Abstract
Wnt signalling is a crucial signalling pathway controlling intestinal homeostasis and cancer. We show here that the JNK MAP kinase pathway and one of its most important substrates, the AP-1 transcription factor c-Jun, modulates Wnt signalling strength in the intestine. Transgenic gut-specific augmentation of JNK signalling stimulated progenitor cell proliferation and migration, resulting in increased villus length. In the crypt, c-Jun protein was highly expressed in progenitor cells and the absence of c-Jun resulted in decreased proliferation and villus length. In addition to several known c-Jun/AP-1 target genes, expression of Wnt target genes Axin2 and Lgr5 were stimulated by JNK activation, suggesting a cross talk of JNK to Wnt signalling. Expression of the Wnt pathway component TCF4 was controlled by JNK activity, and chromatin immunoprecipitation and reporter assays identified tcf4 as a direct c-Jun target gene. Consequently, increased JNK activity accelerated tumourigenesis in a model of colorectal carcinogenesis. As c-jun is a direct target of the TCF4/β-catenin complex, the control of tcf4 expression by JNK/c-Jun leads to a positive feedback loop that connects JNK and Wnt signalling. This mechanism regulates the physiological function of progenitor cells and oncogenic transformation.
Keywords: c-Jun, colon cancer, intestinal stem cell, JNK, TCF4
Introduction
The intestinal tissue consists of adjoining villi and crypts. The villus protrudes into the intestinal lumen and contains terminally differentiated cells. Villi consist of three main epithelial cell types that fulfil the main functions of the gut. Enteroendocrine cells release gastrointestinal hormones, enterocytes absorb nutrients and goblet cells secrete a protective mucus barrier. The crypt is formed by an invagination of the epithelial sheet into the underlying connective tissue. Most of the cells present in the crypts are immature, with the notable exception of differentiated Paneth cells, which are located at the crypt base and secrete antibacterial peptides into the crypt lumen (Humphries and Wright, 2008).
Although it has been clear that intestinal crypts harbour the stem cells of this tissue, the exact location of these cells has remained controversial (Barker et al, 2008). Using long-term label retention, a classical assay for stem cells, the cell located at position +4 relative to the crypt bottom, above the terminally differentiated Paneth cells, was suggested to be the intestinal stem cell (Potten et al, 1974). The stem cell properties of +4 cells have recently been substantiated. Bmi-1 was identified as a marker for +4 stem cells and lineage tracing studies using a knock-in allele driving an inducible creER construct from the Bmi-1 promoter showed that +4 stem cells are capable of giving rise to all intestinal cell lineages (Sangiorgi and Capecchi, 2008).
In addition, using clonal marking techniques also the crypt base columnar (CBC) cells, which are located at the crypt bottom in between paneth cells, were suggested to have stem cell like properties (Cheng and Leblond, 1974a, 1974b). CBC cells are easily identified due to their elongated shape and wedge shaped nuclei, in contrast to the +4 cells, which are difficult to distinguish morphologically from their neighbouring cells. An important finding that lends molecular support for the stem cell nature of CBC cells was the identification of Lgr5, also known as Gpr49, as a CBC cell marker gene. Moreover, elegant lineage-tracing experiments using a knock-in of an inducible creER allele showed that CBC cells could differentiate into all the intestinal cell lineages (Barker et al, 2007). Thus, there seem to co-exist two spatially separated stem cell compartments in the intestine. Whether the +4 and the CBC stem cells fulfill similar or different functions, or whether the molecular pathways regulating these two cell population are similar or distinct, is not clear.
The Wnt signalling pathway controls the proliferation and mediates developmental signals between cells. In the absence of Wnt signal, in unstimulated cells, a degradation complex consisting of the adenomatous polyposis coli (APC) tumour-suppressor protein, axin, and the glycogen synthase kinase, phosphorylates β-catenin marking it for subsequent ubiquitination and degradation. On Wnt ligand binding to its Frizzled receptor, a signalling cascade (termed canonical Wnt signalling) is triggered that destabilizes the degradation complex, allowing unphosphorylated β-catenin levels to accumulate and translocate to the nucleus where β-catenin functions as a co-factor for transcription factors of the T-cell factor/lymphoid-enhancing factor (TCF/LEF) family (Giles et al, 2003; Radtke and Clevers, 2005).
Wnt signalling can also activate β-catenin-independent pathways. An important pathway for non-canonical Wnt signal transduction is the activation of the c-Jun N-terminal kinases (JNK) (Veeman et al, 2003). JNKs are serine/threonine kinases that belong to the group of MAP kinases, which are activated by a plethora of extracellular signals and are essential mediators of signal transduction (Davis, 2000). JNK was originally identified, as the name suggests, as an activity that phosphorylated the c-Jun N-terminus (Derijard et al, 1994). The proto-oncoprotein c-Jun belongs to the AP-1 group of transcription factors, which is a crucial regulator of cellular proliferation, apoptosis and tumourigenesis (Shaulian and Karin, 2001; Eferl and Wagner, 2003). Using a leucine zipper interaction interface, c-Jun heterodimerizes and forms functional transcription factors with a number of interacting partners, including all members of the Fos and ATF families of proteins (Mechta-Grigoriou et al, 2001). AP-1 activity is strongly induced in response to numerous signals, including growth factors, cytokines and extracellular stresses (Davis, 2000). AP-1 stimulation is mediated, in part, through the phosphorylation of c-Jun by the JNKs (Davis, 2000). c-Jun N-terminal phosphorylation at the serine residues 63 and 73 and threonine residues 91 and 93 within its transactivation domain is thought to increase the transcription of target genes, one of which is the c-jun gene itself (Angel et al, 1988).
The Wnt signalling pathway is believed to be the major signalling pathway controlling intestinal homeostasis and cancer. c-jun is a well-characterized Wnt target gene (Mann et al, 1999; Staal et al, 2004) and absence of c-jun significantly delays tumourigenesis in mice heterozygous for a nonsense mutation at codon 850 of the Apc gene (ApcMin/+), which develop multiple intestinal neoplasias due to excessive canonical Wnt signalling (Moser et al, 1993; Nateri et al, 2005). Although Wnt signalling seems to be essential for intestinal stem cell function and maintenance, little is known about the contribution and function of other signalling pathways. In this study, we show that the JNK MAP kinase signalling pathway and its main substrate, the c-Jun transcription factor, have an important role in intestinal progenitor cells and oncogenic transformation.
Results
Transgenic JNK pathway activation in the gut
Loss-of-function studies in the mouse have yielded important clues about JNK function (Yang et al, 1997; Kuan et al, 1999; Sabapathy et al, 1999; Chang et al, 2003), but the interpretation of the mutant phenotypes has been complicated by genetic redundancy as the JNK protein family is encoded by three genes jnk1, jnk2 and jnk3 (Gupta et al, 1996; Weston and Davis, 2002). It is conceivable that essential roles of MAP kinase signalling have been masked by redundancy and have remained undetected. Therefore, we devised a strategy that allows to reproducibly activate JNK signalling in mice.
To investigate the significance of JNK activation in the intestine, we generated transgenic mice allowing the overexpression of a constitutively active JNKK2-JNK1 fusion protein (Zheng et al, 1999). A cassette encoding a fusion protein of β-galactosidase and NeoR (β-geo) flanked by loxP sites prevents the expression of constitutively active JNK1 (JNKK2-JNK1) before Cre-mediated recombination (β-geo–JNKK2-JNK1) (Figure 1A). Floxed single transgenic mice were crossed with Villin-cre transgenic mice previously shown to provide efficient gut-specific Cre activity (el Marjou et al, 2004).
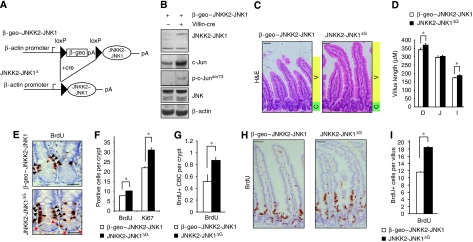
Figure 1.
JNK signalling increases progenitor cell proliferation and villus length. (A) Scheme of the β-geo–JNKK2-JNK1 construct before and after Cre recombination. When these single transgenic mice are crossed to Cre transgenic mice, the β-geo cassette is excised and the JNKK2-JNK1 cDNA is expressed. (B) Protein lysates from unrecombined β-geo–JNKK2-JNK1 and JNKK2-JNK1ΔG intestines were analysed for JNK1, c-Jun, ser 73 phosphorylated c-Jun (p-c-Junser73), total JNK and β-actin (loading control) expression. (C) Haematoxylin and eosin staining of duodenum epithelium from β-geo–JNKK2-JNK1 and JNKK2-JNK1ΔG mice. Green shading denotes proliferative zone (crypt, C) and yellow shading marks the zone of differentiation (villus, V). All animals were killed between 8–12 weeks. Scale bar represents 50 μM. (D) Quantification of the villus length from the base of the villus to the villus apex of β-geo–JNKK2-JNK1 and JNKK2-JNK1ΔG intestines. Histogram represents the villus length as mean ± s.e.m. in different regions of the gut (D=duodenum, J=jejunum and I=Ileum) (*P⩽0.05; student's t test). (E) Immunohistochemistry for BrdU on representative crypts of β-geo–JNKK2-JNK1 and JNKK2-JNK1ΔG intestines 2 h BrdU post-injection. Black arrowheads represent BrdU+ proliferative progenitors whereas red arrow heads indicate BrdU+ columnar base cells (CBC). Scale bar represents 30 μM. (F) Quantification of BrdU+ and Ki67+ cells in β-geo–JNKK2-JNK1 and JNKK2-JNK1ΔG crypts (*P⩽0.05; student's t test). (G) BrdU+ CBC quantification in β-geo–JNKK2-JNK1 and JNKK2-JNK1ΔG crypts (*P⩽0.05; student's t test). (H) Immunohistochemistry for BrdU on representative sections of β-geo–JNKK2-JNK1 and JNKK2-JNK1ΔG intestines 24 h BrdU post-injection. (I) Quantification of BrdU+ cells in the villi of β-geo–JNKK2-JNK1 and JNKK2-JNK1ΔG intestines 24 h BrdU post-injection (*P⩽0.05; student's t test). (n and P values are detailed in Supplementary Table S1).
In the absence of Cre recombinase, JNKK2-JNK1 fusion protein was not detectable in the intestine of β-geo–JNKK2-JNK1 single transgenic mice (Figure 1B), but JNKK2-JNK1 expression was induced in β-geo–JNKK2-JNK1;Villin-cre+ double transgenic mice (designated JNKK2-JNK1ΔG mice). c-Jun N-terminal phosphorylation was stimulated in JNKK2-JNK1ΔG mice, and presumably because of the autoregulation of the c-jun promoter by c-Jun, led to increased c-Jun protein levels (Figure 1B). JNKK2-JNK1ΔG mice developed normally and were indistinguishable from their control littermates. Histological analysis showed that the gross morphology of the intestine was normal in JNKK2-JNK1ΔG mice, and that all four differentiated cell types of the intestine (paneth cells, enterocytes, goblet cells and enteroendocrine cells) were present (Figure 1C and Supplementary Figure 1).
JNK signalling increases intestinal cell proliferation and villus length
Although intestinal tissue architecture and cell differentiation was unaffected by increased JNK signalling, we noted that the average villus length seemed to be increased in JNKK2-JNK1ΔG mice, which was corroborated by morphometric quantification (Figure 1C, D; and Supplementary Figure 2). Average villus length was slightly but consistently increased throughout the intestine in JNKK2-JNK1ΔG mice compared with control mice, albeit not to the same extent. This suggested that JNK signalling increased the total number of cells present per villus unit.
JNK/c-Jun signalling controls proliferation in a number of cell types (Johnson et al, 1993; Hess et al, 2004), therefore we determined whether the increase in villus length might be caused by a pro-proliferative role of JNK signalling. At the crypt–villus junction, rapidly proliferating transit-amplifying (TA) cells are present that are capable of differentiating towards the intestinal cell lineages. Both bromodeoxyuridine (BrdU) labelling (Figure 1E) and immunohistochemistry (IHC) for the proliferation marker Ki67 (data not shown) indicated that the number of cycling transit-amplifying cells per crypt was increased by augmented JNK signalling in the small intestine (Figure 1E black arrowheads and Figure 1F; Supplementary Figure 2) and also in the colon (Supplementary Figure 3). In contrast to several other stem cell populations, including the +4 cells, CBC cells are frequently cycling and incorporate BrdU (Figure 1E red arrowheads). Activation of the JNK pathway in JNKK2-JNK1ΔG mice significantly increased the percentage of CBC cells incorporating BrdU by 50% (Figure 1G).
JNK signalling also has well-established functions in the regulation of cell death and cell migration (Shaulian and Karin, 2002; Xia and Karin, 2004). However, survival of intestinal cells was not altered in JNKK2-JNK1ΔG mice (Supplementary Figure 4). In contrast, long-term BrdU labelling showed an increased rate of migration across the crypt–villus axis (Figure 1H, I). Thus, it seems that stimulation of JNK signalling leads to increased cell number and villus length by regulating proliferation and migration of progenitor cells.
JNK signalling controls both AP-1 and Wnt target genes
To investigate in more detail the function of JNK signalling in intestinal homeostasis, we carried out IHC for a crucial substrate of the JNK family of kinases, the c-Jun transcription factor. Staining for c-Jun protein was detected at the apex of the villi (data not shown), but in the crypt c-Jun protein was highly expressed in CBC cells (Figure 2A red arrowheads).
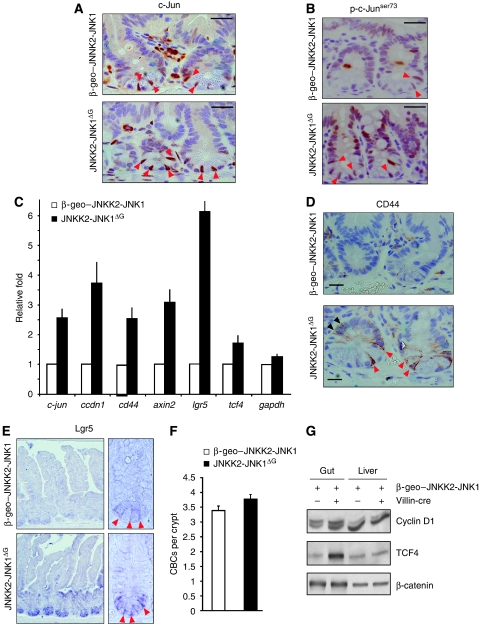
Figure 2.
Wnt target gene activation in JNKK2-JNK1ΔG mice. (A, B) Immunohistochemistry for c-Jun (A) or p-c-Junser73 (B) on representative crypts of β-geo–JNKK2-JNK1 and JNKK2-JNK1ΔG intestines. Red arrowheads represent c-Jun positively labelled columnar base cells (CBC). Scale bar represents 30 μM. (C) qRT–PCR analysis of c-jun, ccdn1, cd44, axin2, lgr5, tcf4 and gapdh transcripts in JNKK2-JNK1ΔG intestines compared with β-geo–JNKK2-JNK1. The data are normalized to β-actin and represented as fold induction over β-geo–JNKK2-JNK1 mice. (D) Immunohistochemistry for CD44 on representative crypts of β-geo–JNKK2-JNK1 and JNKK2-JNK1ΔG intestines. Red arrowheads indicate CD44 staining inCBC whereas black arrowheads indicate CD44+ proliferative progenitors. Scale bar represents 10 μM. (E) Lgr5 in situ hybridization in comparable regions from β-geo–JNKK2-JNK1 and JNKK2-JNK1ΔG intestines. (F) Quantification of CBCs in β-geo–JNKK2-JNK1 and JNKK2-JNK1ΔG crypts. (G) Western blot analysis of protein lysates from unrecombined β-geo–JNKK2-JNK1 and JNKK2-JNK1ΔG intestine and liver for cyclinD1, TCF4 and β-catenin expression.
Moreover, phosphorylated c-Jun at serine 73 (p-c-Junser73) as a readout for JNK activity was detected in both CBC and TA progenitor cells and was elevated in JNKK2-JNK1ΔG mice compared with controls (Figure 2B).
Importantly, staining for phosphorylated c-Jun was absent in junAA homozygous mutant mice, in which the JNK phosphoacceptor serines 63 and 73 are mutated to alanines and which served as a negative control for the IHC (Behrens et al, 1999) (Supplementary Figure 5). The expression of c-Jun and p-c-Junser73 indicates that JNK activity is present in progenitor cells and increased in JNKK2-JNK1ΔG mice.
To understand the molecular mechanism of how JNK signalling controls intestinal stem cell function, we investigated the expression of known target genes of the JNK/c-Jun signalling pathway. c-Jun autoregulates the transcription of the c-jun gene and accordingly c-jun mRNA levels were increased in JNKK2-JNK1ΔG intestine (Figure 2C)(Angel et al, 1988). The expression of cyclinD1 (ccnd1) is regulated by both JNK and Wnt signalling (Tetsu and McCormick, 1999; Wisdom et al, 1999; Wulf et al, 2001), and cyclinD1 expression was also increased in JNKK2-JNK1ΔG mice. CD44 is expressed in the crypt and induced in intestinal tumours (Wielenga et al, 1999; Sansom et al, 2004). cd44 is a c-Jun target gene both in the intestine and in neurons (Raivich et al, 2004; Nateri et al, 2005), and augmentation of JNK signalling significantly induced cd44 mRNA expression (Figure 2C). IHC showed that CD44-protein expression was greatly augmented in CBC cells and transient amplifying cells in the crypts of JNKK2-JNK1ΔG mice (Figure 2D arrowheads).
As Wnt signalling is a key pathway in intestinal stem cells, we also investigated the regulation of bona fide Wnt target genes. Unexpectedly, JNK signalling also substantially increased the expression of the classical Wnt target genes axin2 and lgr5 (also called gpr49) (Jho et al, 2002; Van der Flier et al, 2007). Lgr5 is highly expressed in CBC cells and has been used as a marker gene to define the CBC cells (Barker et al, 2007). It was conceivable that the increased lgr5 mRNA abundance was due to an increased number of CBC cells, or alternatively, activation of JNK signalling might change the expression pattern of lgr5 and stimulate lgr5 expression inappropriately in differentiated cell types. However, in-situ hybridization for lgr5 mRNA showed that the cell type specificity of lgr5 expression was not altered in JNKK2-JNK1ΔG mice, which as in controls was largely confined to the bottom of the crypt. Instead, lgr5 levels in CBC cells were noticeably increased in JNKK2-JNK1ΔG mice (Figure 2E arrowheads). Moreover, quantification showed normal numbers of CBC cells in JNKK2-JNK1ΔG mice (Figure 2F). Therefore, activation of JNK signalling stimulates both AP-1 and Wnt target gene expression in progenitor cells.
Wnt target gene induction in the gut is mainly controlled by the TCF4 transcription factor. tcf4 (encoded by the tcf7l2 gene) mRNA levels were augmented in JNKK2-JNK1ΔG mice (Figure 2C), suggesting a potential mechanism of JNK-mediated Wnt target gene induction. Western blot analysis showed higher levels of cyclinD1 and TCF4 protein in the gut of JNKK2-JNK1ΔG mice (Figure 2F). The expression of cyclinD1 and TCF4 in the liver, where Villin-cre is not active and the JNKK2-JNK1 fusion protein is not expressed (data not shown), was comparable (Figure 2G).
Decreased progenitor cell proliferation and villus length in the absence of c-Jun
The expression of c-Jun in progenitor cells indicated a potential role of c-Jun in this cell population. To directly test the importance of c-Jun in intestinal homeostasis, we used mice with a tissue-specific inactivation of c-jun in the gut (c-junf/f; villin-cre+ mice or c-junΔG mice)(Behrens et al, 2002; el Marjou et al, 2004; Nateri et al, 2005). IHC staining for c-Jun was not detected in the intestine of c-junΔG mice, confirming the deletion of the floxed c-jun gene in progenitor cells and the specificity of the IHC staining (Figure 3A arrowheads). As in JNKK2-JNK1ΔG mice, the gross intestinal architecture and histological appearance of c-junΔG intestine was normal. However, the average villus length in c-junΔG mice was reduced (Figure 3B). Morphometric quantification showed that there was subtle, but reproducible and statistically significant reduction of villus length in the absence of c-jun (Figure 3C). Not all regions of the gut were affected to the same extent by increased JNK signalling, possibly because of the different histological structure and biology of distinct regions of the intestine. Inactivation of c-jun also led to an appreciable reduction in the percentage of cells incorporating BrdU (Figure 3D, E) whereas c-jun deletion had no significant effect on CBC cell number (Figure 3F, G). Moreover, quantitative PCR and western blot analysis showed diminished levels of cyclinD1, TCF4 and Lgr5 mRNA, and TCF4 and cyclinD1 protein in c-junΔG gut (Figure 3H, I). Thus, inactivation of c-jun leads to the opposite phenotype than augmentation of JNK signalling.
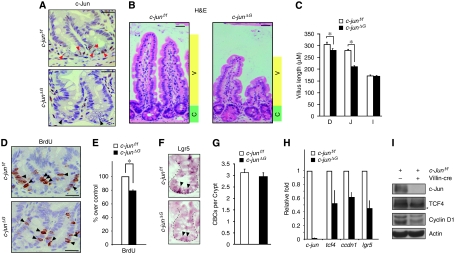
Figure 3.
Absence of c-Jun decreases crypt cell proliferation and villus length. (A) Immunohistochemistry for c-Jun on representative crypts from c-junf/f and c-junΔG intestines. Red arrow heads represent c-Jun positively labelled columnar base cells (CBC), black arrow heads represent c-Jun negative CBCs. Scale bar represents 30 μM. (B) Haematoxylin and eosin staining of jejunum epithelium from c-junf/f and c-junΔG mice. Green shading denotes proliferative zone (crypt, C) and yellow shading marks the zone of differentiation (villus, V). All animals were killed between 8–12 weeks. Scale bar represents 50 μM. (C) Quantification of the villus length from the base of the villus to the villus apex of c-junf/f and c-junΔG intestines. Histogram represents the villus length as mean ± s.e.m. in different regions of the gut (D=duodenum, J=jejunum and I=Ileum) (*P⩽0.05; student's t test). (D) Immunohistochemistry for BrdU on representative crypts from c-junf/f and c-junΔG intestines. Black arrowheads represent BrdU+ proliferative progenitors. Scale bar represents 25 μM. (E) Quantification of BrdU+ cells is represented in the histogram and expressed as % of positive cells per crypt, considering c-junf/f as 100% (*P⩽0.05; student's t test). (F) Lgr5 in situ hybridization in comparable crypts from c-junf/f and c-junΔG intestines. (G) Quantification of CBCs in c-junf/f and c-junΔG crypts. (H) qRT–PCR analysis of c-jun, ccdn1 and tcf4 transcripts in c-junΔG intestines compared with c-junf/f. The data are normalized to β-actin and represented as fold induction over c-junf/f mice. (I) Western analysis of protein lysates from c-junf/f and c-junΔG intestines for c-Jun, TCF4, cyclinD1 and β-actin (loading control). * indicates non-specific band. (n and P values are detailed in Supplementary Table S1).
tcf4 is a direct c-Jun target gene
The deregulation of tcf4 expression in JNKK2-JNK1ΔG mice suggested a potential mechanism of cross talk between JNK and Wnt signalling. Therefore we examined the regulation of TCF4 by JNK signalling in more detail. Treatment of HCT116 colon cancer cells with the JNK activator anisomycin (Ans) stimulated c-Jun and TCF4 expression (Figure 4A), whereas a pharmacological JNK inhibitor (JNKi) reduced c-jun and tcf4 mRNA and protein levels (Figure 4B, C). Thus, tcf4 expression like c-jun expression, is controlled by JNK activity. c-Jun overexpression resulted in increased TCF4 protein levels (Figure 4D). Moreover, to investigate whether endogenous c-Jun is required for tcf4 expression, we generated a stable polyclonal HCT116 cell line expressing a lentivirus-mediated c-jun knockdown shRNA hairpin construct and a matched control cell line expressing an irrelevant shRNA hairpin. c-jun depletion significantly reduced tcf4 mRNA and TCF4 protein (Figure 4E, F). Moreover, also cyclinD1 and, importantly, axin2 mRNA levels were noticeably reduced (Figure 4E). Consequently the JNK/c-Jun pathway was required for efficient proliferation of HCT116 tumour cells as both c-jun knock down and pharmacological JNK inhibition reduced growth of these cells (Supplementary Figure 6).
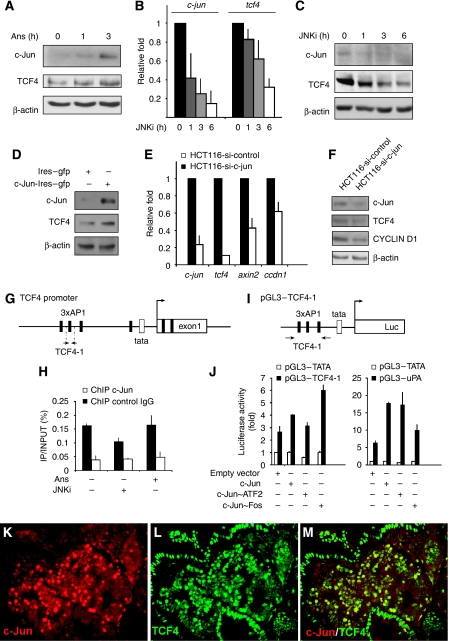
Figure 4.
tcf4 is a direct c-Jun target gene. (A) Western blot analysis of c-Jun and TCF4 protein levels in HCT116 cells treated with anisomycin (Ans) for the indicated time periods. (B) qRT–PCR analysis of c-jun and tcf4 transcripts in HCT116 cells treated with SP600125 (JNKi) for the indicated time periods. (C) Western analysis of protein lysates from HCT116 cells treated with SP600125 (JNKi) for c-Jun, TCF4 and β-actin (loading control). (D) Western blot analysis of c-Jun and TCF4 protein levels in HCT116 transfected with an expression plasmid for c-Jun (c-Jun-Ires–gfp) or empty vector (Ires–gfp). (E) qRT–PCR analysis of c-jun, tcf4, axin2 and ccdn1 transcripts in HCT116-si-c-jun (stable cell line that express an shRNA against c-jun) or HCT116-si-control (expressing an irrelevant shRNA) (F) Western blot analysis of c-Jun, TCF4, cyclinD1 and β-actin (loading control) in HCT116-si-c-jun and HCT116-si-control cell lines. (G) Schematic representation of TCF4 promoter. Black rectangles denote AP1 consensus binding sites. Arrows indicate approximate positions of qPCR primers used in ChIPs. (H) ChIP was carried out using HCT116 cells treated for 2 h with JNK inhibitor (JNKi) or Ans and c-Jun binding to the TCF4-1 region was determined by qPCR. Data is represented as IP/INPUT (%). Rabbit IgG antibody was used as an isotype control. (I) Schematic representation of pGL3–TCF4-1. (J) HCT116 cells were transfected with pGL3–TCF4-1, pGL3–uPA or pGL3–TATA together with c-Jun, c-Jun∼ATF2 and c-Jun∼Fos overexpression vector (or empty vector as control). Data represent luciferase activity relative to pGL3–TATA+empty-vector-transfected cells. (K–M) Double immunofluorescence for c-Jun (red; K) and TCF4 (green; L) and the merge image (M) on paraffin section from ApcMin/+ tumour.
To investigate the regulation of tcf4 expression, we screened the tcf4 promoter for the presence of AP-1/c-Jun binding sites. Computational transcription factor binding site predictions identified two clusters of AP-1/c-Jun binding sites. At position −996 to −833 bp relative to the TATA box, three AP-1 sites were predicted closely adjacent to each other, and a second cluster of binding sites within the first exon of the tcf4 gene (Figure 4G). To investigate the relevance of these sites, c-Jun chromatin immunoprecipitation (ChIP) assays were carried out. c-Jun ChIP showed efficient binding to the cluster of AP-1 sites in the tcf4 promoter, but not to the cluster in the tcf4 coding region (Supplementary Figure 7). These findings are in agreement with a genome-wide ChIP-on-chip analysis, which identified the tcf4 promoter region as a target for phosphorylated c-Jun (Hayakawa et al, 2004). c-Jun autoregulates its transcription through two proximal binding sites (Stein et al, 1992; Nateri et al, 2005) and c-jun is probably the best characterized gene regulated by JNK signalling (see also Figure 4A–C). The regulation of c-Jun binding to the tcf4 promoter was similar to c-Jun interaction with the c-jun promoter. JNK activation by Ans-stimulated c-Jun binding to both promoters whereas pharmacological JNK inhibition (JNKi) reduced it (Figure 4H; Supplementary Figure 7).
To further analyse the regulation of the tcf4 promoter, a 375 bp fragment containing the three AP-1/c-Jun sites of the tcf4 promoter was fused to a luciferase reporter gene (tcf4–luc, Figure 4I). AP-1 is a dimeric transcription factor that is made up of the members of the Jun, Fos and ATF protein families. Different dimer combinations have different DNA-binding specificities: c-Jun/ATF2 dimers (and c-Jun homodimers) preferentially bind to the 8-bp CRE site, whereas c-Jun/c-Fos dimers preferentially bind to 7-bp TRE sites. To investigate potential AP-1 dimer specificity in tcf4–luciferase regulation, we made use of tethered single-chain AP-1 dimers (Bakiri et al, 2002). We carried out a side-by-side comparison in HCT116 cells of the tcf4–luc construct with a classical AP-1 reporter construct, urokinase plasminogen activator-1 (uPA)–luciferase, that contains a well-characterized CRE AP-1 site, to have a benchmark for the extent of activation that can be achieved. c-Jun overexpression activated uPA–luciferase 3-fold, compared with 1.5-fold for the tcf4–luciferase construct (Figure 4J). In agreement with the presence of a CRE site in the uPA–luciferase, c-Jun/ATF2 dimers activated this construct to similar degree as c-Jun, but a c-Jun/c-Fos dimer was less efficient. Interestingly, Jun/ATF2 dimers were very inefficient on tcf4–luciferase, but activation through c-Jun/c-Fos dimer was substantial and comparable to uPA–luciferase activation by Jun/ATF2 dimer. Thus, the tcf4 promoter fragment containing the c-Jun binding site identified by ChIP does mediate transcriptional induction in response to AP-1 activation. In addition, strong activation of Wnt signalling in adenomas of aged ApcMin/+ heterozygous mice resulted in c-Jun expression, and TCF4 was found to be highly expressed in c-Jun-expressing tumour cells (Figure 4K–M). These data indicate that tcf4 is a direct target gene of JNK/c-Jun.
Activation of JNK signalling accelerates colitis-induced tumourigenesis, but not tumourigenesis triggered by mutant APC
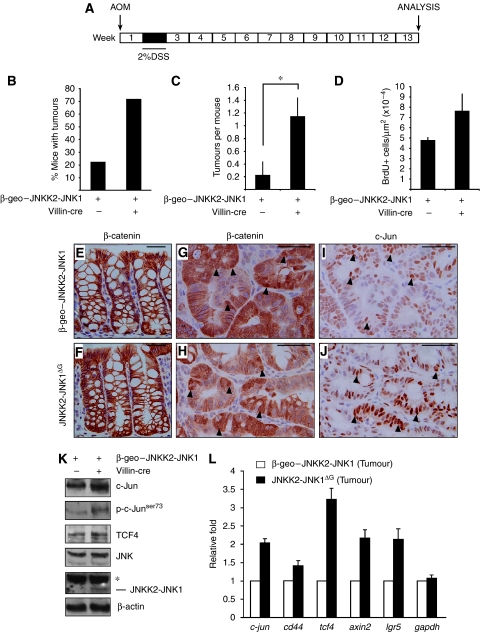
Chronic inflammation (colitis) increases the risk of colon-cancer development in humans (Itzkowitz and Yio, 2004). In mice, administration of azoxymethane (AOM) followed by dextran sodium sulphate (DSS) ingestion causes severe colonic inflammation and the subsequent development of colitis-associated tumours (Figure 5A). AOM/DSS-induced tumours most frequently show mutations of the β-catenin gene in its GSK-3β phosphorylation consensus motif, thereby leading to constitutive activation of the Wnt signalling pathway (Takahashi and Wakabayashi, 2004). Using this protocol, control mice develop tumours in the colon approximately 20 weeks after the treatment (Neufert et al, 2007). However, even at 13 weeks, some JNKK2-JNK1ΔG mice showed distress and weight loss. Quantification showed that both tumour incidence and tumour number was increased in AOM/DSS-treated JNKK2-JNK1ΔG mice compared with controls (Figure 5B, C). Tumour cell proliferation was significantly increased by augmented JNK signalling (Figure 5D). In adjacent colonic tissue, β-catenin was mainly detected at the cell membrane (Figure 5E, F), but tumours in both control and JNKK2-JNK1ΔG mice contained cells showing nuclear accumulation of β-catenin protein (Figure 5G, H arrowheads). c-Jun levels were increased in JNKK2-JNK1ΔG adenomas compared with control tumours (Figure 5I, J). Co-staining for β-catenin and c-Jun showed a tendency that c-Jun protein expression was increased in tumour cells with nuclear β-catenin, but the percentage of tumour cells showing nuclear accumulation of β-catenin was comparable in JNKK2-JNK1ΔG mice and control tumours (Supplementary Figure 8). Biochemical analysis of tumour material showed that the JNKK2-JNK1 transgene was expressed in adenomas and the levels of phosphorylated c-Jun and TCF4 were augmented (Figure 5K). Presumably as a consequence, the transcription of both AP-1 target genes like c-jun and cd44 and of the Wnt target genes axin-2 and lgr5 was increased (Figure 5L). Thus, activation of JNK/c-Jun signalling leads to increased susceptibility to colitis-induced tumourigenesis.
Figure 5.
JNK overexpression accelerates tumourigenesis in the AOM/DSS model of colon carcinoma. (A) Schematic representation of the experimental design followed. (B) Quantification of incidence of tumours. Data represent the % of mice with tumours (JNKK2-JNK1 (n=9) and JNKK2-JNK1ΔG (n=7)). (C) Quantification of number of AOM/DSS-induced tumours in β-geo–JNKK2-JNK1 and JNKK2-JNK1ΔG mice (*P⩽0.05; student's t test). (D) Quantification of BrdU+ cells per tumour area. (E–J) Immunohistochemistry for β-catenin in large bowel crypts adjacent to tumours (E, F) and β-catenin (G,H) and c-Jun (I,J) staining on AOM/DSS-induced tumours of β-geo–JNKK2-JNK1 and JNKK2-JNK1ΔG mice. Black arrowheads indicate some nuclear β-catenin or c-Jun positively labelled cells. Scale bar represents 50 μM. (K) Western blot analysis of c-Jun, p-c-Junser73, TCF4, JNK, JNKK2-JNK1 and β-actin as control in JNKK2-JNK1ΔG and β-geo–JNKK2-JNK1 AOM/DSS-induced colorectal tumours. (L) qRT–PCR analysis of c-jun, cd44, tcf4, axin2, lgr5 and gapdh transcripts in JNKK2-JNK1ΔG and β-geo–JNKK2-JNK1 AOM/DSS-induced colorectal tumours. The data are normalized to β-actin and represented as fold induction over β-geo–JNKK2-JNK1 mice tumour. (n and P values are detailed in Supplementary Table S1).
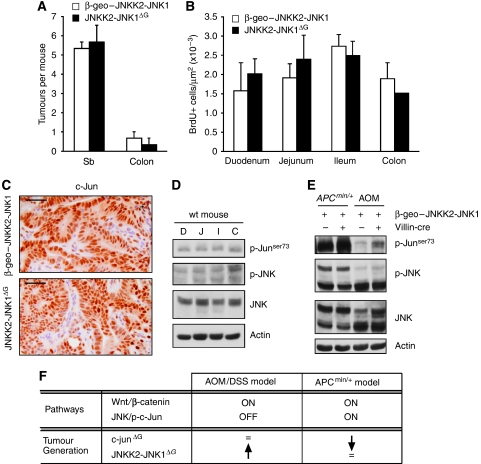
To analyse JNK function in intestinal tumourigenesis in more detail, we analysed the effect of JNK activation in tumours triggered by mutant Apc. Unexpectedly, the presence of the JNKK2-JNK1ΔG transgene did neither alter tumour number nor tumour cell proliferation in an ApcMin/+ genetic background (Figure 6A, B). Moreover, c-Jun protein levels in ApcMin/+:JNKK2-JNK1ΔG compound-mutant tumours were comparable to ApcMin/+ tumours (Figure 6C). Thus, augmentation of JNK signalling has differential effects in two different models of intestinal tumourigenesis.
Figure 6.
JNK overexpression in the APCMin/+ model of intestinal tumourigenesis. (A) Quantification of number of tumours in β-geo–JNKK2-JNK1 and JNKK2-JNK1ΔG mice in an APCMin/+ background. (B) Quantification of BrdU+ cells per area in tumours found in different intestinal regions. (C) Immunohistochemistry for c-Jun staining on APC-induced tumours of β-geo–JNKK2-JNK1 and JNKK2-JNK1ΔG mice. Scale bar represents 50 μM. (D) Western blot analysis of p-c-Junser73, p-JNK, JNK and β-actin in different intestinal regions in wild-type mouse (D: duodenum, J: jejunum, I: ileum and C: colon). (E)Western blot analysis of p-c-Junser73, p-JNK, JNK and β-actin in JNKK2-JNK1ΔG and β-geo–JNKK2-JNK1 tumours obtained in the two models used: AOM/DSS and APCMin/+.(F) Summary table with the proposed mechanism for tumour promotion/tumour suppression mediated by JNK/cJun pathway modulation in different intestinal tumourigenic models.
To gain insights into the differential function of JNK signalling in intestinal tumourigenesis, we considered the possibility that there might be differences in JNK activity in different regions of the gut. Colitis-induced tumours arise in the colon, whereas most of the adenomas in ApcMin/+ mice form in the small intestine. However, the levels of active JNK and phosphorylated c-Jun were similar throughout the intestine (Figure 6D). In contrast, there was a striking difference in JNK activity between colitis-induced and ApcMin/+ tumours. Different JNK isoenzymes were expressed and activated in ApcMin/+ tumours and colitis-induced tumours. Presumably, as a consequence, the levels of phosphorylated c-Jun were very high in ApcMin/+ tumours compared with colitis-induced tumours. (Figure 6E). Expression of the JNKK2-JNK1ΔG transgene increased the low levels of c-Jun phosphorylation in colitis-induced tumours, but had no appreciable effect in ApcMin/+ tumours (Figure 6E). These observations suggest that the differential effects of JNK activation in the two intestinal tumour models might be because of relative differences in endogenous JNK pathway activation status.
Discussion
Cross talk of JNK and Wnt signalling
In this study, we show that JNK signalling regulates intestinal homeostasis and tumourigenesis, and we propose that this at least in part is due to a cross talk of JNK to Wnt signalling. Recently, it has been shown that JNK2, and to a lesser extent the related JNK1 isoenzyme, can induce nuclear translocation of β-catenin by directly phosphorylating the protein (Wu et al, 2008). We have not obtained evidence for the increased nuclear localization of β-catenin in JNKK2-JNK1ΔG intestine (Figure 5E, F; Supplementary Figure 8). A potential explanation for this could be the fact that we used an activated form of JNK1, but not JNK2, in our study.
Our data suggest that in addition to β-catenin, JNK signalling also regulates TCF4, the second component of this transcription factor co-activator complex. TCF4 and c-Jun are the key transcription factors that mediate the effects of Wnt and JNK signalling, respectively. TCF/β-catenin strongly activates c-jun trancription in both hematopoietic cells and colon-cancer cells (Mann et al, 1999; Staal et al, 2004; Nateri et al, 2005). We show that the interaction between Wnt and JNK signalling is reciprocal, and that the JNK/c-Jun pathway regulates the expression of the tcf4 gene. Thus, the mutual regulation of c-Jun and TCF4 form a positive feedback loop that links Wnt and JNK signalling. This mechanism seems to operate both in physiological and pathological conditions. High JNK activity not only stimulated the expression of AP-1 target genes, but also increases the strength of Wnt signalling, and caused augmented progenitor-cell proliferation and longer villi. Similarly, JNKK2-JNK1ΔG mice were more susceptible to inflammation-induced colon cancer, presumably because of the concomitant activation of both AP-1 and Wnt target genes by JNK signalling. TCF4 protein levels were significantly reduced in the absence of c-Jun, suggesting a mechanism for the reduction of intestinal cancer development observed in ApcMin/+ mice lacking c-jun (Nateri et al, 2005). It is noteworthy that an important role for JNK signalling has recently been described in Drosophila gut. Also in this organism JNK signalling controls the proliferation of intestinal stem cells (Biteau et al, 2008).
Function of JNK in intestinal cancer
The function of JNK signalling in cancer is ambiguous. On one hand, JNK activity is strongly activated by oncogenes and JNK activity is elevated in certain tumour types (Derijard et al, 1994; Engelberg, 2004). However, loss-of-function mutations in JNK-pathway components (JNK3 and MKK4) are found in human tumours, indicating a tumour suppressing role for JNK (Teng et al, 1997; Yoshida et al, 1999, 2001). The genetic and mechanistic basis for these different roles of JNK in tumour biology is a key question that remains unresolved. The realization that JNK inhibition may be of therapeutic benefit has led to the development of small molecule inhibitors of JNK (Manning and Davis, 2003; Karin and Gallagher, 2005), but an improved understanding of the apparently contradictory functions of JNK will be required to validate JNK inhibition as a therapeutic strategy for cancer.
This study suggests that JNK signalling can have a pro-tumourigenic function in certain forms of intestinal cancer, however, another study found that mice lacking jnk1 develop spontaneous intestinal tumours (Tong et al, 2007). The reasons for these apparent discrepancies are not clear. As in this study we used intestine-specific gene activation and inactivation, respectively, the phenotypes observed are due to a cell-autonomous function of JNK/c-Jun in intestinal epithelial cells. In contrast, jnk1-mutant mice are conventional knockouts that lack JNK1 in all cells. It is therefore conceivable that JNK1 function in non-intestinal cell types, for example in immune cells by causing inflammation or reduced immune surveillance, contributes to tumour development. In line with this, jnk1-mutant mice have been reported to show reduced immunity and jnk1-deficient T cells show abnormal T helper cell differentiation and cytokine production (Dong et al, 1998; Constant et al, 2000). Moreover, there is cross talk between JNK isoenzymes. Fibroblasts lacking jnk2 unexpectedly show higher JNK activity and c-Jun phosphorylation (Sabapathy and Wagner, 2004; Sabapathy et al, 2004). As neither JNK activity nor the level of c-Jun phosphorylation was analysed in jnk1-deficient intestines, it is unclear whether a similar phenomenon occurs in the absence of JNK1 (Tong et al, 2007).
c-Jun was reported to be expressed only at the tip of the villus (Hasselblatt et al, 2008). However, it is unlikely that the expression of c-Jun in differentiated intestinal cells contributes to its function in intestinal tumour formation. We have confirmed c-Jun expression at the tip of the villus (data not shown), but our analysis shows that c-Jun is also highly expressed in CBC stem cells and it is likely that the function of c-Jun in intestinal homeostasis and tumourigenesis are because of c-Jun function in stem cells. It should be noted that c-Jun was recently identified as one of the 95 genes that form the core transcriptome of Lgr5-positive CBC stem cells (van der Flier et al, 2009), thereby providing an independent confirmation for the stem cell specific c-Jun expression described in this study.
Our observations that JNK signalling is differentially activated in colitis-induced and ApcMin/+ tumours highlights the different molecular mechanisms of tumour formation in these two models of intestinal cancerogenesis. Moreover, it may provide a molecular explanation for the discrepancies that have been reported with regard to the function of c-Jun during intestinal tumourigenesis. c-Jun is required for ApcMin/+-triggered tumourigenesis (Nateri et al, 2005), but mice lacking c-jun show normal colitis-induced intestinal-tumour formation (Hasselblatt et al, 2008). The differential requirement for c-Jun is directly opposite to the phenotypes observed for JNKK2-JNK1ΔG mice. During colitis-induced tumourigenesis, in which JNK/c-Jun signalling is low, JNK activation accelerates tumourigenesis, whereas in ApcMin/+-tumours, in which the JNK/c-Jun pathway is highly active, transgenic JNK activation has no effect. This suggests that loss-of-function of the JNK/c-Jun pathway only reduces tumour formation in tumours with high pathway activity, whereas gain-of-function of the JNK/c-Jun pathway only accelerates tumourigenesis in tumours with low JNK activity (Figure 6F). We speculate that the different status of JNK/c-Jun pathway activation in colitis-induced and ApcMin/+-induced intestinal tumours may explain the differential requirements for JNK activity and c-Jun in different model of intestinal cancer.
Materials and methods
Mouse lines
The β-geo–JNKK2-JNK1 transgenic construct was electroporated into embryonic stem cells, stable transfectants were selected and mice generated according to standard protocols (Behrens et al, 1999). β-gal staining was used to ensure high transgene expression in ES cells and Southern blot analysis was used to confirm single-copy integration (data not shown). c-junf/f and Villin-cre mice have been described before (Behrens et al, 2002; el Marjou et al, 2004; Nateri et al, 2005).
Immunohistochemical staining and immunofluorescence
Mice were injected intraperitoneally with 100 mg/kg BrdU (Sigma), 1.5 h (or 24 h where indicated) before killing. Mice were killed by cervical dislocation and the small intestines were dissected out into ice-cold PBS and the faecal contents flushed out with ice-cold PBS. The intestines were cut longitudinally into pieces of similar size, opened out and fixed overnight in 10% neutral buffered formalin, briefly washed with PBS and transferred into 70% ethanol, rolled, processed and embedded into paraffin. Sections of the size of 4 μm were cut for haematoxylin and eosin staining, immunohistochemistry and immunofluorescence. For immunohistochemistry, antibodies against BrdU (Becton Dickinson), c-Jun (Becton Dickinson), CD44 (Chemicon) and β-catenin (Becton Dickinson) were used. Anti-TCF4 (Abcam) and anti-c-Jun (Santa Cruz Biotechnology) were used for immunofluorescence as described before (Nateri et al, 2005). For the morphometric analysis of the villi, the distance between the base of the villus and the villus apex was measured using AxioVision digital image processing software. A minimum of 40 crypt–villus units in comparable intestinal regions were quantified for at least four mice of each genotype. To quantify the BrdU, Ki67 or CBC-BrdU positive cells per crypt, 100 full crypts were scored from at least three mice of each genotype. Data are represented as mean±s.e.m. (P⩽0.05 was considered statistically significant in student's t test).
In situ hybridization
In situ hybridization was carried out as previously described (Barker et al, 2007). All ISH were carried out by the CRUK in situ hybridization service at LRI.
Western blot analysis
Mice were killed by cervical dislocation and the intestines were removed and flushed extensively with cold PBS. The first 3 cm of duodenum was taken and frozen in liquid nitrogen. Cell lysates were homogenized in RIPA lysis buffer (NEB) supplemented with protease inhibitor (Sigma). Immunoblots were carried out as previously described (Nateri et al, 2005). Antibodies against monoclonal anti-human-JNK1 (Becton Dickinson), c-Jun (Becton Dickinson) , p-c-Junser73 (Cell Signalling), SAPK/JNK (Cell Signalling), cyclinD1 (Cell Signalling), TCF4 (Upstate), β-catenin (Becton Dickinson) and β-actin (Sigma) were used. The proteins on the gels were transferred to nitrocellulose membranes, and the membranes were immunoblotted with various antibodies as indicated. HCT116 western-blot analysis was carried out as described above.
qRT–PCR analysis
For quantitative real-time–PCR analysis, total mRNA was isolated from dissected intestines or HCT116 cells using RNeasy Mini-kit according to the manufacturer's instructions (Qiagen). For quantitative real-time–PCRs, cDNA was synthesized using Invitrogen Superscript reagents according to the manufacturer's instructions. Quantitative real-time PCR was accomplished with SYBR Green incorporation (Platinum Quantitative PCR SuperMix-UDG w/ROX, Invitrogen) using an ABI7900HT real-time PCR system (Applied Bioscience), and the data were analysed using the SDS 2.3 software. Results were normalized to those obtained with β-actin and results are presented as fold induction over control mice. The following primers were used for mouse tissue qRT–PCR analysis:
F-c-JUN: 5′-TGAAAGCTGTGTCCCCTGTC-3′
R-c-JUN: 5′-ATCACAGCACATGCCACTTC-3′
F-CD44: 5′-CTCCTGGCACTGGCTCTGA-3′
R-CD44: 5′-CTGCCCACACCTTCTCCTACTATT-3′
F-CCDN1 : 5′-GTGCGTGCAGAAGGAGATTGT-3′
R-CCDN1: 5′-CTCACAGACCTCCAGCATCCA-3′
F-TCF7L2: 5′-GAGAGTGCAGCCATCAACCAG-3′
R-TCF7L2: 5′-GTGATCGGAGGAAGCGAAAGG-3′
F-AXIN2: 5′- GGTTCCGGCTATGTCTTTGC-3′
R-AXIN2: 5′-CAGTGCGTCGCTGGATAACTC-3′
F-GRP49: 5′-CGGAGGAAGCGCTACAGAAT-3′
R-GRP49: 5′-CTGGGTGGCACGTAGCTGAT-3′
F-β-actin: 5′-ATGCTCCCCGGGCTGTAT-3′
R-β-actin: 5′-CATAGGAGTCCTTCTGACCCATTC-3′
F- GAPDH: 5′- TGAAGCAGGCATCTGAGGG -3′
R-GAPDH: 5′-CGAAGGTGGAAGAGTGGGAG -3′
The following primers sequences were used for HCT116 qRT–PCR analysis:
F-TCF7L2-h: 5′-TGAAGGCAGCTGCCTCAGC-3′
R-TCF7L2-h: 5′-GTGGGTGGCCTCAGCGAGC-3′
F-c-JUN-h: 5′-TCGACATGGAGTCCCAGGA-3′
R-c-JUN-h: 5′-GGCGATTCTCTCCAGCTTCC-3′
F-β-actin-h: 5′-GGATGCAGAAGGAGATCACTG-3′
R-β-actin-h: 5′-CGATCCACACGGAGTACTTG-3′
Chromatin Immunoprecipitation
ChIP analysis was carried out as described previously (Nelson et al, 2006). Cells were treated 2 h before collection with 50 μM JNKi (SP600125, Calbiochem) or 25 ng/ml Ans (Sigma). Immunoprecipitations were carried out with conjugated agarose beads for anti-c-Jun (Santa-Cruz Biotechnology) or rabbit IgG as isotype control. The oligonucleotide sequences used to amplify the tcf4 DNA fragments by qPCR were:
Ch-dist-TCF4-H (FW): 5′-TCATTGTAGATGACCAGGAACTTTG-3′
Ch-dis-TCF4-H (RV): 5′-AAAGATCAACCCTCCACTTTCAGA-3′
Reporter gene assay
The region containing the distal 3xAP1 consensus binding sites of the tcf7l2 promoter (from −996 to −833 bp relative to the TATA box) was cloned in pGL3-enhancer (Promega). Expression plasmid for c-Jun has been described before (Nateri et al, 2005). Expression plasmid for c-Jun∼ATF2 or c-Jun∼Fos have been described before (Bakiri et al, 2002). HCT116 cells were transfected using Lipofectamine reagent (Invitrogen). Transient transfections of the experimental samples and controls of firefly and renilla luciferase reporters were carried out and measured using the Dual-Luciferase Reporter Assay System, 36 h post-transfection (Promega). Data are expressed as fold induction after being normalized using tk-renilla luciferase (mean±s.e.m.; n=3).
AOM/DSS model of colon carcinoma
The AOM/DSS model used has been previously described by Neufert et al (2007). Mice were injected intraperitoneally with 10 mg/kg body weight of AOM (Sigma) dissolved in physiological saline. Seven days later, 2% DSS was given in the drinking water over 7 days, followed by regular water until the end of the experiment. Body weight was measured every week, and the animals were killed 13 weeks after AOM injection for histological analysis by staining with c-Jun, β-catenin and BrdU antibodies. The number of tumours, incidence of tumours and BrdU-positive cells per area were determined. For biochemical characterization dissected tumours were snap frozen in liquid nitrogen and used for western blot and qRT–PCR analysis.
Supplementary Material
Supplementary Information
Review Process File
Acknowledgments
We are grateful to the Animal Unit, Equipment Park and the Experimental Pathology Lab at the London Research Institute and PreCOS at Nottingham University for technical help and advice on histology. We thank M Mitchell for computational promoter analysis and I Tomlinson and EF Wagner for critical reading of the paper. RS was financially supported by a Marie Curie Intraeuropean Fellowship (MEIF-CT-2006-041119). The London Research Institute is funded by Cancer Research UK.
References
- Angel P, Hattori K, Smeal T, Karin M (1988) The jun proto-oncogene is positively autoregulated by its product, Jun/AP1. Cell 55: 875–885 [DOI] [PubMed] [Google Scholar]
- Bakiri L, Matsuo K, Wisniewska M, Wagner EF, Yaniv M (2002) Promoter specificity and biological activity of tethered AP-1 dimers. Mol Cell Biol 22: 4952–4964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N, van de Wetering M, Clevers H (2008) The intestinal stem cell. Genes Dev 22: 1856–1864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H (2007) Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449: 1003–1007 [DOI] [PubMed] [Google Scholar]
- Behrens A, Sibilia M, David JP, Mohle-Steinlein U, Tronche F, Schutz G, Wagner EF (2002) Impaired postnatal hepatocyte proliferation and liver regeneration in mice lacking c-jun in the liver. EMBO J 21: 1782–1790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens A, Sibilia M, Wagner EF (1999) Amino-terminal phosphorylation of c-Jun regulates stress-induced apoptosis and cellular proliferation. Nat Genet 21: 326–329 [DOI] [PubMed] [Google Scholar]
- Biteau B, Hochmuth CE, Jasper H (2008) JNK activity in somatic stem cells causes loss of tissue homeostasis in the aging Drosophila gut. Cell Stem Cell 3: 442–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Jones Y, Ellisman MH, Goldstein LS, Karin M (2003) JNK1 is required for maintenance of neuronal microtubules and controls phosphorylation of microtubule-associated proteins. Dev Cell 4: 521–533 [DOI] [PubMed] [Google Scholar]
- Cheng H, Leblond CP (1974a) Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. I. Columnar cell. Am J Anat 141: 461–479 [DOI] [PubMed] [Google Scholar]
- Cheng H, Leblond CP (1974b) Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. Am J Anat 141: 537–561 [DOI] [PubMed] [Google Scholar]
- Constant SL, Dong C, Yang DD, Wysk M, Davis RJ, Flavell RA (2000) JNK1 is required for T cell-mediated immunity against Leishmania major infection. J Immunol 165: 2671–2676 [DOI] [PubMed] [Google Scholar]
- Davis RJ (2000) Signal transduction by the JNK group of MAP kinases. Cell 103: 239–252 [DOI] [PubMed] [Google Scholar]
- Derijard B, Hibi M, Wu IH, Barrett T, Su B, Deng T, Karin M, Davis RJ (1994) JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell 76: 1025–1037 [DOI] [PubMed] [Google Scholar]
- Dong C, Yang DD, Wysk M, Whitmarsh AJ, Davis RJ, Flavell RA (1998) Defective T cell differentiation in the absence of Jnk1. Science 282: 2092–2095 [DOI] [PubMed] [Google Scholar]
- Eferl R, Wagner EF (2003) AP-1: a double-edged sword in tumorigenesis. Nat Rev Cancer 3: 859–868 [DOI] [PubMed] [Google Scholar]
- el Marjou F, Janssen KP, Chang BH, Li M, Hindie V, Chan L, Louvard D, Chambon P, Metzger D, Robine S (2004) Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis 39: 186–193 [DOI] [PubMed] [Google Scholar]
- Engelberg D (2004) Stress-activated protein kinases-tumor suppressors or tumor initiators? Semin Cancer Biol 14: 271–282 [DOI] [PubMed] [Google Scholar]
- Giles RH, van Es JH, Clevers H (2003) Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta 1653: 1–24 [DOI] [PubMed] [Google Scholar]
- Gupta S, Barrett T, Whitmarsh AJ, Cavanagh J, Sluss HK, Dérijard B, Davis RJ (1996) Selective interaction of JNK protein kinase isoforms with transcription factors. EMBO J 15: 2760–2770 [PMC free article] [PubMed] [Google Scholar]
- Hasselblatt P, Gresh L, Kudo H, Guinea-Viniegra J, Wagner EF (2008) The role of the transcription factor AP-1 in colitis-associated and beta-catenin-dependent intestinal tumorigenesis in mice. Oncogene 27: 6102–6109 [DOI] [PubMed] [Google Scholar]
- Hayakawa J, Mittal S, Wang Y, Korkmaz KS, Adamson E, English C, Ohmichi M, McClelland M, Mercola D (2004) Identification of promoters bound by c-Jun/ATF2 during rapid large-scale gene activation following genotoxic stress. Mol Cell 16: 521–535 [DOI] [PubMed] [Google Scholar]
- Hess J, Angel P, Schorpp-Kistner M (2004) AP-1 subunits: quarrel and harmony among siblings. J Cell Sci 117: 5965–5973 [DOI] [PubMed] [Google Scholar]
- Humphries A, Wright NA (2008) Colonic crypt organization and tumorigenesis. Nat Rev Cancer 8: 415–424 [DOI] [PubMed] [Google Scholar]
- Itzkowitz SH, Yio X (2004) Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol 287: G7–G17 [DOI] [PubMed] [Google Scholar]
- Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F (2002) Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol 22: 1172–1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RS, van Lingen B, Papaioannou VE, Spiegelmann BM (1993) A null mutation at the c-jun locus causes embryonic lethality and retarded cell growth in culture. Genes Dev 7: 1309–1317 [DOI] [PubMed] [Google Scholar]
- Karin M, Gallagher E (2005) From JNK to pay dirt: jun kinases, their biochemistry, physiology and clinical importance. IUBMB Life 57: 283–295 [DOI] [PubMed] [Google Scholar]
- Kuan CY, Yang DD, Samanta Roy DR, Davis RJ, Rakic P, Flavell RA (1999) The Jnk1 and Jnk2 protein kinases are required for regional specific apoptosis during early brain development. Neuron 22: 667–676 [DOI] [PubMed] [Google Scholar]
- Mann B, Gelos M, Siedow A, Hanski ML, Gratchev A, Ilyas M, Bodmer WF, Moyer MP, Riecken EO, Buhr HJ, Hanski C (1999) Target genes of beta-catenin-T cell-factor/lymphoid-enhancer-factor signaling in human colorectal carcinomas. Proc Natl Acad Sci USA 96: 1603–1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning AM, Davis RJ (2003) Targeting JNK for therapeutic benefit: from junk to gold? Nat Rev Drug Discov 2: 554–565 [DOI] [PubMed] [Google Scholar]
- Mechta-Grigoriou F, Gerald D, Yaniv M (2001) The mammalian Jun proteins: redundancy and specificity. Oncogene 20: 2378–2389 [DOI] [PubMed] [Google Scholar]
- Moser AR, Mattes EM, Dove WF, Lindstrom MJ, Haag JD, Gould MN (1993) ApcMin, a mutation in the murine Apc gene, predisposes to mammary carcinomas and focal alveolar hyperplasias. Proc Natl Acad Sci USA 90: 8977–8981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nateri AS, Spencer-Dene B, Behrens A (2005) Interaction of phosphorylated c-Jun with TCF4 regulates intestinal cancer development. Nature 437: 281–285 [DOI] [PubMed] [Google Scholar]
- Nelson JD, Denisenko O, Bomsztyk K (2006) Protocol for the fast chromatin immunoprecipitation (ChIP) method. Nat Protoc 1: 179–185 [DOI] [PubMed] [Google Scholar]
- Neufert C, Becker C, Neurath MF (2007) An inducible mouse model of colon carcinogenesis for the analysis of sporadic and inflammation-driven tumor progression. Nat Protoc 2: 1998–2004 [DOI] [PubMed] [Google Scholar]
- Potten CS, Kovacs L, Hamilton E (1974) Continuous labelling studies on mouse skin and intestine. Cell Tissue Kinet 7: 271–283 [DOI] [PubMed] [Google Scholar]
- Radtke F, Clevers H (2005) Self-renewal and cancer of the gut: two sides of a coin. Science 307: 1904–1909 [DOI] [PubMed] [Google Scholar]
- Raivich G, Bohatschek M, Da Costa C, Iwata O, Galiano M, Hristova M, Nateri AS, Makwana M, Riera-Sans L, Wolfer DP, Lipp HP, Aguzzi A, Wagner EF, Behrens A (2004) The AP-1 transcription factor c-Jun is required for efficient axonal regeneration. Neuron 43: 57–67 [DOI] [PubMed] [Google Scholar]
- Sabapathy K, Hochedlinger K, Nam SY, Bauer A, Karin M, Wagner EF (2004) Distinct roles for JNK1 and JNK2 in regulating JNK activity and c-Jun-dependent cell proliferation. Mol Cell 15: 713–725 [DOI] [PubMed] [Google Scholar]
- Sabapathy K, Jochum W, Hochedlinger K, Chang L, Karin M, Wagner EF (1999) Defective neural tube morphogenesis and altered apoptosis in the absence of both JNK1 and JNK2. Mech Dev 89: 115–124 [DOI] [PubMed] [Google Scholar]
- Sabapathy K, Wagner EF (2004) JNK2: a negative regulator of cellular proliferation. Cell Cycle 3: 1520–1523 [DOI] [PubMed] [Google Scholar]
- Sangiorgi E, Capecchi MR (2008) Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet 40: 915–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansom OJ, Reed KR, Hayes AJ, Ireland H, Brinkmann H, Newton IP, Batlle E, Simon-Assmann P, Clevers H, Nathke IS, Clarke AR, Winton DJ (2004) Loss of Apc in vivo immediately perturbs Wnt signaling, differentiation, and migration. Genes Dev 18: 1385–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaulian E, Karin M (2001) AP-1 in cell proliferation and survival. Oncogene 20: 2390–2400 [DOI] [PubMed] [Google Scholar]
- Shaulian E, Karin M (2002) AP-1 as a regulator of cell life and death. Nat Cell Biol 4: E131–E136 [DOI] [PubMed] [Google Scholar]
- Staal FJ, Weerkamp F, Baert MR, van den Burg CM, van Noort M, de Haas EF, van Dongen JJ (2004) Wnt target genes identified by DNA microarrays in immature CD34+ thymocytes regulate proliferation and cell adhesion. J Immunol 172: 1099–1108 [DOI] [PubMed] [Google Scholar]
- Stein B, Angel P, van Dam H, Ponta H, Herrlich P, van der Eb A, Rahmsdorf HJ (1992) Ultraviolet-radiation induced c-jun gene transcription: two AP-1 like binding sites mediate the response. Photochem Photobiol 55: 409–415 [DOI] [PubMed] [Google Scholar]
- Takahashi M, Wakabayashi K (2004) Gene mutations and altered gene expression in azoxymethane-induced colon carcinogenesis in rodents. Cancer Sci 95: 475–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng DH, Perry WL 3rd, Hogan JK, Baumgard M, Bell R, Berry S, Davis T, Frank D, Frye C, Hattier T, Hu R, Jammulapati S, Janecki T, Leavitt A, Mitchell JT, Pero R, Sexton D, Schroeder M, Su PH, Swedlund B et al. (1997) Human mitogen-activated protein kinase kinase 4 as a candidate tumor suppressor. Cancer Res 57: 4177–4182 [PubMed] [Google Scholar]
- Tetsu O, McCormick F (1999) Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 398: 422–426 [DOI] [PubMed] [Google Scholar]
- Tong C, Yin Z, Song Z, Dockendorff A, Huang C, Mariadason J, Flavell RA, Davis RJ, Augenlicht LH, Yang W (2007) c-Jun NH2-terminal kinase 1 plays a critical role in intestinal homeostasis and tumor suppression. Am J Pathol 171: 297–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Flier LG, Sabates-Bellver J, Oving I, Haegebarth A, De Palo M, Anti M, Van Gijn ME, Suijkerbuijk S, Van de Wetering M, Marra G, Clevers H (2007) The Intestinal Wnt/TCF Signature. Gastroenterology 132: 628–632 [DOI] [PubMed] [Google Scholar]
- van der Flier LG, van Gijn ME, Hatzis P, Kujala P, Haegebarth A, Stange DE, Begthel H, van den Born M, Guryev V, Oving I, van Es JH, Barker N, Peters PJ, van de Wetering M, Clevers H (2009) Transcription factor achaete scute-like 2 controls intestinal stem cell fate. Cell 136: 903–912 [DOI] [PubMed] [Google Scholar]
- Veeman MT, Axelrod JD, Moon RT (2003) A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell 5: 367–377 [DOI] [PubMed] [Google Scholar]
- Weston CR, Davis RJ (2002) The JNK signal transduction pathway. Curr Opin Genet Dev 12: 14–21 [DOI] [PubMed] [Google Scholar]
- Wielenga VJ, Smits R, Korinek V, Smit L, Kielman M, Fodde R, Clevers H, Pals ST (1999) Expression of CD44 in Apc and Tcf mutant mice implies regulation by the WNT pathway. Am J Pathol 154: 515–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisdom R, Johnson RS, Moore C (1999) c-Jun regulates cell cycle progression and apoptosis by distinct mechanisms. EMBO J 18: 188–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Tu X, Joeng KS, Hilton MJ, Williams DA, Long F (2008) Rac1 activation controls nuclear localization of beta-catenin during canonical Wnt signaling. Cell 133: 340–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulf GM, Ryo A, Wulf GG, Lee SW, Niu T, Petkova V, Lu KP (2001) Pin1 is overexpressed in breast cancer and cooperates with Ras signaling in increasing the transcriptional activity of c-Jun towards cyclin D1. EMBO J 20: 3459–3472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Karin M (2004) The control of cell motility and epithelial morphogenesis by Jun kinases. Trends Cell Biol 14: 94–101 [DOI] [PubMed] [Google Scholar]
- Yang DD, Kuan CY, Whitmarsh AJ, Rincón M, Zheng TS, Davis RJ, Rakic P, Flavell RA (1997) Absence of excitotoxicity-induced apoptosis in the hippocampus of mice lacking the Jnk3 gene. Nature 389: 865–870 [DOI] [PubMed] [Google Scholar]
- Yoshida BA, Dubauskas Z, Chekmareva MA, Christiano TR, Stadler WM, Rinker-Schaeffer CW (1999) Mitogen-activated protein kinase kinase 4/stress-activated protein/Erk kinase 1 (MKK4/SEK1), a prostate cancer metastasis suppressor gene encoded by human chromosome 17. Cancer Res 59: 5483–5487 [PubMed] [Google Scholar]
- Yoshida S, Fukino K, Harada H, Nagai H, Imoto I, Inazawa J, Takahashi H, Teramoto A, Emi M (2001) The c-Jun NH2-terminal kinase3 (JNK3) gene: genomic structure, chromosomal assignment, and loss of expression in brain tumors. J Hum Genet 46: 182–187 [DOI] [PubMed] [Google Scholar]
- Zheng C, Xiang J, Hunter T, Lin A (1999) The JNKK2-JNK1 fusion protein acts as a constitutively active c-Jun kinase that stimulates c-Jun transcription activity. J Biol Chem 274: 28966–28971 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information
Review Process File