Abstract
The blood-brain barrier (BBB) efficiently restricts penetration of therapeutic agents to the brain from the periphery. Therefore, discovery of new modalities allowing for effective delivery of drugs and biomacromolecules to the central nervous system (CNS) is of great need and importance for treatment of neurodegenerative disorders. This manuscript focuses on three relatively new strategies. The first strategy involves inhibition of the drug efflux transporters expressed in BBB by Pluronic® block copolymers, which allows for the increased transport of the substrates of these transporters to the brain. The second strategy involves the design of nanoparticles conjugated with specific ligands that can target receptors in the brain microvasculature and carry the drugs to the brain through the receptor mediated transcytosis. The third strategy involves artificial hydrophobization of peptides and proteins that facilitates the delivery of these peptides and proteins across BBB. This review discusses the current state, advantages and limitations of each of the three technologies and outlines their future prospects.
Keywords: blood-brain barrier, drug efflux, drug delivery, fatty acylation, nanogel, nanoparticles, Pluronic block copolymers, poloxamer
1. Introduction
Tremendous attention, efforts, and hope are focused on the development of novel drug delivery systems. The principal reason for the extremely rapid growth of research and technology in this field is the realization that substantial improvement of current therapies will necessitate the use of therapeutic modalities allowing for efficient and site-specific transport of drugs to the target tissues affected by the disease. This necessity arises primarily due to the enormous barriers that a drug molecule must overcome before it reaches its target site within the body. One of the most challenging barriers in the body is the blood–brain barrier (BBB) that significantly restricts the entry of compounds to the brain from the periphery. This impedes the use of many low molecular weight drugs as well as biomacromolecules, such DNA and proteins, for treatment of neurological diseases, especially at early stages of the disease when the BBB remains intact. The low permeability of the BBB is attributed, in large part, to the brain microvessel endothelial cells (BMVEC), which form tight extracellular junctions and have low pinocytic activity (1, 2). Passive diffusion of substances across the BMVEC may occur depending on the lipophilicity and molecular weight of these substances. However, a large number of compounds is rapidly effluxed from the brain into the blood by extremely effective efflux pumps expressed in the BBB (3–5). These efflux systems include P-glycoprotein (Pgp) and Multidrug Resistance Proteins (MRPs). There is also an enzymatic barrier to drug transport in BMVECs. Specifically, activity of many enzymes that participate in the metabolism and inactivation of endogenous compounds, such as γ-glutamyl transpeptidase, alkaline phosphatase, and aromatic acid decarboxylase is elevated in cerebral microvessels (6, 7). Aside from approaches that cause short-term disruption of the BBB, drug delivery systems need to improve the transcellular routes of drug transport through the BMVEC. Therefore, discovery of new modalities allowing for effective drug delivery to the central nervous system (CNS) is of great need and importance for treatment of neurodegenerative disorders. A number of earlier publications and extensive reviews on drug and biomacromolecule delivery to the brain are available in the literature (8–18). The present mini-review describes three relatively new approaches for improving drug transport through the BBB: i) inhibition of drug efflux transporters in BBB by amphiphilic block copolymers (Pluronic®), ii) using receptor-mediated transport of drugs encapsulated into nanoparticles, and iii) artificial hydrophobization of peptides and proteins by fatty acid residues.
2. Inhibition of drug efflux systems in BBB by Pluronic® block copolymers
One emerging strategy to enhance drug delivery to the CNS is the co-administration with a drug of a pharmacological modulator that inhibits drug efflux transport systems in BMVEC. One promising example of such pharmacological modulators is represented by a class of Pluronic® block copolymers (also known under non-proprietary name “poloxamers”). These block copolymers consist of hydrophilic ethylene oxide (EO) and hydrophobic propylene oxide (PO) blocks arranged in a basic A-B-A tri-block structure: EOn/2-POm-EOn/2. The block copolymers with various numbers of hydrophilic EO (n) and hydrophobic PO (m) units are characterized by distinct hydrophilic-lipophilic balance (HLB). Due to their amphiphilic character these copolymers display surfactant properties including ability to interact with hydrophobic surfaces and biological membranes. In aqueous solutions at concentrations above critical micelle concentration (CMC) these copolymers self-assemble into micelles.
Studies in multidrug resistant (MDR) cancer cells, polarized intestinal epithelial cells, Caco-2, and polarized BMVEC monolayers provided compelling evidence that selected Pluronic® block copolymers can inhibit drug efflux transport systems (19–26). Specifically, in primary cultured BMVEC monolayers, used as an in vitro model of BBB, the inhibition of Pgp efflux system was associated with an increased accumulation and permeability of the Pgp probe, rhodamine 123 (22). It was found that Pluronic® block copolymers also increase accumulation and transport of the MRP probe, fluorescein, in these cells, thus suggesting possible inhibition by the block copolymers of MRPs or MRP-like transporters present in the BBB (27). Furthermore, these studies suggested that co-administration with the block copolymers increases the permeability of a broad spectrum of drugs in the BBB.
The effects of Pluronic® block copolymers on Pgp and MRPs drug efflux transporters in the BMVEC were most apparent at concentrations below the critical micellization concentration CMC (21, 22). Particularly, exposure of the BMVEC to low concentrations of P85 (ca. from 0.001%wt to 0.01%wt) resulted in increased rhodamine 123 accumulation, consistent with the inhibition of the Pgp efflux transport protein. At higher concentrations of Pluronic® P85 (e.g. 1 % wt.) the inhibition of Pgp efflux system was diminished, and rhodamine 123 intracellular levels were decreased. It was suggested that “unimers”, i.e. single block copolymer molecules, are responsible for the inhibition of Pgp and MRPs efflux transport system (CMC for Pluronic® P85 is 0.03%wt (28)). Incorporation of the probe into the micelles formed at high concentrations of the block copolymer, decreases its availability to the cells and reduces the transport of this probe in BMVEC (21, 22).
Recent findings suggest that effects of Pluronic® on drug efflux transport proteins involve interactions of the block copolymers with the cell membranes (24, 29). The hydrophobic PO chains of Pluronic® immerse into the membrane hydrophobic areas, resulting in alterations of the membrane structure, and decrease of its microviscosity (“membrane fluidization”) At relatively low concentrations (e.g. 0.01 %), of Pluronic® inhibits the Pgp ATPase activity, possibly, due to conformational changes in the transport protein induced by the immersed copolymer chains in the Pgp-expressing membranes (24). In particular, Pluronic® P85 displayed the effects characteristic of a mixed type enzyme inhibitor - decreasing maximal reaction rate, Vmax and increasing Michaelis constant, Km for ATP as well as Pgp-specific substrates such as vinblastine (a detailed study is in preparation). The magnitude of these effects for vinblasine was as high as over 200-fold Vmax/Km change (interestingly, MRP1 ATPase activity was affected less, which could explain somewhat smaller effects of Pluronic® on this transporter). In contrast, at the high concentrations (e.g. 1 %), binding of Pluronic® to the membrane actually results in restoration of Pgp ATPase activity. This could be due to the segregation of the block copolymer molecules in the 2D clusters in the membrane, which diminishes its interactions with the transport proteins.
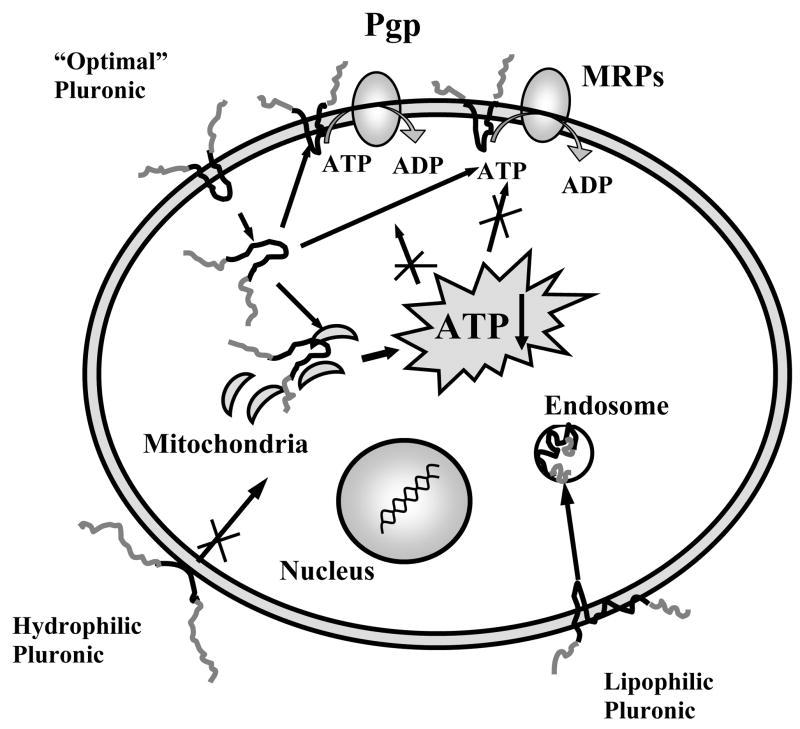
Various drug resistance mechanisms, including drug transport and detoxification systems, require consumption of energy to sustain their function in the barrier cells. Because of this fact, mechanistic studies have focused on the effects of Pluronic® block copolymers on metabolism and energy conservation in BMVEC (24). The basis for such studies was the earlier reports that Pluronic® block copolymers can affect mitochondria function and energy conservation in the cells (30). A recent study have demonstrated that exposure to Pluronic® P85 induced significant decrease in ATP levels in BMVEC monolayers (24). The observed energy depletion was due to inhibition of the cellular metabolism rather than a loss of ATP in the environment. The study by Rapoport et al. suggested that Pluronic® P85 can be transported into the cells and decrease the activity of electron transport chains in the mitochondria (31). Remarkably the ATP depletion induced by Pluronic® appears to be tightly linked to the specific cell genotype, since this effect is observed selectively in the cells that overexpress Pgp (as well as MRPs) (32, 33). The explanation of this relationship still needs to be found, although it was speculated that inhibition of ATP production in high energy-consuming cells, such as cells overexpressing Pgp, results in the rapid exhaustion of intracellular ATP, i.e. ATP depletion (32). Overall, the energy depletion (decreasing ATP pool available for drug transport proteins) and membrane interactions (inhibiting of ATPase activity of drug transport proteins) are critical factors collectively contributing to a potent inhibition of the drug efflux systems by Pluronic® (Figure 1) (24).
Fig. 1.
Schematic illustrating two-fold effects of Pluronic® block copolymers with intermediate lipophilicity on Pgp and MRPs drug efflux system. These effects include (a) decrease in membrane viscosity (“fluidization”) resulting in inhibition of Pgp and MRPs ATPase activity, and (b) ATP depletion in BMVEC. Extremely lipophilic or hydrophilic Pluronic® block copolymers do not cross the cellular membranes and do not cause energy depletion in the cells.
It was demonstrated that a fine balance between hydrophilic (EO) and lipophilic (PO) components in the Pluronic® molecule should be accomplished to potent enable inhibition of the drug efflux systems (29). Overall, the most efficacious block copolymers are those with intermediate lengths of PO block and relatively hydrophobic structure (HLB < 20), such as Pluronic® P85 or L61 (23). Hydrophilic block copolymers, which have an extended EO block, do not incorporate into lipid bilayers and practically do not transport into the cells. As a result, they have little effect on either Pgp ATPase activity or ATP levels, which explains their negligible effect on Pgp efflux pump in BBMEC (29). Very lipophilic block copolymers with long PO blocks anchor in the plasma membranes and remain there for an extended period of time. As a result, although they are potent inhibitors of Pgp ATPase, they are not efficiently transported into the cell, do not cause ATP depletion and have little net effect on Pgp efflux system in BMVEC. In contrast, the block copolymers displaying intermediate lipophilicity transport across the membrane, spread throughout the cytoplasm and reach mitochondria and nuclei. They inhibit Pgp ATPase activity and decrease ATP intracellular levels, which combined results in effective inhibition of drug efflux transport systems and enhanced drug transport to the brain (24, 29).
Effect of Pluronic® P85 on drug transport into the brain was evaluated in animal experiments (25). Brain delivery of a Pgp substrate, digoxin, administered intravenously in the wild-type mice expressing functional Pgp, was greatly enhanced in the presence of Pluronic® P85. It was found that the digoxin brain/plasma ratios in the Pluronic® treated animals were practically the same as those in the knockout mice, an animal model that is deficient in both mdr1a and mdr1b isoforms of Pgp. This suggests that co-administration of Pluronic® with the drug in mice resulted in inhibition of Pgp in the BBB of the wild-type animals (25).
One possible concern in these studies is that that by virtue of inhibiting the ATP in BMVEC, the copolymer may display toxic effects on the BBB. However, the ATP depletion was found to be transient; following removal of the block copolymers from BMVEC monolayers the initial ATP levels were restored (24). Although there were significant decreases in cellular ATP following Pluronic® treatment, even during peak depletion of ATP by Pluronic® there was no evidence of loss of barrier functions of BBB as demonstrated using 3H-mannitol as a permeability marker both in vitro and in vivo (22, 25). Moreover, Pluronic® does not affect the glucose transporter, GLUT1, and only slightly inhibits lactate transporter, MCT1, the two transporters playing an important role in the brain metabolism (in preparation). A histochemical examination of the tissue sections obtained from animals treated with Pluronic® revealed no pathological changes in the BBB. Importantly, no cerebral toxicity of any kind has been observed in the human Phase I studies of SP1049C, a Pluronic®-based formulation of doxorubicin to treat MDR tumors (34). After completion of phase I clinical trials, SP1049C is undergoing several phase II clinical trials. It is possible, that this formulation, evaluated in human trials, can be adopted for the use with CNS drugs to enhance drug delivery to the brain.
3. Receptor-mediated delivery of nanoparticles to the brain
While the transport of many small molecules to the brain is a difficult task, the transport of biomacromolecules such as DNA or proteins across the BBB presents an even more formidable challenge. Nevertheless, some naturally occurring peptides can effectively pass this barrier due to receptor-mediated transport (transcytosis) (18, 35–37). Furthermore, homing peptides exhibiting specific targeting to the brain can be selected from phage display libraries (38). Therefore, utilizing the specific peptides for targeting of macromolecules and their receptor-mediated transcytosis across BBB could be a successful strategy for improving drug delivery to the brain.
A number of studies proposed approaches to accomplish receptor-mediated transcytosis of biomacromolecules across the BBB (37). For example, coupling of oligonucleotides (ODNs) with OX26 monoclonal antibody to the rat transferrin receptor was used in an attempt to enhance brain uptake of biotinylated ODNs (39, 40) and neurotrophin peptides (41). While these studies demonstrated the potential of receptor-mediated transcytosis across the BBB for delivery of biomacromolecules to the brain, they also revealed some limitations of this approach. In particular, the while ODN-OX26 constructs displayed efficient transport across BMVEC in vitro, their intravenous administration in vivo was much less successful, because of the binding of these constructs with the plasma proteins.
This reinforces the idea that to be useful in drug delivery across the BBB the brain-specific peptides need to combined with an appropriate drug carrier. The need for a carrier is highlighted by the fact that many biomacromolecules have low hydrolytic stability and are subject to degradation by blood proteins or by enzymes encountered in the BBB, which can be overcome by incorporating these biomacromolecules into protective carrier species. The concept of using polymeric drug carriers in combination with the targeting moieties is, generally speaking, very attractive (42). A single unit of a given polymeric drug carrier can incorporate many molecules of drug or biomacromolecules, resulting in high “payloads” per one targeting moiety and/or receptor engaged. Furthermore, by increasing the payload of the carrier, one might improve the efficacy of the delivery while maintaining a relatively low level of involvement of numbers of targeted moieties and receptors.
To allow for efficient transcytosis across the BMVEC the carrier particles have to be small with the size not exceeding ca. 100 nm. The use of nanoparticles as vehicles for drug and gene delivery has been an area of intensive research and development for over a decade (43–46). Some examples, include solid nanoparticles (44, 47, 48), liposomes (49–51) and polymer micelles (52–56). The surface of such carriers is often modified by poly(ethylene glycol) (PEG)1 brush (“PEGylation”) to increase the stability of nanoparticles in dispersion and extend circulation time of nanoparticles in the body (46, 57–59). The targeting moieties, such as peptides, can be attached to the ends of the PEG chains at the external side of the brush to allow for the binding of these moieties with their specific receptors.
One of the early studies of targeted drug delivery to the brain used Pluronic® block copolymers micelles as carriers of solubilized drugs (60, 61). These micelles were conjugated with either antibodies to the brain-specific antigens or insulin as a moiety to target insulin receptors at the lumenal side of BMEC. Both, the antibody-conjgated and insulin-conjugated micelles were shown to effectively deliver a drug incorporated into the micelles to the brain tissue in vivo. Subsequent studies demonstrated that the micelles conjugated with insulin undergo receptor-mediated transport in BMVEC (22). Insulin receptor appears to be promising target for drug delivery to the brain using the carrier technology. A recent study reported targeting of plasmid DNA encapsulated into the PEGylated liposomes using monoclonal antibodies to the human insulin receptor, human epidermal growth factor receptor (EGFR), or rat transferrin receptor (62). The studies using human and rat glioma cells suggested 100- to 200-fold higher levels of gene expression when the insulin receptor was targeted compared to the two other receptor targets used. The same group has demonstrated that immunoliposomes carrying a therapeutic antisense EGFR gene can be successfully delivered to EGFR-dependent brain gliomas in vivo, resulting in gene expression and reduction of the tumor growth (63).
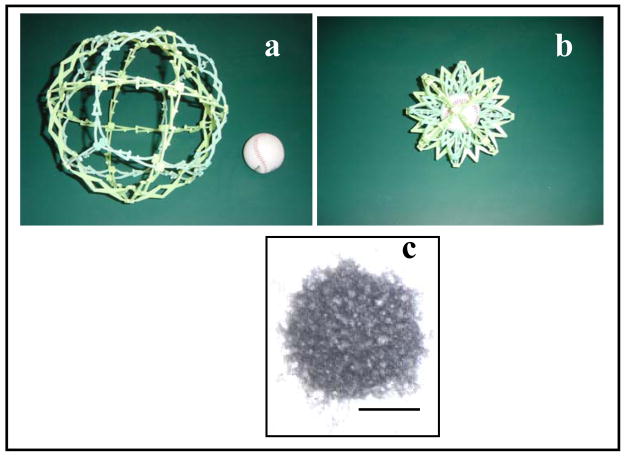
A new family of carrier systems, Nanogel™ was recently developed for targeted delivery of drugs and biomacromolecules to the brain (64, 65). Nanogel™ represents a nanoscale size polymer network of cross-linked ionic polyethyleneimine (PEI) and nonionic poly(ethylene glycol) (PEG) chains (PEG-cl-PEI). Figure 2 shows a schematic the Nanogel™ delivery system. Nanogel™ forms swollen cross-linked networks dispersed in solution (panel a). Upon binding of a macromolecular drug through electrostatic interaction of this drug with PEI chain Nanogel™ collapses resulting in decreased volume and size of the particles (panel b). Because of the effect of PEG chains, the collapsed Nanogel™ forms a stable dispersion with the particles size of ca. 80 nm. Nanogel™ can absorb spontaneously, through ionic interactions, a broad set of biomacromolecules, including negatively charged ODNs. One advantage is that Nanogel™ displays efficient loading of macromolecules (40–60% by weight), resulting in high “payloads” not achieved with conventional carrier systems.
Fig. 2.
Schematic illustration of Nanogel™ principle using a model: (a) swollen Nanogel™ has large pores, through which the drug (“ball”) can enter; (b) binding of a drug to results in Nanogel™ collapse. (c) Transmission electron microphotograph of PEG-cl-PEI Nanogel™ loaded with ODN. Bar = 50 nm. PEG-cl-PEI networks were synthesized by cross-linking of PEI (M≈25000) with double end N,N′-carbonyldiimidazole-activated PEG (Mn≈8000) using the emulsification-solvent evaporation technique (64). Following the synthesis the Nanogel™ particles were fractionated by gel-permeation chromatography and a fraction with an average particle diameter of ca. 250 nm was used for complex formation with phosphorothioate ODN.
Figure 2 panel c presents an electron microscopy of Nanogel™ loaded with the ODN. The ODNs incorporated into the Nanogel™ were protected against degradation by nucleases. However, upon delivery within a target cell the ODNs were released and exhibited specific activity against their molecular targets, as demonstrated using several cell models (64). The study using bovine BMVEC monolayers, as an in vitro model, demonstrated that following incorporation in the Nanogel™ particles the transport of ODNs across BBB was significantly increased compared to the free ODNs transport (64). Furthermore, the Nanogel™-incorporated ODNs were protected from degradation in BMVEC. This study also tested Nanogel™ system for the receptor-mediated delivery of ODNs across BMVEC monolayers. Specifically, to target the receptors displayed at BMVEC the surface of the Nanogel™ particles was modified by either transferrin or insulin using avidin-biotin coupling chemistry. Both peptides were shown to increase transcellular permeability of the Nanogel™ and enhance delivery of ODN across BMVEC monolayers. Recent in vivo studies demonstrated that intravenously administered 3H-ODN encapsulated in Nanogel™ was accumulated in the brain (in preparation). These studies suggest that Nanogel™ is one promising carrier delivery of biomacromolecules to the brain.
4. Artificial hydrophobization of peptides and proteins for delivery to CNS
Aside from the use of brain-specific peptides as targeting moieties there is a tremendous need to enhance delivery of therapeutic peptides and proteins to the brain to treat neurodegenerative disorders. Some examples, include Parkinson’s and Alzheimer’s diseases (66–68), lysosomal diseases (69, 70), and human obesity (18, 71). BBB significantly restricts and controls the exchange of peptides and regulatory proteins between the CNS and the blood. The only peptides that cross the BBB to any appreciable extent utilize receptor-mediated endocytosis (e.g. insulin, insulin-like growth factor, and transferrin) (72). The hydrophilicity, the lack of stability due to enzymatic or chemical degradation, and the lack of transport carriers capable of shuttling proteins across cell membranes, all play a part in precluding most peptides and proteins from uptake into and transport into the brain (73). The attempts were made to covalently modify peptides and proteins to enable their transport to the brain. For example, early studies reported that modification of immunoglobulins with cationic groups (“cationization”) resulted in the enhanced uptake of these proteins into the cells and delivery of these proteins to the brain (74–76).
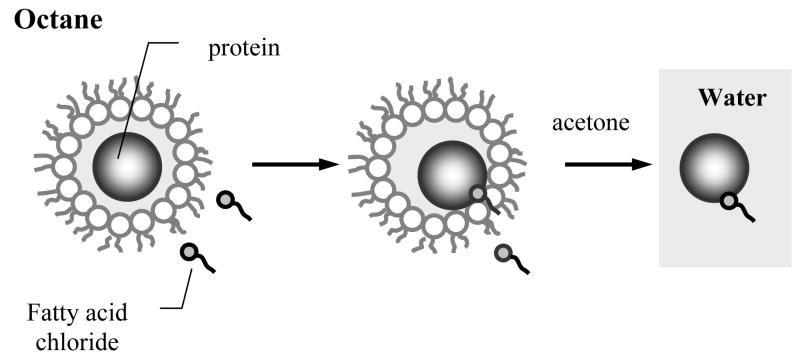
One useful strategy to enhance binding and uptake of proteins in the cells involves artificial hydrophobization of these proteins with fatty acid residues (77–80). This technology involves introduction of a very small number of residues of a fatty acid (e.g. stearic, palmitic, oleic) into the protein molecules, specifically, 1 to 2 residues per protein globule. As a result of such “gentle” modification the protein molecule remains water-soluble but it also acquires hydrophobic anchor groups that can target even very hydrophilic proteins to the cell surfaces. To obtain low and controlled degrees of modification of proteins with water-insoluble reagents a modification procedure has been developed, which utilizes system of reverse micelles of a surfactant, sodium bis-(2-ethylhexyl)sulfosucciate (Aerosol OT) in octane (77). After solubilization in such colloidal system, which has very low content of water (less that 1 % wt.), the protein molecule becomes entrapped into the inner water pool of the reverse micelle, acquiring a monolayer cover of the hydrated surfactant molecules (Figure. 3). The size of the water pool can be easily altered by changing the ratio [H2O]/[Aerosol OT]. The water-insoluble reagent is localized not only in the bulk phase of the organic solvent but also incorporated into the surfactant layer of the micelle coming into contact with the modified group of the protein. Following the completion of the reaction the modified protein is precipitated and the surfactant and excess of the reagent are removed by adding cold acetone. By conducting the reaction in the microheterogeneous environment of the reverse micelles the protein conjugates with strictly controlled and low degree of modification can be obtained. Over a dozen water-soluble proteins (enzymes, antibodies, toxins, cytokines) were modified using this technique with their functional activity being preserved after the modification (77, 81–87).
Fig. 3.
Chemical modification of the protein with a water-insoluble reagent in the reverse micelles of Aerosol OT in octane (77). The protein molecule is entrapped in the reverse micelle is surrounded by a cover of hydrated surfactant molecules. The water-insoluble reagent is located in the bulk organic phase and can be incorporated into the micelle surface layer coming in contact with the reactive group in the protein. After completion of the reaction the reverse micelle system is disintegrated and the protein is precipitated by cold acetone.
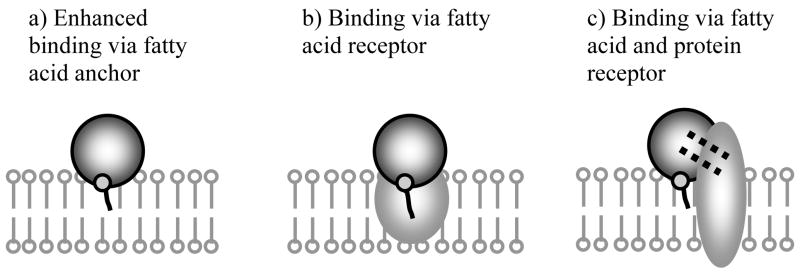
Further, extensive studies of the fatty acylated proteins with artificial lipid membranes and cells were conducted by us and others (77, 82, 84, 87–91). Briefly, the results of these studies are illustrated by the schematic presented in Figure 4. First, in all cases modification of water-soluble proteins with fatty acid residues resulted in enhanced binding of these proteins with lipid membranes due to the anchoring effect of the hydrophobic group. Second, in selected cases when the specific fatty acid binding receptors were present at the cell surface (e.g. hepatocytes) the binding was enhanced to an even greater extent, presumably, due to the targeting of this receptor by the fatty acid residue attached to the protein. Third, when the protein had a receptor expressed at the cell surface, fatty acylation and specific interaction with the receptor combined resulted in a cumulative effect promoting very strong binding of the protein with the cell membrane. In addition to the enhanced binding to the cell surface there was a significant increase in the uptake of the fatty acylated proteins into the cells (78). In selected cased such modification resulted in translocation of the protein across the cell membrane into the cytoplasm (e.g. increased toxicity of ricin A chain (82)). However, in most cases the internalized fatty acylated protein remained entrapped in the endocytic vesicles within the cell and was not released into the cytoplam (87). Nevertheless, when the protein displayed biological effect in the cells, mediated by specific receptor(s), the activity of this protein, as a result of fatty acylation was greatly increased. Specific examples include increase of antiproliferative activity of Staphylococcus aureus enterotoxin A and recombinant α-inerferon by ca. 100- and 1000- respectively (82, 84). Finally, fatty acylated antibodies against virus-specific antigens displayed the ability to inhibit virus reproduction in the cells, whereas unmodified antibodies had practically no effect on the virus growth (81, 92). This effect was also explained by a combination interaction of the modified antibodies with the infected cells involving specific binding with virus antigens (via the antibody active center), and non-specific binding with membranes (via the fatty acid residues) (88). As a result, the modified antibodies disrupted the essential stages of virus reproduction in the cells including the virus particle assembly and budding.
Fig. 4.
Schematic representation of major mechanisms of interaction of fatty acylated proteins with cell membranes: (a) attachment to the lipid membrane by the fatty acid anchor group; (b) binding with fatty acid receptor; (c) two-point attachment via the the fatty acid anchor and specific binding with the protein receptor.
The interest to this technology as related to CNS delivery of proteins was precipitated by the finding that antibodies against brain specific-antigens and antibody Fab fragments, as a result of fatty acylation, acquire the ability to accumulate in the brain after systemic administration (83, 93). Specifically, five days after administration the amounts of Fab fragments modified with stearic acid residues appeared to be ca. 20 times higher in the brain than in other organs (liver or kidney). Furthermore, a neuroleptic drug conjugated with the modified Fab fragments appeared to be more efficiently delivered to the brain than the free drug. Interestingly, fatty acylated Fab fragments of non-specific antibodies did not accumulate in the brain to the extent observed with their brain-specific counterparts. These modified Fab fragments were accumulated mainly in the liver. This suggests that combination of interactions involving both the fatty acid residues and the antigen-binding site is essential for the delivery of the modified antibodies in the brain tissue.
Subsequently, evidence began to mount that delivery of the fatty acylated proteins to the brain might involve enhanced transcytosis of these proteins across BMVEC. In particular, a group of French investigators, using Aerosol OT reversed micelles, synthesized a fatty acylated ribonuclease A (Rnase A) and demonstrated that as a result of such modification the enzyme acquired an ability to cross BMVEC monolayers with little, if any, degradation (90). Specifically, the transport of acylated RNase A across bovine BMVEC monolayers was increased 10-fold compared to the non-modified enzyme. Noteworthy, for successful translocation of RNase A across the BMVEC monolayers a minimal length of the fatty acid residue of 16 carbon atoms (stearoyl residue) was required.
The mechanism of the enhanced transport of fatty acylated proteins in the BBB at present remains unknown. However, the technology involving fatty acylation of peptides and proteins for their delivery to the brain could be very promising. The long-chain fatty acids are present in the body in high amounts and could be much less harmful, compared to many ligands capable of receptor-mediated binding and transport in the BBB, which can also display various side effects. Fatty acid binding proteins (FABPs), which facilitate uptake, transport, and targeting of long-chain fatty acids are expressed in many tissues, particularly, in neural tissue, including brain FABP (B-FABP), and myelin FABP (M-FABP) (94–97). Some involvement of FABP in the transport and biodistribution of the fatty acylated proteins in the body is possible and it needs to be evaluated in subsequent studies. Furthermore, it is noteworthy that fatty acid modification of proteins can actually reduce immunogenicity of these proteins and decrease production of antibodies against them (98). This result suggests a simple strategy for reducing the immunogenicity of foreign proteins and for decreasing the risk of immunological complications in therapy, which could be an additional benefit in the studies of the CNS delivery of therapeutic peptides and proteins. One should expect the rapid increase of the studies using artificially hydrophobized peptides and proteins in the near future.
5. Conclusions
Novel drug delivery systems promise new opportunities in the therapy of acute and chronic brain disease. The transport of the drugs to the brain can be improved by inhibition of drug efflux transport proteins, such as Pgp, which are important gatekeepers in the BBB. Alternative strategies, involve the use of polymer nanocarriers, such as Nanogel™, which can be targeted to the brain by attaching specific peptides to their surface. Finally, a new promising strategy for the brain delivery of peptides and proteins is emerging, which involves artificial hydrophobization of the protein (peptide) molecule with fatty acid residues. Overall, the design of successful formulations for CNS delivery low molecular drugs and biomacromolecules will require clear understanding and careful consideration of the mechanisms for the transport, accumulation and elimination of these drugs in the brain.
Acknowledgments
We acknowledge support from the National Institute for Neurological Disorders and Stroke (NS36229) and National Science Foundation (BES-9907281). We also would like to thank Dr. Valery Yu. Alakhov (Montreal, Canada), Dr. Vladimir P. Chekhonin (Moscow, Russia), Drs. William F. Elmquist and Donald W. Miller (Omaha, NE) for contributions, stimulating discussions and valuable advice concerning various aspects of the studies discussed in this review.
Footnotes
Same as poly(ethylene oxide) or PEO
References
- 1.Pardridge WM, editor. Methodology, biology and pathology. Cambridge: University Press; 1998. Introduction to the blood-brain barrier. [Google Scholar]
- 2.Mayhan WG. Regulation of blood-brain barrier permeability. Microcirculation. 2001;8(2):89–104. doi: 10.1111/j.1549-8719.2001.tb00160.x. [DOI] [PubMed] [Google Scholar]
- 3.Begley D. The blood-brain barrier: principles for targeting peptides and drugs to the central nervous system. J Pharm Pharmacol. 1996;48(2):136–146. doi: 10.1111/j.2042-7158.1996.tb07112.x. [DOI] [PubMed] [Google Scholar]
- 4.Tamai I, Tsuji A. Transporter-mediated permeation of drugs across the blood-brain barrier. J Pharm Sci. 2000;89(11):1371–1388. doi: 10.1002/1520-6017(200011)89:11<1371::aid-jps1>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 5.Fromm MF. P-glycoprotein: a defense mechanism limiting oral bioavailability and CNS accumulation of drugs. Int J Clin Pharmacol Ther. 2000;38(2):69–74. doi: 10.5414/cpp38069. [DOI] [PubMed] [Google Scholar]
- 6.Minn A, Ghersi-Egea JF, Perrin R, Leininger B, Siest G. Drug metabolizing enzymes in the brain and cerebral microvessels. Brain Res Brain Res Rev. 1991;16(1):65–82. doi: 10.1016/0165-0173(91)90020-9. [DOI] [PubMed] [Google Scholar]
- 7.Abbott NJ, Romero IA. Transporting therapeutics across the blood-brain barrier. Mol Med Today. 1996;2(3):106–113. doi: 10.1016/1357-4310(96)88720-x. [DOI] [PubMed] [Google Scholar]
- 8.Pardridge WM. Targeting neurotherapeutic agents through the blood-brain barrier. Arch Neurol. 2002;59(1):35–40. doi: 10.1001/archneur.59.1.35. [DOI] [PubMed] [Google Scholar]
- 9.Haller MF, Saltzman WM. Localized delivery of proteins in the brain: can transport be customized? Pharm Res. 1998;15(3):377–385. doi: 10.1023/a:1011911912174. [DOI] [PubMed] [Google Scholar]
- 10.Jolliet-Riant P, Tillement JP. Drug transfer across the blood-brain barrier and improvement of brain delivery. Fundam Clin Pharmacol. 1999;13(1):16–26. doi: 10.1111/j.1472-8206.1999.tb00316.x. [DOI] [PubMed] [Google Scholar]
- 11.Spector R. Drug transport in the mammalian central nervous system: multiple complex systems. A critical analysis and commentary. Pharmacology. 2000;60(2):58–73. doi: 10.1159/000028349. [DOI] [PubMed] [Google Scholar]
- 12.Lo EH, Singhal AB, Torchilin VP, Abbott NJ. Drug delivery to damaged brain. Brain Res Brain Res Rev. 2001;38(1–2):140–148. doi: 10.1016/s0165-0173(01)00083-2. [DOI] [PubMed] [Google Scholar]
- 13.Bodor N, Buchwaldm P. Barriers to remember: brain-targeting chemical delivery systems and Alzheimer’s disease. Drug Discov Today. 2002;7(14):766–774. doi: 10.1016/s1359-6446(02)02332-2. [DOI] [PubMed] [Google Scholar]
- 14.Loscher W, Potschka H. Role of multidrug transporters in pharmacoresistance to antiepileptic drugs. J Pharmacol Exp Ther. 2002;301(1):7–14. doi: 10.1124/jpet.301.1.7. [DOI] [PubMed] [Google Scholar]
- 15.Pardridge WM. Drug and gene delivery to the brain: the vascular route. Neuron. 2002;36(4):555–558. doi: 10.1016/s0896-6273(02)01054-1. [DOI] [PubMed] [Google Scholar]
- 16.Pardridge WM. Drug and gene targeting to the brain with molecular Trojan horses. Nat Rev Drug Discov. 2002;1(2):131–139. doi: 10.1038/nrd725. [DOI] [PubMed] [Google Scholar]
- 17.Cornford EM, Hyman S. Blood-brain barrier permeability to small and large molecules. Adv Drug Deliv Rev. 1999;36(2–3):145–163. doi: 10.1016/s0169-409x(98)00082-9. [DOI] [PubMed] [Google Scholar]
- 18.Banks WA, Lebel CR. Strategies for the delivery of leptin to the CNS. J Drug Target. 2002;10(4):297–308. doi: 10.1080/10611860290031895. [DOI] [PubMed] [Google Scholar]
- 19.Alakhov VY, Moskaleva EY, Batrakova EV, Kabanov AV. Hypersensitization of multidrug resistant human ovarian carcinoma cells by pluronic P85 block copolymer. Bioconjug Chem. 1996;7(2):209–216. doi: 10.1021/bc950093n. [DOI] [PubMed] [Google Scholar]
- 20.Venne A, Li S, Mandeville R, Kabanov A, Alakhov V. Hypersensitizing effect of pluronic L61 on cytotoxic activity, transport, and subcellular distribution of doxorubicin in multiple drug- resistant cells. Cancer Res. 1996;56(16):3626–3629. [PubMed] [Google Scholar]
- 21.Miller DW, Batrakova EV, Waltner TO, Alakhov V, Kabanov AV. Interactions of pluronic block copolymers with brain microvessel endothelial cells: evidence of two potential pathways for drug absorption. Bioconjug Chem. 1997;8(5):649–657. doi: 10.1021/bc970118d. [DOI] [PubMed] [Google Scholar]
- 22.Batrakova EV, Han HY, Miller DW, Kabanov AV. Effects of pluronic P85 unimers and micelles on drug permeability in polarized BBMEC and Caco-2 cells. Pharm Res. 1998;15(10):1525–1532. doi: 10.1023/a:1011942814300. [DOI] [PubMed] [Google Scholar]
- 23.Batrakova EV, Lee S, Li S, Venne A, Alakhov V, Kabanov A. Fundamental relationships between the composition of pluronic block copolymers and their hypersensitization effect in MDR cancer cells. Pharm Res. 1999;16(9):1373–1379. doi: 10.1023/a:1018942823676. [DOI] [PubMed] [Google Scholar]
- 24.Batrakova EV, Li S, Vinogradov SV, Alakhov VY, Miller DW, Kabanov AV. Mechanism of pluronic effect on P-glycoprotein efflux system in blood-brain barrier: contributions of energy depletion and membrane fluidization. J Pharmacol Exp Ther. 2001;299(2):483–493. [PubMed] [Google Scholar]
- 25.Batrakova EV, Miller DW, Li S, Alakhov VY, Kabanov AV, Elmquist WF. Pluronic P85 enhances the delivery of digoxin to the brain: in vitro and in vivo studies. J. Pharmacol. Exp. Ther. 2001;296(2):551–557. [PubMed] [Google Scholar]
- 26.Batrakova EV, Li S, Alakhov VY, Elmquist WF, Miller DW, Kabanov AV. Sensitization of cells overexpressing multidrug resistant protein by Pluronic P85. Pharm Res. 2003 doi: 10.1023/a:1026179132599. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller DW, Batrakova EV, Kabanov AV. Inhibition of multidrug resistance-associated protein (MRP) functional activity with pluronic block copolymers. Pharm Res. 1999;16(3):396–401. doi: 10.1023/a:1018873702411. [DOI] [PubMed] [Google Scholar]
- 28.Kozlov MY, Melik-Nubarov NS, Batrakova EV, Kabanov AV. Relationship between pluronic block copolymer structure, critical micellization concentration and partitioning coefficients of low molecular mass solutes. Macromolecules. 2000;33(9):3305–3313. [Google Scholar]
- 29.Batrakova EV, Li S, Alakhov VY, Miller DW, Kabanov AV. Optimal structure requirements for Pluronic block copolymers in modifying P-glycoprotein drug efflux transporter activity in bovine brain microvessel endothelial cells. J Pharmacol Exp Ther. 2003;304(2):845–854. doi: 10.1124/jpet.102.043307. [DOI] [PubMed] [Google Scholar]
- 30.Kirillova GP, Mokhova EN, Dedukhova VI, Tarakanova AN, Ivanova VP, Efremova NV, et al. The influence of pluronics and their conjugates with proteins on the rate of oxygen consumption by liver mitochondria and thymus lymphocytes. Biotechnol Appl Biochem. 1993;18(Pt 3):329–339. [PubMed] [Google Scholar]
- 31.Rapoport N, Marin AP, Timoshin AA. Effect of a polymeric surfactant on electron transport in HL-60 cells. Arch Biochem Biophys. 2000;384(1):100–108. doi: 10.1006/abbi.2000.2104. [DOI] [PubMed] [Google Scholar]
- 32.Batrakova EV, Li S, Elmquist WF, Miller DW, Alakhov VY, Kabanov AV. Mechanism of sensitization of MDR cancer cells by Pluronic block copolymers: Selective energy depletion. Br J Cancer. 2001;85(12):1987–1997. doi: 10.1054/bjoc.2001.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kabanov AV, Batrakova E, Alakhov VYu. An essential relationship between ATP depletion and chemosensitizing activity of Pluronic block copolymers. J Contr Release. 2003 doi: 10.1016/s0168-3659(03)00211-6. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ranson M, Ferry D, Kerr D, Radford J, Dickens D, Alakhov V, et al. Results of a Cancer Research Campaighn phase I dose escalation trial of SP1049C in patients with advanced cancer. 5th international symposium on polymer therapeutics: from laboratory to clinical pactice; 2002 3th-5th January 2002; Cardiff, UK: The Welsh School of Pharmacy, Cardiff University; 2002. p. 15. [Google Scholar]
- 35.Zlokovic BV. Cerebrovascular permeability to peptides: manipulations of transport systems at the blood-brain barrier. Pharm Res. 1995;12(10):1395–1406. doi: 10.1023/a:1016254514167. [DOI] [PubMed] [Google Scholar]
- 36.Bickel U, Yoshikawa T, Pardridge WM. Delivery of peptides and proteins through the blood-brain barrier. Adv Drug Deliv Rev. 2001;46(1–3):247–279. doi: 10.1016/s0169-409x(00)00139-3. [DOI] [PubMed] [Google Scholar]
- 37.Pardridge WM. Neurotrophins, neuroprotection and the blood-brain barrier. Curr Opin Investig Drugs. 2002;3(12):1753–1757. [PubMed] [Google Scholar]
- 38.Pasqualini R, Arap W, McDonald DM. Probing the structural and molecular diversity of tumor vasculature. Trends Mol Med. 2002;8(12):563–571. doi: 10.1016/s1471-4914(02)02429-2. [DOI] [PubMed] [Google Scholar]
- 39.Wu D, Song BW, Vinters HV, Pardridge WM. Pharmacokinetics and brain uptake of biotinylated basic fibroblast growth factor conjugated to a blood-brain barrier drug delivery system. J Drug Target. 2002;10(3):239–245. doi: 10.1080/10611860290022679. [DOI] [PubMed] [Google Scholar]
- 40.Boado RJ, Tsukamoto H, Pardridge WM. Drug delivery of antisense molecules to the brain for treatment of Alzheimer’s disease and cerebral AIDS. J Pharm Sci. 1998;87(11):1308–1315. doi: 10.1021/js9800836. [DOI] [PubMed] [Google Scholar]
- 41.Wu D, Boado RJ, Pardridge WM. Pharmacokinetics and blood-brain barrier transport of [3H]-biotinylated phosphorothioate oligodeoxynucleotide conjugated to a vector-mediated drug delivery system. J Pharmacol Exp Ther. 1996;276(1):206–211. [PubMed] [Google Scholar]
- 42.Torchilin VP. Drug targeting. Eur J Pharm Sci. 2000;11 (Suppl 2):S81–S91. doi: 10.1016/s0928-0987(00)00166-4. [DOI] [PubMed] [Google Scholar]
- 43.Moghimi SM, Illum L, Davis SS. Physiopathological and physicochemical considerations in targeting of colloids and drug carriers to the bone marrow. Crit Rev Ther Drug Carrier Syst. 1990;7(3):187–209. [PubMed] [Google Scholar]
- 44.Chavany C, Saison-Behmoaras T, Le Doan T, Puisieux F, Couvreur PCH. Adsorption of oligonucleotides onto polyisohexylcyanoacrylate nanoparticles protects them against nucleases and increases their cellular uptake. Pharm Res. 1994;11(9):1370–1378. doi: 10.1023/a:1018923301967. [DOI] [PubMed] [Google Scholar]
- 45.Calvo P, Gouritin B, Chacun H, Desmaele D, D’Angelo J, Noel JP, et al. Long-circulating PEGylated polycyanoacrylate nanoparticles as new drug carrier for brain delivery. Pharm Res. 2001;18(8):1157–1166. doi: 10.1023/a:1010931127745. [DOI] [PubMed] [Google Scholar]
- 46.Torchilin V. Polymer-coated long-circulating microparticulate pharmaceuticals. J Microencapsul. 1998;15(1):1–19. doi: 10.3109/02652049809006831. [DOI] [PubMed] [Google Scholar]
- 47.Godard G, Boutorine AS, Saison-Behmoaras E, Helene C. Antisense effects of cholesterol-oligodeoxynucleotide conjugates associated with poly(alkylcyanoacrylate) nanoparticles. Eur J Biochem. 1995;232(2):404–410. [PubMed] [Google Scholar]
- 48.Fattal E, Vauthier C, Aynie I, Nakada Y, Lambert G, Malvy C, et al. Biodegradable polyalkylcyanoacrylate nanoparticles for the delivery of oligonucleotides. J Control Release. 1998;53(1–3):137–143. doi: 10.1016/s0168-3659(97)00246-0. [DOI] [PubMed] [Google Scholar]
- 49.Gregoriadis G. Engineering liposomes for drug delivery: progress and problems. Trends Biotechnol. 1995;13(12):527–537. doi: 10.1016/S0167-7799(00)89017-4. [DOI] [PubMed] [Google Scholar]
- 50.Torchilin VP. Affinity liposomes in vivo: factors influencing target accumulation. J Mol Recognit. 1996;9(5–6):335–346. doi: 10.1002/(sici)1099-1352(199634/12)9:5/6<335::aid-jmr309>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 51.Allen TM. Liposomes. Opportunities in drug delivery Drugs. 1997;54(Suppl 4):8–14. doi: 10.2165/00003495-199700544-00004. [DOI] [PubMed] [Google Scholar]
- 52.Kwon GS, Kataoka K. Block copolymer micelles as long-circulating drug vehicles. Adv Drug Delivery Rev. 1995;16(2–3):295–309. [Google Scholar]
- 53.Alakhov VY, Kabanov AV. Block copolymeric biotransport carriers as versatile vehicles for drug delivery. Exp Op Invest Drugs. 1998;7(9):1453–1473. doi: 10.1517/13543784.7.9.1453. [DOI] [PubMed] [Google Scholar]
- 54.Kwon GS, Okano T. Soluble self-assembled block copolymers for drug delivery. Pharm Res. 1999;16(5):597–600. doi: 10.1023/a:1011991617857. [DOI] [PubMed] [Google Scholar]
- 55.Torchilin VP. PEG-based micelles as carriers of contrast agents for different imaging modalities. Adv Drug Deliv Rev. 2002;54(2):235–252. doi: 10.1016/s0169-409x(02)00019-4. [DOI] [PubMed] [Google Scholar]
- 56.Kabanov AV, Alakhov VY. Pluronic block copolymers in drug delivery: from micellar nanocontainers to biological response modifiers. Crit Rev Ther Drug Carrier Syst. 2002;19(1):1–72. doi: 10.1615/critrevtherdrugcarriersyst.v19.i1.10. [DOI] [PubMed] [Google Scholar]
- 57.Gref R, Minamitake Y, Peracchia MT, Trubetskoy V, Torchilin V, Langer R. Biodegradable long-circulating polymeric nanospheres. Science. 1994;263(5153):1600–1603. doi: 10.1126/science.8128245. [DOI] [PubMed] [Google Scholar]
- 58.Stolnik S, Dunn SE, Garnett MC, Davies MC, Coombes AG, Taylor DC, et al. Surface modification of poly(lactide-co-glycolide) nanospheres by biodegradable poly(lactide)-poly(ethylene glycol) copolymers. Pharm Res. 1994;11(12):1800–1808. doi: 10.1023/a:1018931820564. [DOI] [PubMed] [Google Scholar]
- 59.Peracchia MT, Vauthier C, Desmaele D, Gulik A, Dedieu JC, Demoy M, et al. Pegylated nanoparticles from a novel methoxypolyethylene glycol cyanoacrylate-hexadecyl cyanoacrylate amphiphilic copolymer. Pharm Res. 1998;15(4):550–556. doi: 10.1023/a:1011973625803. [DOI] [PubMed] [Google Scholar]
- 60.Kabanov AV, Chekhonin VP, Alakhov VY, Batrakova EV, Lebedev AS, Melik-Nubarov NS, et al. The neuroleptic activity of haloperidol increases after its solubilization in surfactant micelles. Micelles as microcontainers for drug targeting. FEBS Lett. 1989;258(2):343–345. doi: 10.1016/0014-5793(89)81689-8. [DOI] [PubMed] [Google Scholar]
- 61.Kabanov AV, Batrakova EV, Melik-Nubarov NS, Fedoseev NA, Dorodnich TY, Alakhov VY, et al. A new class of drug carriers: micelles of poly(oxyethylene)-poly(oxypropylene) block copolymers as microcontainers for drug targeting from blood in brain. J Controlled Release. 1992;22(2):141–157. [Google Scholar]
- 62.Zhang Y, Boado RJ, Pardridge WM. Marked enhancement in gene expression by targeting the human insulin receptor. J Gene Med. 2003;5(2):157–163. doi: 10.1002/jgm.333. [DOI] [PubMed] [Google Scholar]
- 63.Zhang Y, Jeong Lee H, Boado RJ, Pardridge WM. Receptor-mediated delivery of an antisense gene to human brain cancer cells. J Gene Med. 2002;4(2):183–194. doi: 10.1002/jgm.255. [DOI] [PubMed] [Google Scholar]
- 64.Vinogradov SV, Batrakova EV, Kabanov AV. Poly(ethylene glycol)-polyethyleneimine NanoGel particles: novel drug delivery systems for antisense oligonucleotides. Coll Surf B: Biointerfaces. 1999;16:291–304. [Google Scholar]
- 65.Vinogradov SV, Bronich TK, Kabanov AV. Nanosized cationic hydrogels for drug delivery: preparation, properties and interactions with cells. Adv Drug Deliv Rev. 2002;54(1):135–147. doi: 10.1016/s0169-409x(01)00245-9. [DOI] [PubMed] [Google Scholar]
- 66.Brinton RD. A women’s health issue: Alzheimer’s disease and strategies for maintaining cognitive health. Int J Fertil Womens Med. 1999;44(4):174–85. [PubMed] [Google Scholar]
- 67.Gozes I. Neuroprotective peptide drug delivery and development: potential new therapeutics. Trends Neurosci. 2001;24(12):700–705. doi: 10.1016/s0166-2236(00)01931-7. [DOI] [PubMed] [Google Scholar]
- 68.Kroll RA, Neuwelt EA. Outwitting the blood-brain barrier for therapeutic purposes: osmotic opening and other means. Neurosurgery. 1998;42(5):1083–99. doi: 10.1097/00006123-199805000-00082. discussion 1099–100. [DOI] [PubMed] [Google Scholar]
- 69.Desnick RJ, Schuchman EH. Enzyme replacement and enhancement therapies: lessons from lysosomal disorders. Nat Rev Genet. 2002;3(12):954–966. doi: 10.1038/nrg963. [DOI] [PubMed] [Google Scholar]
- 70.Downs-Kelly E, Jones MZ, Alroy J, Cavanagh KT, King B, Lucas RE, et al. Caprine mucopolysaccharidosis IIID: a preliminary trial of enzyme replacement therapy. J Mol Neurosci. 2000;15(3):251–262. doi: 10.1385/JMN:15:3:251. [DOI] [PubMed] [Google Scholar]
- 71.Banks WA. Is obesity a disease of the blood-brain barrier? Physiological, pathological, and evolutionary considerations. Curr Pharm Des. 2003;9(10):801–809. doi: 10.2174/1381612033455350. [DOI] [PubMed] [Google Scholar]
- 72.Pardridge W. Peptide drug delivery to the brain. Raven Press; New York: 1991. [Google Scholar]
- 73.Lee VHL. Peptide and protein drug delivery. Dekker; New York: 1991. [Google Scholar]
- 74.Pardridge WM, Buciak J, Yang J, Wu D. Enhanced endocytosis in cultured human breast carcinoma cells and in vivo biodistribution in rats of a humanized monoclonal antibody after cationization of the protein. J Pharmacol Exp Ther. 1998;286(1):548–554. [PubMed] [Google Scholar]
- 75.Triguero D, Buciak JL, Pardridge WM. Cationization of immunoglobulin G results in enhanced organ uptake of the protein after intravenous administration in rats and primate. J Pharmacol Exp Ther. 1991;258(1):186–192. [PubMed] [Google Scholar]
- 76.Triguero D, Buciak JB, Yang J, Pardridge WM. Blood-brain barrier transport of cationized immunoglobulin G: enhanced delivery compared to native protein. Proc Natl Acad Sci U S A. 1989;86(12):4761–4765. doi: 10.1073/pnas.86.12.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kabanov AV, Levashov AV, Martinek K. Transformation of water-soluble enzymes into membrane active form by chemical modificationAcad. Ann NY of Sci. 1987;501:63–66. doi: 10.1111/j.1749-6632.1987.tb45685.x. [DOI] [PubMed] [Google Scholar]
- 78.Kabanov AV, Levashov AV, Alakhov VY. Lipid modification of proteins and their membrane transport. Protein Eng. 1989;3(1):39–42. doi: 10.1093/protein/3.1.39. [DOI] [PubMed] [Google Scholar]
- 79.Hashimoto M, Takada K, Kiso Y, Muranishi S. Synthesis of palmitoyl derivatives of insulin and their biological activities. Pharm Res. 1989;6(2):171–176. doi: 10.1023/a:1015992828666. [DOI] [PubMed] [Google Scholar]
- 80.Colsky AS, Peacock JS. Palmitate-derivatized antibodies can specifically “arm” macrophage effector cells for ADCC. J Leukoc Biol. 1991;49(1):1–7. doi: 10.1002/jlb.49.1.1. [DOI] [PubMed] [Google Scholar]
- 81.Kabanov AV, Ovcharenko AV, Melik-Hubarov NS, Bannikov AI, Alakhov V, Kiselev VI, et al. Fatty acid acylated antibodies against virus suppress its reproduction in cells. FEBS Lett. 1989;250(2):238–240. doi: 10.1016/0014-5793(89)80729-x. [DOI] [PubMed] [Google Scholar]
- 82.Alakhov V, Kabanov AV, Batrakova EV, Koromyslova IA, Levashov AV, Severin ES. Increasing cytostatic effects of ricin A chain and Staphylococcus aureus enterotoxin A through in vitro hydrophobization with fatty acid residues. Biotechnol Appl Biochem. 1990;12(1):94–8. [PubMed] [Google Scholar]
- 83.Chekhonin VP, Kabanov AV, Zhirkov YA, Morozov GV. Fatty acid acylated Fab-fragments of antibodies to neurospecific proteins as carriers for neuroleptic targeted delivery in brain. FEBS Lett. 1991;287(1–2):149–152. doi: 10.1016/0014-5793(91)80037-4. [DOI] [PubMed] [Google Scholar]
- 84.Kabanov AV, Alakhov VY, Chekhonin VP. Enhancement of macromolecule penetration into cells and nontraditional drug delivery systems. Glasgow: Harwood Academic Publishers; 1992. pp. 1–77. [Google Scholar]
- 85.Robert S, Domurado D, Thomas D, Chopineau J. Fatty acid acylation of RNase A using reversed micelles as microreactors. Biochem Biophys Res Commun. 1993;196(1):447–454. doi: 10.1006/bbrc.1993.2270. [DOI] [PubMed] [Google Scholar]
- 86.Robert S, Domurado D, Thomas D, Chopineau J. Optimization of RNase A artificial hydrophobization in AOT reversed micelles. Ann N Y Acad Sci. 1995;750:121–124. doi: 10.1111/j.1749-6632.1995.tb19939.x. [DOI] [PubMed] [Google Scholar]
- 87.Slepnev VI, Phalente L, Labrousse H, Melik-Nubarov NS, Mayau V, Goud B, et al. Fatty acid acylated peroxidase as a model for the study of interactions of hydrophobically-modified proteins with mammalian cells. Bioconjug Chem. 1995;6(5):608–615. doi: 10.1021/bc00035a016. [DOI] [PubMed] [Google Scholar]
- 88.Melik-Nubarov NS, Suzdaltseva Yu G, Priss EL, Slepnev VI, Kabanov AV, Zhirnov OP, et al. Interaction of hydrophobized antiviral antibodies with influenza virus infected MDCK cells. Biochem Mol Biol Int. 1993;29(5):939–47. [PubMed] [Google Scholar]
- 89.Ekrami HM, Kennedy AR, Shen WC. Water-soluble fatty acid derivatives as acylating agents for reversible lipidization of polypeptides. FEBS Lett. 1995;371(3):283–286. doi: 10.1016/0014-5793(95)00910-2. [DOI] [PubMed] [Google Scholar]
- 90.Chopineau J, Robert S, Fenart L, Cecchelli R, Lagoutte B, Paitier S, et al. Monoacylation of ribonuclease A enables its transport across an in vitro model of the blood-brain barrier. J Control Release. 1998;56(1–3):231–237. doi: 10.1016/s0168-3659(98)00091-1. [DOI] [PubMed] [Google Scholar]
- 91.Kozlova NO, Bruskovskaya IB, Melik-Nubarov NS, Yaroslavov AA, Kabanov VA. Catalytic properties and conformation of hydrophobized alpha-chymotrypsin incorporated into a bilayer lipid membrane. FEBS Lett. 1999;461(3):141–144. doi: 10.1016/s0014-5793(99)01449-0. [DOI] [PubMed] [Google Scholar]
- 92.Kabanov AV, Ovcharenko AV, Melik-Nubarov NS, Bannikov AI, Lisok TP, Klyushnenkova EV, et al. Effective inhibition of viral reproduction by hydrophobised antiviral antibodies. Biomed Sci. 1990;1(1):63–67. [PubMed] [Google Scholar]
- 93.Chekhonin VP, Ryabukhin IA, Zhirkov YA, Kashparov IA, Dmitriyeva TB. Transport of hydrophobized fragments of antibodies through the blood-brain barrier. Neuroreport. 1995;7(1):129–132. [PubMed] [Google Scholar]
- 94.Zimmerman AW, van Moerkerk HT, Veerkamp JH. Ligand specificity and conformational stability of human fatty acid-binding proteins. Int J Biochem Cell Biol. 2001;33(9):865–876. doi: 10.1016/s1357-2725(01)00070-x. [DOI] [PubMed] [Google Scholar]
- 95.Rademacher M, Zimmerman AW, Ruterjans H, Veerkamp JH, Lucke C. Solution structure of fatty acid-binding protein from human brain. Mol Cell Biochem. 2002;239(1–2):61–68. [PubMed] [Google Scholar]
- 96.Thumser AE, Tsai J, Storch J. Collision-mediated transfer of long-chain fatty acids by neural tissue fatty acid-binding proteins (FABP): studies with fluorescent analogs. J Mol Neurosci. 2001;16(2–3):143–150. doi: 10.1385/JMN:16:2-3:143. discussion 151–157. [DOI] [PubMed] [Google Scholar]
- 97.Veerkamp JH, Zimmerman AW. Fatty acid-binding proteins of nervous tissue. J Mol Neurosci. 2001;16(2–3):133–142. doi: 10.1385/JMN:16:2-3:133. discussion 151–157. [DOI] [PubMed] [Google Scholar]
- 98.Shi Q, Domurado M, Domurado D. Effect of protein chemical hydrophobization on antiglucose oxidase immunoglobulin production in mouse. Pharmacol Toxicol. 1995;76(4):278–285. doi: 10.1111/j.1600-0773.1995.tb00143.x. [DOI] [PubMed] [Google Scholar]