Abstract
The Drosophila gene bicoid functions at the beginning of a gene cascade that specifies anterior structures in the embryo. Its transcripts are localized at the anterior pole of the oocyte, giving rise to a Bicoid protein gradient, which regulates the spatially restricted expression of target genes along the anterior–posterior axis of the embryo in a concentration-dependent manner. The morphogen function of Bicoid requires the coactivity of the zinc finger transcription factor Hunchback, which is expressed in a Bicoid-dependent fashion in the anterior half of the embryo. Whereas hunchback is conserved throughout insects, bicoid homologs are known only from cyclorrhaphan flies. Thus far, identification of hunchback and bicoid homologs rests only on sequence comparison. In this study, we used double-stranded RNA interference (RNAi) to address the function of bicoid and hunchback homologs in embryos of the lower cyclorrhaphan fly Megaselia abdita (Phoridae). Megaselia-hunchback RNAi causes hunchback-like phenotypes as observed in Drosophila, but Megaselia-bicoid RNAi causes phenotypes different from corresponding RNAi experiments in Drosophila and bicoid mutant embryos. Megaselia-bicoid is required not only for the head and thorax but also for the development of four abdominal segments. This difference between Megaselia and Drosophila suggests that the range of functional bicoid activity has been reduced in higher flies.
Body axis formation in insects is best understood in Drosophila. Polarity along the anterior–posterior axis of the Drosophila embryo rests on maternally derived protein gradients, which emanate from prelocalized mRNAs in the pole regions of the egg (1). Anteriorly localized maternal bicoid mRNA encodes a homeodomain protein (Bicoid) that specifies anterior development in a concentration-dependent manner through spatially restricted activation of genes required for segmentation (2, 3). Loss of Bicoid activity results in embryos without a head and thorax and with variable deletions and fusions of segments in the anterior abdominal region. The head and thorax are replaced by duplicated posterior terminal structures of reversed polarity (4). The morphogen-like function of Bicoid requires cooperation with the zinc finger type transcription factor Hunchback in determining the anterior segment pattern; posteriorly, Bicoid complements the homeodomain transcription factor caudal in a partially redundant manner (5–8).
Because hunchback and caudal homologs of the red flour beetle Tribolium castaneum are regulated in a Bicoid-dependent manner in transgenic Drosophila embryos, it has been postulated that a bicoid-like gene is active in the Tribolium embryo (9). In the lower dipteran species Smittia (Chironomidae, Nematocera), irradiation experiments with UV light provide indirect evidence for an anteriorly localized morphogen (10, 11). However, a general role of Bicoid in anterior patterning of insect embryos contrasts with the fact that bicoid orthologs have been found only in cyclorrhaphan flies (12–14). Moreover, different developmental processes between cyclorrhaphan and noncyclorrhaphan Diptera suggest differences in anterior patterning among those species (15–17). Finally, genetic redundancy in patterning the Drosophila embryo led to the proposition that hunchback fulfills a bicoid-like function in lower insects (5, 18). This hypothesis implies that an ancestral bicoid gene performed fewer or different patterning functions than bicoid in Drosophila.
Recently, on the basis of sequence similarity, the most basal bicoid ortholog within the phylogenetic tree of the Diptera (19, 20) was identified in the lower cyclorrhaphan fly Megaselia abdita (Phoridae) (14). Sequence comparison suggests that bicoid emerged by duplication of the Hox class 3 gene zerknüllt, which specifies extraembryonic tissue in insects (14). If bicoid derives from zerknüllt, it must have undergone functional adaptations that might be apparent when comparing bicoid gene functions in Drosophila and Megaselia embryos.
Herein, we present a functional analysis of the Megaselia-bicoid gene (Ma-bcd) and of a newly identified hunchback homolog (Ma-hb) in Megaselia embryos. In the absence of genetic tools for Megaselia, we made use of double-stranded RNA (dsRNA)-mediated gene silencing (RNAi; refs. 21–25), which reduces transcripts in a sequence-specific manner (26–32). The results support the proposal that the range of bicoid function became gradually restricted to anterior body parts in the course of dipteran evolution.
Materials and Methods
Fly Culture and Injection of Embryos.
The M. abdita Schmitz (Phoridae, Aschiza, Cyclorrhapha, Brachycera, Diptera) culture was provided by Klaus Sander (Albert-Ludwigs-Universität, Freiburg, Germany). Animals were kept on wet paper towels sprinkled with aquarium fish food “TetraRubin” (Tetra, Melle, Germany). For injection experiments, 30-min egg depositions at 21°C were used. Eggs were collected on ice, dechorionated in 50% (vol/vol) commercial bleach, attached to a coverslip with heptane glue, air dried on silica gel (Merck) for ≈6–7 min at 19°C and covered with 10S Voltalef oil (Atochem). Drosophila eggs were injected at ≈95% egg length (0% = posterior pole). Megaselia eggs were injected in either the anterior or the posterior pole at ≈95% or 5% egg length, because a distinction of the posterior from the anterior pole of Megaselia eggs was not possible under the dissecting microscope. A transjector (Eppendorf, no. 5246) and femtotips (Eppendorf) were used. The injection volume was below 100 pl as estimated from injections in oil.
Cloning of Ma-hb.
A PCR clone of Ma-hb was obtained with the primer pair AARCACCAYYTNGARTAYCA (12) and TGRCARTAYTTNGTNGCRTA (N is A, C, G, or T; R is G or A; Y is C or T) on genomic Megaselia DNA. Ma-hb cDNA from adult Megaselia females was amplified by 3′ and 5′ rapid amplification of cDNA ends on a template prepared with the Marathon cDNA Amplification Kit (CLONTECH). The predicted ORF spans 1,863 bp; the 5′ untranslated region includes 236 bp; and the 3′ untranslated region includes 53 bp. The Ma-hb sequence is deposited in GenBank under accession no. AJ295635.
RNAi.
Ma-bcd, bicoid, Ma-hb, and hunchback cDNA portions of the ORFs 1–1.5 kilobases in length were PCR amplified with the primer pairs TAATACGACTCACTATAGGGAGACCACTGTTTACGAGAAAATGGC/TAATACGACTCACTATAGGGAGACCACTCAATTGAAACAGTAGGC, TAATACGACTCACTATAGGGAGACCACTCCCATCCGCATCCG/TAATACGACTCACTATAGGGAGACCACGCCTCTCGTCCAGG, TAATACGACTCACTATAGGGAGACCACAATGCAGAATTGGGAATC/TAATACGACTCACTATAGGGAGACCACAATTCTCCTATGGGAAAC, and TAATACGACTCACTATAGGGAGACCACGATGCAGAACTGGGAGAC/TAATACGACTCACTATAGGGAGACCACCATACTTGCGCAGATGCA, respectively. The PCR primers contained a T7 promoter at their 5′ end, which was used for simultaneous sense and antisense RNA synthesis. RNA was phenol extracted, precipitated, and dissolved in water or injection buffer (100 nM NaPO4, pH 7.2/5 mM KCl). All probes were checked on an agarose gel for the formation of dsRNA before injection. If necessary, probes were denatured and reannealed in injection buffer. To this end, the probes were submerged in a 250-ml beaker of water at 95°C for 1 min and allowed to cool to ambient temperature for several hours. For Ma-hb, two additional dsRNA probes covering 1 kilobase of the 5′ or the 3′ half of the cDNA were prepared from equimolar amounts of sense and antisense RNA strands generated with SP6 and T7 RNA polymerase, respectively. Megaselia dsRNA was injected at a concentration of 7–8 μM. Drosophila dsRNA was injected at concentrations of 7–9 μM (hunchback) and 1 or 3 μM (bicoid). bicoid dsRNA injections into Drosophila embryos at a concentration of 7 μM did not increase the phenotype but led to a high percentage of unspecific defects.
Immunocytochemistry.
Hybridization to Megaselia embryos was done with DNA probes at 48°C as described for Drosophila (33) with the following modifications. To burst the vitelline layer, −80°C cold methanol was used in the devitellinization step, and the embryos were heated in methanol for 1 min to +56°C. The temperature shock was repeated two or three times during the following methanol washes. Engrailed and Even-skipped staining was done with the monoclonal antibodies 4D9 (34) and 2B8 (35), respectively. The developmental stage of early embryos was determined by nuclear Hoechst 33258 staining (1 μg/ml).
Results and Discussion
Distribution of bicoid and hunchback Transcripts in Megaselia and Drosophila.
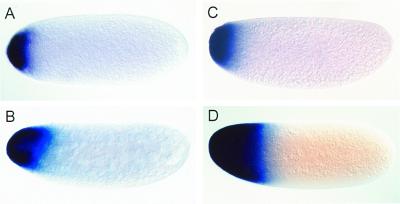
Megaselia and Drosophila eggs are the same size, and embryonic development of the two species is very similar as judged by both morphological criteria and molecular markers (14, 16, 36). In the Drosophila embryo, the maternal bicoid transcripts are localized in a narrow anterior cap, which disappears during the blastoderm stage (ref. 37; Fig. 1 A and B). In young Megaselia embryos containing four nuclei as visualized by Hoechst staining of DNA, Ma-bcd transcripts are also detected in a narrow cap (Fig. 1C). Transcripts in preblastodermal embryos, however, extend further posterior (Fig. 1D), suggesting that prelocalized transcripts have spread from the anterior pole. Thus, the patterns of localized bicoid transcripts in Megaselia and Drosophila are similar at the beginning of development but differ at subsequent stages when bicoid protein (Bicoid) is known to activate its zygotic target genes.
Figure 1.
Expression patterns of bicoid and Ma-bcd in early embryos. (A and B) Transcript distribution of bicoid in Drosophila. (C and D) Transcript distribution of Ma-bcd in Megaselia. Embryos are shown at the four-nuclei stage (A and C) and at preblastoderm stage (B and D).
In Drosophila, Bicoid activates hunchback in the anterior half of the embryo, and the posterior boundary of this expression domain is set by the concentration of Bicoid (2), serving as a measure for Bicoid's morphogenetic activity (38). In view of the different distribution of bicoid transcripts at the time when hunchback expression occurs, we asked whether this difference results in an altered expression pattern of the target gene hunchback. To address this question, we cloned the Megaselia hunchback gene and generated a cDNA by independent 3′ and 5′ rapid amplification approaches (see Materials and Methods). The rapid amplification of cDNA ends products were sequenced and compared with genomic DNA. The sequence alignment shown in Fig. 2 establishes the identity of the hunchback homolog, termed Ma-hb (Fig. 2).
Figure 2.
Alignment of Hunchback sequences from Drosophila melanogaster (Dm-Hb; ref. 54), Drosophila virilis (Dv-Hb; ref. 55), Musca domestica (Md-Hb; ref. 56), and M. abdita (Ma-Hb). Identical amino acids are shaded in gray; dots denote gaps. Asterisks mark the cysteine and histidine residues that are crucial for the formation of the zinc fingers. The numbers to the right refer to the last amino acid in each row. The highest similarity is seen in the zinc finger domains ZFD1 and ZFD2 (boxed) and in the C and D boxes (dashed boxes; ref. 57).
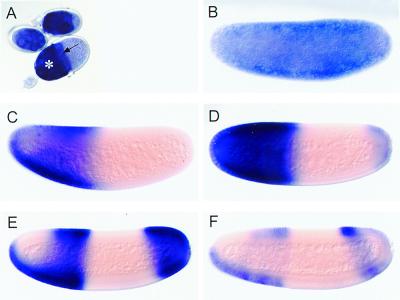
Fig. 3A shows that Ma-hb transcripts accumulate in the nurse cells and the oocyte of ovarian follicles, indicating that they are maternally expressed. At the beginning of embryogenesis, the transcripts are distributed throughout the egg (Fig. 3B). Like in Drosophila, the maternal transcripts subsequently disappear from the posterior half of the embryo (Fig. 3C) and zygotic expression of Ma-hb occurs in the anterior half of the embryo, showing a sharp on/off boundary (Fig. 3D). Transcripts accumulate also in a posterior cap. At blastoderm, the transcripts disappear from the dorsoanterior position and the posterior pole, and expression resolves in three anterior stripes (Fig. 3 E and F). The only notable difference to the hunchback expression pattern in Drosophila is therefore the maintenance of transcripts in an anterior ventral position (Fig. 3E). After gastrulation, Ma-hb is expressed in the central nervous system as found with Drosophila (not shown). These observations indicate that, although Ma-bcd transcripts extend posteriorly, the pattern of zygotic Ma-hb expression is not correspondingly altered. Thus, the blueprint of the anterior portion of the Megaselia embryo, as visualized by Ma-hb expression, is not expanded posteriorly. This finding suggests that the putative gradient of Bicoid protein in Megaselia provides the same spatial information as in Drosophila.
Figure 3.
Expression patterns of Ma-hb RNA in Megaselia. Transcript distribution in nurse cells (A; asterisk), the oocyte (A; arrow), and embryos at preblastoderm (B), progressively older syncytial blastoderm (C–E), and cellular blastoderm stage (F).
RNAi Induced Phenocopies of bicoid and hunchback Mutations in Drosophila.
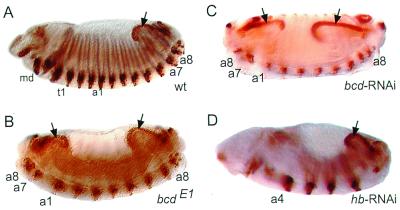
In the absence of genetic tools that allow us to establish gene functions in Megaselia, we adopted the RNAi technique to assess the function of Ma-bcd and Ma-hb. Injection of dsRNA into insect embryos has already been demonstrated to cause phenocopies of zygotically expressed segmentation genes (39–41). However, genes that are expressed and required earlier than the genes of the zygotic segmentation cascade, such as maternally derived bicoid or maternally and zygotically expressed hunchback, have not yet been examined by this technique. To test the suitability of RNAi with respect to bicoid and hunchback function, we injected bicoid dsRNA into Drosophila embryos. These embryos developed as phenocopies corresponding to the phenotypic series of bicoid mutations (ref. 4; Table 1). Strong phenocopies lack head and thorax and exhibit a duplication of posterior structures with reversed polarity. The duplicated structures include the hindgut, spiracles, and abdominal segments 9 to 7. These embryos resemble the phenotype of a null allele of bicoid (Fig. 4 B and C). Because control-injected embryos did not develop as phenocopies (Table 1), we conclude that bicoid RNAi specifically interferes with maternally derived bicoid activity and causes specific and reliable phenocopies of the bicoid mutant phenotype.
Table 1.
Effect of bicoid, hunchback, and Ma-bcd RNAi and buffer injection in Drosophila embryos
| bicoid, 1 μM | bicoid, 3 μM | hunchback, 7–9 μM | Ma-bcd, 8 μM | Injection buffer | |
|---|---|---|---|---|---|
| n | 71 | 73 | 78 | 104 | 100 |
| Percentage not developed | 21 | 22 | n.d. | 8 | 7 |
| Percentage mutant embryos | 37 | 53 | 78 | 0 | 0 |
n, number of embryos injected; n.d., not determined. Percentage of mutant embryos was identified after Engrailed staining.
Figure 4.
RNAi in Drosophila embryos. Wild-type (wt; A), bicoidE1 mutant (B), bicoid RNAi (C), and hunchback RNAi (D) embryos were stained with Engrailed antibody to visualize segments and the hindgut (arrows). For description of phenotypes see text. md, t1, a1, a4, a7, and a8 designate mandibular, prothoracic, and various abdominal segments, respectively. Anterior is to the left; dorsal is up.
Drosophila embryos lacking maternal hunchback activity develop normally, whereas embryos with only one maternal hunchback copy and lacking zygotic hunchback activity fail to develop the thorax, the posterior-most gnathal (labial) segment, and parts of abdominal segment 8, which is fused to abdominal segment 7. In addition, they have a defective central nervous system (refs. 42 and 43; unpublished observations). A weaker phenotype is observed in embryos lacking zygotic hunchback activity, if two maternal hunchback copies are provided. Such embryos lack thoracic segments 2 and 3 in addition to fused abdominal segments 7 and 8 (44). Embryos lacking both the maternal and the zygotic hunchback activities develop two to three segments of abdominal identity in reversed polarity, followed by four segments in normal orientation (43). hunchback RNAi resulted in the deletion of the thorax, variable deletions and fusions in abdominal segments 1 to 3 and 8, and defects in the gnathal segments and in the central nervous system including the brain (Table 1; Fig. 4D). Thus, the phenocopies resembled hunchback mutations, which in strength are intermediary between embryos lacking only zygotic hunchback activity and those that lack hunchback activity completely. Therefore, hunchback RNAi, in contrast to bicoid RNAi, does not cause phenocopies resembling the lack-of-function hunchback phenotype. This finding suggests that small amounts of hunchback mRNA escape RNAi-mediated degradation, providing sufficient hunchback activity to maintain the development of some anterior structures.
Ma-hb RNAi Causes hunchback-Like Phenotypes in Megaselia.
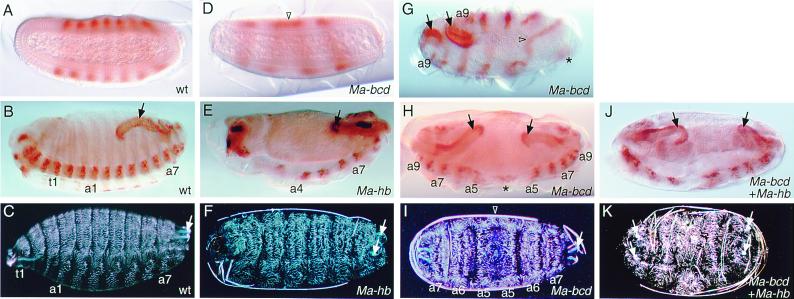
Based on the phenocopies produced by bicoid and hunchback RNAi in Drosophila embryos, we considered RNAi as a tool to address the function of the homologs in Megaselia embryos. Ma-hb RNAi resulted in the deletion of the thorax and variable defects in abdominal segments 1 to 3, and 8, the gnathal segments, and the central nervous system including the brain (Table 2; Fig. 5E). In addition, we found that the cephalopharyngeal head skeleton (which derives from several head segments; ref. 45) and spiracles (which derive from the eighth abdominal segment; ref. 46) were reduced (Fig. 5F). However, duplications of abdominal structures with reversed polarity, which are characteristic for hunchback-deficient Drosophila embryos, were not observed. To confirm the specificity of the RNAi-dependent Ma-hb phenotypes, we examined also phenotypes produced with dsRNA probes covering either the 3′ or the 5′ half of the cDNA, respectively (see Materials and Methods). The phenotypes obtained were not different from those described above. Thus, the Ma-hb phenotypes in Megaselia are similar to the hunchback phenocopies in Drosophila, which, however, corresponded only to a hypomorphic hunchback phenotype.
Table 2.
Effect of Ma-hb, Ma-bcd, Ma-hb + Ma-bcd, and bicoid RNAi in Megaselia embryos
| Ma-hb | Ma-bcd | Ma-hb + Ma-bcd | bicoid | |
|---|---|---|---|---|
| n | 602 | 322 | 108 | 377 |
| Percentage hatched | 47 | 53 | n.d. | 78 |
| Percentage not hatched | 53 | 47 | n.d. | 22 |
| Percentage mutant cuticles | 35 | 26 | 32 | <1 |
n, number of embryos injected; n.d., not determined. Percentage not hatched includes mutant cuticles and cuticles without specific defects.
Figure 5.
RNAi in Megaselia embryos. Wild-type (wt; A–C), Ma-bcd RNAi (D and G–I), Ma-hb RNAi (E and F), and Ma-bcd + Ma-hb RNAi (J and K) embryos stained with Even-skipped antibody, which labels alternate segments (A and D), Engrailed antibody, which serves as a segmental marker (B, E, G, H, and J), and cuticle preparations (C, F, I, and K). Note dorsal fusion of abdominal segments in the symmetry plane (D, G, and I; triangle) and remnants of a putative a4 neuromere (G and H; asterisk). Hindgut (black arrows) and spiracular structures (white arrows) are duplicated in Ma-bcd RNAi embryos. For description of phenotypes, see text; for abbreviations, see legend to Fig. 4.
Ma-bcd RNAi Causes Symmetrical Double Abdomen in Megaselia.
We next asked whether Ma-bcd carries a bicoid-corresponding function in Megaselia. Ma-bcd RNAi caused a reduction of anterior segments including the cephalopharyngeal head skeleton (Table 2; Fig. 5). The strongest phenotypes lack the head, thorax, and three to four abdominal segments, which are replaced by a mirror image duplication of the remaining abdomen (Fig. 5 D, H, and I). Ectopic gastrulation movements at the anterior pole can be delayed with respect to gastrulation movements at the posterior pole, resulting in asymmetric germ band extension (Fig. 5G). The relative positions of the remaining abdominal segments at different developmental stages and the unambiguous identification of the reduced abdominal segment 9 suggest that abdominal segments 1, 2, and 3 and the dorsal part of abdominal segment 4 are missing. Dorsally, the symmetry plane lies most likely in abdominal segment 5, whereas in the nerve cord, it lies in the fourth abdominal neuromere (Fig. 5 G–I). Thus, such embryos resemble a bicaudal (47–49) rather than the bicoid phenotype. Injection of bicoid dsRNA into Megaselia embryos did not produce specific defects. In less than 1% of injected embryos presumably unspecific head defects but no duplications were observed (Table 2). These results indicate that Ma-bcd RNAi in Megaselia embryos causes the specific deletion of the anterior abdominal segments, which is not observed in the corresponding RNAi experiments with Drosophila or with bicoid- or hunchback-deficient Drosophila embryos (4). It is important to note, however, that in Drosophila, a symmetrical bicaudal-like phenotype had been observed when the combined activities of bicoid and hunchback are repressed in the anterior half of the embryos indicating synergistic effects of both genes (5). We therefore asked whether coinjection of Ma-bcd and Ma-hb dsRNAs into Megaselia embryos results in a more than additive extension of anterior deletions as compared with single dsRNA injections. We found that the phenotypes obtained after combined Ma-bcd and Ma-hb dsRNA injections were similar to the sum of effects observed in independent Ma-bcd dsRNA and Ma-hb dsRNA injections. In addition, the ventral nerve cord was more disorganized and interrupted between the symmetrical abdominal halves, and the gut was reduced in size (Fig. 5 E, H, and J). These results suggest only a weak synergistic effect of Ma-bcd and Ma-hb in the abdomen.
Concluding Remarks.
Our findings provide evidence for conserved functions of bicoid and hunchback in Megaselia and Drosophila embryos. However, unlike bicoid mutant and RNAi-treated wild-type embryos of Drosophila, a deletion of three to four abdominal segments was observed after reducing Ma-bcd activity in Megaselia embryos. This result suggests that the activity range of Ma-bcd is extended consistent with the transcript distribution (Fig. 1). Possibly, Ma-bcd is repressing translation of a Megaselia caudal homolog more posteriorly than in the Drosophila embryo (50, 51) and/or Ma-bcd has a stronger influence on the activation of posterior gap genes such as Krüppel and knirps (7, 52). However, the additional requirement for bicoid might not be linked to increased hunchback function in the more primitive fly species, because the relevant expression of this gene is conserved (Fig. 3). It has been proposed that during insect evolution Bicoid gradually assumed morphogenetic functions that are carried out by Hunchback in insects more primitive than Drosophila (1, 5, 18, 53). With respect to Diptera, our results would rather argue in the opposite direction, because we have observed neither an increase of the functional range of hunchback activity in RNAi-treated Megaselia versus Drosophila embryos nor a corresponding reduction of bicoid activity in the lower fly species. Future studies directed at the identification, expression, and function of bicoid progenitors in noncyclorrhaphan Diptera will be required to test this hypothesis.
Acknowledgments
We thank Herbert Jäckle for support and discussions, Gordon Dowe for sequencing, Klaus Sander for the Megaselia culture, and Wendy Gerber, Alexander Prell, and Emanuel Busch for comments on the manuscript. The monoclonal antibodies 4D9 and 2B8 (see Materials and Methods) were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development and maintained by the University of Iowa, Department of Biological Sciences, Iowa City, IA. This work was supported by the Deutsche Forschungsgemeinschaft and by the Max-Planck-Gesellschaft.
Abbreviations
- RNAi
double-stranded RNA interference
- dsRNA
double-stranded RNA
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AJ295635).
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.190095397.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.190095397
References
- 1.St. Johnston D, Nüsslein-Volhard C. Cell. 1992;68:201–220. doi: 10.1016/0092-8674(92)90466-p. [DOI] [PubMed] [Google Scholar]
- 2.Driever W. In: in The Development of Drosophila melanogaster. Bate M, Martinez-Arias A, editors. Vol. 1. Plainview, NY: Cold Spring Harbor Lab. Press; 1993. pp. 301–324. [Google Scholar]
- 3.Rivera-Pomar R, Jäckle H. Trends Genet. 1996;12:478–483. doi: 10.1016/0168-9525(96)10044-5. [DOI] [PubMed] [Google Scholar]
- 4.Frohnhöfer H G, Nüsslein-Volhard C. Nature (London) 1986;324:120–125. [Google Scholar]
- 5.Simpson-Brose M, Treisman J, Desplan C. Cell. 1994;78:855–865. doi: 10.1016/s0092-8674(94)90622-x. [DOI] [PubMed] [Google Scholar]
- 6.Schulz C, Tautz D. Development (Cambridge, UK) 1995;121:1023–1028. doi: 10.1242/dev.121.4.1023. [DOI] [PubMed] [Google Scholar]
- 7.Rivera-Pomar R, Lu X, Perrimon N, Taubert H, Jäckle H. Nature (London) 1995;376:253–256. doi: 10.1038/376253a0. [DOI] [PubMed] [Google Scholar]
- 8.LaRosée A, Häder T, Taubert H, Rivera-Pomar R, Jäckle H. EMBO J. 1997;16:4403–4411. doi: 10.1093/emboj/16.14.4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolff C, Schröder R, Schulz C, Tautz D, Klingler M. Development (Cambridge, UK) 1998;125:3645–3654. doi: 10.1242/dev.125.18.3645. [DOI] [PubMed] [Google Scholar]
- 10.Kalthoff K, Sander K. Wilhelm Roux' Archiv. 1968;161:129–146. doi: 10.1007/BF00585968. [DOI] [PubMed] [Google Scholar]
- 11.Kalthoff K. In: in Determinants of Spatial Organization. Subtelny S, Konigsberg I R, editors. NY: Academic; 1979. pp. 97–126. [Google Scholar]
- 12.Sommer R, Tautz D. Development (Cambridge, UK) 1991;113:419–430. doi: 10.1242/dev.113.2.419. [DOI] [PubMed] [Google Scholar]
- 13.Schröder R, Sander K. Roux's Arch Dev Biol. 1993;203:34–43. doi: 10.1007/BF00539888. [DOI] [PubMed] [Google Scholar]
- 14.Stauber M, Jäckle H, Schmidt-Ott U. Proc Natl Acad Sci USA. 1999;96:3786–3789. doi: 10.1073/pnas.96.7.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sander K. Semin Cell Dev Biol. 1996;7:573–582. [Google Scholar]
- 16.Rohr K B, Tautz D, Sander K. Dev Genes Evol. 1999;209:145–154. doi: 10.1007/s004270050238. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt-Ott U. Dev Genes Evol. 2000;210:373–376. doi: 10.1007/s004270000068. [DOI] [PubMed] [Google Scholar]
- 18.Irish V, Lehmann R, Akam M. Nature (London) 1989;338:646–648. doi: 10.1038/338646a0. [DOI] [PubMed] [Google Scholar]
- 19.McAlpine J F. In: Manual of Nearctic Diptera. McAlpine J F, editor. Vol. 3. Hull, Quebec, Canada: Canadian Government Publishing Centre; 1989. pp. 1397–1518. [Google Scholar]
- 20.Yeates D K, Wiegmann B M. Annu Rev Entomol. 1999;44:397–428. doi: 10.1146/annurev.ento.44.1.397. [DOI] [PubMed] [Google Scholar]
- 21.Montgomery M K, Fire A. Trends Genet. 1998;14:255–258. doi: 10.1016/s0168-9525(98)01510-8. [DOI] [PubMed] [Google Scholar]
- 22.Fire A. Trends Genet. 1999;15:358–363. doi: 10.1016/s0168-9525(99)01818-1. [DOI] [PubMed] [Google Scholar]
- 23.Hunter C P. Curr Biol. 1999;9:R440–R442. doi: 10.1016/s0960-9822(99)80276-0. [DOI] [PubMed] [Google Scholar]
- 24.Sharp P A, Zamore P D. Science. 2000;287:2431–2433. doi: 10.1126/science.287.5462.2431. [DOI] [PubMed] [Google Scholar]
- 25.Bass B L. Cell. 2000;101:235–238. doi: 10.1016/s0092-8674(02)71133-1. [DOI] [PubMed] [Google Scholar]
- 26.Fire A, Xu S, Montgomory M K, Kostas S A, Driver S E, Mello C C. Nature (London) 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 27.Montgomery M K, Xu S, Fire A. Proc Natl Acad Sci USA. 1998;95:15502–15507. doi: 10.1073/pnas.95.26.15502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tuschl T, Zamore P D, Lehmann R, Bartel D P, Sharp P A. Genes Dev. 1999;13:3191–3197. doi: 10.1101/gad.13.24.3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hammond S M, Bernstein E, Beach D, Hannon G J. Nature (London) 2000;404:293–296. doi: 10.1038/35005107. [DOI] [PubMed] [Google Scholar]
- 30.Zamore P D, Tuschl T, Sharp P A, Bartel D P. Cell. 2000;101:25–33. doi: 10.1016/S0092-8674(00)80620-0. [DOI] [PubMed] [Google Scholar]
- 31.Ketting R F, Plasterk R H A. Nature (London) 2000;404:296–298. doi: 10.1038/35005113. [DOI] [PubMed] [Google Scholar]
- 32.Grishok A, Tabara H, Mello C C. Science. 2000;287:2494–2497. doi: 10.1126/science.287.5462.2494. [DOI] [PubMed] [Google Scholar]
- 33.Tautz D, Pfeifle C. Chromosoma. 1989;98:81–85. doi: 10.1007/BF00291041. [DOI] [PubMed] [Google Scholar]
- 34.Patel N H, Martin-Blanco E, Coleman K G, Poole S J, Ellis M C, Kornberg T B, Goodman C S. Cell. 1989;58:955–968. doi: 10.1016/0092-8674(89)90947-1. [DOI] [PubMed] [Google Scholar]
- 35.Patel N H, Condron B G, Zinn K. Nature (London) 1994;367:429–434. doi: 10.1038/367429a0. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt-Ott U, Sander K, Technau G M. Roux's Arch Dev Biol. 1994;203:298–303. doi: 10.1007/BF00457800. [DOI] [PubMed] [Google Scholar]
- 37.Berleth T, Burri M, Thoma G, Bopp D, Richstein S, Frigerio G, Noll M, Nüsslein-Volhard C. EMBO J. 1988;7:1749–1756. doi: 10.1002/j.1460-2075.1988.tb03004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Janody F, Sturny R, Catala F, Desplan C, Dostatni N. Development (Cambridge, UK) 2000;127:279–289. doi: 10.1242/dev.127.2.279. [DOI] [PubMed] [Google Scholar]
- 39.Kennerdell J R, Carthew R W. Cell. 1998;95:1017–1026. doi: 10.1016/s0092-8674(00)81725-0. [DOI] [PubMed] [Google Scholar]
- 40.Misquitta L, Paterson B M. Proc Natl Acad Sci USA. 1999;96:1451–1456. doi: 10.1073/pnas.96.4.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brown S J, Mahaffey J P, Lorenzen M D, Denell R E, Mahaffey J W. Evol Dev. 1999;1:11–15. doi: 10.1046/j.1525-142x.1999.99013.x. [DOI] [PubMed] [Google Scholar]
- 42.Kambadur R, Koizumi K, Stivers C, Nagle J, Poole S J, Odenwald W F. Genes Dev. 1998;12:246–260. doi: 10.1101/gad.12.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lehmann R, Nüsslein-Volhard C. Dev Biol. 1987;119:402–417. doi: 10.1016/0012-1606(87)90045-5. [DOI] [PubMed] [Google Scholar]
- 44.Wimmer E A, Carleton A, Harjes P, Turner T, Desplan C. Science. 2000;287:2476–2479. doi: 10.1126/science.287.5462.2476. [DOI] [PubMed] [Google Scholar]
- 45.Jürgens G. Roux's Arch Dev Biol. 1987;196:141–157. doi: 10.1007/BF00376308. [DOI] [PubMed] [Google Scholar]
- 46.Kuhn D T, Sawyer M, Packert G, Turenchalk G, Mack J A, Sprey T E. Development (Cambridge, UK) 1992;116:11–20. doi: 10.1242/dev.116.1.11. [DOI] [PubMed] [Google Scholar]
- 47.Mohler J, Wieschaus E F. Genetics. 1986;112:803–822. doi: 10.1093/genetics/112.4.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wharton R P, Struhl G. Cell. 1989;59:881–892. doi: 10.1016/0092-8674(89)90611-9. [DOI] [PubMed] [Google Scholar]
- 49.Suter B, Romberg L M, Steward R. Genes Dev. 1989;3:1957–1968. doi: 10.1101/gad.3.12a.1957. [DOI] [PubMed] [Google Scholar]
- 50.Dubnau J, Struhl G. Nature (London) 1996;379:694–699. doi: 10.1038/379694a0. [DOI] [PubMed] [Google Scholar]
- 51.Rivera-Pomar R, Niessing D, Schmidt-Ott U, Gehring W J, Jäckle H. Nature (London) 1996;379:746–749. doi: 10.1038/379746a0. [DOI] [PubMed] [Google Scholar]
- 52.Hoch M, Seifert E, Jäckle H. EMBO J. 1991;10:2267–2278. doi: 10.1002/j.1460-2075.1991.tb07763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sander K. Development (Cambridge, U.K.) 1994. Suppl., 187–191. [Google Scholar]
- 54.Tautz D, Lehmann R, Schnürch H, Schuh R, Seifert E, Kienlin A, Jones K, Jäckle H. Nature (London) 1987;327:383–389. [Google Scholar]
- 55.Treier M, Pfeifle C, Tautz D. EMBO J. 1989;8:1517–1525. doi: 10.1002/j.1460-2075.1989.tb03536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bonneton F, Shaw P J, Fazakerly C, Shi M, Dover G A. Mech Dev. 1997;66:143–156. doi: 10.1016/s0925-4773(97)00100-7. [DOI] [PubMed] [Google Scholar]
- 57.Hülskamp M, Schroeder C, Pfeifle C, Jäckle H, Tautz D. Genetics. 1994;138:125–134. [Google Scholar]