Abstract
Obesity is a major problem from a public health perspective and a difficult practical matter for intensivists. The obesity pandemic has required treating clinicians to develop an appreciation of the substantial pathophysiological effects of obesity on the various organ systems. The important physiological concepts are illustrated by focusing on obstructive sleep apnoea, obesity hypoventilation syndrome, abdominal compartment syndrome and ventilatory management of the obese patient with acute respiratory distress syndrome.
The obesity pandemic has led to an increased appreciation of the special needs of severely over-weight patients. Obesity presents major pathophysiological and technical issues, particularly when patients are critically ill.1–3 Problems in obese patients in the intensive care unit (ICU) may include difficulties with airway maintenance, disordered ventilation and gas exchange, impaired circulation and altered drug pharmacokinetics. Procedures are more challenging, whether non-operative (eg, airway intubation, vascular access, neural blocks, urinary catheterisation) or operative. Safe transport, repositioning, image acquisition and mobilisation can be major challenges requiring careful planning and execution. The problems of obesity are compounded by its common comorbidities which include hypertension, asthma,4 5 hyperlipidaemia and type 2 diabetes mellitus which further increase patient risk.6
Of the many effects of obesity on various organ systems (fig 1), we have chosen to focus on the following four obesity-related syndromes commonly encountered in the ICU that illustrate the diverse effects and multiple mechanisms through which obesity increases morbidity and complicates management:7
obstructive sleep apnoea (OSA), a context in which to discuss airway management;
obesity hypoventilation syndrome with consideration of ventilation and gas exchange abnormalities;
abdominal compartment syndrome and the role of obesity in intra-abdominal hypertension; and
acute respiratory distress syndrome in obese subjects with discussion of the complicating role of obesity in the management of severe parenchymal lung disorders.
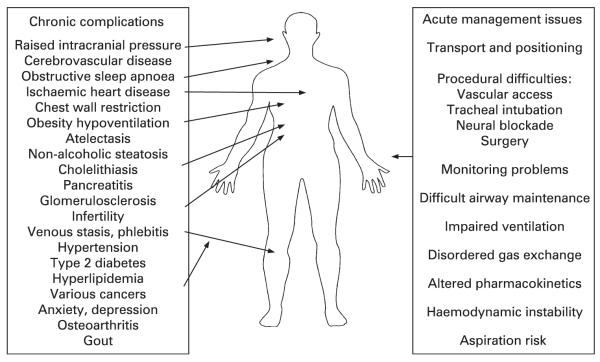
Figure 1.
Obesity-related complications.
OBESITY AND OBSTRUCTIVE SLEEP APNOEA
OSA is a common condition8 characterised by recurrent episodes of upper airway obstruction during sleep, which are associated with arterial oxygen desaturation and repetitive arousals resulting in disrupted sleep and excessive daytime sleepiness.9 10 OSA predisposes to a variety of problems including systemic hypertension11 and vascular disease,12 13 diabetes,14 metabolic syndrome,15 16 depression, gastro-oesophageal reflux17 18 and accident risk.19 20 OSA is important in ICU practice as it is a clinical indicator of a “difficult airway” and can complicate perioperative management increasing the risk of ICU admission, contribute to the evolution of respiratory failure and result in “failed extubation” following mechanical ventilation.
The difficult airway
The anatomical problems that contribute to OSA may contribute to difficult intubation with parameters such as the Mallampati score being helpful to characterise these.21 22 The specific difficulties that obesity poses for intubation (box 1) relate to limited mouth opening and neck mobility23 compounded by difficulties in maintaining airway patency prior to successful intubation. Furthermore, obesity-related decreases in functional residual capacity—particularly when recumbent—result in lower oxygen stores and impaired gas exchange because of atelectasis in dependent lung zones.24 25 Hence, time in apnoea before arterial oxygen desaturation occurs is reduced26 and the prospects of a “can’t intubate, can’t ventilate” scenario are compounded by the minimal time available to resolve the airway issue. Furthermore, both obesity and OSA predispose to gastro-oesophageal reflux, increasing the risk of pulmonary aspiration while the airway remains unprotected.
Box 1. Effect of obesity on physiological parameters.
↑Intra-abdominal pressure: abdominal compartment syndrome can cause renal, hepatic failure and visceral necrosis.
↑Intracranial pressure: associated with raised intra-abdominal and pleural pressures.
↑Central venous pressure: increased by high intra-abdominal and pleural pressures.
↑Pulmonary artery occlusion pressure: increased by high intra-abdominal and pleural pressures.
↑Pulmonary artery pressure: mild to moderate elevations may result from OSA alone.
↓Total lung capacity.
↓Vital capacity.
↓Functional residual capacity: atelectasis and reduced oxygen stores increase propensity for desaturation.
↑Pleural pressure: reflects chest wall compression.
↓Respiratory system compliance: stiffer respiratory system likely from lung and chest wall contribution.
↑Airway resistance: risk of asthma and airway closure.
↓Hypercapnic ventilatory response: contributes to obesity hypoventilation syndrome.
↑Upper airway resistance: contributes to difficult airway and sleep apnoea.
A precise definition of the risk of difficult intubation in morbid obesity and OSA has not been undertaken, but is likely to be high as both conditions are associated with upper airway narrowing.27–29 Some have argued that morbid obesity alone is not commonly associated with intubation difficulty30 given proper patient positioning. However, the risk is likely to increase where other indicators of difficult intubation are also present including mandibular retrusion, limited mouth opening and poor neck extension. The presence of OSA may be an additional indicator of risk of difficult intubation in obese patients.21 Without these additional features it appears reasonable to undertake “rapid sequence” endotracheal intubation with preoxygenation, use of short-acting neuromuscular relaxants and cricoid pressure under experienced supervision and with equipment for difficult intubation available (eg, bougies/introducers). Where additional anatomical features indicating difficult intubation are present, problems may be circumvented by undertaking awake fibreoptic-assisted intubation.31
Airway management problems are not confined to these matters. Tracheostomy is more problematic in morbidly obese patients because of access, difficulty identifying landmarks and increased distance from skin to trachea complicating correct tube placement.32 Hence, even creating a “crash” airway where endotracheal intubation has proved impossible or converting from prolonged endotracheal intubation to tracheostomy is difficult. Furthermore, extubation may be problematic in obese patients with OSA where the conscious state is compromised,33 either through illness or the effect of sedative drugs, and extreme caution needs to be exercised before extubation in such circumstances. Following extubation, continuous positive airway pressure (CPAP) or non-invasive ventilation (NIV) must be available for high-risk patients, including those with OSA, for use during sleep or sedation34 (see section on “OSA and failed extubation” below). A list of the potential factors complicating endotracheal intubation in obesity is shown in box 2.
Box 2. Potential factors complicating endotracheal intubation in obesity.
Anatomically difficult airway.
Rapid desaturation due to reduced functional residual capacity.
Drug distribution and metabolism.
Underlying cardiomyopathy.
Underlying pulmonary hypertension.
Diabetes mellitus affecting autonomic reflexes.
Difficult “crash” airway including cricothyroidotomy or tracheostomy.
Difficult vascular access.
Aspiration risk.
Perioperative management of patients with obesity and OSA
While there is some inconsistency in findings regarding the effects of obesity on postoperative morbidity in non-cardiac patients,35 36 there is substantial evidence to suggest that it is associated with an increased incidence of wound infections and breakdown,37 venous thromboembolism38 and adverse cardiac events,39 particularly where severe. The risk of admission to the ICU increases as does the risk of prolonged ventilation, length of ICU stay and mortality in the ICU, particularly in the surgical ICU40 or with extreme obesity (body mass index (BMI) ≥40 kg/m2). Following cardiac surgery, prolonged ventilation is more common in patients in this high weight range, as are longer lengths of stay in hospital.41
In the early postoperative period the risk of hypoxaemia and/or upper airway obstruction is increased.42 This early postoperative period is a time of particular vulnerability because of the residual effects of anaesthetic, paralytic and analgesic drugs, all predisposing to upper airway obstruction and depressing arousal responses which protect against asphyxia. Not surprisingly, patients with OSA appear to be vulnerable during this period. While data on postoperative risk in OSA are sparse and some suggest few problems for outpatient surgery at least,43 complications are more apparent when the immediate perioperative period is examined, particularly following upper airway surgery and especially in children.44 This finding presumably reflects the compounding influences of drug effects and surgically-induced oedema45 on an anatomically small upper airway. The data regarding perioperative risk for patients with OSA following major surgery are also sparse, but a case-control study of postoperative morbidity following hip or knee arthroplasty demonstrated a substantial increase in the incidence of “serious” complications requiring emergency interventions, ICU admissions and length of stay.46
Patients with a combination of morbid obesity and OSA may be at particular risk of postoperative pulmonary complications, but there are few published data to define this risk. Such constraints have limited the development of practice guidelines for the perioperative management of patients with OSA47 and the recent guidelines were largely based on expert opinion.48 Because of the vulnerability of patients with OSA in the perioperative period, the following principles should be considered:
efforts to identify OSA preoperatively;
use of CPAP therapy perioperatively;
use of regional rather than general anaesthesia if possible;
careful reversal of neuromuscular blockade;
awake extubation following general anaesthesia;
use of regional analgesic techniques rather than sedating systemic analgesics;
use of the lateral rather than supine posture; and
extended close monitoring in the postoperative period.
Many of the principles, along with bed head elevation, appear applicable to the management of obese patients whether or not they have OSA. CPAP therapy is arguably underused in the perioperative period in the management of obese patients, particularly—but not confined to—those with OSA. Its advantages include both pneumatic splinting of the upper airway and recruitment of atelectatic lung. CPAP is well tolerated by patients familiar with it but may be a challenge in CPAP-naïve patients. Other less certain strategies to secure airway patency, such as use of the lateral posture and a nasopharyngeal airway, may be necessary in CPAP-intolerant patients. Successful application of positive pressure in the case of postoperative upper airway obstruction obviates the need for reintubation in some patients.
OSA, obesity and respiratory failure
While most patients with OSA alone do not develop daytime hypercapnia, when other factors that weaken or mechanically load the respiratory muscles or impair gas exchange such as severe obesity (see section on “Obesity hypoventilation syndrome” below)49 or chronic obstructive pulmonary disease (COPD) are present, OSA can contribute to respiratory failure. The term “overlap syndrome”50 was introduced to describe the association of OSA and COPD and the capacity of OSA to exacerbate disturbances in ventilation and gas exchange in patients with COPD. In general, respiratory failure during wakefulness is associated with sleep-related hypoxaemia and hypercapnia in both OSA and COPD and OSA and obesity.51
These associations are important to intensivists as failure to consider OSA and its contribution to respiratory failure will exacerbate difficulties in weaning patients from ventilatory support, prolong length of stay, increase morbidity and, in survivors, lead to recurrent hospital admissions. The possibility of OSA should be considered in patients with recurrent respiratory failure unexplained by degree of impairment of ventilatory capacity during wakefulness. Study of ventilation during sleep will define the problem and determine the most appropriate form of treatment: CPAP in the case of predominant upper airway obstruction and NIV where there is a major “central” (non-obstructive) hypoventilatory component.49 Such treatments must be titrated to optimise gas exchange during sleep, as the degree of adequacy of this is an important determinant of magnitude of improvement in wakeful respiratory function.52
OSA, obesity and failed extubation
Upper airway patency is determined by a balance between forces that narrow the airway and those that dilate it. Patients with OSA have narrow airways and require disproportionate activation of pharyngeal muscles during wakefulness to maintain patency.53 Activation is driven by a combination of influences including the wakeful state itself, ventilatory drive and reflex activation initiated by upper airway mechanoreceptors that respond to intraluminal negative pressure developed during inspiration.9 If the patient is obtunded, there is reduced drive to the upper airway muscles from all these sources. The presence of an endotracheal tube may blunt upper airway reflexes.54 This combination of circumstances may lead to post-extubation upper airway obstruction in patients with narrow upper airways such as those with obesity and/or OSA. In support of this concept, several authors have noted that attempts to fast track extubation after cardiac surgery55 56 and abdominal aneurysm reconstruction57 are less successful in obese patients. The use of non-invasive positive pressure ventilation applied immediately after extubation appears a useful strategy to allow successful weaning and extubation in difficult to wean patients58 or to prevent re-intubation in high-risk patients. Because end-expiratory lung volume can affect upper airway patency, some also advocate the reverse Trendelenburg position after extubation to maximise lung volume and thus pharyngeal patency.59
OSA and cardiac function
Both obesity and OSA have been associated with impairment in left ventricular function. The mechanisms underlying obesity-related cardiomyopathy are unclear. However, some authors have suggested that OSA may contribute through catechola-mine-mediated mechanisms (similar to cocaine-induced or phaeochromocytoma-associated cardiomyopathy).60 Increases in pulmonary artery pressures (PAP) are debated in OSA. Although marked increases in PAP are uncommon from OSA alone, OSA may cause mild to moderate increases in PAP.61 Increased PAP induced by OSA is generally reversible with CPAP treatment. These patients have marked hypoxic vasoreactivity with substantial increases in PAP occurring during mild hypoxaemia. We have observed patients in the ICU with underlying OSA who develop marked increases in PAP in the setting of mild hypoxaemia (eg, secondary to pneumonia). OSA combined with parenchymal lung disease or other daytime blood gas abnormalities (such as obesity hypoventilation syndrome, OHS) have been associated with severe pulmonary hypertension and cor pulmonale in some cases. Severe increases in PAP can occur during sleep (especially REM sleep) if the OSA remains untreated. The haemodynamic management of obese patients with OSA requires consideration of these findings.
OBESITY HYPOVENTILATION SYNDROME (OHS)
The combination of obesity (BMI >30 kg/m2) and hypercapnia (arterial carbon dioxide tension (PaCO2) >45 mm Hg (6 kPa)) during wakefulness in the absence of other known causes of alveolar hypoventilation defines the “obesity hypoventilation syndrome”.49 In severely obese (BMI ≥35 kg/m2) hospitalised patients, up to 31% have hypercapnia with no other cause for it, with the prevalence of OHS increasing with increasing BMI.62 There are several contributing mechanisms including:
excessive loading of respiratory muscles by the mass of centrally deposited fat;
disordered gas exchange, particularly when recumbent, because of atelectasis in dependent lung zones;
obesity-related upper airway narrowing; and
Sleep is a vulnerable period because of diminished drive to respiratory muscles and periods of central (non-obstructive) hypoventilation with associated hypoxaemia and hypercapnia may often last many minutes before eventual arousal.9 Up to 90% of patients with OHS have a combination of central hypoventilation and OSA with concomitant effects on ventilation and gas exchange.65 The degree of daytime hypercapnia appears to be directly related to the degree of sleep-disordered breathing,51 correction of which results in control of wakeful ventilatory failure when off ventilatory support.66 This may be achieved with CPAP in cases of OSA, but NIV is often required to augment carbon dioxide elimination where there is a central component.67 NIV allows inspiratory and expiratory pressure to be independently adjusted with end expiratory pressure to maintain pharyngeal patency (and recruit atelectatic lung, analogous to positive end expiratory pressure (PEEP)) and inspiratory pressure support to control central hypoventilation (analogous to pressure support). In some cases NIV may be necessary initially but conversion to (less expensive) CPAP may be feasible once the respiratory failure is controlled.68
While the possibility of OHS should be actively considered in severely obese patients in the outpatient setting, intensivists frequently diagnose this problem during respiratory and right heart failure.69 There may be a history of snoring and sleepiness from disturbed sleep secondary to sleep-disordered breathing. Intensivists must appreciate the pivotal role of sleep-related hypoventilation to avoid difficulty weaning these patients from ventilatory support and to prevent recurrent respiratory and right heart failure. In patients with OSA, extubation can be considered once ventilatory capacity during wakefulness is adequate; however, ongoing NIV may be required during sleep.70
Studies of ventilation during sleep help to define the problem and are preferably undertaken before patients require hospitalisation. Although the thresholds of severity of sleep-related hypoventilation, independent of daytime respiratory failure, requiring intervention with NIV remain to be defined, more than 1–2% of total sleep time spent below an oxygen saturation of 85% (equivalent arterial oxygen tension (PaO2) of approximately 55 mm Hg (7.3 kPa)) provides an independent justification for this treatment. Subsequent studies of ventilation during sleep on treatment allow a decision about the requirement for and the type (CPAP or NIV) of ongoing treatment. Thus, OHS is an important syndrome in the ICU.
OBESITY AND ABDOMINAL COMPARTMENT SYNDROME
The abdominal compartment syndrome (ACS) has been recognised relatively recently in medical patients in the ICU.71 Trauma surgeons had previously observed that trauma patients who developed anuria frequently improved following decompressive laparotomy. More recent studies have suggested that important increases in intra-abdominal pressure (IAP) can also occur in up to 20% of medical patients in the ICU. Although a variety of processes may contribute to raised IAP including ascites, ileus and intraperitoneal haemorrhage, the best predictor of ACS in multivariate analysis is BMI. Thus, obesity represents a major risk factor for ACS in critically ill patients.
The pathogenesis of ACS reflects the fact that the abdomen can behave like a closed space.72 Under normal conditions IAP is relatively low (close to atmospheric pressure). With deposition of fat and accumulation of fluid during conditions of capillary leak, gradual increases in IAP can occur. With sufficient increases in IAP, critical blood vessels can be compressed leading to ischaemia of abdominal organs. For example, increased IAP can lead to renal failure ostensibly through compression of renal venules. Involvement of other organs such as the liver, with hepatic necrosis, has also been reported.
Increased IAP can also affect cardiopulmonary physiology as it can be transmitted to the intrathoracic compartment, yielding increases in intrapleural pressure. The degree of transmission of abdominal pressure to the pleural space depends on the tension developed in the diaphragm, reflected in transdiaphragmatic pressure. Because the diaphragm can remodel with chronic increases in IAP, medical conditions such as obesity and chronic ascites tend to have a greater influence on pleural pressure than acute conditions such as intraperitoneal haemorrhage.
With regard to increases in pleural pressure, several points deserve emphasis. First, some have debated whether negative transpulmonary pressure (pleural pressure in excess of airway opening pressure) is physiologically possible.73 Several mechanisms explain how increased pleural pressures can be sustained including the development of regional atelectasis, airway closure and/or expiratory flow limitation. Second, there are apparent measurement “artefacts” which can occur in the setting of increased pleural pressures. For example, intravascular pressures—which are generally measured referenced to atmosphere—can appear unexpectedly high in patients with increased IAP; that is, a high central venous pressure or left ventricular end diastolic (wedge) pressure could be observed in the setting of hypovolaemia if the cardiac chambers are being externally compressed. Third, real haemodynamic effects of increased pleural pressure can occur since, for example, right atrial compression can limit venous return from extrathoracic structures including the head. Thus, ACS has been associated with pseudotumor cerebri as well as increases in intracranial pressure in trauma patients.74 Internists are often alarmed by neurosurgical recommendations to perform decompressive laparotomy to address intracranial hypertension. However, this strategy is effective in some cases (fig 2). Optimal patient care therefore requires an understanding of IAP changes on patient physiology and measurements.
Figure 2.
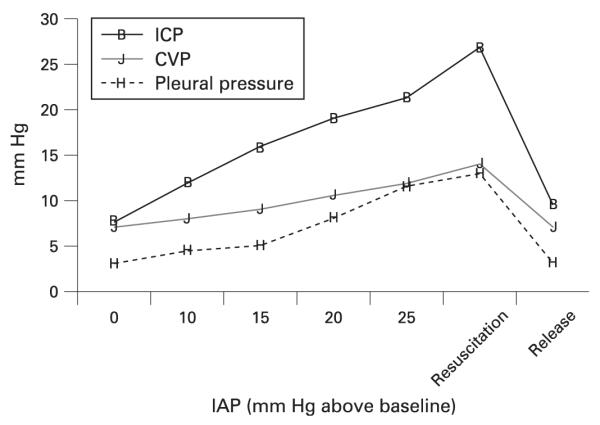
Impact of experimental manipulations of intra-abdominal pressure (IAP) on central venous pressure (CVP), pleural pressure and intracranial pressure (ICP). Note that resuscitation does not improve the intracranial pressure but release of the IAP (abdominal decompression) does lower ICP considerably. Failure to recognise increases in IAP could therefore contribute to mistreatment or poor outcome. Reproduced with permission from Citerio and Berra.95
The diagnosis of ACS requires a high clinical index of suspicion because physical examination is frequently misleading in making this diagnosis.72 A number of methods have been used to estimate IAP including measurement of intragastric pressure, common iliac venous pressure, intraperitoneal pressure (via a paracentesis needle) and bladder pressure (via a Foley catheter). The Foley catheter technique is reasonably well validated and has a relatively low risk. A needle is inserted into the side port of the Foley catheter and this catheter is transduced using the monitoring equipment for the central venous pressure. The technique requires that 50–300 ml of fluid is present in the bladder since an empty bladder may not reflect IAP whereas an overfilled bladder may create a recoil pressure across the bladder wall which again does not reflect the pressure throughout the abdominal cavity. Raising the bag of urine and observing the height of the standing column of urine as a manometer can provide an approximation of the IAP. Thus, estimation of the IAP can be straightforward if the clinician suspects ACS.75
The treatment of ACS is somewhat more challenging. If reversible pathology is present, the underlying cause should clearly be addressed. Large volume paracentesis can be helpful in cases of massive ascites. The authors have observed cases of complete reversal of renal failure following paracentesis in patients with cirrhosis who had been incorrectly diagnosed with hepatorenal syndrome, a condition associated with a very high mortality. Use of the reverse Trendelenburg position can help to reduce pressure on retroperitoneal structures. Gastric decompression, treatment of ileus and fecal disempaction can all be helpful in lowering IAP. Although decompressive laparotomy is considered the most definitive treatment, this procedure comes with obvious risk so surgeons are often reluctant to perform this procedure for such patients.
OBESITY AND ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS)
When total respiratory compliance is considered in obese patients, the effects of obesity on the chest wall must be separated from the effects attributable to decreased lung compliance, as seen in acute lung injury (ALI) and acute respiratory distress syndrome (ARDS). This distinction has major implications for the application of mechanical ventilation in obese patients.
Three important physiological concepts are critical to the selection of mechanical ventilator settings in such patients. First, the transpulmonary pressure (pressure difference between airway opening and pleural space) is the distending pressure across the lung and must be distinguished from transthoracic pressure (difference between pleural and ambient atmospheric) which is the distending pressure across the chest wall. Airway pressures applied during mechanical ventilation reflect the sum of these lung and chest wall components. In experiments designed to distinguish the effects of high inflation pressures using manipulations such as casting of the chest wall, it has clearly been shown that it is excessive transpulmonary pressure that leads to damage of the lung, a phenomenon referred to as ventilator-induced lung injury.76 The transpulmonary pressure is the critical variable determining risk of lung injury since marked increases in airway pressure may be observed with minimal lung stretch and injury when the chest wall is restricted, reflected in raised pleural (hence transthoracic) pressure. Obese patients can frequently be safely ventilated with relatively high applied pressures since transpulmonary pressures are quite low in this setting.77
Second, the recruitability of the lung—which refers to the ability to open collapsed units of the lung—must be considered. Collapse of alveoli can occur due to increases in surface tension (eg, from surfactant dysfunction) or from raised pleural pressures that effectively compress lung units leading to their collapse.78 Such units can sometimes be recruited (reopened) if the applied pressures are sufficient to overcome the opening pressure. Obese patients frequently develop considerable atelectasis, particularly in the posterior dependent lung zones.24
Third, parenchymal heterogeneity is important. This refers to the concept that high shear forces can occur in the lung, particularly at junctions of normal and abnormal lung. Classic physiology studies have estimated effective pressures in excess of 100 cm H2O at junctions of normal and abnormal lung, even when applied pressures are below the generally recommended ceiling of 30 cm H2O.79 Thus, many clinicians attempt to achieve parenchymal homogeneity (eg, by the use of recruitment manoeuvres to open collapsed alveoli80) and thereby minimise shear forces within the ventilated lung. Because obese patients frequently have considerable atelectasis, they would be predicted to have parenchymal heterogeneity even without well-established lung injury.
With these physiological principles in mind, mechanical ventilator settings can be considered. One challenge is that most clinicians do not routinely measure pleural pressure and therefore transpulmonary pressure is not known at the bedside in most cases. The clinician is therefore often in a situation of having to surmise or infer what pleural pressures are prevailing in a given patient. The failure to fully account for the chest wall effects on airway pressures may lead to inappropriate therapy, such as undertitration of PEEP. We have frequently used PEEP values of 20–25 cm H2O in obese patients to maintain oxygenation and parenchymal homogeneity.81
In patients with ALI/ARDS, randomised trials have shown a benefit on mortality with the application of 6 ml/kg tidal volume compared with 12 ml/kg.77 82 One point of major emphasis is that 6 ml/kg must be calculated on the basis of ideal body weight rather than actual measured body weight. The reason for this is that, when a patient gains weight, the lung does not actually change in size appreciably and therefore an individual whose weight increases from 70 kg to 140 kg should receive 420 ml tidal volume (rather than 840 ml). Excessive tidal volumes may be applied to patients because this important concept is overlooked. Because the number of alveoli participating in gas exchange is difficult to predict at the bedside, there is no easy way to tell the proportion of lung participating in each tidal inflation. Thus, a volume targeted approach could lead to regional overdistension even when applied pressures are below the recommended 30 cm H2O threshold.83 On the other hand, pressure targeted approaches could lead to marked increases in tidal volume, particularly in spontaneously breathing patients. Such patients can generate large transpulmonary pressures that may not be obvious to the clinician at the bedside. We believe that a satisfactory “low-stretch” result can be obtained using either volume or pressure targeted strategies, provided adjustments are made during serial bedside assessments. Regardless of how it is achieved, lung stretch should be minimised in patients with ALI/ARDS to prevent worsening lung injury.83 84
A few points relative to ALI/ARDS are noteworthy in obese patients. Such patients often have marked increases in pleural pressure and therefore we believe that the recommended ceiling in airway pressure of 30 cm H2O can be safely exceeded in such patients.73 In some cases heavy sedation and/or paralysis are required to keep such patients below this limit. In addition, when PEEP requirements are high, only very small tidal volumes can be delivered if end-inspiratory plateau pressures are kept below 30 cm H2O. In such cases we would favour allowing inflation pressures to exceed 30 cm H2O, recognising that transpulmonary pressures and therefore lung distension remain low in such cases. Permissive hypercapnia—allowing carbon dioxide values to increase and pH to fall—can also be used in such cases based on strong experimental evidence, although clinical evidence is still evolving.85
Open lung protective ventilation remains controversial since most (but not all) of the clinical trials have shown no outcome benefit to this approach.86–90 The concept relies on the issue of parenchymal homogeneity such that efforts to open collapsed alveoli and then maintain their patency using high PEEP could be beneficial. In this setting, low distending pressures are provided yielding permissive hypercapnia. The intervention, as employed by Amato et al,88 therefore requires recruitment manoeuvres, high PEEP, low tidal volume and permissive hypercapnia. High frequency oscillation techniques have been advocated by some authors to achieve this goal.91–93 Ongoing trials are examining the role of open lung protective ventilation although the existing data are equivocal at best.
CONCLUSIONS
In all these commonly encountered clinical scenarios obesity magnifies patient risk, by predisposing to the underlying condition, contributing to the associated pathophysiological derangements and complicating management from a technical and logistical point of view. Regrettably, the problem of morbid obesity is flourishing and an increasing proportion of patients in the ICU suffer from its pervasive effects, adding to its status as the one of the greatest challenges to the health of communities in the developed world. The recent demonstration of improved long-term outcome following bariatric surgery provides some source for optimism, but is likely to further increase the burden of critically ill obese patients due to rare but serious perioperative complications.94 Thus, practitioners must be aware of these important issues relevant to this special population.
Acknowledgments
Funding: AM is funded by National Institutes of Health RO1-HL73146, SCOR P50 HL060292 and AG024837 and has received consulting and/or research funding from Respironics, Cephalon, Restore Medical, Apnex Medical, Itamar Medical, Novartis, Inspiration Medical, NMT Medical and Pfizer. DH is supported by National Health and Medical Research Council grants 403953 and 303218 and an Australian Research Council Discovery Grant and has received consulting and/or research funding from ResMed and Inspiration Medical.
Footnotes
Competing interests: None.
REFERENCES
- 1.McTigue K, Larson JC, Valoski A, et al. Mortality and cardiac and vascular outcomes in extremely obese women. JAMA. 2006;296:79–86. doi: 10.1001/jama.296.1.79. [DOI] [PubMed] [Google Scholar]
- 2.Bercault N, Boulain T, Kuteifan K, et al. Obesity-related excess mortality rate in an adult intensive care unit: a risk-adjusted matched cohort study. Crit Care Med. 2004;32:998–1003. doi: 10.1097/01.ccm.0000119422.93413.08. [DOI] [PubMed] [Google Scholar]
- 3.Morris AE, Stapleton RD, Rubenfeld GD, et al. The association between body mass index and clinical outcomes in acute lung injury. Chest. 2007;131:342–8. doi: 10.1378/chest.06-1709. [DOI] [PubMed] [Google Scholar]
- 4.Yim S, Fredberg JJ, Malhotra A. Continuous positive airway pressure for asthma: not a big stretch? Eur Respir J. 2007;29:226–8. doi: 10.1183/09031936.00160206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beuther DA, Sutherland ER. Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. Am J Respir Crit Care Med. 2007;175:661–6. doi: 10.1164/rccm.200611-1717OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El-Solh A, Sikka P, Bozkanat E, et al. Morbid obesity in the medical ICU. Chest. 2001;120:1989–97. doi: 10.1378/chest.120.6.1989. [DOI] [PubMed] [Google Scholar]
- 7.Duarte AG, Justino E, Bigler T, et al. Outcomes of morbidly obese patients requiring mechanical ventilation for acute respiratory failure. Crit Care Med. 2007;35:732–7. doi: 10.1097/01.CCM.0000256842.39767.41. [DOI] [PubMed] [Google Scholar]
- 8.Young T, Peppard P, Gottlieb D. The epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 9.Malhotra A, White DP. Obstructive sleep apnoea. Lancet. 2002;360:237–45. doi: 10.1016/S0140-6736(02)09464-3. [DOI] [PubMed] [Google Scholar]
- 10.Eckert DJ, Malhotra A. Pathophysiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:144–53. doi: 10.1513/pats.200707-114MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peppard P, Young T, Palta M, et al. Prospective study of the association between sleep disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 12.Hung J, Whitford EG, Parsons RW, et al. Association of sleep apnoea with myocardial infarction in men. Lancet. 1990;336:261–4. doi: 10.1016/0140-6736(90)91799-g. [DOI] [PubMed] [Google Scholar]
- 13.Yaggi HK, Concato J, Kernan WN, et al. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–41. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 14.Punjabi N, Sorkin J, Katzel L, et al. Sleep-disordered breathing and insulin resistance in middle-aged and overweight men. Am J Respir Crit Care Med. 2002;165:677–82. doi: 10.1164/ajrccm.165.5.2104087. [DOI] [PubMed] [Google Scholar]
- 15.Vgontzas AN, Bixler EO, Chrousos GP. Sleep apnea is a manifestation of the metabolic syndrome. Sleep Med Rev. 2005;9:211–24. doi: 10.1016/j.smrv.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Sharafkhaneh A, Giray N, Richardson P, et al. Association of psychiatric disorders and sleep apnea in a large cohort. Sleep. 2005;28:1405–11. doi: 10.1093/sleep/28.11.1405. [DOI] [PubMed] [Google Scholar]
- 17.Harding SM. Sleep related gastroesophageal reflux. The tip of the iceberg is showing. J Clin Sleep Med. 2007;3:514–5. [PMC free article] [PubMed] [Google Scholar]
- 18.Eastwood PR, Katagiri S, Shepherd KL, et al. Modulation of upper and lower esophageal sphincter tone during sleep. Sleep Med. 2007;8:135–43. doi: 10.1016/j.sleep.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 19.Teran-Santos J, Jimenez-Gomez A, Cordero-Guevara J. The association between sleep apnea and the risk of traffic accidents. Cooperative Group Burgos-Santander. N Engl J Med. 1999;340:847–51. doi: 10.1056/NEJM199903183401104. [DOI] [PubMed] [Google Scholar]
- 20.George CF. Reduction in motor vehicle collisions following treatment of sleep apnoea with nasal CPAP. Thorax. 2001;56:508–12. doi: 10.1136/thorax.56.7.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hiremath AS, Hillman DR, James AL, et al. Relationship between difficult tracheal intubation and obstructive sleep apnoea. Br J Anaesth. 1998;80:606–11. doi: 10.1093/bja/80.5.606. [DOI] [PubMed] [Google Scholar]
- 22.Kim JA, Lee JJ. Preoperative predictors of difficult intubation in patients with obstructive sleep apnea syndrome. Can J Anaesth. 2006;53:393–7. doi: 10.1007/BF03022506. [DOI] [PubMed] [Google Scholar]
- 23.Williamson R. Nasotracheal intubation for head and neck surgery. Anaesthesia. 2003;58:1129–31. doi: 10.1046/j.1365-2044.2003.03494.x. [DOI] [PubMed] [Google Scholar]
- 24.Suratt PM, Wilhoit SC, Hsiao HS, et al. Compliance of chest wall in obese subjects. J Appl Physiol. 1984;57:403–7. doi: 10.1152/jappl.1984.57.2.403. [DOI] [PubMed] [Google Scholar]
- 25.Pelosi P, Croci M, Ravagnan I, et al. Total respiratory system, lung, and chest wall mechanics in sedated-paralyzed postoperative morbidly obese patients. Chest. 1996;109:144–51. doi: 10.1378/chest.109.1.144. [DOI] [PubMed] [Google Scholar]
- 26.Juvin P, Lavaut E, Dupont H, et al. Difficult tracheal intubation is more common in obese than in lean patients. Anesth Analg. 2003;97:595–600. doi: 10.1213/01.ANE.0000072547.75928.B0. [DOI] [PubMed] [Google Scholar]
- 27.Welch K, Foster G, Ritter C, et al. A novel volumetric magnetic resonance imaging paradigm to study upper airway anatomy. Sleep. 2002;25:532–42. [PubMed] [Google Scholar]
- 28.Haponik E, Smith P, Bohlman M, et al. Computerized tomography in obstructive sleep apnea: correlation of airway size with physiology during sleep and wakefulness. Am Rev Respir Dis. 1983;127:221–6. doi: 10.1164/arrd.1983.127.2.221. [DOI] [PubMed] [Google Scholar]
- 29.Horner RL, Shea SA, McIvor J, et al. Pharyngeal size and shape during wakefulness and sleep in patients with obstructive sleep apnoea. Q J Med. 1989;72:719–35. [PubMed] [Google Scholar]
- 30.Brodsky JB, Lemmens HJ, Brock-Utne JG, et al. Morbid obesity and tracheal intubation. Anesth Analg. 2002;94:732–6. doi: 10.1097/00000539-200203000-00047. [DOI] [PubMed] [Google Scholar]
- 31.Benumof JL. Obstructive sleep apnea in the adult obese patient: implications for airway management. J Clin Anesth. 2001;13:144–56. doi: 10.1016/s0952-8180(01)00232-x. [DOI] [PubMed] [Google Scholar]
- 32.El-Solh AA. Clinical approach to the critically ill, morbidly obese patient. Am J Respir Crit Care Med. 2004;169:557–61. doi: 10.1164/rccm.200309-1256CC. [DOI] [PubMed] [Google Scholar]
- 33.Epstein SK. Extubation failure: an outcome to be avoided. Crit Care. 2004;8:310–2. doi: 10.1186/cc2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rennotte MT, Baele P, Aubert G, et al. Nasal continuous positive airway pressure in the perioperative management of patients with obstructive sleep apnea submitted to surgery. Chest. 1995;107:367–74. doi: 10.1378/chest.107.2.367. [DOI] [PubMed] [Google Scholar]
- 35.Dindo D, Muller MK, Weber M, et al. Obesity in general elective surgery. Lancet. 2003;361:2032–5. doi: 10.1016/S0140-6736(03)13640-9. [DOI] [PubMed] [Google Scholar]
- 36.Pikarsky AJ, Saida Y, Yamaguchi T, et al. Is obesity a high-risk factor for laparoscopic colorectal surgery? Surg Endosc. 2002;16:855–8. doi: 10.1007/s004640080069. [DOI] [PubMed] [Google Scholar]
- 37.Gendall KA, Raniga S, Kennedy R, et al. The impact of obesity on outcome after major colorectal surgery. Dis Colon Rectum. 2007;50:2223–37. doi: 10.1007/s10350-007-9051-0. [DOI] [PubMed] [Google Scholar]
- 38.Kucher N, Tapson VF, Goldhaber SZ. Risk factors associated with symptomatic pulmonary embolism in a large cohort of deep vein thrombosis patients. Thromb Haemost. 2005;93:494–8. doi: 10.1160/TH04-09-0587. [DOI] [PubMed] [Google Scholar]
- 39.Bamgbade OA, Rutter TW, Nafiu OO, et al. Postoperative complications in obese and nonobese patients. World J Surg. 2007;31:556–61. doi: 10.1007/s00268-006-0305-0. [DOI] [PubMed] [Google Scholar]
- 40.Goulenok C, Monchi M, Chiche JD, et al. Influence of overweight on ICU mortality: a prospective study. Chest. 2004;125:1441–5. doi: 10.1378/chest.125.4.1441. [DOI] [PubMed] [Google Scholar]
- 41.Wigfield CH, Lindsey JD, Munoz A, et al. Is extreme obesity a risk factor for cardiac surgery? An analysis of patients with a BMI > or = 40. Eur J Cardiothorac Surg. 2006;29:434–40. doi: 10.1016/j.ejcts.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 42.Rose DK, Cohen MM, Wigglesworth DF, et al. Critical respiratory events in the postanesthesia care unit. Patient, surgical, and anesthetic factors. Anesthesiology. 1994;81:410–8. doi: 10.1097/00000542-199408000-00020. [DOI] [PubMed] [Google Scholar]
- 43.Sabers C, Plevak DJ, Schroeder DR, et al. The diagnosis of obstructive sleep apnea as a risk factor for unanticipated admissions in outpatient surgery. Anesth Analg. 2003;96:1328–35. doi: 10.1213/01.ANE.0000061585.09157.66. [DOI] [PubMed] [Google Scholar]
- 44.Sanders JC, King MA, Mitchell RB, et al. Perioperative complications of adenotonsillectomy in children with obstructive sleep apnea syndrome. Anesth Analg. 2006;103:1115–21. doi: 10.1213/01.ane.0000244318.77377.67. [DOI] [PubMed] [Google Scholar]
- 45.Terris DJ, Clerk AA, Norbash AM, et al. Characterization of postoperative edema following laser-assisted uvulopalatoplasty using MRI and polysomnography: implications for the outpatient treatment of obstructive sleep apnea syndrome. Laryngoscope. 1996;106(2 Pt 1):124–8. doi: 10.1097/00005537-199602000-00002. [DOI] [PubMed] [Google Scholar]
- 46.Gupta RM, Parvizi J, Hanssen AD, et al. Postoperative complications in patients with obstructive sleep apnea syndrome undergoing hip or knee replacement: a case-control study. Mayo Clin Proc. 2001;76:897–905. doi: 10.4065/76.9.897. [DOI] [PubMed] [Google Scholar]
- 47.Turner K, VanDenkerkhof E, Lam M, et al. Perioperative care of patients with obstructive sleep apnea: a survey of Canadian anesthesiologists. Can J Anaesth. 2006;53:299–304. doi: 10.1007/BF03022219. [DOI] [PubMed] [Google Scholar]
- 48.Gross JB, Bachenberg KL, Benumof JL, et al. Practice guidelines for the perioperative management of patients with obstructive sleep apnea: a report by the American Society of Anesthesiologists Task Force on Perioperative Management of patients with obstructive sleep apnea. Anesthesiology. 2006;104:1081–93. doi: 10.1097/00000542-200605000-00026. quiz 1117–8. [DOI] [PubMed] [Google Scholar]
- 49.Olson AL, Zwillich C. The obesity hypoventilation syndrome. Am J Med. 2005;118:948–56. doi: 10.1016/j.amjmed.2005.03.042. [DOI] [PubMed] [Google Scholar]
- 50.Flenley DC. Sleep in chronic obstructive lung disease. Clin Chest Med. 1985;6:651–61. [PubMed] [Google Scholar]
- 51.O’Donoghue FJ, Catcheside PG, Ellis EE, et al. Sleep hypoventilation in hypercapnic chronic obstructive pulmonary disease: prevalence and associated factors. Eur Respir J. 2003;21:977–84. doi: 10.1183/09031936.03.00066802. [DOI] [PubMed] [Google Scholar]
- 52.Jones DJ Meecham, Paul EA, Jones PW, et al. Nasal pressure support ventilation plus oxygen compared with oxygen therapy alone in hypercapnic COPD. Am J Respir Crit Care Med. 1995;152:538–44. doi: 10.1164/ajrccm.152.2.7633704. [DOI] [PubMed] [Google Scholar]
- 53.Mezzanotte WS, Tangel DJ, White DP. Waking genioglossal EMG in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanisms) J Clin Invest. 1992;89:1571–9. doi: 10.1172/JCI115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Larminat V, Montravers P, Dureuil B, et al. Alteration in swallowing reflex after extubation in intensive care unit patients. Crit Care Med. 1995;23:486–90. doi: 10.1097/00003246-199503000-00012. [DOI] [PubMed] [Google Scholar]
- 55.Parlow JL, Ahn R, Milne B. Obesity is a risk factor for failure of “fast track” extubation following coronary artery bypass surgery. Can J Anaesth. 2006;53:288–94. doi: 10.1007/BF03022217. [DOI] [PubMed] [Google Scholar]
- 56.Borracci RA, Dayan R, Rubio M, et al. Operating room extubation (ultra fast-track anesthesia) in patients undergoing on-pump and off-pump cardiac surgery. Arch Cardiol Mex. 2006;76:383–9. [PubMed] [Google Scholar]
- 57.Stone WM, Larson JS, Young M, et al. Early extubation after abdominal aortic reconstruction. J Cardiothorac Vasc Anesth. 1998;12:174–6. doi: 10.1016/s1053-0770(98)90327-5. [DOI] [PubMed] [Google Scholar]
- 58.Girault C, Daudenthun I, Chevron V, et al. Noninvasive ventilation as a systematic extubation and weaning technique in acute-on-chronic respiratory failure: a prospective, randomized controlled study. Am J Respir Crit Care Med. 1999;160:86–92. doi: 10.1164/ajrccm.160.1.9802120. [DOI] [PubMed] [Google Scholar]
- 59.Heinzer RC, Stanchina ML, Malhotra A, et al. Lung volume and continuous positive airway pressure requirements in obstructive sleep apnea. Am J Respir Crit Care Med. 2005;172:114–7. doi: 10.1164/rccm.200404-552OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kaneko Y, Floras J, Usui K, et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med. 2003;348:1233–41. doi: 10.1056/NEJMoa022479. [DOI] [PubMed] [Google Scholar]
- 61.Sajkov D, Wang T, Saunders NA, et al. Continuous positive airway pressure treatment improves pulmonary hemodynamics in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165:152–8. doi: 10.1164/ajrccm.165.2.2010092. [DOI] [PubMed] [Google Scholar]
- 62.Nowbar S, Burkart KM, Gonzales R, et al. Obesity-associated hypoventilation in hospitalized patients: prevalence, effects, and outcome. Am J Med. 2004;116:1–7. doi: 10.1016/j.amjmed.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 63.Koenig SM. Pulmonary complications of obesity. Am J Med Sci. 2001;321:249–79. doi: 10.1097/00000441-200104000-00006. [DOI] [PubMed] [Google Scholar]
- 64.Eckert DJ, Jordan AS, Merchia P, et al. Central sleep apnea: pathophysiology and treatment. Chest. 2007;131:595–607. doi: 10.1378/chest.06.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rapoport DM, Garay SM, Epstein H, et al. Hypercapnia in the obstructive sleep apnea syndrome. A reevaluation of the “Pickwickian syndrome”. Chest. 1986;89:627–35. doi: 10.1378/chest.89.5.627. [DOI] [PubMed] [Google Scholar]
- 66.Mokhlesi B, Tulaimat A, Evans AT, et al. Impact of adherence with positive airway pressure therapy on hypercapnia in obstructive sleep apnea. J Clin Sleep Med. 2006;2:57–62. [PMC free article] [PubMed] [Google Scholar]
- 67.Rapoport DM, Sorkin B, Garay SM, et al. Reversal of the “Pickwickian syndrome” by long-term use of nocturnal nasal-airway pressure. N Engl J Med. 1982;307:931–3. doi: 10.1056/NEJM198210073071507. [DOI] [PubMed] [Google Scholar]
- 68.Piper AJ, Sullivan CE. Effects of short-term NIPPV in the treatment of patients with severe obstructive sleep apnea and hypercapnia. Chest. 1994;105:434–40. doi: 10.1378/chest.105.2.434. see comments. [DOI] [PubMed] [Google Scholar]
- 69.de Llano LA Perez, Golpe R, Piquer M Ortiz, et al. Short-term and long-term effects of nasal intermittent positive pressure ventilation in patients with obesityhypoventilation syndrome. Chest. 2005;128:587–94. doi: 10.1378/chest.128.2.587. [DOI] [PubMed] [Google Scholar]
- 70.El-Solh AA, Aquilina A, Pineda L, et al. Noninvasive ventilation for prevention of post-extubation respiratory failure in obese patients. Eur Respir J. 2006;28:588–95. doi: 10.1183/09031936.06.00150705. [DOI] [PubMed] [Google Scholar]
- 71.Malbrain ML. Abdominal pressure in the critically ill: measurement and clinical relevance. Intensive Care Med. 1999;25:1453–8. doi: 10.1007/s001340051098. [DOI] [PubMed] [Google Scholar]
- 72.Malbrain ML, Cheatham ML, Kirkpatrick A, et al. Results from the International Conference of Experts on Intra-abdominal Hypertension and Abdominal Compartment Syndrome. I. Definitions. Intensive Care Med. 2006;32:1722–32. doi: 10.1007/s00134-006-0349-5. [DOI] [PubMed] [Google Scholar]
- 73.Talmor D, Sarge T, O’Donnell CR, et al. Esophageal and transpulmonary pressures in acute respiratory failure. Crit Care Med. 2006;34:1389–94. doi: 10.1097/01.CCM.0000215515.49001.A2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sugerman HJ, Felton WL, 3rd, Salvant JB, Jr, et al. Effects of surgically induced weight loss on idiopathic intracranial hypertension in morbid obesity. Neurology. 1995;45:1655–9. doi: 10.1212/wnl.45.9.1655. [DOI] [PubMed] [Google Scholar]
- 75.Loring SH, Yoshino K, Kimball WR, et al. Gravitational and shear-associated pressure gradients in the abdomen. J Appl Physiol. 1994;77:1375–82. doi: 10.1152/jappl.1994.77.3.1375. [DOI] [PubMed] [Google Scholar]
- 76.Dreyfuss D, Soler P, Basset G, et al. High inflation pressure pulmonary edema. Respective effects of high airway pressure, high tidal volume, and positive endexpiratory pressure. Am Rev Respir Dis. 1988;137:1159–64. doi: 10.1164/ajrccm/137.5.1159. [DOI] [PubMed] [Google Scholar]
- 77.Malhotra A. Low-tidal-volume ventilation in the acute respiratory distress syndrome. N Engl J Med. 2007;357:1113–20. doi: 10.1056/NEJMct074213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rimensberger PC, Pristine G, Mullen BM, et al. Lung recruitment during small tidal volume ventilation allows minimal positive end-expiratory pressure without augmenting lung injury. Crit Care Med. 1999;27:1940–5. doi: 10.1097/00003246-199909000-00037. [DOI] [PubMed] [Google Scholar]
- 79.Mead J, Takishima T, Leith D. Stress distribution in lungs: a model of pulmonary elasticity. J Appl Physiol. 1970;28:596–608. doi: 10.1152/jappl.1970.28.5.596. [DOI] [PubMed] [Google Scholar]
- 80.Talmor D, Sarge T, Legedza A, et al. Cytokine release following recruitment maneuvers. Chest. 2007;132:1434–9. doi: 10.1378/chest.07-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pelosi P, Quintel M, Malbrain ML. Effect of intra-abdominal pressure on respiratory mechanics. Acta Clin Belg. 2007;(1 Suppl):78–88. doi: 10.1179/acb.2007.62.s1.011. [DOI] [PubMed] [Google Scholar]
- 82.ARDSNET Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network (ARDSNET) N Engl J Med. 2000;342:1301–8. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 83.Terragni PP, Rosboch G, Tealdi A, et al. Tidal hyperinflation during low tidal volume ventilation in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2007;175:160–6. doi: 10.1164/rccm.200607-915OC. [DOI] [PubMed] [Google Scholar]
- 84.Hager DN, Krishnan JA, Hayden DL, et al. Tidal volume reduction in patients with acute lung injury when plateau pressures are not high. Am J Respir Crit Care Med. 2005;172:1241–5. doi: 10.1164/rccm.200501-048CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kavanagh BP, Laffey JG. Hypercapnia: permissive and therapeutic. Minerva Anestesiol. 2006;72:567–76. [PubMed] [Google Scholar]
- 86.Meade MO, Cook DJ, Arabi Y, et al. A multinational randomized controlled trial of a lung open ventilation strategy in ALI/ARDS: preliminary results. JAMA. 2008;299:637–45. doi: 10.1001/jama.299.6.637. [DOI] [PubMed] [Google Scholar]
- 87.Villar J, Kacmarek RM, Perez-Mendez L, et al. A high positive end-expiratory pressure, low tidal volume ventilatory strategy improves outcome in persistent acute respiratory distress syndrome: a randomized, controlled trial. Crit Care Med. 2006;34:1311–8. doi: 10.1097/01.CCM.0000215598.84885.01. [DOI] [PubMed] [Google Scholar]
- 88.Amato MB, Barbas CS, Medeiros DM, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338:347–54. doi: 10.1056/NEJM199802053380602. [DOI] [PubMed] [Google Scholar]
- 89.Brower RG, Lanken PN, MacIntyre N, et al. Higher versus lower positive endexpiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351:327–36. doi: 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
- 90.Mercat A, Richard JC, Brochard L, et al. Comparison of two strategies for setting PEEP in ALI/ARDS (ExPress study) JAMA. 2008;299:646–55. [Google Scholar]
- 91.Slutsky AS, Drazen JM, Ingram RH, Jr, et al. Effective pulmonary ventilation with small-volume oscillations at high frequency. Science. 1980;209:609–71. doi: 10.1126/science.6771872. [DOI] [PubMed] [Google Scholar]
- 92.Kacmarek RM, Malhotra A. High-frequency oscillatory ventilation: what large-animal studies have taught us. Crit Care Med. 2005;33(3 Suppl):S148–54. doi: 10.1097/01.CCM.0000156786.43935.A0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Derdak S, Mehta S, Stewart TE, et al. High-frequency oscillatory ventilation for acute respiratory distress syndrome in adults: a randomized, controlled trial. Am J Respir Crit Care Med. 2002;166:801–8. doi: 10.1164/rccm.2108052. [DOI] [PubMed] [Google Scholar]
- 94.Sjostrom L, Narbro K, Sjostrom CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–52. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 95.Citerio G, Berra L. Intra-abdominal hypertension and the central nervous system. In: Ivatury RR, Cheatham ML, Malbrain MLNG, et al., editors. Abdominal compartment syndrome. Landes Bioscience; Georgetown: 2006. p. 147. [Google Scholar]