Abstract
Objective
Determining language lateralization is important for the presurgical evaluation of patients with medically intractable epilepsy. The Wada test has been the gold standard for lateralization of language dominance before epilepsy surgery. However, it is an invasive test with risk, and have some limitations.
Methods
We compared the volumetric analysis with Wada test, and studied the clinical potential of volumetric analysis to assess language laterality in large surgical candidates with temporal lobe epilepsy (TLE). To examine the efficacy of volumetric analysis to determine language lateralization during presurgical evaluation, we compared the volumetric analysis of the bilateral planum temporale with the results of Wada test in 59 patients with chronic intractable TLE (rTLE, n=32; lTLE, n=27) who underwent epilepsy surgery. We measured the gray matter volumes of planum temporale (PT) of each patients using the VoxelPlus2 program (Mevisys, Daejeon, Korea).
Results
Overall congruence of the volumetric analysis with the Wada test was 97.75% in rTLE patients and 81.5% in lTLE patients. There were more significant leftward asymmetry of the PT in rTLE patients than lTLE patients. In lTLE patients, relatively high proportion (37%) of the patients showed bilateral or right hemispheric language dominance.
Conclusion
These results provide evidence that the volumetric analysis of the PT could be used as an alternatives in language lateralization. Also, the results of the Wada test suggested that there was considerable plasticity of language representation in the brains of patients with intractable TLE and it was associated with an earlier age of brain injury.
Keywords: Cerebral dominance, Temporal lobe epilepsy, Wada, Volume
INTRODUCTION
The determination of language lateralization is important in the presurgical evaluation of patients with medically intractable epilepsy. Surgical treatment offers the possibility of successful seizure control in a selected group of patients, particularly in those with temporal lobe epilepsy (TLE). However, it also puts them at risk for the development of new cognitive deficits. Post-operative aphasia, simple naming, or other word-finding difficulties has been reported in 6 to 30% of patients after left temporal lobectomy18,23,29). The risk of postoperative language and memory deficits is related directly to the preoperative lateralization of language functions in the brain4). The intracarotid amobarbital procedure (IAP), or the Wada test, is the most commonly used method at present and represents the gold standard in the assessment of language lateralization. However, this test has major shortcomings due to its invasiveness, lack of standardization, absence of spatial resolution, and difficulties related to application and interpretation3,5,27). Therefore, non-invasive alternatives to the Wada test are continually evaluated. Repetitive magnetic stimulation is a non-invasive alternative that works by deactivating the language cortex. The majority of other promising alternatives including functional magnetic resonance imaging (fMRI), positron emission tomography (PET), single photon emission computerized tomography (SPECT), transcranial Doppler, and near infrared spectroscopy are based on brain activation and detect hemodynamic responses to language cortex activation. Magnetoencephalography directly measures event-related physiological brain activation. Some techniques provide localization of language functions, while the Wada test is strictly a lateralization method. The fMRI will likely serve as the most widespread alternative due to broad availability1). But, there are very few studied comparisons between volumetric analysis and the IAP.
The planum temporale (PT), which constitutes part of Wernicke's area, was postulated to be an important area for language processing with speculation that this region facilitates the conversion of auditory phonemes to visual graphemes. The PT is located in the posterior superior temporal gyrus (Brodmann's area 22) and is associated with auditory phonological processing32). The 3D gradient echo magnetic resonance imaging (MRI) allows acquisition of high resolution consecutive millimeter sections. This allows in vivo quantitative analysis of the PT22,34). Steinmetz et al.34) used this technique to study the relationship of PT asymmetry to hand preference. The left-handers had a less significant leftward asymmetry of the PT as compared to the right-handers35). In 1994, Foundas et al.10) measured the PT on MRI scans of patients with selective hemispheric anesthesia or Wada testing for language lateralization. All individuals with language area lateralized to the left hemisphere (right-handed individuals) had leftward asymmetry in the PT, and one subject with language area lateralized to the right hemisphere (left-handed individual) exhibited a strong rightward asymmetry of the PT. This suggested that MRI-determined PT asymmetries are associated with language dominance and may predict language laterality10). Therefore, we compared language-related lateralization by the IAP test and with volumetric analysis to test potential of PT volumetry to detect language lateralization in patients with intractable temporal lobe epilepsy.
MATERIALS AND METHODS
Patients
This study included 59 patients with TLE who underwent temporal lobectomy at our institution from March 2004 to December 2007. Preoperative evaluations included detailed clinical and neurologic examinations, video-EEG monitoring, high-resolution MRI, SPECT, PET, neuropsychological examination, the IAP, and volumetric analysis of the PT. Patients were grouped according to seizure onset in subjects with right (rTLE, n=32) or left (lTLE, n=27) TLE. Clinical and demographic data of patients are summarized in Table 1.
Table 1.
Clinical details of patients groups
TLE : temporal lobe epilepsy, NMD : neuronal migration disorder
Intracarotid amobarbital procedure (IAP)
The patients were prepared for an intracarotid injection of anesthetic, typically thiopental sodium, after the cerebral angiogram. The cerebral hemispheres suspected to harbor the epileptogenic focus were always studied first. Paralysis of the contralateral arm and the arrest of finger wiggling usually indicated that the hemisphere has been anesthetized and that language and memory testing could begin. In general, language testing was performed first. The patient was asked to name objects, follow simple commands, repeat sentences, and count or name the days of the week. We recorded whether the patients showed correct naming, dysarthria or global aphasia. So, if the patients showed dysarthria when right side injection, and global aphasia when left side injection, we determined that the patients showed left language dominance. Memory items were presented in order to test memory reserves in the non-anesthetized hemisphere. Memory for the presented items was tested after complete recovery of neurological functions. To account for the different number of items used in some presentation, a percentage of retention (number of objects recalled or recognized divided by the number displayed to the patient) was calculated for the right and left hemisphere memory scores. It recommended that both injections were spaced by at least 30 minutes to avoid residual amobarbital effects in the hemisphere where functions were to be tested1,8).
MRI scan acquisition and measurements
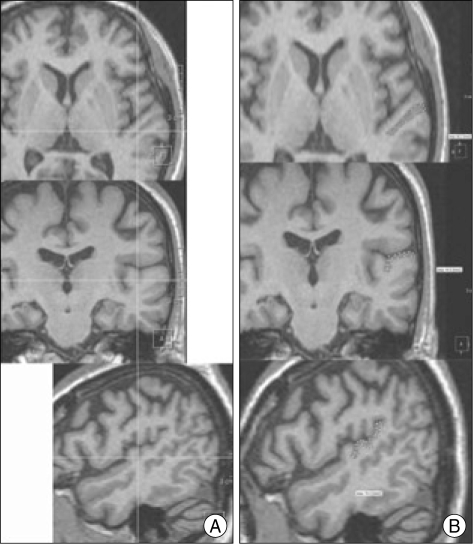
Volumetric MR images were acquired in all patients on a Siemens 1.5 Tesla system. T1 weighted images were obtained as a series of 104 to 124 coronal image sections of 2.0 mm thickness. The gray matter volumes in the PT were measured with the VoxelPlus2 program (Mevisys, Daejeon, Korea). VoxelPlus2 allows the simultaneous measurement of images in all three orthogonal planes and this enhances the ability to discern whether specific tissues are part of a particular structure (Fig. 1). Galaburda13) noted that "coronal, axial, and sagittal views were all necessary for the appropriate measurement of the PT" (p. 457). The gray matter of each of the regions of interest (ROI) was traced with a mouse-driven, computer guided cursor and the sagittal, coronal, and axial planes were simultaneously viewed. Boundaries were marked and saved in all three planes, allowing for accurate identification of the boundaries of each region. Therefore, the full gray matter volume could be measured through this three-dimensional approach, as described by Honeycutt19). The examiner of volumetry did not know results of wada test.
Fig. 1.
Display of all three orthogonal views (A). Cross-hairs indicate the same location on each of the views. The planum temporale is highlighted on all three views (B).
The planum temporale (PT) measurements
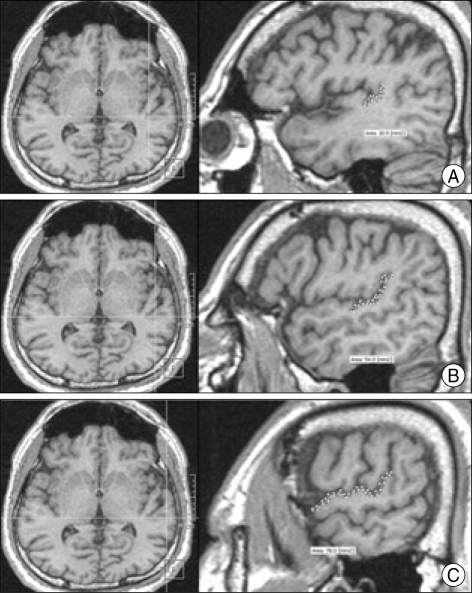
The PT is the flat plane that forms the surface of the superior temporal gyrus caudal to Heschl's gyrus (HG). The anatomic delineation of the PT on the 3D MR scan was defined by methodologies established by Steinmetz et al.34) and refined by Kulynych et al.22). The anterior border is defined by the transverse sulcus caudal to HG, and the posterior border is defined by the caudal extent of the posterior horizontal ramus as it bifurcates into the posterior ascending and descending rami. The medial tip of the roughly triangular PT is the retroinsular point where the anterior and posterior borders of the PT coincide. We defined the lateral border of the PT as the superolateral margin of the bulging rim of the superior temporal convolution. We drew the PT in the sagittal plane from medial to lateral with visualization of the axial plane according to these definitions (Fig. 2).
Fig. 2.
T1-weighted MR images demonstrating the borders of the left planum temporale in serial sagittal planes from medial to lateral side. The upper two images (A) demonstrate the most medial part of the planum temporale and the middle two images (B) demonstrate the middle part of the planum temporale. The lower two images (C) demonstrate the lateral part of the planum temporale. The horizontal line in the left axial images indicates the position of the sagittal slice.
The normal brain anatomy was characterized by one transverse gyrus on the left and two transverse gyri on the right supratemporal plane according to Pfeifer28). However, this is typically seen in only 50% of brains. Additionally, HG sometimes appears to be divided into anterior and posterior portions by an additional "transverse sulcus" running along the HG edge. These portions are characterized as two parts of HG and not as part of the PT since they appear to originate from a common medial stem. This is consistent with Pfeifer's anatomic descriptions. However, if two transverse gyri originate separately from the retroinsular region and are separated by HG, the posterior gyrus is regarded as part of the PT regardless of side.
Analyses
Asymmetry quotients (AQs) were calculated as (Left-Right)/{(Left+Right)/2} in accordance with calculations established by previous investigators. An AQ greater than or equal to 0.025 was defined as leftward asymmetry and an AQ of less than or equal to -0.025 was defined as rightward asymmetry. Symmetry is defined as greater than 5% interhemispheric differences, with a value range between -0.025 and +0.025. This is consistent with previous studies10-12,14,24). AQs were calculated for the PT in all patients.
Interobserver correlation
The volume of the PT was measured according to previous designations with the VoxelPlus2. The Planimetric volumes of the PT are listed in Table 2. The volumetric differences in the PT area measurements between two observers were not significant (p>0.05).
Table 2.
Volumes of the planum temporale in right- and left-temporal lobe epilepsy patients
*analyzed by paired t-test. TLE : temporal lobe epilepsy
RESULTS
Intracarotid amobarbital procedure (IAP)
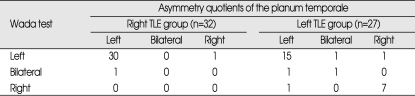
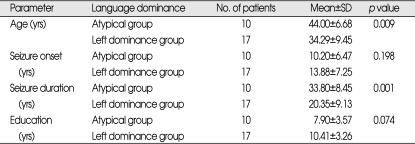
The side of speech dominance was clearly elucidated in all individuals. Language lateralization was distributed as follows in the rTLE group : 96.8% (31 of 32 individuals) had left hemispheric language dominance and 3.2% (1 of 32) had bilateral language dominance. Corresponding values in the lTLE group were 63% (17 of 27) with left hemispheric language dominance, 7.4% (2 of 27) with bilateral language dominance, and 29.6% (8 of 27) with right hemispheric language dominance (Table 3). The lTLE group showed the high incidence of atypical (right hemispheric or bilateral) language dominance pattern (about 37%). These incidences of atypical language dominance pattern in lTLE group was correlated with the age at the time of operation and the seizure duration (Table 4). In the atypical language dominance group, the mean age at the time of operation was older and the seizure duration was longer than the left language dominance group. But, there was no significant difference between two groups with respect to the age of seizure onset and education.
Table 3.
Language dominance in Wada test and volumetry
TLE : temporal lobe epilepsy
Table 4.
The comparison between left hemispheric language dominance group and atypical language dominance group in left temporal lobe epilepsy patients
SD : standard deviation
The volume of the planum temporale (PT)
The volume of the PT in rTLE and lTLE patients is shown in Table 2. The volume of the left PT was bigger than that of the right PT in rTLE patients (p<0.001). But, there was no significant difference between the right and left PT volumes in the lTLE patients (p=0.061).
Language lateralization in TLE patients according to the asymmetry quotients (AQs) of the Planum Temporale (PT)
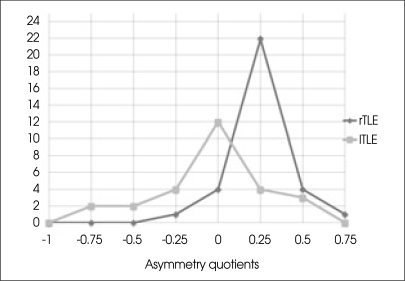
We calculated AQs after the PT measurements in all patients. The results of the volumetric analysis and the congruence between volumetric language dominance and the Wada test can be seen in Table 3. The figures in bold are representing that the results of the language lateralization for both Wada test and volumetry are the same. Overall congruence with the Wada test was 93.75% (30 of 32 individuals) in rTLE patients and 85.1% (23 of 27) in lTLE patients. Left hemispheric language dominance was present in 96.8% (31 of 32) of patients in the rTLE group, and right dominance was seen in 3.2% (1 of 32) of patients in the rTLE group. In the lTLE group, left hemispheric language dominance was present in 63% (17 of 27) of patients, bilateral dominance was present in 7.4% (2 of 27) of patients, and right dominance was present in 29.6% (8 of 27) of the patients. There was a significant leftward asymmetry of the planum temporale noted in rTLE patients (Fig. 3).
Fig. 3.
Asymmetry quotients in right and left temporal lobe epilepsy patients. This shows a significant leftward asymmetry in right temporal lobe epilepsy patients.
DISCUSSION
The determination of language dominance is important in epilepsy surgery to prevent postoperative language deficits. The intracarotid amobarbital procedure or the Wada test has been the gold standard for pre-surgical lateralization of language dominance36). However, the Wada test is an invasive test that involves an arteriogram. Previous studies have reported the risk of carotid artery dissection at 0.7%25). Further, the Wada test is not standardized across clinical centers31), has difficulties in application and interpretation3), and exhibits the waning drug effect17). Therefore, noninvasive procedures are preferred. Several studies have compared the IAP with fMRI in patients with epilepsy. In 2003, Adcock et al.2) studied 12 healthy right-handed controls and 19 right-handed preoperative patients with TLE, and found concordance between the Wada test and the fMRI results. This suggested that the fMRI method may be implemented in a similar way to the Wada test. Benke et al.6) compared the fMRI with the Wada test in 68 patients with chronic intractable right and left temporal lobe epilepsy and reported that the overall congruence between fMRI and the Wada test was 89.3% in rTLE patients and 72.5% in lTLE patients. In 1998, Choi et al.7) reported MRI-based sulcal, gyral patterns and volumetric asymmetry of inferior frontal gyrus correlate with speech lateralization of Wada test. Our volumetric analysis of the planum temporale revealed an overall congruence with the Wada test of 97.75% in rTLE patients and 81.5% in lTLE patients (Table 3). Left speech was correctly allocated in 96.8% of rTLE and in 88.2% of lTLE patients. Our results suggest that volumetry of the planum temporale as well as fMRI could serve as alternatives for the Wada test in determination of language dominance.
The PT lies between the HG and the posterior border of sylvian fissure. Flechsig9) first recognized the gross left-right asymmetry of this postero-lateral part of the supratemporal plane. Correlation between PT asymmetry and functional cerebral lateralization is a subject of extensive discussion15,16). Quantitative anatomic data have reported an asymmetry excess of the left PT in 63%14) to 82%37) of brains studied post mortem. The PT is covered by the auditory cortex, and therefore planum temporale asymmetry was thought to represent a structural substrate for speech lateralization. Geschwind and Levitsky16) found highly significant differences between the left and right hemispheres in the planum temporale. Steinmetz et al.34) first reported MR-derived PT planimetric measurements in 10 human cadaver brains and found that the left PT was larger in 8 brains. Foundas et al.10) measured the PT on MRI scans of right- and left-handed patients with selective hemispheric anesthesia or Wada testing performed for language lateralization. All subjects with language lateralized to the left hemisphere (right-handed patients) had a leftward asymmetry of the PT and one subject with language lateralized to the right hemisphere (left-handed patient) had a strong rightward asymmetry of the PT. This suggested that PT asymmetries determined by MR were associated with language dominance and could predict language laterality. To our knowledge, our study is the first to compare PT asymmetries determined by MR volumetry with the Wada test in temporal lobe epilepsy patients. A potential drawback of MR volumetry is the time involved in measurement. It takes approximately 3 to 4 hours for an experienced rater to measure both the right and left PT. However, calculation of the planum temporale volume using software for volumetry is easy and noninvasive.
Previous studies have suggested that fMRI measures may be useful in the presurgical evaluation of patients with TLE2), demonstrated that bilateral language-related fMRI activations were detected more frequently in lTLE patients than in healthy controls or in patients with rTLE20,21,33). Our results were concordant with those seen in previous studies. Left language dominance was seen in 96.8% of rTLE patients and 63% of lTLE patients (Table 3). Approximately 37% of lTLE patients showed bilateral or right hemispheric language dominance. This finding concurs with previous studies demonstrating a close relationship between atypical language dominance and lTLE patients26,30,38) and suggests that pathological processes in epilepsy exert a common set of influences on the development of language lateralization. There may be considerable plasticity of language representation in the brains of patients with intractable TLE. Sakai32) suggested that cortical plasticity for second language acquisition was guided toward first language specialization of the left inferior frontal gyrus, at least at the age of 13. Springer et al.33) suggested that atypical language dominance in the epilepsy group was associated with an earlier age of brain injury and with weaker right hand dominance. In our study, the seizure duration of the atypical language dominance group was significantly longer than the left hemispheric language dominance group in lTLE patients (Table 4). Thus, we suggested that atypical language dominance in the epilepsy group was associated with the seizure duration.
CONCLUSION
The present study confirms the usefulness of language lateralization using volumetric analysis in presurgical evaluation in temporal lobe epilepsy patients. We suggest that volumetric analysis of the PT can be an effective alternative to the Wada test in the determination of language dominance. Our data identified a close relationship between atypical language dominance and lTLE patients, and suggested the presence of considerable cerebral plasticity of language representation in the brains of patients with intractable TLE.
References
- 1.Abou-Khalil B. An update on determination of language dominance in screening for epilepsy surgery : the wada test and newer noninvasive alternatives. Epilepsia. 2007;48:442–455. doi: 10.1111/j.1528-1167.2007.01012.x. [DOI] [PubMed] [Google Scholar]
- 2.Adcock JE, Wise RG, Oxbury JM, Oxbury SM, Matthews PM. Quantitative fMRI assessment of the differences in lateralization of language-related brain activation in patients with temporal lobe epilepsy. Neuroimage. 2003;18:423–438. doi: 10.1016/s1053-8119(02)00013-7. [DOI] [PubMed] [Google Scholar]
- 3.Baxendale S, Thompson P, Duncan J, Richardson M. Is it time to replace the Wada test? Neurology. 2003;60:354–355. doi: 10.1212/wnl.60.2.354. author reply 354-355. [DOI] [PubMed] [Google Scholar]
- 4.Bell BD, Davies KG, Haltiner AM, Walters GL. Intracarotid amobarbital procedure and prediction of postoperative memory in patients with left temporal lobe epilepsy and hippocampal sclerosis. Epilepsia. 2000;41:992–997. doi: 10.1111/j.1528-1157.2000.tb00284.x. [DOI] [PubMed] [Google Scholar]
- 5.Benbadis SR, Dinner DS, Chelune GJ, Piedmonte M, Lüders HO. Objective criteria for reporting language dominance by intracarotid amobarbital procedure. J Clin Exp Neuropsychol. 1995;17:682–690. doi: 10.1080/01688639508405158. [DOI] [PubMed] [Google Scholar]
- 6.Benke T, Köylü B, Uisani P, Karner E, Brenneis C, Bartha L, et al. Language lateralization in temporal lobe epilepsy : a comparison between fMRI and the Wada Test. Epilepsia. 2006;47:1308–1319. doi: 10.1111/j.1528-1167.2006.00549.x. [DOI] [PubMed] [Google Scholar]
- 7.Choi C, Choi HY, Bae SH. MRI-based lateralization of anterior speech area. J Korean Neurosurg Soc. 1998;27:1385–1394. [Google Scholar]
- 8.Cohen-Gadol AA, Westerveld M, Alvarez-Carilles J, Spencer DD. Intracarotid Amytal memory test and hippocampal magnetic resonance imaging volumetry : validity of the Wada test as an indicator of hippocampal integrity among candidates for epilepsy surgery. J Neurosurg. 2004;101:926–931. doi: 10.3171/jns.2004.101.6.0926. [DOI] [PubMed] [Google Scholar]
- 9.Flechsig P. Bemerkungen über die Hörsphäre des menschlichen Gehirns. Neurol Zentralbl. 1908;27:2–7. [Google Scholar]
- 10.Foundas AL, Eure KF, Luevano LF, Weinberger DR. MRI Asymmetries of Broca's Area : the pars triangularis and pars opercularis. Brain Lang. 1998;64:282–296. doi: 10.1006/brln.1998.1974. [DOI] [PubMed] [Google Scholar]
- 11.Foundas AL, Faulhaber JR, Kulunych JJ, Browing CA, Weinberger DR. Hemispheric and sex-linked differences in Sylvian fissure morphology : a quantitative approach using volumetric magnetic resonance imaging. Neuropsychiatry Neuropsychol Behav Neurol. 1999;12:1–10. [PubMed] [Google Scholar]
- 12.Foundas AL, Leonard CM, Gilmore R, Fennell E, Heilman KM. Planum temporale asymmetry and language dominance. Neuropsychologia. 1994;32:1225–1231. doi: 10.1016/0028-3932(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 13.Galaburda AM. The planum temporale. Arch Neurol. 1993;50:457. doi: 10.1001/archneur.1993.00540050011007. [DOI] [PubMed] [Google Scholar]
- 14.Galaburda AM, Corsiglia J, Rosen GD, Sherman GF. Planum temporale asymmetry, reappraisal since Geschwind and Levitsky. Neuropsychologia. 1987;25:853–868. [Google Scholar]
- 15.Galaburda AM, LeMay M, Kemper TL, Geschwind N. Right-left asymmetries in the brain. Science. 1978;199:852–856. doi: 10.1126/science.341314. [DOI] [PubMed] [Google Scholar]
- 16.Geschwind N, Levitsky W. Human brain : left-right asymmetries in temporal speech region. Science. 1968;161:186–187. doi: 10.1126/science.161.3837.186. [DOI] [PubMed] [Google Scholar]
- 17.Hart J, Jr, Lesser RP, Fisher RS, Schwerdt P, Bryan RN, Gordon B. Dominant-side intracarotid amobarbital spares comprehension of word meaning. Arch Neurol. 1991;48:55–58. doi: 10.1001/archneur.1991.00530130063021. [DOI] [PubMed] [Google Scholar]
- 18.Hermann BP, Wyler AR, Somes G, Clement L. Dysnomia after left anterior temporal lobectomy without functional mapping : frequency and correlates. Neurosurgery. 1994;35:52–56. doi: 10.1227/00006123-199407000-00008. discussion 56-57. [DOI] [PubMed] [Google Scholar]
- 19.Honeycutt NA, Musick A, Barta PE, Pearlson GD. Measurement of the planum temporale (PT) on magnetic resonance imaging scans : temporal PT alone and with parietal extension. Psychiatry Res. 2000;98:103–116. doi: 10.1016/s0925-4927(00)00043-3. [DOI] [PubMed] [Google Scholar]
- 20.Knecht S, Deppe M, Ebner A, Henningsen H, Huber T, Jokeit H, et al. Noninvasive determination of language lateralization by functional transcranial Doppler sonography : a comparison with the Wada test. Stroke. 1998;29:82–86. doi: 10.1161/01.str.29.1.82. [DOI] [PubMed] [Google Scholar]
- 21.Knecht S, Dräger B, Deppe M, Bobe L, Lohmann H, Flöel A, et al. Handedness and hemispheric language dominance in healthy humans. Brain . 2000;123(Pt 12):2512–2518. doi: 10.1093/brain/123.12.2512. [DOI] [PubMed] [Google Scholar]
- 22.Kulynych JJ, Vladar K, Jones DW, Weinberger DR. Three-dimensional surface rendering in MRI morphometry : a study of the planum temporale. J Comput Assist Tomogr. 1993;17:529–535. doi: 10.1097/00004728-199307000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Langfitt JT, Rausch R. Word-finding deficits persist after left anterotemporal lobectomy. Arch Neurol. 1996;53:72–76. doi: 10.1001/archneur.1996.00550010090021. [DOI] [PubMed] [Google Scholar]
- 24.Leonard CM, Voeller KK, Lombardino LJ, Morris MK, Hynd GW, Alexander AW, et al. Anomalous cerebral structure in dyslexia revealed with magnetic resonance imaging. Arch Neurol. 1993;50:461–469. doi: 10.1001/archneur.1993.00540050013008. [DOI] [PubMed] [Google Scholar]
- 25.Loddenkemper T, Morris HH, 3rd, Perl J., 2nd Carotid artery dissection after the intracarotid armobarbital test. Neurology. 2002;59:1797–1798. doi: 10.1212/01.wnl.0000035635.30367.b6. [DOI] [PubMed] [Google Scholar]
- 26.Loring DW, Meador KJ, Lee GP, Murro AM, Smith JR, Flanigin HF, et al. Cerebral language lateralization : evidence from intracarotid amobarbital testing. Neuropsychologia. 1990;28:831–838. doi: 10.1016/0028-3932(90)90007-b. [DOI] [PubMed] [Google Scholar]
- 27.Meador KJ, Loring DW. The Wada test : controversies, concerns, and insights. Neurology. 1999;52:1535–1536. doi: 10.1212/wnl.52.8.1535. [DOI] [PubMed] [Google Scholar]
- 28.Pfeifer RA. Myelogenetisch-anatomische Untersuchungen über das kortikale Ende der Hörleitung. Abh Math-Physik K1 Sächs Akad Wiss Leipzig. 1920;37:1–54. [Google Scholar]
- 29.Pilcher WH, Roberts DW, Flanigan HF. Complications of epilepsy surgery. In: Engel J Jr, editor. Surgical Treatment of the Epilepsies. New York: Raven Press; 1993. pp. 565–581. [Google Scholar]
- 30.Rasmussen T, Milner B. The role of early left-brain injury in determining lateralization of cerebral speech functions. Ann NY Acad Sci. 1977;299:355–369. doi: 10.1111/j.1749-6632.1977.tb41921.x. [DOI] [PubMed] [Google Scholar]
- 31.Rausch R, Silfvenius H, Wieser HG, Dodrill CD, Meador KJ, Jones-Gotman M. Intraarterial amobarbital procedures. In: Engel J Jr, editor. Surgical Treatment of the Epilepsies. New York: Raven Press; 1993. pp. 341–357. [Google Scholar]
- 32.Sakai KL. Language acquisition and brain development. Science. 2005;310:815–819. doi: 10.1126/science.1113530. [DOI] [PubMed] [Google Scholar]
- 33.Springer JA, Binder JR, Hammeke TA, Swanson SJ, Frost JA, Bellgowan PS, et al. Language dominance in neurologically normal and epilepsy subject : a functional MRI study. Brain. 1999;122:2033–2046. doi: 10.1093/brain/122.11.2033. [DOI] [PubMed] [Google Scholar]
- 34.Steinmetz H, Rademacher J, Huang YX, Hefter H, Zilles K, Thron A, et al. Cerebral asymmetry : MR planimetry of the human planum temporale. J Comput Assist Tomogr. 1989;13:996–1005. [PubMed] [Google Scholar]
- 35.Steinmetz H, Volkmann J, Jäncke L, Freund HJ. Anatomical left-right asymmetry of language-related temporal cortex is different in left- and right-handers. Ann Neurol. 1991;29:315–319. doi: 10.1002/ana.410290314. [DOI] [PubMed] [Google Scholar]
- 36.Wada JA. A new method for the determination of the side of cerebral speech dominance : a preliminary report on the intracarotid injection of sodium amytal in man. Medical Biology. 1949;14:221–222. [Google Scholar]
- 37.Wada JA, Clarke R, Hamm A. Cerebral hemispheric asymmetry in humans. Cortical speech zone in 100 adult and 100 infant brains. Arch Neurol. 1975;32:239–246. doi: 10.1001/archneur.1975.00490460055007. [DOI] [PubMed] [Google Scholar]
- 38.Woods RP, Dodrill CB, Ojemann GA. Brain injury, handedness and speech lateralization in a series of amobarbital studies. Ann Neurol. 1988;23:510–518. doi: 10.1002/ana.410230514. [DOI] [PubMed] [Google Scholar]