Abstract
Background
For patients with axillary lymph node-negative breast cancer, benefits from adjuvant therapy are smaller than in node-positive disease and thus more selective use is warranted, prompting development of risk profiling to identify those most likely to benefit. Examination of the magnitude and changes in the hazard of failure over time in node-negative breast cancer may also be informative in this regard.
Methods
Among 9,444 participants from five randomized trials (accrual 1982–1998) investigating chemotherapy and tamoxifen for node-negative breast cancer, we estimated recurrence hazards over time by tumor estrogen receptor (ER) status and adjuvant treatment.
Results
In patients treated by surgery only, we observed the previously noted larger hazard peak followed by a rapid decrease in ER-negative patients and smaller but more persistent hazard in ER-positive patients. After approximately 48 months, the ER-positive hazard is greater. For adjuvant treatment, while tamoxifen decreases the early hazard in ER-positive patients to that of the chemotherapy-treated ER-negative group, in later follow-up (beyond 5 years) the hazard for ER-positive patients again exceeds that of ER-negative patients. Adding chemotherapy to tamoxifen in ER-positive patients results in large early hazard reduction, but in later follow-up the hazard converges with those of patients treated by surgery only or tamoxifen.
Conclusions
Recurrence hazards over time reveal changes in risk that may have biologic and therapeutic strategy relevance. In ER-negative tumors, a large early chemotherapy benefit is followed by a consistently low recurrence hazard over time. In ER-positive patients, the chemotherapy benefit appears concentrated mostly in earlier follow-up, and a greater recurrence risk remains.
Keywords: Lymph node-negative, Estrogen receptor, Systemic adjuvant therapy, Hazard, Prognosis
Introduction
Although prognosis for breast cancer with negative axillary lymph nodes is often favorable, there is considerable heterogeneity in outcomes and substantial benefit of adjuvant therapy for many patients [1, 2]. Efforts to prospectively profile recurrence risk and consequently breast cancer mortality risk have been undertaken, with the goal of more judicious use of adjuvant therapy, in particular cytotoxic chemotherapy [3, 4].
One challenging aspect of early stage breast cancer is the remarkably long period over which individuals remain at risk of recurrence, with events continuing to occur many years after initial diagnosis and treatment [5, 6]. Moreover, over long-term (>10 years) follow-up, patterns emerge that suggest a changing failure risk relative to early follow-up for some classes of patients. For example, in several predominantly lymph node-positive clinical cohorts, those with estrogen receptor (ER)-positive tumors had better prognosis than those with ER-negative tumors initially, while at later follow-up time, the relative risk of failure changed, with ER-positive patients having greater recurrence risk [7-9]. A similar pattern has been noted in the Surveillance, Epidemiology, and End Results (SEER) registry cohort with respect to breast cancer mortality [10]. In contrast to the more widely used survival curve, the hazard function, heuristically equaling the probability of failure in a small period of time given no failure thus far, more readily reveals time-varying differences in risk. For example, the crossing in hazard of failure between ER-positive and ER-negative tumors is to occur at about 4 years, while for the same patients, recurrence-free survival (an aggregate of failure risk over follow-up time) remains more favorable for ER-positive patients through about 10 years [8, 9].
In this investigation, we examine recurrence hazards in patients from clinical trials exclusively in lymph node-negative breast cancer [11, 12]. Using this unique cohort consisting of patients receiving surgery alone as well as surgery followed by adjuvant chemotherapy and hormonal therapy, we explore the magnitude of treatment effects as a function of time, and the role that both treatment and ER play in short and long-term recurrence risk. Because both the estimation and interpretation of the hazard function can be complex, we also comment on conceptual and methodological challenges in working directly with the hazard function as a data summary.
Materials and methods
Patient cohort
The National Surgical Adjuvant Breast and Bowel Project (NSABP) is a National Cancer Institute-sponsored cooperative clinical trials group with participating institutions throughout North America. Women from five randomized trials that evaluated systemic adjuvant therapy for histologically lymph node-negative breast cancer comprised the cohort for this study. These trials accrued patients between 1981 and 1998. Among women with ER-negative tumors (<10 femtomoles per milligram (fmol/mg) of cytosol protein), three successive trials evaluated (1) surgery alone compared to surgery followed by 12 cycles of sequential methotrexate and 5-fluorouracil (MF) [13], (2) six cycles of MF compared to six cycles of the conventional regimen of cyclophosphamide, methotrexate, and 5-fluorouracil (CMF) [14], and (3) CMF compared to the two-drug regimen consisting of adriamycin and cyclophosphamide (AC) given for four cycles (using a factorial design, patients in this trial were also randomized within each chemotherapy arm to tamoxifen or placebo (double-blind)) [15]. For women with ER-positive tumors (≥10 fmol/mg of cytosol protein), two trials evaluated (1) placebo compared to tamoxifen (double-blind) for 5 years [16, 17] and (2) either tamoxifen alone, tamoxifen plus MF, or tamoxifen plus CMF [18]. Further details of the trial designs and findings can be found in published primary reports.
Institutional Review Boards at study sites approved the trials, and the participants provided written informed consent prior to randomization. For this analysis, we excluded patients with missing tumor size or absence of follow-up information after randomization. The resultant cohort consists of 9,444 patients. Results in this report reflect data reported to the NSABP data-coordinating center as of March 31, 2005 (December 31, 2003 for Protocol B-13). Median follow-up times range from 102 to 182 months among the five trials.
Endpoints and statistical methods
In these analyses, we estimate the cause-specific hazard for recurrence, treating competing events (second primary cancers, deaths prior to recurrence/second primary) as censored observations (i.e., the hazard for the recurrence-free interval endpoint). Hazards were estimated using an extension of the familiar Cox proportional hazards model [19, 20]. Typically, the Cox model is used to obtain hazard ratios as measures of effect for covariates (treatment, tumor size, etc.) and the hazard function itself is not estimated. Furthermore, in the standard model implementation, all covariate effects are time-invariant, that is, the influence of tumor characteristics such as ER are not permitted to vary over time. Similarly, treatment effects are also assumed to produce a constant hazard reduction throughout follow-up time. However, in the Cox model, hazard function estimates can be obtained [21] and extensions have been developed to allow time-varying covariate effects [22-24]. Here, we use a simple approach in order to permit hazard shapes to naturally vary by ER and treatment group. Specifically, we model the hazard separately within ER status by treatment group strata defined by the trials, resulting in seven distinct groups: ER-negative: surgery only (N = 383), MF (N = 1,277), and CMF/AC (combined, with or without tamoxifen, N = 2,542), ER-positive: surgery only (with placebo, N = 1,450), tamoxifen (N = 2,223), tamoxifen + MF (N = 784), and tamoxifen + CMF (N = 785). Other covariates (age, tumor size, progesterone receptor level) are modeled in the usual fashion, with appropriate checking for proportionality and diagnostic methods to determine the correct functional form [25]. For the hazard estimates obtained from the model, kernel density smoothing is used to produce smoothed plots [26, 27]. As a check on the model-based estimates, the plots are compared to simple discrete-time estimates based on ratios of events over number of patients at risk in yearly intervals (as shown in Saphner et al. [7]). All hazard and survival curve estimates are generated at the mean of covariate values within the respective ER status group. In plots contrasting outcomes across ER groups, we standardized estimates to common mean covariate values of the ER-positive group. Time-fixed treatment hazard ratios were estimated separately in an early and a late follow-up time interval (0–7 years and >7 years, respectively), with the partition chosen by visual inspection of the hazard plots and likelihood ratio tests under parametric survival models to characterize the behavior of the hazard in the later interval.
Results
Hazards in patients treated by surgery without systemic adjuvant therapy
For those undergoing surgery without subsequent systemic adjuvant therapy, distinct patterns in the failure hazard are apparent (Fig. 1). The hazard for patients with ER-negative tumors reaches a peak around 18 months and then diminishes rapidly. For the ER-positive group, the hazard peak appears slightly later, and is smaller in magnitude, but has a less rapid decrease. After approximately 40 months, the hazard for the ER-positive group is greater and remains so through 144 months.
Fig. 1.
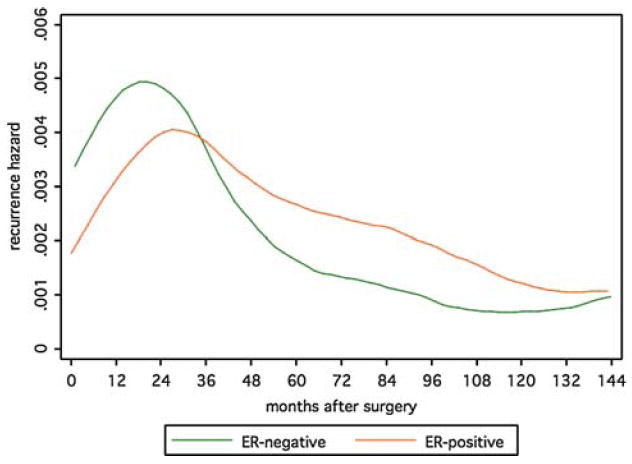
Recurrence hazards by ER-status for patients receiving surgery without systemic adjuvant therapy
Note that ER-negative patients are younger at diagnosis and have larger tumors (Table 1), and that the curves shown are standardized to a common set of values across ER groups. Unadjusted curves (not shown) differ slightly more, reflecting the influence of these characteristics on differences in failure rates between ER-negative and ER-positive tumors.
Table 1.
Patient and tumor characteristics by ER status
| ER− (N = 4,202) | ER+ (N = 5,242) | All patients (N = 9,444)
|
|||
|---|---|---|---|---|---|
| N (%) | N (%) | N | % | Pa | |
| Age at diagnosis | |||||
| <40 | 894 (21.3) | 497 (9.5) | 1,381 | 14.6 | <0.001 |
| 40–49 | 1,526 (36.3) | 1,455 (27.8) | 2,981 | 31.6 | |
| 50–59 | 1,141 (27.2) | 1,554 (29.6) | 2,695 | 28.5 | |
| ≥60 | 651 (15.5) | 1,736 (33.1) | 2,387 | 25.3 | |
| Menopausal status | |||||
| Pre/peri-menopausal | 2,286 (54.4) | 1,964 (37.5) | 4,250 | 45.0 | <0.001 |
| Post-menopausal | 1,916 (45.6) | 3,278 (62.5) | 5,194 | 55.0 | |
| Tumor size | |||||
| ≤2.0 cm | 2,299 (54.7) | 3,308 (63.1) | 5,607 | 59.4 | <0.001 |
| 2.1–4.0 cm | 1,609 (38.3) | 1,710 (32.6) | 3,319 | 35.1 | |
| ≥4.1 cm | 294 (7.0) | 224 (4.3) | 518 | 5.5 | |
| Tumor progesterone receptors (fmol/mg) | |||||
| 0–9 | 3,264 (77.7) | 937 (17.9) | 4,201 | 44.5 | <0.001 |
| 10–49 | 458 (10.9) | 493 (9.4) | 951 | 10.1 | |
| ≥50 | 480 (11.4) | 3,812 (72.7) | 4,292 | 45.4 | |
Comparison of frequency distributions by ER status
Hazards under systemic adjuvant therapy
Patients with ER-negative tumors
In ER-negative breast cancer, cytotoxic chemotherapy had a substantial effect on reducing the hazard of recurrence in a regimen-dependent manner (Fig. 2). The MF chemotherapy regimen produced a less marked hazard reduction than the CMF and AC regimens in earlier follow-up, although at later follow-up the difference in hazard is not apparent. The hazards at later time points do however suggest that chemotherapy continues to be of benefit among those who remain at risk to fail in later follow-up, indicating that not all of the benefit from chemotherapy is accrued early.
Fig. 2.
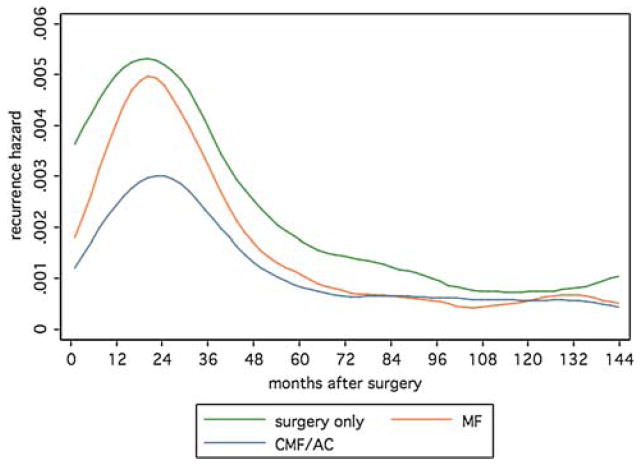
Recurrence hazards for surgery and adjuvant systemic therapy in patients with ER-negative tumors
Patients with ER-positive tumors
In ER-positive breast cancer, systemic adjuvant therapy reduces recurrence (Fig. 3a). With tamoxifen, the early recurrence hazard was reduced, and the addition of chemotherapy to tamoxifen reduced the hazard further to the lowest level among all patient groups (Fig. 3a). In later follow-up, the relative benefit of adjuvant treatment over surgery alone appears to diminish. These patterns indicate a degree of time-dependence (i.e., non-proportionality) in the treatment effect for both tamoxifen and tamoxifen plus chemotherapy relative to surgery only, as well as with chemotherapy over tamoxifen alone. Because of the large benefit accrued early in follow-up, these attenuating effects are not readily apparent in the recurrence-free survival curves (Fig. 3b). Twelve-years recurrence-free survival percentages are 70.2% for surgery only, 80.9% for tamoxifen, 86.3% for tamoxifen plus MF, and 89.4% for tamoxifen plus CMF. While these curves are actually still diverging, the rate of divergence is not consistent with the proportional hazards assumption (Fig. 3b).
Fig. 3.
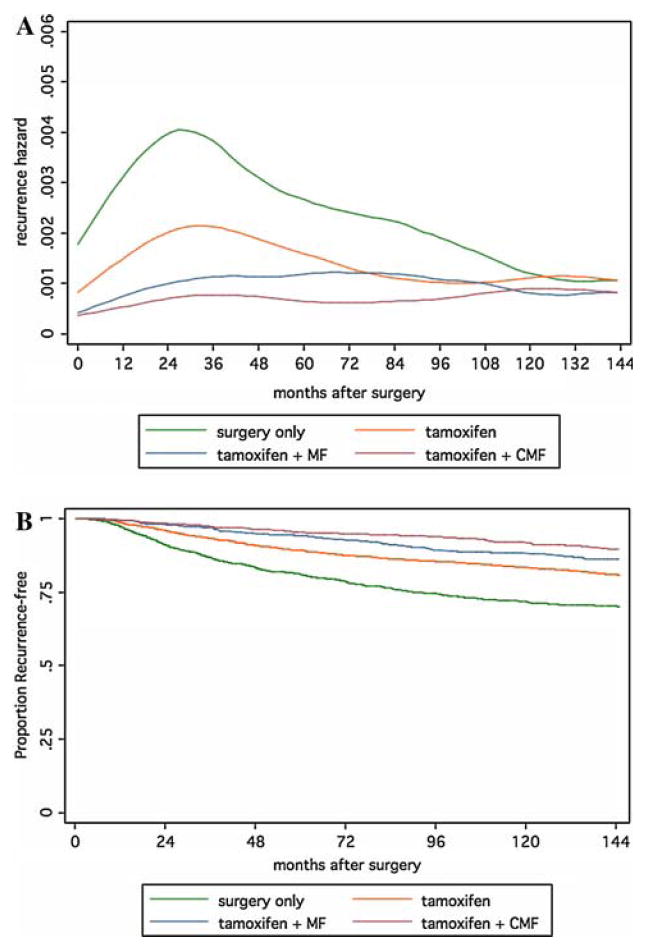
Recurrence hazards (top) and recurrence-free survival curves (bottom) for surgery and adjuvant systemic therapy in patients with ER-positive tumors
Treatment effect estimates within time intervals
A straightforward way to examine time-dependence in the effects of adjuvant treatment is to simply partition the time interval and estimate the effects within each interval. Beginning at approximately 7 years from randomization, recurrence rates in patients receiving adjuvant therapy begin to appear relatively constant compared to the initial wave of failures (Figs. 2, 3a). Thus we chose this time point as a partition and estimated treatment effects within ER groups for the early interval (0–7 years) and late interval (>7 years). We note that this choice is not unique and that partitions earlier (6 years) and later (8 years) produce materially similar results.
In ER-negative patients, 86% of the observed recurrence events occur in the first interval. However, for these patients, relative hazard reductions for chemotherapy over surgery are similar in the early and late interval, although the relative benefit of CMF/AC over MF appears diminished in the later interval (Table 2). For ER-positive patients, 69% of the recurrence events occur in the first interval, and benefits for both tamoxifen alone and accompanied by chemotherapy are substantial. Both treatment regimens remain superior to surgery alone in the late interval, although the relative benefit of adding chemotherapy to tamoxifen is diminished (Table 2).
Table 2.
Relative hazards for adjuvant systemic therapy
| 0–7 Years
|
> 7 Years
|
|||
|---|---|---|---|---|
| Hazard ratioa | 95% CI | Hazard ratioa | 95% CI | |
| ER-negative | ||||
| Surgery alone | 1.00 | – | 1.00 | – |
| MF | 0.75 | 0.59–0.94 | 0.56 | 0.33–0.94 |
| CMF/AC | ||||
| versus surgery | 0.54 | 0.43–0.68 | 0.52 | 0.31–0.88 |
| versus MF | 0.72 | 0.61–0.85 | 0.92 | 0.61–1.40 |
| ER-positive | ||||
| Surgery alone | 1.00 | – | 1.00 | – |
| Tamoxifen | 0.60 | 0.51–0.70 | 0.73 | 0.57–0.95 |
| Tamoxifen + CMF | ||||
| versus surgery alone | 0.26 | 0.19–0.36 | 0.68 | 0.46–1.00 |
| versus tamoxifen | 0.42 | 0.31–0.58 | 0.92 | 0.65–1.31 |
| versus tamoxifen + MF | 0.62 | 0.43–0.88 | 0.95 | 0.64–1.40 |
From standard Cox model (i.e., time-invariant covariate effects) for failures within the time interval shown
Comparisons of systemic adjuvant therapy effects by ER status
The greater frequency of late recurrences in ER-positive patients (31% of recurrence events) compared to ER-negative patients (14% of recurrence events) prompted a comparison of those patients who received systemic adjuvant therapy by ER status. Although tamoxifen produces a substantial early benefit in ER-positive patients, reducing failure hazard to a level commensurate of that in ER-negative patients receiving cytotoxic chemotherapy, after 4 years the hazard for the ER-positive patients exceed that of the ER-negative group (Fig. 4a). The addition of chemotherapy produces a substantial decrease in early failures in ER-positive patients, but the hazard at later time points appears to be similar to that of tamoxifen-treated patients, and again exceeds that of ER-negative chemotherapy-treated patients (Fig. 4a). However, because of the substantially lower early hazard in ER-positive patients, cumulative probability of failure as indicated by the recurrence-free survival curves show an advantage for ER-positive chemotherapy-treated patients until later in follow-up, when the survival curves begin to converge (Fig. 4b). The 12-years recurrence-free survival percentage is 84.4% for CMF/AC (ER-negative), compared to 80.9% for tamoxifen (ER-positive) and 89.4% for tamoxifen plus CMF (ER-positive).
Fig. 4.
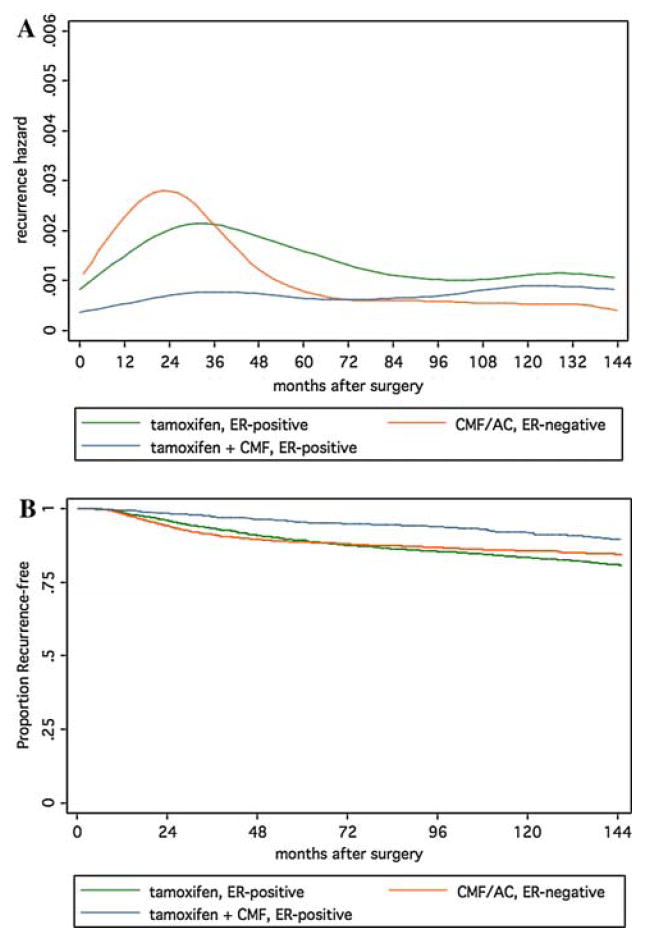
Recurrence hazards (top) and recurrence-free survival curves (bottom) by ER-status for patients receiving systemic adjuvant therapy
Because the recurrence hazards in the ER-positive and ER-negative groups cross in the early period, a single time-invariant treatment effect estimate is not appropriate to use to compare these groups. In the later period, the ER-negative group has significantly lower recurrence hazard than both the tamoxifen-treated (HR = 0.37, 95% CI 0.21–0.65) and tamoxifen plus-CMF-treated (HR = 0.45, 95% CI 0.26–0.76) ER-positive groups.
Although these estimates were adjusted for covariates, we also examined hazard estimates separately by age and tumor size (not shown). Among patients aged <50 years at diagnosis, the early hazard for the tamoxifen group was similar in magnitude to that of the CMF/AC group. In patients aged 60 or older at diagnosis, the advantage of tamoxifen plus-CMF over tamoxifen was smaller than in younger patients, as previously reported [12]. With respect to tumor size, chemotherapy groups (either ER-negative or ER-positive) had superior outcomes to ER-positive women receiving tamoxifen only even among those with tumors of 2 cm or less.
Discussion
In this study, we have investigated the dynamics in the recurrence hazard after surgery and different adjuvant therapies from a cohort of over 9,400 randomized trial participants. Overall, the study confirms that in lymph node-negative breast cancer patients (a) the high early failure risk for patients with ER-negative tumors diminishes with time, (b) there is a lower but more persistent failure risk in patients with ER-positive tumors, and (c) there is a substantial benefit for adjuvant systemic therapy in both types of tumors.
In patients with ER-negative tumors, differences in hazards by treatment support the idea that the largest absolute benefit of adjuvant treatment is manifest in the avoidance of early relapse, due to the highly non-constant hazard rate that declines dramatically with time even in patients receiving surgery alone (Fig. 1). However, there also appears to be a sustained benefit for chemotherapy, with a consistently lower failure rate remaining late in follow-up (Fig. 2). Whether these late failures are due to heterogeneous tumors (or misclassified cases) with respect to ER (i.e., not pure ER-negative) is unknown. Nonetheless, the possibility of ‘cure’ would appear more achievable in ER-negative breast cancer, if early recurrences could be further reduced.
Although we confirm previous observations that ER-positive disease is more favorable than its ER-negative counterpart with respect to cumulative recurrence risk (i.e., recurrence-free survival) over a period of over 10 years, our findings also illustrate the unique therapeutic challenge presented by ER-positive tumors. Even under adjuvant chemo-hormonal therapy, the persistent elevated hazard beyond 10 years after diagnosis validates the need for long-term therapeutic maneuvers. While a recent study suggested limited differential benefit for various improvements in cytotoxic chemotherapy among ER-positive patients [28], our results illustrate a large early hazard reduction with the addition of chemotherapy to tamoxifen, as did a recent update of these trials [12]. However, because the prognosis of lymph node-negative patients is relatively favorable under tamoxifen, the absolute benefit of chemotherapy is more modest than in node-positive disease. Recent efforts have been directed towards prospectively identifying which patients are (or are not) likely to require more aggressive adjuvant therapy management [3, 4]. In NSABP Protocol B-20, which compared the addition of chemotherapy to tamoxifen in ER-positive patients, patients with a high Oncotype DX™ recurrence risk score incurred a large benefit from chemotherapy, while those with low-risk scores did not [4]. Furthermore, the effect of chemotherapy on recurrence-free survival appeared mostly within the first 5 years among the high-risk patients, suggesting, as does this analysis, that the largest effect of chemotherapy is on early failure in ER-positive breast cancers. Other studies of gene profile risk scores also indicate an attenuating prognostic effect of risk profile scores as follow-up time increases [29], an observation commensurate with the well-known phenomenon of important prognostic factors diminishing in effect among patients who have been failure-free for an extended period [8, 30].
With respect to hormonal treatments, the persistent recurrence hazard at later time points even among the chemotherapy-treated patients (which essentially equals that of tamoxifen-treated patients) indicates the need for improved disease control even in “low-risk” patients. Recent trials evaluating aromatase inhibitors after 5 years of tamoxifen in post-menopausal women have shown impressive results, with recurrence hazard reductions of over 50% [31-33]. It is interesting to note that since all participants were event-free on tamoxifen through 5 years, those trials implicitly consist of patients for whom recurrence events would occur only near or in the later follow-up period of our study. Thus, reduction of recurrence many years after onset would seem to depend more on extended hormonal therapy with new agents than on chemotherapy. Much remains to be learned with respect to how to optimize the use of the growing number of these new effective hormonal therapy agents [34].
The hazard function is a dynamic quantity for assessing failure risk over time, but its interpretation can be subtle and at times elusive or even misleading [35]. For example, it is important to note that the hazard function roughly represents, at any given time considered, the probability of failure for the group of individuals remaining, and it is not necessarily representative of this risk for any one individual over the entire time period. So, while the observed hazard for the group may be decreasing, the actual risk of failure for a given individual (their “hazard potential” [36], which is, for practical purposes, unobservable) may be decreasing, constant, or even increasing. In fact, the average hazard of a heterogeneous group of individuals, some with constant high probability of failure and others with similarly constant low failure probability, will decrease over time as the high-risk individuals fail early while the low-risk individuals tend to remain longer without failure [37]. This heterogeneity, or what is known in statistical parlance as a mixture of distributions, can be a major contributor to apparent time-dependent patterns in the hazard [38]. Added to this heterogeneity is the well-known instability in the hazard estimate, making it difficult to distinguish distinct hazard change-points from random variations, particularly in later follow-up [27,39].
Consequently, apparent patterns in the recurrence hazard such as a distinct second peak several years after surgery, as reported in some studies, must be weighed against many factors influencing the estimate, despite the intriguing concept conjectured to support such a pattern [40-42]. In our cohort of lymph node-negative patients, failure rates are small in absolute magnitude, particularly in later time intervals, and a second hazard peak is not apparent (discounting variations around 12 years, where failure events number in single digits). Examining the data separately by ER status may account for this, as the distinct patterns between these groups can produce such patterns when combined (not shown). However, if recurrence events are in part stimulated by growth factors produced after surgery as conjectured, then differences in recurrence patterns by ER status might point to differential response to this influence by ER or characteristics with which it is correlated. We plan to investigate hazard patterns further in lymph node-positive patients, with particular interest in trials comparing pre-operative and post-operative chemotherapy [43], which may have direct bearing on recurrence patterns over time.
With regard to the methods used here, we have followed earlier investigations that aimed to portray changes in the risk of failure over time via hazard estimation, revealing information that is less apparent than that which can be obtained from a survival curve [7-9]. By estimating hazards in separate treatment group strata, we effectively permit treatment effects (relative hazards) to vary over time. This is in contrast to clinical reports on these trials, where time-fixed treatment effects (hazard ratios) were estimated [11-18]. Previous methodological work on these same data have also noted the time-varying treatment effect and suggested other approaches [44, 45]. However, even when treatment effects are not constant over time, the standard analysis via the Cox model with a time-fixed treatment hazard ratio provides a reasonable summary that can be considered an average treatment effect over time, provided that the hazards are not strongly non-proportional (e.g., causing survival curves to cross or rapidly converge over time). This condition holds within ER status groups in these trials.
In summary, examination of the recurrence hazard in lymph node-negative breast cancer under systemic adjuvant therapy offers clues for both biological and clinical research to improve outcomes. While ER-negative tumors present both increased risk of early recurrence and the greater potential for elimination of recurrence risk (e.g.,“cure”), there remains enough variation in outcomes over an extended follow-up period to support efforts to profile ER-negative patients for potential failure risk and identify candidates for enhanced adjuvant therapy efforts. In ER-positive tumors, the challenge for long-term disease control that addresses heterogeneity is more glaring when examined on the hazard scale. Ongoing and future developments towards better molecular profiling of tumors will undoubtedly be critical for risk classification, but long-term adjuvant therapy will likely continue to have a role. Given the complexity and dynamics of the “lifespan” of breast cancer, long-term observations of outcomes such as those presented here will be required to firmly establish the clinical utility of any such developments.
Acknowledgments
Supported by a grant from the Susan G. Komen for the Cure Foundation (JJD, VMD) and Public Health Service grants NCI-U10-CA-12027, NCI-U10-CA-69651, NCI-U10-CA-69974, NCI-U10-CA-37377, and NCI P30-CA-14599 from the US National Cancer Institute.
Contributor Information
James J. Dignam, Department of Health Studies, The University of Chicago, 5841 South Maryland Avenue, MC 2007, Chicago, IL 60637, USA, jdignam@health.bsd.uchicago.edu, National Surgical Adjuvant Breast and Bowel Project (NSABP) Biostatistical Center, Pittsburgh, PA, USA
Vanja M. Dukic, Department of Health Studies, The University of Chicago, 5841 South Maryland Avenue, MC 2007, Chicago, IL 60637, USA
Stewart J. Anderson, National Surgical Adjuvant Breast and Bowel Project (NSABP) Biostatistical Center, Pittsburgh, PA, USA, Department of Biostatistics, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA, USA
Eleftherios P. Mamounas, NSABP Operation Center, Pittsburgh, PA, USA, Altman Cancer Center, Canton, OH, USA
D. Lawrence Wickerham, NSABP Operation Center, Pittsburgh, PA, USA, Allegheny General Hospital, Pittsburgh, PA, USA.
Norman Wolmark, NSABP Operation Center, Pittsburgh, PA, USA, Allegheny General Hospital, Pittsburgh, PA, USA.
References
- 1.National Institutes of Health Consensus Development Panel. National Institutes of Health Consensus Development Conference statement: adjuvant therapy for breast cancer, November 1–3, 2000. J Natl Cancer Inst Monogr. 2001;30:5–15. [PubMed] [Google Scholar]
- 2.Early Breast Cancer Trialists’ Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 3.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 4.Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24:3726–3734. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- 5.Robbins GF, Berg J. Curability of patients with invasive breast carcinoma based on a 30-year study. World J Surg. 1977;1:284–286. doi: 10.1007/BF01556838. [DOI] [PubMed] [Google Scholar]
- 6.Karrison T, Ferguson D, Meier P. Dormancy of mammary carcinoma after mastectomy. J Natl Cancer Inst. 1999;91:80–85. doi: 10.1093/jnci/91.1.80. [DOI] [PubMed] [Google Scholar]
- 7.Saphner T, Tormey D, Gray R. Annual hazard rates of recurrence for breast cancer after primary therapy. J Clin Oncol. 1996;14:2738–2746. doi: 10.1200/JCO.1996.14.10.2738. [DOI] [PubMed] [Google Scholar]
- 8.Hilsenbeck SG, Ravdin PM, de Moor CA, et al. Time-dependence of hazard ratios for prognostic factors in primary breast cancer. Breast Cancer Res Treat. 1998;52:227–237. doi: 10.1023/a:1006133418245. [DOI] [PubMed] [Google Scholar]
- 9.Hess K, Pusztai L, Buzdar A, et al. Estrogen receptors and distinct patterns of breast cancer relapse. Breast Cancer Res Treat. 2003;78:105–118. doi: 10.1023/a:1022166517963. [DOI] [PubMed] [Google Scholar]
- 10.Anderson WF, Chen RE, Jatoi I, et al. Effect of estrogen receptor expression and histopathology on annual hazard rates of death from breast cancer. Breast Cancer Res Treat. 2006;100:121–126. doi: 10.1007/s10549-006-9231-y. [DOI] [PubMed] [Google Scholar]
- 11.Fisher B, Jeong J-H, Anderson S, et al. Treatment of axillary lymph node-negative, estrogen receptor-negative breast cancer: updated findings from National Surgical Adjuvant Breast and Bowel Project clinical trials. J Natl Cancer Inst. 2004;96:1823–1831. doi: 10.1093/jnci/djh338. [DOI] [PubMed] [Google Scholar]
- 12.Fisher B, Jeong J-H, Bryant J, et al. Treatment of lymph node-negative, estrogen receptor-positive breast cancer: long-term findings from National Surgical Adjuvant Breast and Bowel Project clinical trials. Lancet. 2004;364:858–868. doi: 10.1016/S0140-6736(04)16981-X. [DOI] [PubMed] [Google Scholar]
- 13.Fisher B, Redmond C, Dimitrov NV, et al. A randomized clinical trial evaluating sequential methotrexate and fluorouracil in the treatment of patients with node-negative breast cancer who have estrogen-receptor-negative tumors. N Engl J Med. 1989;320:473–478. doi: 10.1056/NEJM198902233200801. [DOI] [PubMed] [Google Scholar]
- 14.Fisher B, Dignam J, Mamounas EP, et al. Sequential methotrexate and fluorouracil for the treatment of node-negative breast cancer patients with estrogen receptor-negative tumors: eight-year results from National Surgical Adjuvant Breast and Bowel Project (NSABP) B-13 and first report of findings from NSABP B-19 comparing methotrexate and fluorouracil with conventional cyclophosphamide, methotrexate, and fluorouracil. J Clin Oncol. 1996;14:1982–1992. doi: 10.1200/JCO.1996.14.7.1982. [DOI] [PubMed] [Google Scholar]
- 15.Fisher B, Anderson S, Tan-Chiu E, et al. Tamoxifen and chemotherapy for axillary node-negative, estrogen receptor-negative breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-23. J Clin Oncol. 2001;19:931–942. doi: 10.1200/JCO.2001.19.4.931. [DOI] [PubMed] [Google Scholar]
- 16.Fisher B, Costantino J, Redmond C, et al. A randomized clinical trial evaluating tamoxifen in the treatment of patients with node-negative breast cancer who have estrogen-receptor positive tumors. N Engl J Med. 1989;320:479–484. doi: 10.1056/NEJM198902233200802. [DOI] [PubMed] [Google Scholar]
- 17.Fisher B, Dignam J, Bryant J, et al. Five versus more than five years of tamoxifen for lymph node-negative breast cancer: findings from the National Surgical Adjuvant Breast and Bowel Project B-14 randomized trial. J Natl Cancer Inst. 1996;88:1529–1542. doi: 10.1093/jnci/93.9.684. [DOI] [PubMed] [Google Scholar]
- 18.Fisher B, Dignam JJ, Wolmark N, et al. Tamoxifen and chemotherapy for lymph node-negative, estrogen-receptor positive breast cancer. J Natl Cancer Inst. 1997;89:1673–1682. doi: 10.1093/jnci/89.22.1673. [DOI] [PubMed] [Google Scholar]
- 19.Cox DR. Regression models and life tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 20.Prentice RL, Kalbfleisch JD, Peterson AV, et al. The analysis of failure times in the presence of competing risks. Biometrics. 1978;34:541–554. [PubMed] [Google Scholar]
- 21.Kalbfleisch J, Prentice R. The statistical analysis of failure time data. Wiley; New York: 1980. [Google Scholar]
- 22.Sargent D. A flexible approach to time-varying coefficients in the Cox regression setting. Lifetime Data Anal. 1997;3:13–25. doi: 10.1023/a:1009612117342. [DOI] [PubMed] [Google Scholar]
- 23.Gray RJ. Flexible methods for analyzing survival data using splines, with applications to breast cancer prognosis. J Am Stat Assoc. 1992;87:942–951. [Google Scholar]
- 24.Hess KR. Assessing time-by-covariate interactions in proportional hazards regression models using cubic spline functions. Stat Med. 1994;13:1045–1062. doi: 10.1002/sim.4780131007. [DOI] [PubMed] [Google Scholar]
- 25.Grambsch PM, Therneau TM, Fleming TR. Diagnostic plots to reveal functional form for covariates in multiplicative intensity models. Biometrics. 1995;51:1469–1482. [PubMed] [Google Scholar]
- 26.Müller HG, Wang JL. Hazard rates estimation under random censoring with varying kernels and bandwidths. Biometrics. 1994;50:61–76. [PubMed] [Google Scholar]
- 27.Hess KR, Serachitopol DM, Brown BW. Hazard function estimators: a simulation study. Stat Med. 1999;18:3075–3088. doi: 10.1002/(sici)1097-0258(19991130)18:22<3075::aid-sim244>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 28.Berry DA, Cirrincione C, Henderson IC, et al. Estrogen-receptor status and outcomes of modern chemotherapy for patients with node-positive breast cancer. JAMA. 2006;295:1658–1667. doi: 10.1001/jama.295.14.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cardoso F, Van’t Veer L, Rutgers E, et al. Clinical application of the 70-gene profile: the MINDACT trial. J Clin Oncol. 2008;26:729–735. doi: 10.1200/JCO.2007.14.3222. [DOI] [PubMed] [Google Scholar]
- 30.Bryant J, Fisher B, Gündüz N, et al. S-phase fraction combined with other patient and tumor characteristics for the prognosis of node-negative, estrogen-receptor-positive breast cancer. Breast Cancer Res Treat. 1998;51:239–253. doi: 10.1023/a:1006184428857. [DOI] [PubMed] [Google Scholar]
- 31.Goss PE, Ingle JN, Martino S, et al. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA. 17. J Natl Cancer Inst. 2005;97:1262–1271. doi: 10.1093/jnci/dji250. [DOI] [PubMed] [Google Scholar]
- 32.Goss PE, Ingle JN, Pater JL, et al. Late extended adjuvant treatment with letrozole improves outcome in women with early-stage breast cancer who complete 5 years of tamoxifen. J Clin Oncol. 2008;26:1948–1955. doi: 10.1200/JCO.2007.11.6798. [DOI] [PubMed] [Google Scholar]
- 33.Mamounas EP, Jeong JH, Wickerham DL, et al. Benefit from exemestane as extended adjuvant therapy after 5 years of adjuvant tamoxifen: intention-to-treat analysis of the National Surgical Adjuvant Breast and Bowel Project B-33 trial. J Clin Oncol. 2008;26:1965–1971. doi: 10.1200/JCO.2007.14.0228. [DOI] [PubMed] [Google Scholar]
- 34.Lin NU, Winer EP. Advances in adjuvant endocrine therapy for postmenopausal women. J Clin Oncol. 2008;26:798–805. doi: 10.1200/JCO.2007.15.0946. [DOI] [PubMed] [Google Scholar]
- 35.Aalen O, Gjessing H. Understanding the shape of the hazard rate: a process point of view. Stat Sci. 2001;16:1–22. [Google Scholar]
- 36.Singpurwulla N. The hazard potential: introduction and overview. J Am Stat Assoc. 2006;101:1705–1717. [Google Scholar]
- 37.Lawless J. Statistical models and methods for lifetime data. Wiley; New York: 1982. [Google Scholar]
- 38.Berry D. Breast cancer heterogeneity may explain peaks in recurrence. Int J Surg. 2005;3:287. doi: 10.1016/j.ijsu.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 39.Dignam JJ, Dukic V. Time-varying patterns of recurrence risk for Chinese breast cancer patients. In: Yin W, Di G, Zhou L, et al., editors. Breast Cancer Res Treat. 2008. Jun 21, Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 40.Demicheli R, Abbattista A, Miceli R, et al. Time distribution of the recurrence risk for breast cancer patients undergoing mastectomy: further support about the concept of tumor dormancy. Breast Cancer Res Treat. 1996;41:177–185. doi: 10.1007/BF01807163. [DOI] [PubMed] [Google Scholar]
- 41.Demicheli R, Valagussa P, Bonadonna G. Does surgery modify growth kinetics of breast cancer micrometastases? Br J Cancer. 2001;85:490–492. doi: 10.1054/bjoc.2001.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baum M, Cuzick J, Howell A, et al. An exploration of relapse data by hazard rate as a means of developing biological insights into the natural history and treatment of breast cancer. J Clin Oncol; 2005 ASCO annual meeting proceedings; 2005. p. 612. [Google Scholar]
- 43.Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26:778–785. doi: 10.1200/JCO.2007.15.0235. [DOI] [PubMed] [Google Scholar]
- 44.Jeong JH, Jung SH, Wieand S. A parametric model for long-term follow-up data from phase III breast cancer clinical trials. Stat Med. 2003;22:339–352. doi: 10.1002/sim.1349. [DOI] [PubMed] [Google Scholar]
- 45.Dukic V, Dignam J. Bayesian hierarchical multiresolution model for the study of time-dependent patterns of failure in early stage breast cancer. Bayesian Anal. 2007;2:591–610. doi: 10.1214/07-BA223. [DOI] [PMC free article] [PubMed] [Google Scholar]


