr;18(4):157–164. doi: 10.1016/j.tcb.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu J, Zhang L. No PUMA, no death: implications for p53-dependent apoptosis. Cancer Cell. 2003;4:248–249. doi: 10.1016/s1535-6108(03)00249-6. [DOI] [PubMed] [Google Scholar]
- 7.Halazonetis TD, Gorgoulis VG, Bartek J. An oncogene-induced DNA damage model for cancer development. Science. 2008 Mar 7;319(5868):1352–1355. doi: 10.1126/science.1140735. [DOI] [PubMed] [Google Scholar]
- 8.Oren M. Decision making by p53: life, death and cancer. Cell Death Differ. 2003 Apr;10(4):431–442. doi: 10.1038/sj.cdd.4401183. [DOI] [PubMed] [Google Scholar]
- 9.Mizushima N. Autophagy: process and function. Genes Dev. 2007 Nov 15;21(22):2861–2873. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- 10.Kim I, Rodriguez-Enriquez S, Lemasters JJ. Selective degradation of mitochondria by mitophagy. Archives of biochemistry and biophysics. 2007 Jun 15;462(2):245–253. doi: 10.1016/j.abb.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008 Jan 11;132(1):27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lum JJ, Bauer DE, Kong M, Harris MH, Li C, Lindsten T, et al. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell. 2005 Jan 28;120(2):237–248. doi: 10.1016/j.cell.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 13.Colell A, Ricci JE, Tait S, Milasta S, Maurer U, Bouchier-Hayes L, et al. GAPDH and autophagy preserve survival after apoptotic cytochrome c release in the absence of caspase activation. Cell. 2007 Jun 1;129(5):983–997. doi: 10.1016/j.cell.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 14.Zhang H, Bosch-Marce M, Shimoda LA, Tan YS, Baek JH, Wesley JB, et al. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J Biol Chem. 2008 Apr 18;283(16):10892–10903. doi: 10.1074/jbc.M800102200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Mathew R, Karantza-Wadsworth V, White E. Role of autophagy in cancer. Nature reviews. 2007 Dec;7(12):961–967. doi: 10.1038/nrc2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maiuri MC, Tasdemir E, Criollo A, Morselli E, Vicencio JM, Carnuccio R, et al. Control of autophagy by oncogenes and tumor suppressor genes. Cell Death Differ. 2009 Jan;16(1):87–93. doi: 10.1038/cdd.2008.131. [DOI] [PubMed] [Google Scholar]
- 17.Scarlatti F, Granata R, Meijer AJ, Codogno P. Does autophagy have a license to kill mammalian cells? Cell Death Differ. 2008 Jul 4;16:12–20. doi: 10.1038/cdd.2008.101. [DOI] [PubMed] [Google Scholar]
- 18.Berry DL, Baehrecke EH. Growth arrest and autophagy are required for salivary gland cell degradation in Drosophila. Cell. 2007 Dec 14;131(6):1137–1148. doi: 10.1016/j.cell.2007.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007 Sep;8(9):741–752. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- 20.Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005 Sep 23;122(6):927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 21.Maiuri MC, Le Toumelin G, Criollo A, Rain JC, Gautier F, Juin P, et al. Functional and physical interaction between Bcl-X(L) and a BH3-like domain in Beclin-1. Embo J. 2007 May 16;26(10):2527–2539. doi: 10.1038/sj.emboj.7601689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yousefi S, Perozzo R, Schmid I, Ziemiecki A, Schaffner T, Scapozza L, et al. Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis. Nature cell biology. 2006 Oct;8(10):1124–1132. doi: 10.1038/ncb1482. [DOI] [PubMed] [Google Scholar]
- 23.Yee KS, Vousden KH. Contribution of membrane localization to the apoptotic activity of PUMA. Apoptosis. 2008 Jan;13(1):87–95. doi: 10.1007/s10495-007-0140-2. [DOI] [PubMed] [Google Scholar]
- 24.Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. Embo J. 2000 Nov 1;19(21):5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Melino G, Bernassola F, Ranalli M, Yee K, Zong WX, Corazzari M, et al. p73 induces apoptosis via PUMA transactivation and Bax mitochondrial translocation. J Biol Chem. 2004;279:8076–8083. doi: 10.1074/jbc.M307469200. [DOI] [PubMed] [Google Scholar]
- 26.Ullman E, Fan Y, Stawowczyk M, Chen HM, Yue Z, Zong WX. Autophagy promotes necrosis in apoptosis-deficient cells in response to ER stress. Cell Death Differ. 2008 Feb;15(2):422–425. doi: 10.1038/sj.cdd.4402234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elmore SP, Qian T, Grissom SF, Lemasters JJ. The mitochondrial permeability transition initiates autophagy in rat hepatocytes. Faseb J. 2001 Oct;15(12):2286–2287. doi: 10.1096/fj.01-0206fje. [DOI] [PubMed] [Google Scholar]
- 28.Kundu M, Thompson CB. Macroautophagy versus mitochondrial autophagy: a question of fate? Cell Death Differ. 2005 Nov;12(Suppl 2):1484–1489. doi: 10.1038/sj.cdd.4401780. [DOI] [PubMed] [Google Scholar]
- 29.Erlich S, Mizrachy L, Segev O, Lindenboim L, Zmira O, Adi-Harel S, et al. Differential interactions between Beclin 1 and Bcl-2 family members. Autophagy. 2007 Nov-Dec;3(6):561–568. doi: 10.4161/auto.4713. [DOI] [PubMed] [Google Scholar]
- 30.Zhu JH, Horbinski C, Guo F, Watkins S, Uchiyama Y, Chu CT. Regulation of autophagy by extracellular signal-regulated protein kinases during 1-methyl-4-phenylpyridinium-induced cell death. The American journal of pathology. 2007 Jan;170(1):75–86. doi: 10.2353/ajpath.2007.060524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xue L, Fletcher GC, Tolkovsky AM. Autophagy is activated by apoptotic signalling in sympathetic neurons: an alternative mechanism of death execution. Molecular and cellular neurosciences. 1999 Sep;14(3):180–198. doi: 10.1006/mcne.1999.0780. [DOI] [PubMed] [Google Scholar]
- 32.Priault M, Salin B, Schaeffer J, Vallette FM, di Rago JP, Martinou JC. Impairing the bioenergetic status and the biogenesis of mitochondria triggers mitophagy in yeast. Cell Death Differ. 2005 Dec;12(12):1613–1621. doi: 10.1038/sj.cdd.4401697. [DOI] [PubMed] [Google Scholar]
- 33.Crighton D, Wilkinson S, O’Prey J, Syed N, Harrison PR, Gasco M, et al. DRAM, a p53-induced modulator of autophagy, is critical for apoptosis. Cell. 2006;14:121–134. doi: 10.1016/j.cell.2006.05.034. [DOI] [PubMed] [Google Scholar]
- 34.Tasdemir E, Maiuri MC, Galluzzi L, Vitale I, Djavaheri-Mergny M, D’Amelio M, et al. Regulation of autophagy by cytoplasmic p53. Nature cell biology. 2008 Jun;10(6):676–687. doi: 10.1038/ncb1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kroemer G, Levine B. Autophagic cell death: the story of a misnomer. Nat Rev Mol Cell Biol. 2008 Dec;9(12):1004–1010. doi: 10.1038/nrm2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Espert L, Denizot M, Grimaldi M, Robert-Hebmann V, Gay B, Varbanov M, et al. Autophagy is involved in T cell death after binding of HIV-1 envelope proteins to CXCR4. The Journal of clinical investigation. 2006 Aug;116(8):2161–2172. doi: 10.1172/JCI26185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kunchithapautham K, Rohrer B. Apoptosis and autophagy in photoreceptors exposed to oxidative stress. Autophagy. 2007 Sep-Oct;3(5):433–441. doi: 10.4161/auto.4294. [DOI] [PubMed] [Google Scholar]
- 38.Chu CT, Zhu J, Dagda R. Beclin 1-independent pathway of damage-induced mitophagy and autophagic stress: implications for neurodegeneration and cell death. Autophagy. 2007 Nov-Dec;3(6):663–666. doi: 10.4161/auto.4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meier P, Vousden KH. Lucifer’s labyrinth--ten years of path finding in cell death. Mol Cell. 2007 Dec 14;28(5):746–754. doi: 10.1016/j.molcel.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 40.Kissova I, Plamondon LT, Brisson L, Priault M, Renouf V, Schaeffer J, et al. Evaluation of the roles of apoptosis, autophagy, and mitophagy in the loss of plating efficiency induced by Bax expression in yeast. J Biol Chem. 2006 Nov 24;281(47):36187–36197. doi: 10.1074/jbc.M607444200. [DOI] [PubMed] [Google Scholar]
- 41.Nakano K, Vousden KH. PUMA, a novel pro-apopototic gene, is induced by p53. Mol Cell. 2001;7:683–694. doi: 10.1016/s1097-2765(01)00214-3. [DOI] [PubMed] [Google Scholar]
- 42.Phillips AC, Bates S, Ryan KM, Helin K, Vousden KH. Induction of DNA synthesis and apoptosis are separable functions of E2F-1. Genes and Dev. 1997;11:1853–1863. doi: 10.1101/gad.11.14.1853. [DOI] [PubMed] [Google Scholar]
- 43.Boya P, Gonzalez-Polo RA, Casares N, Perfettini JL, Dessen P, Larochette N, et al. Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol. 2005 Feb;25(3):1025–1040. doi: 10.1128/MCB.25.3.1025-1040.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: PUMA induces perinuclear accumulation and loss of mitochondria in different cell types.
A) hTERT-RPE or wild type MEF cells were transfected with Flag-tagged PUMA expression plasmid and maintained in the presence of z-VAD-fmk to inhibit apoptosis. 24 hours later cells were fixed and subject to immunofluorescence analysis. PUMA was detected using an anti-Flag antibody whilst mitochondria were stained using anti-Tom20 antibody.
B) hTERT-RPE cells were co-transfected with a combination of pEGFP plasmid and PUMA expression plasmid and maintained in z-VAD-fmk. 24 hours later, cells were fixed and immunostained with anti-Tom20 antibody. GFP positivity indicated cells that co-expressed PUMA and showed loss of mitochondria (detected with anti-Tom20).
C) Perinuclear accumulation of mitochondria induced by PUMA or Bax is independent of z-VAD-fmk. U2OS cells were transfected with GFP-PUMA or Bax expression plasmid and maintained either in the absence of presence of z-VAD-fmk. 8 hours later, cells were fixed and subject to immunofluorescence analysis. Mitochondria were stained using anti-Tom20 antibody. Results are presented as mean percentage of cells displaying perinuclear accumulation of mitochondria. DAPI staining was used to confirm that these cells have not yet started to display nuclear condensation. There was no mitochondrial clustering in cells not expressing PUMA or Bax.
Supplementary Figure 2: PUMA and Bax expression leads to an accumulation of autophagosomes
A) U2OS cells were infected with GFP-LC3 expressing Adenovirus and then mock transfected or transfected with PUMA expression plasmid. Cells were maintained in the presence of z-VAD-fmk throughout the experiment. 24 hours later, cells were fixed, stained for PUMA and observed under a fluorescence microscope.
B) Saos-2 TetOn Bax cells were infected with GFP-LC3 expressing Adenovirus, left for 16 hours and then treated with doxycycline. Cells were maintained in the presence of z-VAD-fmk throughout the experiment. 24 hours later, cells were fixed and immunostained with anti-Tom20 antibody to visualize the mitochondria. Cells were then observed under a fluorescence microscope to compare the colocalization of GFP-LC3 puncta with mitochondria.
C) Electron micrographs of Saos-2 TetOn PUMA and Bax cells induced with doxycycline for 24 hours in the presence of z-VAD-fmk.
Supplementary Figure 3: GFP-LC3 puncta formation and cytochrome c release occur coincidentally in cells over-expressing Bax and PUMA.
A) Saos-2 TetOn Bax cells were infected with GFP-LC3 expressing Adenovirus, left for 16 hours and then treated with doxycycline in the presence of z-VAD-fmk. At the indicated time points, cells were fixed and immunostained with anti-cytochrome c antibody. The results were quantified as percentage of GFP positive cells exhibiting puncta compared to the percentage of GFP positive cells expressing diffuse cytochrome c.
B) As above using Saos-2 TetOn PUMA cells.


