Abstract
Background
Chagas disease (CD) or American trypanosomiasis is caused by a hemoflagellate protozoan, Trypanosoma cruzi (TC). This organism has been isolated from more than 100 mammalian species and several insect vectors demonstrating a wide host distribution and low host specificity.
Methods
A 23 year old male chimpanzee died acutely and a complete necropsy was performed to evaluate gross and microscopic pathologic changes. After observation of trypanosomal amastigotes in the myocardium, PCR and immunohistochemistry was employed to confirm the diagnosis of TC.
Results
Gross findings were consistent with mild congestive heart failure. Microscopic findings included multifocal myocardial necrosis associated with severe lymphocytic to mixed inflammatory infiltrates, edema, and mild chronic interstitial fibrosis. Multifocal intracytoplasmic amastigotes morphologically consistent with TC were observed in cardiac myofibers. TC was confirmed by PCR and immunohistochemistry.
Conclusion
We report, to the best of our knowledge, the first fatal spontaneous case of TC infection in a chimpanzee.
Keywords: Ape, nonhuman primate, protozoa, fatal case, Trypanosoma cruzi
Introduction
CD or American trypanosomiasis is caused by TC, a protozoan parasite belonging to the order Kinetoplastida, family Trypanosomatidae. Arthropod vectors (Reduviidae, assassin bugs or cone nosed bugs or kissing bugs), particularly Triatoma spp. act as invertebrate hosts during the disease transmission from one animal to another. CD naturally occurs in Central and South America, and the southern United States, within the natural habitat of kissing bugs [15, 31]. TC is widely distributed and has low host specificity. The main reservoir hosts are dogs, raccoons, opossums, armadillos and rodents [2, 5, 11, 13, 14, 23, 29, 33]. Triatoma spp vectors transmit the infectious stages of the protozoa through their feces, but vertical transmission of TC has been reported in primary hosts [1, 20, 30]. Old world monkeys (i.e. Macaca mulatta, Macaca silenus, Macaca nigra) and lemurs (i.e. Lemur catta) may become infected when transported to the areas of vector persistence or through artificial experimentation [6, 12, 24]. In primates TC can spread by blood to blood exposure, intercourse and trans-placental transmission. TC infection in nonhuman primates (NHP) may remain sub-clinical for years with occasional symptoms of anorexia, lymphadenopathy, fever, hepatosplenomegaly, eyelid edema, drowsiness, heart failure and sudden death [17]. Humans may get the disease from handling the infected blood and tissues. Reactivation of CD can occur in immune compromised patients [4, 7] resulting in cardiomyopathy, central nervous system alternations, and the presence trypamastigotes in the blood during the acute phase. Reactivation of TC has also been reported during experimental SIV infection of rhesus macaques [16]. In humans TC is a common cause of cardiomyopathy and has two clinical phases. In the cardiac form of the acute phase changes are minimal, but the chronic phase is characterized by irreversible cardiomyopathy leading to cardiac dysfunction and death [18]. We document a case of a chimpanzee dying acutely with CD.
Methods
Animal
A 23 year old, male chimpanzee died acutely and with a clinical history of abnormal ECG and high calcium and low potassium two months before death. A complete necropsy with histopathology was performed to evaluate the cause of death. The chimpanzee was housed in an indoor-outdoor, metal and concrete cage and fed a standard commercial monkey chow (Teklad©, PMI Nutrition International, LLC, Brentwood, MO 63144); water was available ad libitum. Fresh fruits and vegetables were fed daily. All procedures and care were approved by the Southwest National Primate Research Center Institutional Animal Care and Use Committee and in compliance with national animal care standards.
Serological testing
TC serological testing was performed using an in vitro enzyme immunoassay (EIA) for the qualitative detection of antibody to TC (Abbott Laboratories, Abbott Park, IL). This particular assay differentiates TC from the non pathogenic trypanosome, Trypanosoma rangeli [22].
PCR
DNA was isolated from both ventricles by using proteinase K digestion with phenol/chloroform extraction. The precipitated DNA was washed with cold 70% ethanol, dried, and resuspended in sterile water. Concentration of the DNA was measured by using a Nanodrop™ ND-1000. Touchdown PCR was employed to reduce non-specific amplification, denaturation at 94°C for 10 minutes followed by 10 cycles of “touchdown PCR”, denaturing at 94°C 30 seconds, annealing for 15 seconds, decreasing 1°C per cycle from 64°C to 55°C, and extending at 72°C for 20 seconds. Subsequent amplification carried out with 30 cycles of 94 °C for 30 s, 54 °C for 15 s, and 72°C for 20 s. A final extension was held at 72°C for 7 minutes, and stored at 4°C. A beta-globin locus was also amplified to verify DNA quality. A total of 300 ng of DNA was amplified in a 50 μL reaction y using AmpliTaq Gold (Applied Biosystems, Foster City, CA). Reactions conditions were 0.2 mmol/L MgCl2, 200 μM each dNTP, 0.6 μM each primer and 1 U Taq DNA polymerase. The primers used anneal to the TC ∼ 330 bp minicircle fragment were AAATAATGTACGGGKGAGATGCATGA (121) and GGTTCGATTGGGGTTGGTGTAATATA (122) [25]. A 5 μl sample of the reaction was analyzed on a 1.5% agarose gel electrophoresis and visualized with ethidium bromide and U.V. transillumination.
Immunohistochemical assay (IHC)
IHC was carried out using TC primary antibodies raised in rabbit anti-TC serum (LAB AIIR/IOC, Oswaldo Cruz Foundation, Rio de Janeiro, Brazil) and rabbit ABC Staining System (sc-2018), according to package instructions (Santa Cruz Biotechnology, Inc, CA, USA). IHC was performed on several areas of the heart using formalin fixed paraffin sections, cut at 4 μm and mounted on Polysine (ESCO™) coated glass slides. Target Retrieval (Dako™) was used to recover/unmask the antigenicity in formalin fixed tissue. Tissue sections were incubated with biotinylated goat anti-rabbit IgG followed by peroxidase labeled Avidin biotin-HRP using diaminobenzidine chromogen as substrate. Sections were counter stained with Mayer's hematoxylin, dehydrated, cleared, coverslipped and then analyzed by light microscope. Appropriate positive and negative controls were used.
Results
Gross pathology
The chimpanzee was thin with minimal abdominal fat. Several peritoneal adhesions were observed in the abdomen. The spleen was enlarged approximately three times normal size. There was hypertrophy of the left ventricular wall of the heart and a moderate amount of yellow to orange fluid was evident in both ventricular walls and the septum. The thickness of the left ventricular wall was 2.5 cm, the right ventricular wall 0.7 cm and the septum 2.0 cm. The liver was pale tan and minimally enlarged. Nearly 20 ml of yellow to red fluid was present in the scrotum. The cause of death was not evident at necropsy but the lesions were consistent with mild congestive heart failure.
Microscopic observations
There was moderate to severe multifocal infiltration of lymphocytes (Fig. 1) and a minimal mixed population of inflammatory cells in the left ventricle. Necrosis of myofibers, mild interstitial fibrosis and separation of myofibers with clear spaces were observed. Nests of amastigotes morphologically consistent with TC were seen within muscle fibers primarily in the interventricular septum, but also in both ventricles. The amastigotes were 2-4 um in diameter, round to oval with a nucleus and a variably distinct rod shaped kinetoplast arranged parallel to the nucleus. The lung was markedly congested and multifocally alveolar spaces were filled with proteinaceous fluid (edema). Heart failure cells were not observed. The spleen and liver were unremarkable.
Figure 1.
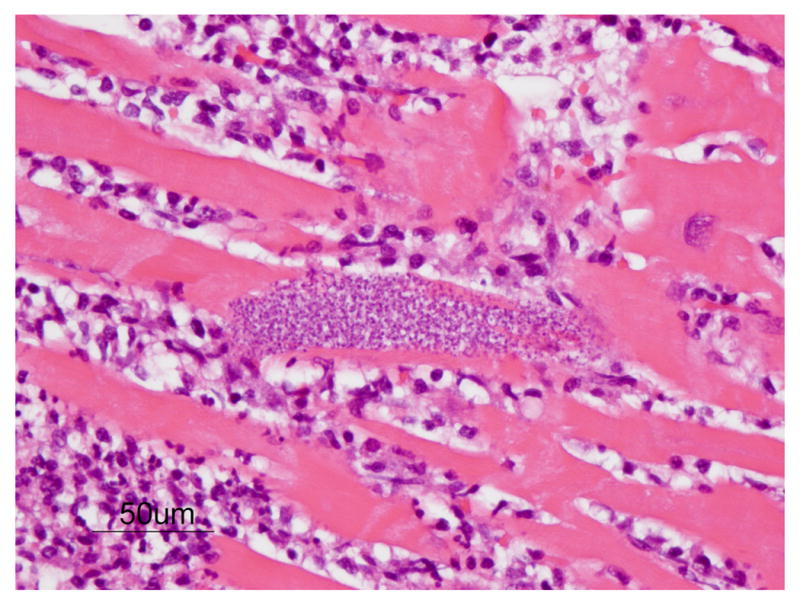
Amastigotes (pseudocyst) of TC in the myocardium of the chimpanzee. Nest of organisms seen within myocardial fibers morphologically consistent with TC. Note the typical, predominantly lymphocytic, inflammatory response. (H&E, Bar = 50 μm)
Serological testing
The chimpanzee was negative for CD specific antibodies in routine serological testing with an in vitro enzyme immunoassay a month before death.
PCR
A band length of >300bp was observed on agarose gel electrophoresis of PCR reactions (Fig. 2). This was consistent with the expected size of the TC ∼ 330 bp minicircle fragment. Similar sized bands were also seen in PCR reaction produced from formalin fixed myocardial tissue (data not shown).
Figure 2.
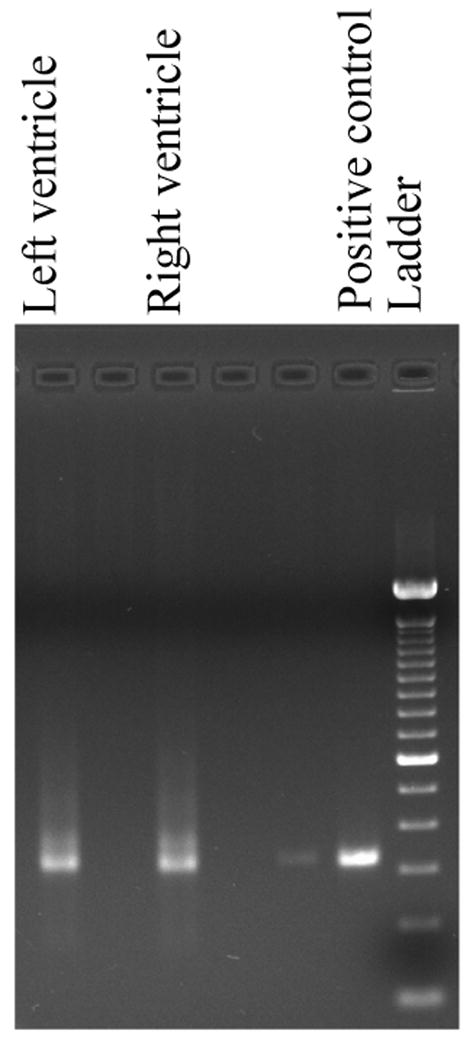
Agarose gel electrophoresis image showing PCR positive bands for mini-circle fragment of the kinetoplastid DNA of TC. Note the >300bp size bands in both ventricles of heart along with the positive control and DNA ladder.
Immunohistochemistry
Nests of amastigotes were readily visualized with immunohistochemistry (Fig. 3).
Figure 3.
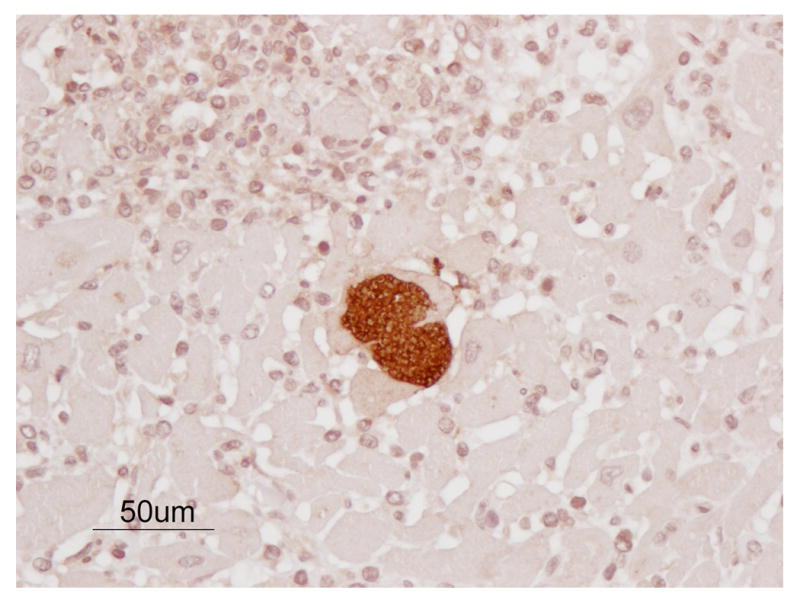
Amastigotes (pseudocyst) of TC in the myocardium of the chimpanzee. Nest of organisms seen within myocardial fibers morphologically consistent with TC. Note the typical, predominantly lymphocytic, inflammatory response. (IHC, Bar = 50 μm)
Discussion
CD is the leading cause of heart disease Central and South America, is becoming recognized as a serious human health concern in the United States [15]. Since the first report of an infected baboon in 1984, CD has been described in baboons and a crab-eating macaque at the same facility as this chimpanzee [9, 10, 32, 34]. Transmission is believed to be the result of catching and eating infected invertebrate hosts, or fecal contamination of wounds created during feeding of these same infected invertebrate hosts. Control of the invertebrate hosts and culling affected NHP are key to limiting infection [32], however the use of outdoor enclosures makes elimination of the disease unlikely.
The fact that the chimpanzee was previously negative for CD specific antibodies in routine serological testing a month before death indicates the acute nature of the disease. The observation of an abnormal ECG made two months before death, whereas TC serology was negative one month before death, could suggest that the cardiac alterations were not related to the TC infection. Although cardiomyopathy is commonly encountered in chimpanzees [27], and could account for some portion of the cardiac fibrosis seen in this case, the length of time required for seroconversion to TC, the incidence of false negative serologic results, particularly in debilitated NHP, and the timecourse of cardiac lesion development have not been established in chimpanzees. It remains possible that the abnormal ECG was an early symptom of CD. TC infected NHP show similar cardiac changes as other species, including humans. Acute CD has been extensively described in rhesus monkeys [3, 8, 19, 21, 26, 28]. Detailed study of CD in chimpanzees offers a unique opportunity to further understand the pathogenesis of the disease.
Acknowledgments
We wish to thank Marie Silva, Michaelle Hohmann, Denise Trejo, M.G. Bonecini-Almeida, and personnel of the Armed Forces Institute of Pathology for their individual contributions to the publication.
Funding: This research was funded in part by NIH/NCRR grant P51 RR013986 to the Southwest National Primate Research Center, Southwest Foundation for Biomedical Research. Chimpanzees were housed in facilities constructed with support from Research Facilities Improvement Program Grant C06 RR016228 from the National Center for Research Resources, NIH.
References
- 1.Azogue E, La Fuente C, Darras C. Congenital Chagas' disease in Bolivia: epidemiological aspects and pathological findings. Trans R Soc Trop Med Hyg. 1985;79:176–180. doi: 10.1016/0035-9203(85)90328-1. [DOI] [PubMed] [Google Scholar]
- 2.Barr SC, Schmidt SP, Brown CC, Klei TR. Pathologic features of dogs inoculated with North American Trypanosoma cruzi isolates. Am J Vet Res. 1991d;52:2033–2039. [PubMed] [Google Scholar]
- 3.Bonecini-Almeida MDB, Galvão-Castro B, Pessoa MHR, Pirmez C, Laranja FS. Experimental Chagas' disease in rhesus monkeys. I. Clinical, parasitological, hematological and anatomo-pathological studies in the acute and indeterminate phase of the disease. Mem Inst Oswaldo Cruz. 1990;85:163–171. doi: 10.1590/s0074-02761990000200004. [DOI] [PubMed] [Google Scholar]
- 4.Braz LM, Amato Neto V, Okay TS. Reactivation of Trypanosoma cruzi infection in immunosuppressed patients: contributions for the laboratorial diagnosis standardization. Rev Inst Med Trop Sao Paulo. 2008 Jan-Feb;50(1):65–6. doi: 10.1590/s0036-46652008000100015. [DOI] [PubMed] [Google Scholar]
- 5.Burkholder JE, Allison TC, Kelly VP. Trypanosoma cruzi (Chagas) (Protozoa: Kinetoplastida) in invertebrate, reservoir, and human hosts of the lower Rio Grande valley of Texas. J Parasitol. 1980;66:305–311. [PubMed] [Google Scholar]
- 6.Carvalho CM, Andrade MC, Xavier SS, Mangia RH, Britto CC, Jansen AM, Fernandes O, Lannes-Vieira J, Bonecini-Almeida MG. Chronic Chagas' disease in rhesus monkeys (Macaca mulatta): evaluation of parasitemia, serology, electrocardiography, echocardiography, and radiology. Am J Trop Med Hyg. 2003 Jun;68(6):683–91. [PubMed] [Google Scholar]
- 7.Cordova E, Boschi A, Ambrosioni J, Cudos C, Corti M. Reactivation of Chagas disease with central nervous system involvement in HIV-infected patients in Argentina, 1992-2007. Int J Infect Dis. 2008 Mar 10; doi: 10.1016/j.ijid.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Enders B, Weinmann F, Nagle CA, Hein B, Zwisler O. Experimental Chagas' disease in primates Macaca fascicularis and Cebus apella: preclinical trials and evaluation of a living non-replicating T. cruzi vaccine. Behring Inst Mitt. 1982;71:132–137. [Google Scholar]
- 9.Gleiser CA, Yaeger RG, Ghidoni JJ. Trypanosoma cruzi infection in a colony-born baboon. J Am Vet Med Assoc. 1986 Nov 1;189(9):1225–6. No abstract available. [PubMed] [Google Scholar]
- 10.Grieves JL, Hubbard GB, Williams JT, VandeBerg JL, Dick EJ, Jr, Lopez-Alvarenga JC, Schlabritz-Loutsevich NE. Trypanosoma cruzi and pregnancy outcome in nonhuman primates: retrospective study (Papio hamadryas spp.) and a case report (Macaca fascicularis) J Med Primatol. doi: 10.1111/j.1600-0684.2008.00302.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grogl M, Kuhn RE, Davis DS, Green GE. Antibodies to Trypanosoma cruzi in coyotes in Texas. J Parasitol. 1984;70:189–191. [PubMed] [Google Scholar]
- 12.Hall CA, Polizzi C, Yabsley MJ, Norton TM. Trypanosoma cruzi prevalence and epidemiologic trends in lemurs on St. Catherines Island, Georgia. J Parasitol. 2007 Feb;93(1):93–6. doi: 10.1645/GE-936R.1. [DOI] [PubMed] [Google Scholar]
- 13.John DT, Hoppe KL. Trypanosoma cruzi from wild raccoons in Oklahoma. Am J Vet Res. 1986;47:1056–1059. [PubMed] [Google Scholar]
- 14.Karsten V, Davis C, Kuhn R. Trypanosoma cruzi in wild raccoons and opossums in North Carolina. J Parasitol. 1992;78:547–549. [PubMed] [Google Scholar]
- 15.Kirchhoff LV. American trypanosomiasis (Chagas' disease) – a tropical disease now in the United States. New Engl J Med. 1993;329:639–644. doi: 10.1056/NEJM199308263290909. [DOI] [PubMed] [Google Scholar]
- 16.Kunz E, Mätz-Rensing K, Stolte N, Hamilton PB, Kaup FJ. Reactivation of a Trypanosoma cruzi infection in a rhesus monkey (Macaca mulatta) experimentally infected with SIV. Vet Pathol. 2002 Nov;39(6):721–5. doi: 10.1354/vp.39-6-721. [DOI] [PubMed] [Google Scholar]
- 17.Laranja FS, Días E, Nobrega G, Miranda A. Chagas' disease: a clinical, epidemiologic and pathologic study. Circulation. 1956;14:1035–1060. doi: 10.1161/01.cir.14.6.1035. [DOI] [PubMed] [Google Scholar]
- 18.Marin-Neto JA, Cunha-Neto E, Maciel BC, Simões MV. Pathogenesis of chronic Chagas heart disease. Circulation. 2007 Mar 6;115(9):1109–23. doi: 10.1161/CIRCULATIONAHA.106.624296. [DOI] [PubMed] [Google Scholar]
- 19.Marsden PD, Seah SKK, Draper CC, Pettitt LE, Miles MA, Voller A. Experimental Trypanosoma cruzi infections in rhesus monkeys. II. The early chronic phase. Trans R Soc Trop MedHyg. 1976;70:247–251. doi: 10.1016/0035-9203(76)90049-3. [DOI] [PubMed] [Google Scholar]
- 20.Miles MA. Trypanosoma cruzi-milk transmission of infection and immunity from mother to young. Parasitology. 1972;65:1–9. doi: 10.1017/s0031182000044188. [DOI] [PubMed] [Google Scholar]
- 21.Miles MA, Marsden PD, Pettitt LE, Draper CC, Watson S, Seah SK, Hutt MS, Fowler JM. Experimental Trypanosoma cruzi infection in rhesus monkeys. Electrocardiographic and histopathological findings. Trans R Soc Trop MedHyg. 1979;73:528–532. doi: 10.1016/0035-9203(79)90044-0. [DOI] [PubMed] [Google Scholar]
- 22.Pan AA, Rosenberg GB, Hurley MK, Schock GJ, Chu VP, Aiyappa A. Clinical evaluation of an EIA for the sensitive and specific detection of serum antibody to Trypanosoma cruzi (Chagas' disease) J Infect Dis. 1992;165:585–8. doi: 10.1093/infdis/165.3.585. [DOI] [PubMed] [Google Scholar]
- 23.Pung OJ, Banks CW, Jones DN, Krissinger MW. Trypanosoma cruzi in wild raccoons, opossums, and triatomine bugs in southeast Georgia. U.S.A. J Parasitol. 1995;81:324–326. [PubMed] [Google Scholar]
- 24.Pung OJ, Spratt J, Clark CG, Norton TM, Carter J. Trypanosoma cruzi infection of free-ranging lion-tailed macaques (Macaca silenus) and ring-tailed lemurs (Lemur catta) on St. Catherine's Island, Georgia, USA. J Zoo Wildl Med. 1998 Mar;29(1):25–30. [PubMed] [Google Scholar]
- 25.Schijman AG, Vigliano C, Burgos J, Favaloro R, Perrone S, Laguens R, Levin MJ. Early diagnosis of recurrence of Trypanosoma cruzi infection by polymerase chain reaction after heart transplantation of a chronic Chagas' heart disease patient. J Heart Lung Transplant. 2000 Nov;19(11):1114–7. doi: 10.1016/s1053-2498(00)00168-6. [DOI] [PubMed] [Google Scholar]
- 26.Seah SKK, Marsden PD, Voller A, Pettitt LE. Experimental Trypanosoma cruzi infection in rhesus monkeys—the acute phase. Trans R Soc Trop MedHyg. 1974;68:63–69. doi: 10.1016/0035-9203(74)90254-5. [DOI] [PubMed] [Google Scholar]
- 27.Seiler BM, Dick EJ, Jr, Guardado-Mendoza R, VandeBerg JL, Williams JT, Mubiru JN, Hubbard GB. Spontaneous Heart Disease in the Adult Chimpanzee (Pan troglodytes) J Med Primatol. 2008 doi: 10.1111/j.1600-0684.2008.00307.x. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szarfman A, Gerecht D, Draper CC, Marsden PD. Tissuereacting immunoglobulin in rhesus monkeys infected with Trypanosoma cruzi: a follow-up study. Trans R Soc Trop MedHyg. 1981;75:114–116. doi: 10.1016/0035-9203(81)90024-9. [DOI] [PubMed] [Google Scholar]
- 29.Telford SR, Jr, Forrester DJ. Hemoparasites of raccoons (Procyon lotor) in Florida. J Wildl Dis. 1991;27:486–490. doi: 10.7589/0090-3558-27.3.486. [DOI] [PubMed] [Google Scholar]
- 30.Torrico F, Alonso-Vega C, Suarez E, Rodriguez P, Torrico MC, Dramaix M, Truyens C, Carlier Y. Maternal Trypanosoma cruzi infection, pregnancy outcome, morbidity, and mortality of congenitally infected and non-infected newborns in Bolivia. Am J Trop Med Hyg. 2004;70(2):201–219. [PubMed] [Google Scholar]
- 31.World Health Organization. Chagas disease. Progress towards elimination of transmission, Argentina. Wkly Epidemiol Rec. 1996;71:12–15. [PubMed] [Google Scholar]
- 32.Williams JT, Dick EJ, Jr, VandeBerg JL, Hubbard GB. Natural Chagas disease in four baboons. J Med Primatol. 2008 doi: 10.1111/j.1600-0684.2008.00308.x. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yaeger RG. The prevalence of Trypanosoma cruzi infection in armadillos collected at a site near New Orleans. La Am J Trop Med Hyg. 1988;38:323–326. doi: 10.4269/ajtmh.1988.38.323. [DOI] [PubMed] [Google Scholar]
- 34.Zabalgoitia M, Ventura J, Anderson L, Carey KD, Williams JT, Vandeberg JL. Morphologic and functional characterization of Chagasic heart disease in non-human primates. Am J Trop Med Hyg. 2003 Feb;68(2):248–52. [PubMed] [Google Scholar]


