Abstract
Several cases of abdominal pregnancy have been described in nonhuman primates. These previous occurrences have been mummified fetuses found in the abdominal cavity. This report describes a case of abdominal pregnancy in a timed-bred rhesus monkey with delivery of a live term infant. The mother died 14 days later from complications of septic peritonitis. At necropsy, the monkey had an intestinal adenocarcinoma that may have allowed leakage of intestinal contents into the abdomen. The second case of pregnancy complication was a rhesus monkey found to have gestational diabetes that later developed pre-eclampsia. She was treated for 5 days with a regimen similar to that used in women, and a live infant was delivered at day 157 of gestation by Caesarian section. These cases of high-risk pregnancy underscore the value of timed-breeding and careful monitoring of pregnant monkeys and the similarities between pregnancy complications in women and in nonhuman primates.
Keywords: obstetric, surgery, medicine, non-human, primate
Introduction
It is possible to optimize monkey-breeding programs to achieve over a 90% pregnancy success rate and large primate facilities are likely to encounter unusual and challenging obstetrical cases [17]. This report describes two such cases at the Harlow Center for Biological Psychology (HCBP), which maintains a breeding colony of approximately 500 rhesus monkeys (Macaca mulatta). Each year up to 100 infants are born, most generated from timed-matings, which facilitates careful monitoring of the gravid female’s pregnancy and health status [5]. Almost all of the pregnancies and deliveries are natural and uneventful, but occasionally intervention is required, as in a case of gestational diabetes and pre-eclampsia and an ectopic, abdominal pregnancy.
Risk factors for gestational diabetes in women include obesity, history of type II diabetes in a first order relative, gestational diabetes diagnosed in a first order relative or a previous diabetic pregnancy. Increased glucose transfer across the placenta from hyperglycemic maternal blood can cause increased fetal size at term (macrosomia) [11]. Recently, oral hypoglycemic agents such as sulfonourea drugs, metformin and thiazoladine diones have been studied as potential treatments for gestational diabetes [20]. Diagnosis of gestational diabetes in women is usually based on the results of an oral glucose tolerance test administered between 24 to 28 weeks of pregnancy. Most pregnant nonhuman primates are not screened for gestational diabetes, especially if they are group-housed and reports of gestational diabetes in nonhuman primates have historically been rare [12, 23].
Pre-eclampsia is a metabolic disease of undetermined etiology that affects pregnant women and their fetuses, consisting of proteinuria, edema, hypertension, and inadequate invasion of the spiral arteries of the placenta into the uterine endometrium. Eclampsia is the occurrence of seizures superimposed on pre-eclampsia [9]. Pre-eclampsia also predisposes women to hemorrhage and disseminated intravascular coagulation (DIC) [13]. There have been a few isolated reports of pre-eclampsia/eclampsia in nonhuman primates including 3 chimpanzees, 2 orangutans, one each of a Stuhlman’s monkey, a rhesus monkey and a zoo-housed patas monkey. Pre-eclampsia was identified in 6 of 98 pregnancies in one year in patas monkeys bred for research [15]. The management of pre-eclampsia and eclampsia in women has been validated by controlled clinical trials. Low-dose aspirin therapy is used to prevent thrombosis and coagulopathy, antihypertensive drugs are used to lower maternal blood pressure and magnesium sulfate is used to prevent and/or treat seizures [6, 7]. The efficacy of these treatments in nonhuman primates has not been determined.
Abdominal pregnancy is another complex phenomenon that constitutes a high-risk pregnancy in women. Abdominal pregnancy results when an egg is fertilized in the oviduct, but instead of being carried into the uterus, the pre-implantation embryo moves retrograde into the abdominal cavity. [1, 16]. Most pregnancies are nonviable, but rarely, placentation will be adequate and the fetus will be carried to term, with delivery of a live infant through an abdominal incision. Live births from human abdominal pregnancies are often malformed and frequently die in the perinatal period [24]. Several instances of abdominal pregnancies resulting in non-viable fetuses have been reported in nonhuman primates including an owl monkey (Aotus trivergatus) [3], a squirrel monkey (Saimiri sciureus) [14], an Assamese macaque (Macaca assamensis) [2] and a baboon (Papio cynocephalus anubis) [19]. This is the first report of an abdominal pregnancy in a nonhuman primate that resulted in delivery of a live infant.
Case #1: Abdominal Pregnancy in a Rhesus Macaque
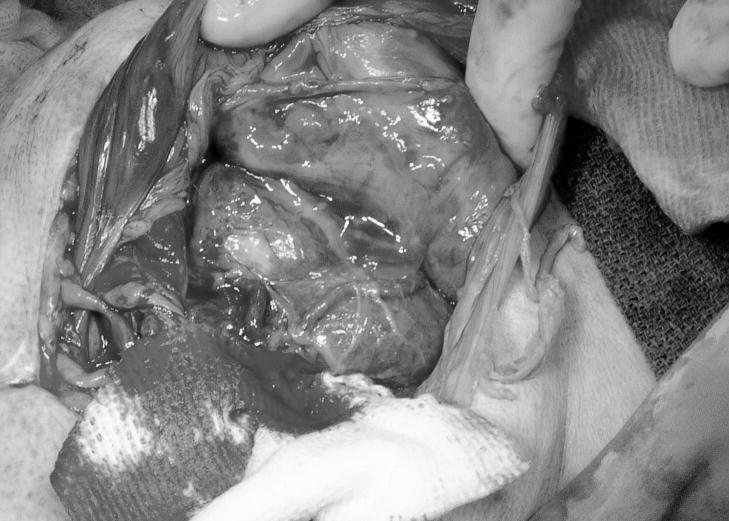
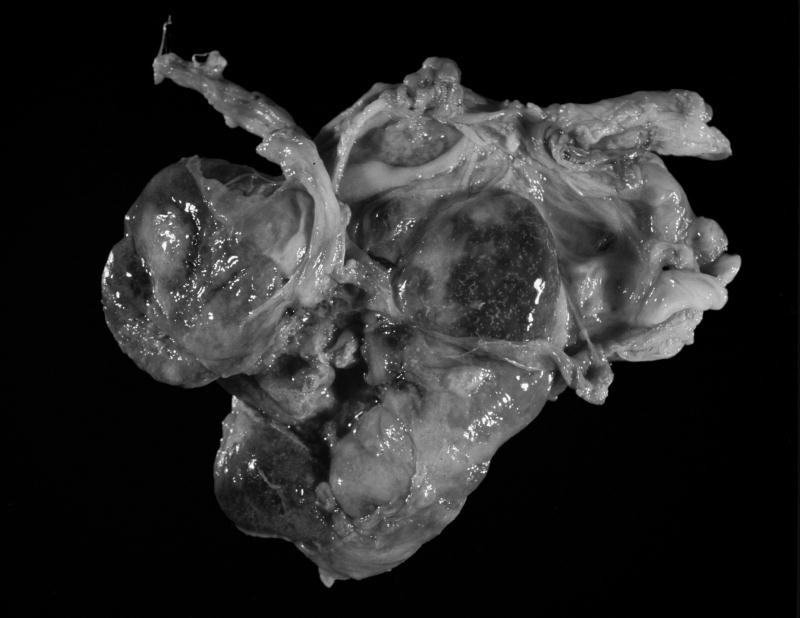
A 14 yr old, multiparous female rhesus macaque was presented for veterinary evaluation due to evident non-productive labor despite the presence of an open cervix. The female had been time-bred and was judged to be >170 d of gestation based on records of her breeding dates, her last observed menstrual cycle and recorded evidence of implantation bleeding. The female macaque appeared alert but in abdominal discomfort. Ultrasound evaluation indicated that the fetus was viable and fetal movement was noted through the abdominal wall. The decision was made to deliver the fetus by Caesarian section and the female macaque was pre-anesthetized with ketamine hydrochloride (10 mg/kg, intramuscularly). Isoflurane was delivered by mask until endotracheal (ET) intubation could be achieved. Then the animal was maintained on isoflurane per ET tube throughout the procedure. A midline abdominal incision was made preparatory to exteriorizing the uterus and delivering the fetus. Inspection of the abdomen revealed a 515 g, viable female infant within the abdominal cavity. The infant was attached by a normal umbilical cord to a large intra-abdominal placenta that was adhered to the anterior uterine serosa, several segments of the large and small bowel, the abdominal wall, and the greater omentum, with many anastamoses between omental and placental vessels (Figure 1A–B).
Figure 1.
A. The monkey’s abdomen after delivery of the fetus.
B. Placenta after removal from the abdomen. The large placenta has extensive anastamoses with the omental vasculature.
The infant was delivered and given supportive care. The placenta was dissected free of its attachments to the abdominal wall and to the abdominal viscera where possible without incurring excessive maternal bleeding. Anastamoses between placental and omental vessels were identified and ligated using 4′0 vicryl suture. After extensive and careful dissection, small placental remnants were still visible within the abdominal cavity, but it was decided that the amount of bleeding associated with the removal of these small fragments would be greater than the risk associated with leaving the remaining cells to die in situ. The abdominal incision was closed in layers. Buprenorphine hydrochloride (Buprenex, Rechitt and Colman Pharmaceuticals, Richmond VA) was administered for pain prior to recovery. Immediate recovery from surgery was uneventful.
The monkey was eating and drinking the next day and accepted the infant. She continued to receive pain medication (Buprenorphine HCl, 0.005 mg/kg, IM BID and generic acetaminophen 15 mg/kg PO, BID) and antibiotics (Ceftriaxone Sodium, Rocephin, Roche Laboratories, Inc., Nutley, NJ). She became anorexic and lethargic 5 days after surgery, and 2 days later after developed abdominal distention that continued to worsen despite antibiotic therapy. Methotrexate was administered intramuscularly on the 14th. Day post surgery (15 mg based on estimated body surface area) to kill any remaining intra-abdominal placental cells and an exploratory laparotomy was scheduled for the next morning. At surgery, the placental cells were dead and the placental remnants were easily removed, but there was evidence of septic peritonitis. Approximately 600 ml of red-brown fluid was removed from the abdominal cavity. The abdomen was closed in layers. The monkey was stable throughout surgery, but she did not recover from the procedure and died later that afternoon. The infant was fostered to a multiparous female and she is now part of the breeding colony. At necropsy, there was evidence of disseminated bacteremia and Enterococcus species was grown from the abdominal cavity, the kidney and the lung. Histopathologically, there was extensive pulmonary edema, hemorrhage and fibrinous pneumonia consistent with bacterial sepsis. The monkey also had adenomyosis of the uterus, severe lymphocytic enteritis, and an adenocarcinoma of the colon.
Case #2: Gestational Diabetes and Pre-eclampsia in a Rhesus Macaque
A multiparous female rhesus macaque was evaluated using standard CBC and serum chemical analysis at 81 d of gestation because of vaginal bleeding that might indicate a spontaneous abortion. This monkey had no prior experimental manipulations expected to alter her glucose metabolism. Her serum glucose concentration was 171 mg/dl. Serum cholesterol and triglyceride concentration values were 60 and 96 mg/dl respectively. A urine sample was obtained and the serum glucose concentration was repeated after a 16 hr. fast. The monkey was glycosuric (2000 mg/dl), but not proteinuric, and her serum glucose concentration was still elevated beyond normal values for her age, species, and gender (152 mg/dl). A moderately restricted diet was instituted; including a prohibition on enrichment treats containing high sugar concentrations. Urine samples were obtained weekly for determination of glucose concentration. A weekly urine sample obtained at 152 d of gestation indicated that she was no longer glycosuric, but was proteinuric (100 mg/dl) and ketonuric (15 mg/dl). Medical treatment for pre-eclampsia was instituted. The monkey was administered low dose aspirin 20 mg PO, BID, to prevent thrombocytopathia and DIC, magnesium sulfate (MgSO4) 2 g in a volume of 4 ml, IM once/d for seizure prophylaxis, and the beta blocker propranolol, 5 mg BID, for hypertension. Elective Caesarian section at day 157 of gestation delivered a live; 9 d premature infant. The infant weighed 600 g at delivery. The monkey received buprenorphine 0.01 mg/kg IM, BID and flunixin megulamine 0.5 mg/kg, IM, once/d (Flumeg, Phoenix Pharmaceuticals, Inc. St. Joseph, MO) for postoperative pain control and systemic antibiotics, Cefazolin, 25 mg/kg, IM, BID, (Marsam Pharmaceuticals Inc., Cherry Hill, NJ) to reduce the chances of postoperative infection. She recovered well, and was able to care for her infant 24 hrs after surgery. Treatment for pre-eclampsia was continued for 2 d after delivery.
Discussion
These two cases of very different pathological processes associated with pregnancy highlight some of the challenges faced by primate veterinarians. Sonography has greatly improved the diagnosis of abdominal pregnancy in human beings. But routine sonograms of pregnant monkeys are uncommon, especially where the breeding animals are kept in large, sometimes naturalistic social groups. Many of the female rhesus macaques at the HCBP are time-mated according to experimental protocols, so dates for breeding, last menses, and occurrence of implantation bleeding are known [5]. Without timed mating, it is doubtful that this monkey’s abdominal pregnancy would have been recognized while the fetus was still viable.
In women with abdominal pregnancies that end either in delivery or elective termination, methotrexate is routinely administered to kill the residual placental cells left in the abdominal cavity [1]. In this case, the placental cells were dying out at the time of the second surgery, but the monkey had peritonitis due to leakage of intestinal bacteria (Enterococcus sp.) into the peritoneum, where either the placental or tumor cells had invaded through the intestinal serosa and then died, leaving small defects through which bacteria from the intestinal lumen could escape. This occurred despite initial aggressive parenteral antibiotic therapy with a broad spectrum, third generation cephalosporin drug (Ceftriaxone). Immunosuppression may be part of the pathophysiology of abdominal pregnancy in women, because peritoneal defenses including resident macrophages would normally clear fertilized eggs or early embryos that might be flushed retrograde into the abdominal cavity [1, 16]. Failure of peritoneal immune defense mechanisms are thought to be part of the pathogenesis of endometriosis, only instead of embryonic cells, it is endometrial cells that are allowed to continue to grow in the abdominal cavity [18].
Another circumstance relevant to this case is the co-occurrence of an intestinal adenocarcinoma along with the abdominal pregnancy. Intestinal adenocarcinoma, usually located in the terminal ileum, is one of the most common tumors in aged rhesus macaques [21]. It is less common to find an intestinal tumor in the colon, and in an animal less than 15 yrs of age. Malignancies are also associated with a failure of the immune system to clear abnormal cells from the host milieu [8]. It is possible that the same failure in immune surveillance that allowed the developing embryo and fetus to remain viable in the abdominal cavity also allowed the growth of the tumor.
The prevalence of gestational diabetes in macaque monkeys is difficult to assess, but many markers of insulin resistance worsen with increasing age, and calorie restriction will ameliorate the age-related rise in insulin resistance [10]. Another probable indicator of insulin resistance in pregnant rhesus monkeys is the birth weight of their infants. Pregnancy itself is a cause of insulin resistance. In human beings, parity is a risk factor for giving birth to macrosomic infants [4]. The birth weight of rhesus monkey infants has been rising at the HCBP over 6 generations of captive breeding, as well as at other primate breeding facilities [17]. Colony management procedures selecting for reproductive fecundity in the breeder females may have inadvertently resulted in increased risk for gestational insulin resistance among a subset of older females.
The rate of occurrence of pre-eclampsia/eclampsia in macaque monkeys is even less well known. The screening methodologies used in women are impractical in large breeding groups, and though possible in time-bred animals, would be labor intensive for personnel, and represent a significant handling stress for the monkey [22]. The diagnosis of eclampsia represents an additional challenge because it requires the occurrence of seizures, which are often self-reported by human patients. Diagnosis of eclampsia in nonhuman primates requires that seizures be observed by personnel working with the animals or documented by EEG as part of a research protocol [15].
Acknowledgments
The breeding program at the HCBP is supported in part by NIH grants to CLC (AI46521, AI067518, HD39386.
We would like to acknowledge the role of the care staff and students who assist in the daily care of the animals.
Literature Cited
- 1.Alto WA. Abdominal pregnancy. Am Fam Physician. 1990;41:209–214. [PubMed] [Google Scholar]
- 2.Bosu WT, Barker IK. An abdominal mummified fetus in a Macaca assamensis. J Med Primatol. 1980;9:71–75. doi: 10.1159/000460123. [DOI] [PubMed] [Google Scholar]
- 3.Bunte RM, Hildebrandt PK. Abdominal mummified fetus in an owl monkey. J Am Vet Med Assoc. 1975;167:667–668. [PubMed] [Google Scholar]
- 4.Catalano PM, Kirwan JP, Haugel-de Mouzon S, King J. Gestational diabetes and insulin resistance: role in short- and long-term implications for mother and fetus. J Nutr. 2003;133:1674S–1683S. doi: 10.1093/jn/133.5.1674S. [DOI] [PubMed] [Google Scholar]
- 5.Czaja JA, Eisele SG, Goy RW. Cyclical changes in the sexual skin of female rhesus: relationships to mating behavior and successful artificial insemination. Fed Proc. 1975;34:1680–1684. [PubMed] [Google Scholar]
- 6.Duley L, Gulmezoglu AM. Magnesium sulfate compared with lytic cocktail for women with eclampsia. Int J Gynaecol Obstet. 2002;76:3–8. doi: 10.1016/s0020-7292(01)00559-8. [DOI] [PubMed] [Google Scholar]
- 7.Duley L, Henderson-Smart DJ, Knight M, King JF. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev. 2004:CD004659. doi: 10.1002/14651858.CD004659. [DOI] [PubMed] [Google Scholar]
- 8.Dunn GP, Bruce AT, Ikeda BH, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 9.Gregg AR. Hypertension in pregnancy. Obstet Gynecol Clin North Am. 2004;31:223–241. doi: 10.1016/j.ogc.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 10.Gresl TA, Colman RJ, Roecker EB, Havighurst TC, Huang Z, Allison DB, Bergman RN, Kemnitz JW. Dietary restriction and glucose regulation in aging rhesus monkeys: a follow-up report at 8.5 yr. Am J Physiol Endocrinol Metab. 2001;281:E757–765. doi: 10.1152/ajpendo.2001.281.4.E757. [DOI] [PubMed] [Google Scholar]
- 11.Jones CW. Gestational diabetes and its impact on the neonate. Neonatal Netw. 2001;20:17–23. doi: 10.1891/0730-0832.20.6.17. [DOI] [PubMed] [Google Scholar]
- 12.Kessler MJ, Howard CF, Jr, London WT. Gestational diabetes mellitus and impaired glucose tolerance in an aged Macaca mulatta. J Med Primatol. 1985;14:237–244. [PubMed] [Google Scholar]
- 13.Lew M, Klonis E. Emergency management of eclampsia and severe pre-eclampsia. Emerg Med (Fremantle) 2003;15:361–368. doi: 10.1046/j.1442-2026.2003.00475.x. [DOI] [PubMed] [Google Scholar]
- 14.McClure HM, Chang J. Ectopic pregnancy in a squirrel monkey. J Am Vet Med Assoc. 1975;167:654–655. [PubMed] [Google Scholar]
- 15.Palmer AE, London WT, Sly DL, Rice JM. Spontaneous preeclamptic toxemia of pregnancy in the patas monkey (Erythrocebus patas) Lab Anim Sci. 1979;29:102–106. [PubMed] [Google Scholar]
- 16.Pisarska MD, Carson SA. Incidence and risk factors for ectopic pregnancy. Clin Obstet Gynecol. 1999;42:2–8. doi: 10.1097/00003081-199903000-00004. quiz 55–56. [DOI] [PubMed] [Google Scholar]
- 17.Price KC, Hyde JS, Coe CL. Matralineal transmission of birth weight in the rhesus monkey (Macaca mulatta) across several generations. Obstet Gynecol. 1999;94:128–134. doi: 10.1016/s0029-7844(99)00269-0. [DOI] [PubMed] [Google Scholar]
- 18.Rier SE, Coe CL, Lemieux AM, Martin DC, Morris R, Lucier GW, Clark GC. Increased tumor necrosis factor-alpha production by peripheral blood leukocytes from TCDD-exposed rhesus monkeys. Toxicol Sci. 2001;60:327–337. doi: 10.1093/toxsci/60.2.327. [DOI] [PubMed] [Google Scholar]
- 19.Schlabritz-Loutsevitch NE, Hubbard GB, Frost PA, Cummins LB, Dick EJ, Jr, Nathanielsz PW, McDonald TJ. Abdominal pregnancy in a baboon: a first case report. J Med Primatol. 2004;33:55–59. doi: 10.1046/j.1600-0684.2003.00044.x. [DOI] [PubMed] [Google Scholar]
- 20.Slocum JM, Sosa ME. Use of antidiabetes agents in pregnancy: current practice and controversy. J Perinat Neonatal Nurs. 2002;16:40–53. doi: 10.1097/00005237-200209000-00005. quiz 42 p following 84. [DOI] [PubMed] [Google Scholar]
- 21.Uno H, Alsum P, Zimbric ML, Houser WD, Thomson JA, Kemnitz JW. Colon cancer in aged captive rhesus monkeys (Macaca mulatta) Am J Primatol. 1998;44:19–27. doi: 10.1002/(SICI)1098-2345(1998)44:1<19::AID-AJP2>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 22.Villar J, Say L, Shennan A, Lindheimer M, Duley L, Conde-Agudelo A, Merialdi M. Methodological and technical issues related to the diagnosis, screening, prevention, and treatment of pre-eclampsia and eclampsia. Int J Gynaecol Obstet. 2004;85(Suppl 1):S28–41. doi: 10.1016/j.ijgo.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 23.Wagner JD, Jayo MJ, Bullock BC, Washburn SA. Gestational diabetes mellitus in a cynomolgus monkey with group A streptococcal metritis and hemolytic uremic syndrome. J Med Primatol. 1992;21:371–374. [PubMed] [Google Scholar]
- 24.White RG. Advanced abdominal pregnancy--a review of 23 cases. Ir J Med Sci. 1989;158:77–78. doi: 10.1007/BF02942151. [DOI] [PubMed] [Google Scholar]