Summary
There is growing consensus that the summed activity of multiple nodes of a distributed cortical network supports face recognition in humans, including “core” ventral occipito-temporal cortex (VOTC) regions [1-3], as well as “extended” regions outside VOTC [4, 5]. Surprisingly, many individuals with congenital prosopagnosia – a lifelong impairment in face processing [6-9] -- exhibit normal BOLD activation in the “core” VOTC regions [10] (but see [11]). Interestingly, these same individuals evince a reduction in the structural integrity of the white matter tracts connecting VOTC to anterior temporal and frontal cortices [12] which form part of the “extended” face network. These findings suggest that the profound impairment in congenital prosopagnosia may arise not from a dysfunction of the core VOTC areas per se but from a failure to propagate signals between the intact VOTC and the extended nodes of the network. Here, using the fMR adaptation paradigm with famous and unknown faces, we show that individuals with congenital prosopagnosia evince normal adaptation effects in VOTC, indicating sensitivity to facial identity, but, unlike controls, show no differential activation for familiar versus unknown faces outside VOTC, particularly in the precuneus/posterior cingulate cortex and the anterior paracingulate cortex. These results indicate that normal BOLD activation in VOTC is insufficient to subserve intact face recognition, and support the hypothesis that disrupted information propagation between VOTC and the extended face processing network underlies the functional impairment in congenital prosopagnosia.
Keywords: prosopagnosia, face processing, ventral visual cortex, neural circuit, neurodevelopmental disorders
Results
We adopted a rapid event-related fMR adaptation technique, which utilizes the change in the fMRI signal (BOLD) following repeated presentation of images to ‘tag’ response properties of neurons [13]. Subjects performed a same/different identity judgment on a pair of sequentially presented photographs of famous and unknown people (see figure 1a and supplementary Experimental Procedures), a task known to engage multiple regions of the face circuit. Each subject participated in 2 separate runs, each lasting 624 sec and containing 28 trials of each condition. Stimuli were presented in a counterbalanced rapid event-related design with ‘Fixation only’ trials embedded among experimental trials. We compared the BOLD profile of the congenital prosopagnosia (N = 6) and control (N = 12) subjects to examine two key aspects of the neural signal (see supplementary Experimental Procedures for more details). The first aspect concerns the specificity of the underlying neural representations of faces: comparing the signal reduction for repeated (‘same picture’) versus non-repeated faces (‘different picture’) in the two groups serves as a marker of sensitivity to facial identity. Typically, under such conditions, the fusiform face area, FFA, and other posterior regions exhibit a clear reduction in the magnitude of the BOLD signal (adaptation) (e.g. [14-19]). The second aspect concerns the neural representation of familiarity. In typical individuals, familiar faces elicit a selective response in regions outside VOTC, presumably to activate associated semantic, biographical and personal representation [20]. If the functional integrity of this distributed cortical network is compromised, the prediction is that the CP group would evince the expected BOLD reduction in core regions but no familiarity signal beyond VOTC.
Figure 1.
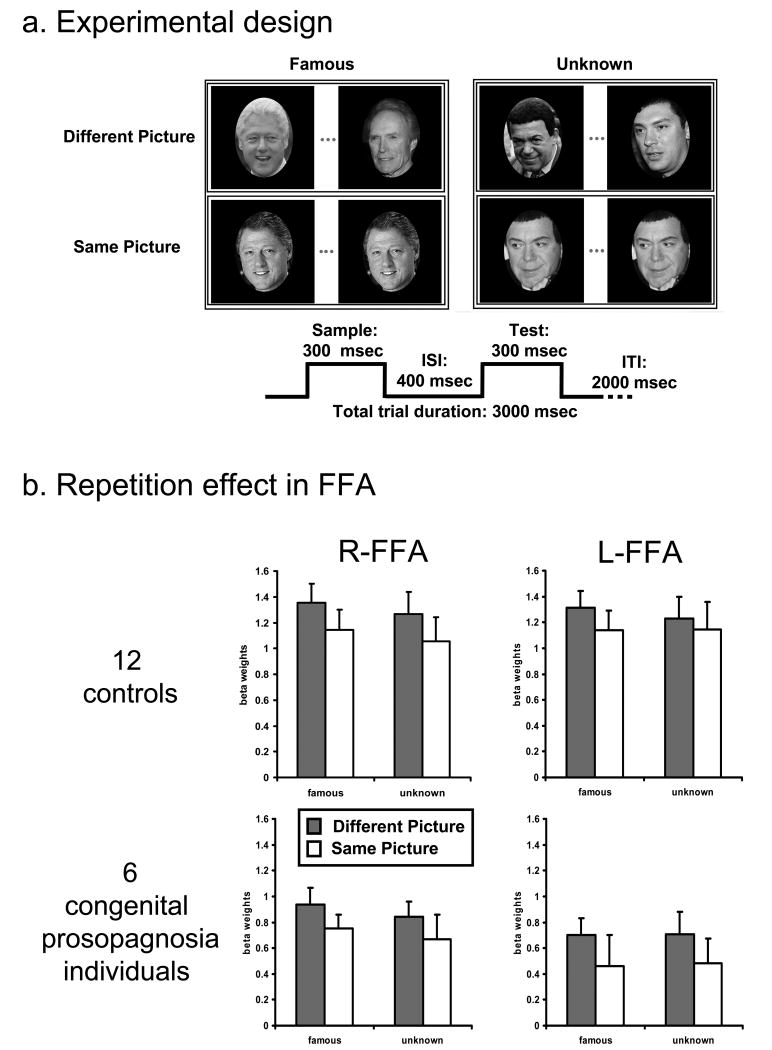
a. Experimental design of the face identity repetition experiment and schematic depiction of a trial In each trial, two faces were presented sequentially and subjects performed a ‘same/different’ identity task. In half of the trials, both pictures were of famous individuals and, in the other half, they were of unknown individuals. All conditions were counterbalanced. On each trial, lasting 3000 msec, the pictures were presented consecutively for 300msec each with an ISI of 200 msec.
b. Repetition effects in FFA Top row: Activation profiles showing the repetition effect (reduced signal for ‘same picture’ compared to ‘different picture’ condition) for 12 control subjects. The y-axis denotes the averaged beta weights (parameter estimates) and error bars indicate standard error of the mean (SEM) across subjects. Bottom row: Activation profiles showing the repetition effect for the congenital prosopagnosia group. Although the signal magnitude was greater in controls compared to the congenital prosopagnosia subjects, there were no interactions with group, indicating that both groups were equally affected by the repetition manipulation.
We first compared the performance of the two groups on the task completed during the scan. While the congenital prosopagnosia group performed less accurately than the controls in deciding whether the sequentially-displayed pair of faces shared identity or not (mean±SEM CP: 92.7±1.0%, controls: 95.3±0.7%; F(1,16)=4.51, p<0.05), their overall accuracy was still relatively high. There was a main effect of repetition (p<0.0001) and of familiarity (p<0.002), with better performance in trials of famous faces and of two identical faces, but no interaction with group (repetition × group F(1,16)=1.7, p>0.2; F<1, p>0.2 for all other interactions). Individuals with congenital prosopagnosia responded significantly more slowly than controls (mean±SEM congenital prosopagnosia group: 832±63ms controls: 694±30ms; F(1,16)=5.90, p<0.03) and, as above, there were main effects of repetition (p<0.0001) and familiarity (p<0.03), but no significant interactions (F<1, p>0.3 for all interactions). These findings confirm the behavioral impairment in congenital prosopagnosia (see [7] for other data confirming the diagnosis) and indicate that the two groups were equally affected by the repetition manipulation and by the familiarity of the faces.
To explore the underlying neural profile, using an independent face localizer scan, we identified in each individual in each hemisphere, regions of interest (ROIs), showing a selective response for faces compared with all other stimuli. Consistent with previous studies (e.g. [2, 4, 10]), these foci included the right and left FFA and OFA (occipital face area), composed of the lateral occipital sulcus (LOS) and the inferior occipital gyrus (IOG). These ROIs were identifiable in the majority of subjects and the Talairach coordinates of the ROIs were similar across the groups (see supplementary Experimental Procedures and supplementary Table 1 for details).
The peak activation (beta weight) from each ROI for each experimental condition was extracted for each participant using a deconvolution analysis (see Supplementary Experimental Procedures, figure 1b and figure S1 for FFA and OFA activation) and subjected to a repeated measures ANOVA with group (congenital prosopagnosia, controls) as a between-subject factor and region (FFA, OFA), hemisphere (right, left), familiarity (famous, unknown) and repetition type (different/same picture) as within-subject factors. This analysis revealed a significant repetition effect that was modulated by cortical region (region × repetition (F(1,13)=16.214, p<0.002) but, critically, did not interact with group; although present in both FFA and OFA, the reduction in BOLD signal for different versus same picture was more marked for the FFA than the OFA (FFA: p<0.0002; OFA: p<0.02). This adaptation effect in the control individuals replicates many previous findings (e.g. [15-18]), some of which also show the greater reduction in FFA than in OFA [17] and some of which also show modulation of the repetition effect by familiarity [14]. Importantly, the presence of an adaptation signal in individuals with congenital prosopagnosia, of equivalent strength to that of the controls, is consistent with results indicating normal face-selective activation in VOTC in these individuals [10, 21]. Furthermore, the repetition index (different versus same picture), calculated in FFA and OFA for famous and unknown faces, for each individual with congenital prosopagnosia is within the range of controls, corroborating the results of the ANOVA and replicating the result at the individual subject level (see Figure S2).
Overall, the magnitude of the BOLD signal was greater in the control than in the congenital prosopagnosia group (F(1,13)=10.617, p<0.006) but this did not interact with any of the critical experimental conditions. In fact, the only interaction involving group (3 way: region × hemisphere × group (F(1,13)=5.5, p<0.04)), revealed that the signal in controls was larger than that of the congenital prosopagnosia group in both the left and right FFA, to a greater extent on the left (right FFA: p<0.03; left FFA p<0.003), and there were no group differences in the OFA (p=0.15, p=0.4 in right and left OFA respectively). Finally, the repetition suppression was more pronounced for famous compared with unknown faces (familiarity × repetition: F(1,13)=4.36, p=0.06) and the magnitude of activation was larger overall for familiar than unknown faces (F(1,13)=4.85, p<0.05). Details of the signal magnitude difference between the congenital prosopagnosia group and controls at the individual subject level can be found in supplementary figure S3. The key result from all these analyses is that there are no interactions with group and, thus, we conclude that the impact of stimulus repetition and stimulus familiarity is equivalent across the groups in the VOTC regions. Importantly, these findings uncover the adaptation effect in congenital prosopagnosia under more sensitive and taxing conditions than those employed previously (i.e. in an event-related design here versus previously in a block design in which signal attenuation could be due reduced attention [10]) and provide strong confirmation of a normal neural profile in the core regions of the face network in congenital prosopagnosia individuals.
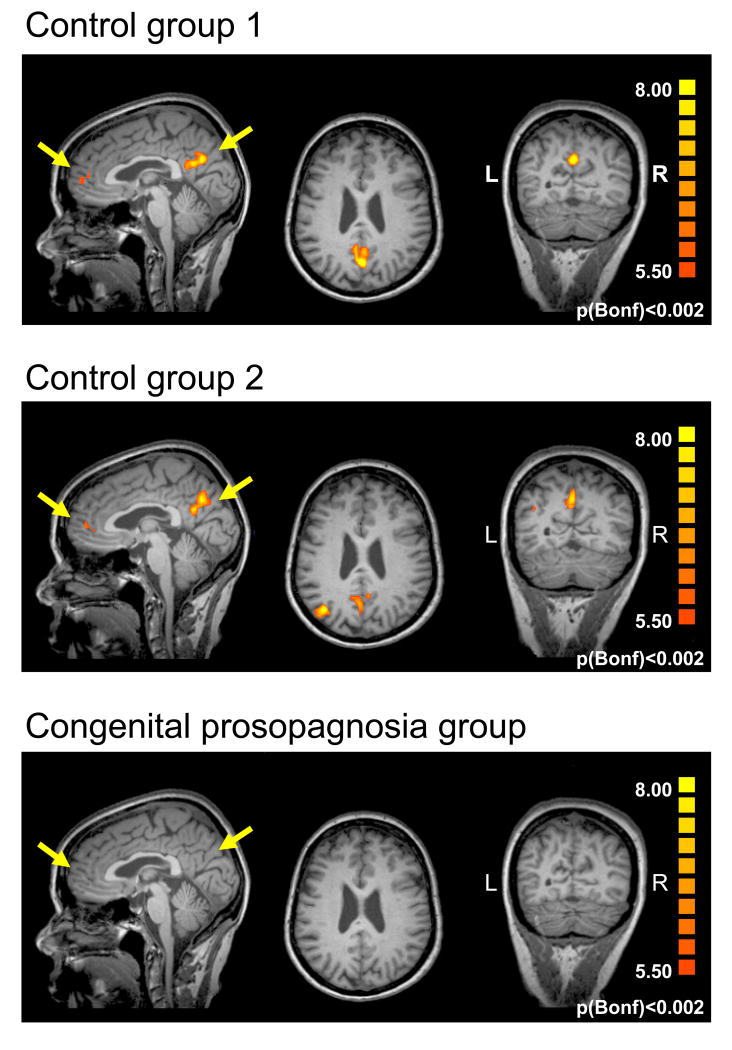
Given the normal VOTC activation profile in parallel with the ongoing behavioral impairment, we explored the differential impact of familiarity on the BOLD signal of the two groups across the entire cortex, by contrasting all trials containing famous vs. unknown faces using a multi-subject GLM analysis (see Supplementary Experimental Procedures). To equate statistical power for the two groups, we split the control group into two groups of six. Since it is not possible to conduct random effects analysis with such small groups, fixed effects analyses were applied using a stringent statistical threshold of p<0.002 (Bonferroni corrected) and a minimum cluster size of 4 contiguous voxels. Using this conservative approach, we found two main foci of activation in both control groups: one in the precuneus/posterior cingulate cortex, mostly in the left hemisphere but also in the right hemisphere (Tal coord.: x=-2, y=-57, z=24; x=-2, y=-62, z=29 in control groups 1, and 2 respectively) and a second focus in the anterior paracingulate cortex (Tal coord.: x=-7, y=56, z=13; x=-5, y=-48, z=7 in control groups 1, and 2 respectively), as shown in Figure 2. There was a third significant focus of activation in the left parietal cortex in control group 2 (Tal coord.: x=-37, y=-72, z=33). The first two activation foci have been identified previously in normal individuals in studies examining cortical activation for famous faces and are attributed to the retrieval of episodic memories and of personal traits and attitudes, respectively [20]. In contrast, in the congenital prosopagnosia group, there was no region whatsoever evincing a famous/unknown difference. When a much more lenient threshold of p<0.005 was applied with no correction for multiple comparisons nor for false discovery rate (FDR), some activity (whose reliability is dubious) emerged in the congenital prosopagnosia group in the vicinity of the precuneus/posterior cingulate cortex. Even under these very liberal conditions, however, no activity was uncovered in the anterior paracingulate region. Thus, while congenital prosopagnosia individuals exhibit normal activation profiles in VOTC, they, unlike the control participants, do not show any familiarity-related activation outside VOTC. Importantly, the absence of this familiarity signature cannot be attributed to low statistical power as it is evident in each of the control subgroups.
Figure 2. Activation foci exhibiting a familiarity effect outside VOTC.
a. A statistical test contrasting all famous and unknown faces was conducted separately for two subgroups of controls with 6 participants in each and for the congenital prosopagnosia group (multi subject GLM, fixed effects, p<0.002 Bonferroni corrected). The analysis revealed selective activation for famous compared to unknown faces in the left precuneus/posterior cingulate cortex and the anterior paracingulate cortex in both control groups but not in the congenital prosopagnosia group. The average activation across each control subgroup is overlaid on sagittal, coronal and axial slices of one individual subject. Note the absence of familiarity selective activation in the CP group (lower panel). (L=left hemisphere, R=right hemisphere).
Discussion
Two clear findings emerge from this study: the first is that individuals with congenital prosopagnosia demonstrate normal face-selective activation of posterior cortical visual regions of the distributed circuit that mediates face processing, even under especially sensitive experimental conditions. Many recent studies have reported that posterior VOTC is sensitive to facial identity as reflected in the BOLD reduction to repeated over non-repeated faces (e.g. [16, 17]) and some studies have shown modulation of this reduction by face familiarity [14]. We replicate and confirm this finding in controls and, critically, show a statistically equivalent repetition effect in the congenital prosopagnosia individuals. The profound behavioral impairment in congenital prosopagnosia, therefore, cannot be attributed to perturbation in these VOTC regions. We note, however, that for reasons that remain to be determined, some congenital prosopagnosia individuals exhibit abnormal activation profiles in VOTC [11, 22, 23]. The second perhaps more important finding is the dramatic absence of activation in congenital prosopagnosia in the extended regions of the face circuit. Taken together, these findings may account for the fact that, despite the lack of overt sense of recognition, individuals with congenital prosopagnosia respond more quickly and more accurately to familiar than to unfamiliar faces i.e. show ‘implicit’ effects of recognition [24]. Thus, regions in VOTC may be sensitive to face familiarity but this information apparently fails to activate regions of the extended face network, thereby precluding explicit recognition.
Indeed, the necessity of activating these extended regions is confirmed by recent studies showing that regions, such as the anterior temporal lobe, but not FFA, show distinct patterns of BOLD activation in response to individual faces [25], are critically involved in normal configural face processing [26] and can give rise to face processing deficits too [27-29]. Moreover, regions such as the precuneus/posterior cingulate cortex and the anterior paracingulate cortex likely play a role in representing some knowledge of faces, consistent with the stronger activation for familiar versus unknown faces in these regions obtained using various paradigms (e.g. generally famous faces [30], personally familiar faces [20] and visually familiar faces [31]). Moreover, others have even implicated the precuneus/posterior cingulate region in the acquisition of face familiarity [32] and this is also consistent with studies showing selective activation for familiar voices in this region [33].
Taken together, these findings suggest that congenital prosopagnosia may result from a failure of information propagation between VOTC and other cortical regions that form a distributed neural network supporting face processing [4, 5, 20, 34]. The alteration of white matter fiber tracts that project through the core face processing regions to the anterior temporal lobe and frontal cortex in congenital prosopagnosia, as well as the reduction in volume of the portion of the fusiform gyrus, located anterior to the FFA [35], and the behavioral evidence showing implicit familiarity processing in these individuals are all clearly consistent with this account, too. This converging evidence provides, for the first time, a comprehensive account of the neural basis underlying congenital prosopagnosia. Furthermore, the results indicate that the multiplicity of face-selective regions revealed in studies with human and non-human primates [36, 37] play a coordinated and functionally necessary role in a network whose joint activity supports the recognition of familiar individuals. We stress that the present findings do not undermine the integral role of core regions such as the FFA in face processing, a finding which is strongly supported by numerous lesion studies (e.g. [1]). Rather, our work points out that these core regions, while necessary, may not be sufficient for successful recognition and that regions such as the prerecuneus/posterior cingulate, and anterior paracingulate cortex are also involved. The findings from congenital prosopagnosia stand in contrast with the neural profile in acquired prosopagnosia, in which the lesion is typically more localized, affecting a particular node in the face network, usually (although not always) the FFA. Of course, damage to one such node can affect propagation of information through the face circuit, rendering the disconnection account plausible for both the congenital and acquired [38] forms of prosopagnosia.
Finally, it is important to note that the present findings have implications that extend beyond congenital prosopagnosia. Interestingly, this condition bears some similarities to other neurodevelopmental disorders, such as developmental dyslexia and congenital amusia. As in congenital prosopagnosia, in these other disorders, the impairments affect a particular domain (reading, auditory pattern analysis) even though the affected individuals have intact sensory and intellectual functions, and the motivation and opportunities for acquiring the relevant skill are normal. Also, as in congenital prosopagnosia, these other disorders have a familial component, implicating some genetic basis [39-41]. A disconnection explanation has also been offered for these disorders; for example, developmental dyslexia has been attributed to reduced connectivity between temporal and parietal regions [42], which may be present from birth or may arise, as in a recent study, as a consequence of brain radiation treatment in early childhood [43]. A similar disconnection account, this time between frontal and auditory cortex, has been offered for congenital amusia or ‘tone deafness’, [44]. The similarities among these disorders suggest that many complex cognitive tasks may be subserved by distributed networks, linking together disparate cortical regions, and that a disruption, resulting from a developmental alteration, acquired lesion, or neurological disease that disconnects the nodes of the circuit can give rise to profound cognitive impairments.
Experimental Procedures
See supplementary Experimental Procedures for details
Supplementary Material
Acknowledgments
We thank Grace Lee Leonard for her substantial help with stimulus preparation and testing, and Michal Tanzer for help with data analysis. We also thank Cibu Thomas, Mayu Nishimura and Suzy Scherf for valuable comments on this manuscript, and Cibu Thomas for his help in testing participants KE and WS. This work was supported by a NIMH 54246 grant to MB.
Footnotes
This paper is dedicated to the memory of BE who participated enthusiastically in many of our studies.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barton JJ. Disorders of face perception and recognition. Neurol Clin. 2003;21:521–548. doi: 10.1016/s0733-8619(02)00106-8. [DOI] [PubMed] [Google Scholar]
- 2.Rossion B, Caldara R, Seghier M, Schuller AM, Lazeyras F, Mayer E. A network of occipito-temporal face-sensitive areas besides the right middle fusiform gyrus is necessary for normal face processing. Brain. 2003;126:2381–2395. doi: 10.1093/brain/awg241. [DOI] [PubMed] [Google Scholar]
- 3.Pitcher D, Walsh V, Yovel G, Duchaine B. TMS evidence for the involvement of the right occipital face area in early face processing. Curr Biol. 2007;17:1568–1573. doi: 10.1016/j.cub.2007.07.063. [DOI] [PubMed] [Google Scholar]
- 4.Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends Cogn Sci. 2000;4:223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- 5.Ishai A. Let's face it: it's a cortical network. Neuroimage. 2008;40:415–419. doi: 10.1016/j.neuroimage.2007.10.040. [DOI] [PubMed] [Google Scholar]
- 6.Bentin S, Deouell LY, Soroker N. Selective visual streaming in face recognition: evidence from developmental prosopagnosia. Neuroreport. 1999;10:823–827. doi: 10.1097/00001756-199903170-00029. [DOI] [PubMed] [Google Scholar]
- 7.Behrmann M, Avidan G, Marotta JJ, Kimchi R. Detailed exploration of face-related processing in congenital prosopagnosia: 1. Behavioral findings. J Cogn Neurosci. 2005;17:1130–1149. doi: 10.1162/0898929054475154. [DOI] [PubMed] [Google Scholar]
- 8.Duchaine BC, Dingle K, Butterworth E, Nakayama K. Normal greeble learning in a severe case of developmental prosopagnosia. Neuron. 2004;43:469–473. doi: 10.1016/j.neuron.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Dobel C, Bolte J, Aicher M, Schweinberger SR. Prosopagnosia without apparent cause: overview and diagnosis of six cases. Cortex. 2007;43:718–733. doi: 10.1016/s0010-9452(08)70501-x. [DOI] [PubMed] [Google Scholar]
- 10.Avidan G, Hasson U, Malach R, Behrmann M. Detailed exploration of face-related processing in congenital prosopagnosia: 2. Functional neuroimaging findings. J Cogn Neurosci. 2005;17:1150–1167. doi: 10.1162/0898929054475145. [DOI] [PubMed] [Google Scholar]
- 11.Bentin S, Degutis JM, D'Esposito M, Robertson LC. Too many trees to see the forest: performance, event-related potential, and functional magnetic resonance imaging manifestations of integrative congenital prosopagnosia. J Cogn Neurosci. 2007;19:132–146. doi: 10.1162/jocn.2007.19.1.132. [DOI] [PubMed] [Google Scholar]
- 12.Thomas C, Avidan G, Humphreys K, Jung K, Gao F, Behrmann M. Reduced structural connectivity in ventral visual cortex in congenital prosopagnosia. Nat Neurosci. 2009;12:29–31. doi: 10.1038/nn.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grill-Spector K, Malach R. fMR-adaptation: a tool for studying the functional properties of human cortical neurons. Acta Psychol (Amst) 2001;107:293–321. doi: 10.1016/s0001-6918(01)00019-1. [DOI] [PubMed] [Google Scholar]
- 14.Henson R, Shallice T, Dolan R. Neuroimaging evidence for dissociable forms of repetition priming. Science. 2000;287:1269–1272. doi: 10.1126/science.287.5456.1269. [DOI] [PubMed] [Google Scholar]
- 15.Eger E, Schyns PG, Kleinschmidt A. Scale invariant adaptation in fusiform face-responsive regions. Neuroimage. 2004;22:232–242. doi: 10.1016/j.neuroimage.2003.12.028. [DOI] [PubMed] [Google Scholar]
- 16.Rotshtein P, Henson RN, Treves A, Driver J, Dolan RJ. Morphing Marilyn into Maggie dissociates physical and identity face representations in the brain. Nat Neurosci. 2005;8:107–113. doi: 10.1038/nn1370. [DOI] [PubMed] [Google Scholar]
- 17.Gilaie-Dotan S, Malach R. Sub-exemplar shape tuning in human face-related areas. Cereb Cortex. 2007;17:325–338. doi: 10.1093/cercor/bhj150. [DOI] [PubMed] [Google Scholar]
- 18.Dricot L, Sorger B, Schiltz C, Goebel R, Rossion B. The roles of “face” and “non-face” areas during individual face perception: evidence by fMRI adaptation in a brain-damaged prosopagnosic patient. Neuroimage. 2008;40:318–332. doi: 10.1016/j.neuroimage.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 19.Davies-Thompson J, Gouws A, Andrews TJ. An image-dependent representation of familiar and unfamiliar faces in the human ventral stream. Neuropsychologia. 2009 doi: 10.1016/j.neuropsychologia.2009.01.017. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gobbini MI, Haxby JV. Neural systems for recognition of familiar faces. Neuropsychologia. 2007;45:32–41. doi: 10.1016/j.neuropsychologia.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 21.Hasson U, Avidan G, Deouell LY, Bentin S, Malach R. Face-selective activation in a congenital prosopagnosic subject. J Cogn Neurosci. 2003;15:419–431. doi: 10.1162/089892903321593135. [DOI] [PubMed] [Google Scholar]
- 22.Hadjikhani N, De Gelder B. Neural basis of prosopagnosia: An fMRI study. Hum Brain Mapp. 2002;16:176–182. doi: 10.1002/hbm.10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Minnebusch DA, Suchan B, Koster O, Daum I. A bilateral occipitotemporal network mediates face perception. Behav Brain Res. 2009;198:179–185. doi: 10.1016/j.bbr.2008.10.041. [DOI] [PubMed] [Google Scholar]
- 24.Avidan G, Behrmann M. Implicit familiarity processing in congenital prosopagnosia. Journal of Neuropsychology. 2008;2:141–164. doi: 10.1348/174866407x260180. [DOI] [PubMed] [Google Scholar]
- 25.Kriegeskorte N, Formisano E, Sorger B, Goebel R. Individual faces elicit distinct response patterns in human anterior temporal cortex. Proc Natl Acad Sci U S A. 2007;104:20600–20605. doi: 10.1073/pnas.0705654104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams MA, Savage G, Halmagyi M. Abnormal configural face perception in a patient with right anterior temporal lobe atrophy. Neurocase. 2006;12:286–291. doi: 10.1080/13554790601026379. [DOI] [PubMed] [Google Scholar]
- 27.Damasio AR, Tranel D, Damasio H. Face agnosia and the neural substrates of memory. Annu Rev Neurosci. 1990;13:89–109. doi: 10.1146/annurev.ne.13.030190.000513. [DOI] [PubMed] [Google Scholar]
- 28.Evans JJ, Heggs AJ, Antoun N, Hodges JR. Progressive prosopagnosia associated with selective right temporal lobe atrophy. A new syndrome? Brain. 1995;118(Pt 1):1–13. doi: 10.1093/brain/118.1.1. [DOI] [PubMed] [Google Scholar]
- 29.Glosser G, Salvucci AE, Chiaravalloti ND. Naming and recognizing famous faces in temporal lobe epilepsy. Neurology. 2003;61:81–86. doi: 10.1212/01.wnl.0000073621.18013.e1. [DOI] [PubMed] [Google Scholar]
- 30.Eger E, Schweinberger SR, Dolan RJ, Henson RN. Familiarity enhances invariance of face representations in human ventral visual cortex: fMRI evidence. NeuroImage. 2005;26:1128–1139. doi: 10.1016/j.neuroimage.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 31.Gobbini MI, Haxby JV. Neural response to the visual familiarity of faces. Brain Res Bull. 2006;71:76–82. doi: 10.1016/j.brainresbull.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 32.Kosaka H, Omori M, Iidaka T, Murata T, Shimoyama T, Okada T, Sadato N, Yonekura Y, Wada Y. Neural substrates participating in acquisition of facial familiarity: an fMRI study. Neuroimage. 2003;20:1734–1742. doi: 10.1016/s1053-8119(03)00447-6. [DOI] [PubMed] [Google Scholar]
- 33.Shah NJ, Marshall JC, Zafiris O, Schwab A, Zilles K, Markowitsch HJ, Fink GR. The neural correlates of person familiarity. A functional magnetic resonance imaging study with clinical implications. Brain. 2001;124:804–815. doi: 10.1093/brain/124.4.804. [DOI] [PubMed] [Google Scholar]
- 34.Fairhall SL, Ishai A. Effective connectivity within the distributed cortical network for face perception. Cereb Cortex. 2007;17:2400–2406. doi: 10.1093/cercor/bhl148. [DOI] [PubMed] [Google Scholar]
- 35.Behrmann M, Avidan G, Gao F, Black S. Structural imaging reveals anatomical alterations in inferotemporal cortex in congenital prosopagnosia. Cereb Cortex. 2007;17:2354–2363. doi: 10.1093/cercor/bhl144. [DOI] [PubMed] [Google Scholar]
- 36.Tsao DY, Schweers N, Moeller S, Freiwald WA. Patches of face-selective cortex in the macaque frontal lobe. Nat Neurosci. 2008;11:877–879. doi: 10.1038/nn.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rajimehr R, Young JC, Tootell RB. An anterior temporal face patch in human cortex, predicted by macaque maps. Proc Natl Acad Sci U S A. 2009;106:1995–2000. doi: 10.1073/pnas.0807304106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fox CJ, Iaria G, Barton JJ. Disconnection in prosopagnosia and face processing. Cortex. 2008;44:996–1009. doi: 10.1016/j.cortex.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 39.Grueter M, Grueter T, Bell V, Horst J, Laskowski W, Sperling K, Halligan PW, Ellis HD, Kennerknecht I. Hereditary prosopagnosia: the first case series. Cortex. 2007;43:734–749. doi: 10.1016/s0010-9452(08)70502-1. [DOI] [PubMed] [Google Scholar]
- 40.McGrath LM, Smith SD, Pennington BF. Breakthroughs in the search for dyslexia candidate genes. Trends in molecular medicine. 2006;12:333–341. doi: 10.1016/j.molmed.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 41.Peretz I, Cummings S, Dube MP. The genetics of congenital amusia (tone deafness): a family-aggregation study. Am J Hum Genet. 2007;81:582–588. doi: 10.1086/521337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klingberg T, Hedehus M, Temple E, Salz T, Gabrieli JD, Moseley ME, Poldrack RA. Microstructure of temporo-parietal white matter as a basis for reading ability: evidence from diffusion tensor magnetic resonance imaging. Neuron. 2000;25:493–500. doi: 10.1016/s0896-6273(00)80911-3. [DOI] [PubMed] [Google Scholar]
- 43.Rauschecker AM, Deutsch GK, Ben-Shachar M, Schwartzman A, Perry LM, Dougherty RF. Reading impairment in a patient with missing arcuate fasciculus. Neuropsychologia. 2009;47:180–194. doi: 10.1016/j.neuropsychologia.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hyde KL, Zatorre RJ, Griffiths TD, Lerch JP, Peretz I. Morphometry of the amusic brain: a two-site study. Brain. 2006;129:2562–2570. doi: 10.1093/brain/awl204. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.