Abstract
Objective
The purpose of this study was to identify independent predictors of mortality and functional recovery in patients with primary intracerebral hemorrhage (PICH) and to improve functional outcome in these patients.
Methods
Data were collected retrospectively on 585 patients with supratentorial PICH admitted to the Stroke Unit at our hospital between 1st January 2004 and the 31st July 2008. Using multivariate logistic regression analysis, the associations between all selected variables and 30-day mortality and 90-day functional recoveries after PICH was evaluated.
Results
Ninety-day functional recovery was achieved in 29.1% of the 585 patients and 30-day mortality in 15.9%. Age (OR=7.384, p=0.000), limb weakness (OR=6.927, p=0.000), and hematoma volume (OR=5.293, p=0.000) were found to be powerful predictors of 90-day functional recovery. Furthermore, initial consciousness (OR=3.013, p=0.014) hematoma location (lobar, OR=2.653, p=0.003), ventricular extension of blood (OR=2.077, p=0.013), leukocytosis (OR=2.048, p=0.008), alcohol intake (drinker, OR=1.927, p=0.023), and increased serum aminotransferase (OR=1.892, p=0.035) were found to be independent predictors of 90-day functional recovery after PICH. On the other hand, a pupillary abnormality (OR=4.532, p=0.000) and initial unconsciousness (OR=3.362, p=0.000) were found to be independent predictors of 30-day mortality after PICH.
Conclusion
The predictors of mortality and functional recovery after PICH identified during this analysis may assist during clinical decision-making, when advising patients or family members about the prognosis of PICH and when planning intervention trials.
Keywords: Intracerebral hemorrhage, Mortality, Outcome
INTRODUCTION
Primary intracerebral hemorrhage (PICH) accounts for 10-15% of all strokes and 78-88% of intracerebral hemorrhage35). Unlike the declining mortalities of subarachnoid hemorrhage and arteriovenous malformation associated with improvements in surgical and critical care techniques, morbidity and mortality after PICH have remained substantially unchanged for decades29). PICH is defined as bleeding into the brain parenchyma without a definite secondary cause. This intraparenchymal bleeding results from the rupture of any of the small penetrating arteries that originate from basilar arteries or from the anterior, middle, or posterior cerebral arteries28). Despite the increasing prevalence of ischemic stroke attributed to a rapid westernization of lifestyle, PICH is still relatively common in South Korea and in other Far Eastern countries23,33). Several studies have identified predictors of outcome in PICH patients4,9,16,19,27,31,36,45,46), but the majority of these have been performed in western countries or Japan. Therefore, the factors related to death and functional recovery after PICH in Koreans have not been well defined. To evaluate effective treatment strategies for PICH and to optimize the managements of individual patients in accord with established prognostic factors, it is essential that reliable predictors of death and functional outcome be identified. Using well-established potential prognostic variables of PICH obtained from the literature and the results of our analysis, we sought to identify independent predictors of death and functional recovery after PICH in the Korean population.
MATERIALS AND METHODS
Data collection
We retrospectively studied consecutive patients admitted to the Stroke Unit at our hospital (a university hospital, serving a population of 1,200,000 people) between 1st January 2004 and the 31st July 2008 with a diagnosis of PICH. For the purpose of this study, patients aged 40 and older were selected. We excluded patients younger than 40, because of the likelihood of a secondary cause of PICH. We also excluded patients with infratentorial hemorrhages, because in these patients small changes in size and location are believed to have a greater impact on survival than is the case for supratentorial hemorrhages. We extracted all patient-related data from a computerized database (PACS; mview™, Marosis Corporation). Three clinical research coordinators independently extracted and recorded patient information using a structured form. To preserve patient confidentiality, patient identifiers were omitted from the collated data set. The Ethics Committee at our hospital approved this study. The inclusion criteria applied were; a diagnosis of PICH verified by computerized tomography (CT) or magnetic resonance imaging (MRI) and admission to the stroke unit within 24 hours of symptom onset. As recommended by the Stroke Council of the American Heart Association (SCAHA)6), selected patients underwent conventional angiography to identify secondary causes of intracerebral hemorrhage. Patients with hemorrhage secondary to head trauma, a ruptured cerebral aneurysm, an arteriovenous malformation, a tumor, bleeding diathesis, or a hemorrhagic infarction were excluded. Patients with a severe neurological handicap (Modified Rankin Scale43) score 4, 5) due to previous stroke were also excluded. After applying these selection criteria, 585 patients were included in this study.
Selection of prognostic variables
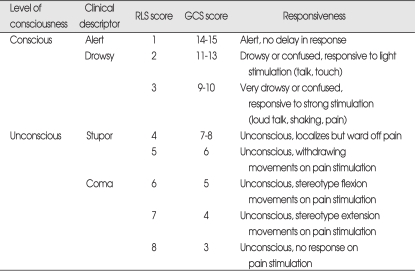
Of the baseline variables recorded, we selected the following because according to literature on the subject they were considered the most relevant potential prognostic indicators;4,9,16,27,31,36,46) age, sex, cigarette smoking and alcohol intake histories, body mass index (BMI), level of consciousness, pupillary abnormalities, limb weakness, Glasgow Coma Scale (GCS)38), hemorrhage side, location of hemorrhage, volume of hematoma, midline shift, presence of ventricular extension of hemorrhage (IVH), white blood cell (WBC) count, serum aspartate aminotransferase (AST), serum alanine aminotransferase (ALT), serum glucose, and platelet count. Blood pressure at admission was not taken into account as a prognostic variable, because it is often markedly elevated during the first 1-2 days after a severe stroke. Analysis was performed to search for relations between the selected prognostic variables and outcome. Historical features were dichotomized as present or absent; these included history of current smoking (≥10 cigarettes/day, ≥6 months), and alcohol intake (≥46 g/day, 3 days/week)18). Clinical variables included BMI (weight in kilograms divided by height in meters squared, kg/m2), pupillary abnormalities at admission (scored as 0, 1, or 2 for pupil reactivity), initial GCS score (dichotomized as 3-8 and 9-15), and limb weakness (moderate or severe hemiparesis (0-3/5)). Level of consciousness at admission was assessed using the Reaction Level Scale (RLS)37) or the GCS. For statistical analysis, patients were dichotomized as conscious or unconscious on admission (Table 1). We also extracted and dichotomized laboratory parameters on admission as elevated or not increased. These parameters included; AST, ALT, serum glucose, WBC count, and platelet count (decreased or not). All CT variables were available for analysis, that is, hemorrhage side (left, right, or central), location of hemorrhage (basal ganglia, thalamus, or lobar), volume of hematoma (mL), lateral shift of cerebral midline structures (<5 mm and ≥5 mm), and ventricular extension of the hemorrhage. Hematoma volume was estimated from the CT scans by using the formula A×B×C/2, where A is greatest diameter on the largest hemorrhage slice, B is the maximal diameter perpendicular to this, and C is the vertical depth of the hematoma24). Hematoma volumes were classified as; <30 mL, 30-59 mL, and ≥60 mL.
Table 1.
Admission consciousness levels according to the Reaction Level Scale (RLS) and the Glasgow Coma Scale (GCS)
Hospital course and assessment of outcome
Patients were treated according to the treatment algorithm used at the author's institution. Surgical intervention was undertaken in selected patients with a moderate or large amount of lobar hemorrhage and clinical deterioration, in patients with basal ganglionic or thalamic hemorrhage when hematoma volume was >30 mL, the hematoma was expanding, or when there was evidence of progressive neurological deterioration. Surgical approaches were individualized based on the site and size of the PICH. The techniques utilized included craniotomy (CO), craniectomy (CE), CT-guided stereotactic hematoma evacuation, and/or extraventricular drainage (EVD). Patients with deep seated PICH usually underwent stereotctic evacuation when hematoma volume was 30-60 mL on preoperative CT scan. After initial aspiration and evacuation of hematoma were performed, the patient was taken for repeated CT scan to check catheter placement. Injection of 6,000 U of urokinase was done through the catheter to facilitate aspiration and the CT scan was repeated 12 hours later. This process was repeated every 12 hours until 80% of hematoma was evacuated. However, patients with hemorrhages (10-30 mL) and severe neurological deficit (limb weakness) were also treated by stereotactic evacuation. Otherwise, when hematoma volume was >60 mL, hematoma evacuation was performed via craniotomy or decompressive craniectomy. The attending neurosurgeon decided whether to implant the bone flap (CO or CE) depending on the intra-operative presence of cerebral swelling after removal of PICH. EVD was done alone or combined with open surgery or stereotactic evacuation for intracranial pressure (ICP) control, particularly in the setting of hydrocephalus or intraventricular hemorrhage. When an intraventricular catheter was used to monitor ICP, CSF drainage could be accomplished by intermittent drainage for short periods in response to ICP elevation.
All patients (medical and surgical) were cared for in a neurosurgical intensive care unit until they were considered stable enough to move to a general unit. Treatment was administered according to current practices at university hospitals, but was not rigidly regimented, and primary attending neurosurgeons were allowed to exercise medical judgment. Hypertension was regulated early in the course of therapy, by setting a target maximal systolic blood pressure (SBP) to maintain SBP at under this level. During the first 24 hours, Labetalol was initial agent to keep the SBP <160 mmHg, with the understanding that sometimes labetalol can lead to bradycardia, which can be dose limiting, so we moved to hydralazine. We also used continuous intravenous antihypertensive therapy, using nicardipine or nitroprusside in the event that we were unable to control blood pressure despite using labetalol and hydralazine. During the next 48 to 72 hours, target SBP were usually set at 140 mmHg and we initiated oral antihypertensives after we achieved to maintain SBP at under this level. We also used a hypertonic agent (mannitol) when the CT scan indicated mass effect or clinical symptoms showed IICP signs. Mannitol was administered initially 0.6 to 1.0 g/kg intravenously, followed by 0.25-0.5 g/kg every 4 hours for 4 days, tapered in the next 2 days. Replacement therapies such as fresh frozen plasma, prothrombin complex concentrate, and factor IX concentrate were used to enhance hemostasis in coagulopathic PICH patients, such as those treated with warfarin. Cryoprecipitate was specifically used to enhance hemostasis in patients with hypofibrinogenemia, and aminocaproic acid, tranexamic acid, and activated recombinant factor VII (rFVIIa) were used in patients with normal coagulation. Treatment included invasive monitoring of ICP when indicated. In the presence of an ICP monitor (infrared parenchymal catheter, Camino, Integra Life Science Corporation, Plainsboro, NJ, USA), ICP elevation was defined as >20 mmHg and cerebral perfusion pressure was maintained at 70 to 100 mmHg. However, we surprisingly found that ICP monitoring was performed in a few patients, who usually showed a GCS score of 8 or lower.
Patients were followed up for 90 days after PICH, and outcomes were assessed as mortality at 30 days after symptom onset (post-PICH). The functional statuses of patients that survived for more than 90 days post-PICH were assessed using the Modified Rankin Scale (MRS)43) as follows : no symptom (MRS 0), symptomatic but no disability (MRS 1), mild disability (MRS 2), moderate disability with independent walking (MRS 3), severe disability (MRS 4), bedridden state (MRS 5), and death (MRS 6). For statistical purposes, patients were assigned to two outcome category groups, namely, the "functional recovery" group (MRS 0, 1, 2, 3), members of which were functionally independent, and the "nonfunctional recovery" group (MRS 4, 5, 6) who were functionally dependent or worse. Information on mortality and functional outcome were categorized using medical records, information obtained from family members by phone interviews, and by direct examinations of patients at our outpatient department after discharge.
Statistical analysis
Our primary aim was to investigate associations between selected variables and 30 day mortality and 90 day functional recovery. Univariate analysis was used initially to identify possible relations between outcome and each of the potential prognostic factors using the Chi-square test. Statistical significance was set at p<0.05. Subsequently, multivariate logistic regression analysis was used to identify those variables that were independently associated with functional recovery (FR) and mortality. p values of <0.05 were considered statistically significant, and the analysis was performed using SPSS Ver. 12.0 for Windows.
RESULTS
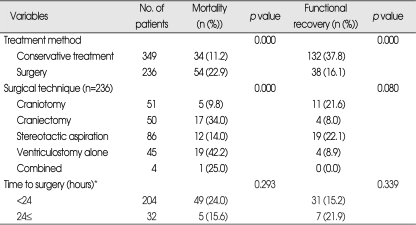
Five-hundreds and eighty-five eligible records were identified and analysed during the course of this study. Of the 585 patients, 288 (49.2%) were male and 297 (50.8%) were female. Mean patient age was 63±12 years (range 40 to 97 years). The mortality rate at 30 days after PICH was 15.9% (n=93), and 99 deaths (16.9%) occurred at ≤90 days. The FR rate at 90 days was 29.1% (n=170). Hematoma locations were basal ganglia in 55.2%, thalamus in 26.7%, and lobar in 18.1%. The mean admission GCS score was 11 (range from 3 to 15). Of the 585 patients, 236 (40.3%) underwent surgery and 349 (59.7%) received initial conservative treatment. The most frequently used surgical technique was CT-guided stereotactic hematoma evacuation (36.4%).
Thirty-day mortality
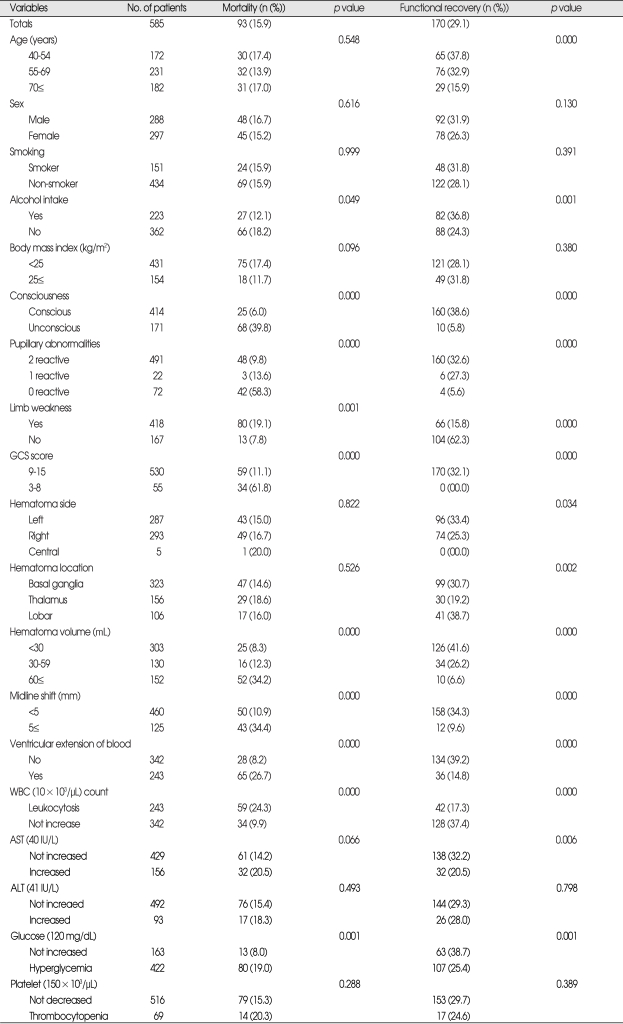
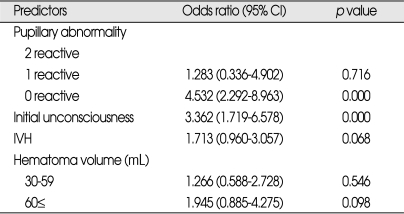
Univariate analysis showed that following variables were significantly associated with death during the first thirty days after PICH (Table 2); unconsciousness at admission, bilateral nonreactive pupils, limb weakness, a GCS score at admission of ≤8, a hematoma volume >60 mL, midline shift >5 mm, ventricular extension of blood, a WBC count >10×103/µL (leukocytosis), serum glucose level >120 mg/dL (hyperglycemia), and no history of alcohol intake (non-drinker). It was observed that those treated surgically group (a hematoma volume >60 mL and/or GCS score at admission of ≤8) had higher 30-day mortality (22.9% versus 11.2%) than those treated conservatively (p=0.000). Of the 45 patients that underwent EVD alone, 19 (42.2%) died within 30 days (p=0.000). However, time elapsed from onset to surgery (dichotomized as <24 hours and 24 hours≤) was not found to be related to outcome (Table 3). Multivariate logistic regression analysis was performed on variables identified by univariate analysis results and on those mentioned in the literature. Results are presented in Table 4. Multivariate analysis showed that a pupillary abnormality (bilateral nonreactive) and initial unconsciousness were independently associated with 30-day mortality, and that ventricular extension of blood was of borderline significance (p=0.068, OR=1.713). GCS score was found to be a highly significant predictor of 30 day mortality by univariate analysis, but because it was dependent on initial level of consciousness and possibly with hematoma volume, we did not include GCS score as a variable in the multivariate analysis to avoid multi-collinearity.
Table 2.
Univariate analysis of predictors of 30-day mortality and 90-day functional recovery after PICH (n=585)
PICH : primary intracerebral henorrhage
Table 3.
Mortality and functional recovery rates of the different treatment methods adopted
*the time elapsed from symptom onset to surgery
Table 4.
Multivariate analysis and predictors of 30-day mortality after PICH
PICH : primary intracerebral hemorrhag, IVH : ventricular extension of blood, CI : confidence interval
Ninety-day functional recovery
At 90 days, there were 486 survivors (83.1%), and of these 170 (29.1% of all patients) achieved FR (MRS 0, 1, 2, 3) and 316 (54.0%) had moderately severe to severe disability (MRS 4, 5). Of the selected variables, following were found to be significantly associated with FR by univariate analysis (Table 2); an age of ≤54, alcohol consumption (drinker), maintained initial consciousness, bilateral reactive pupils, the absence of limb weakness, a GCS score of >9, hematoma side (left), hematoma location (lobar), a hematoma volume of <30 mL, a midline shift of <5 mm, the absence of ventricular extension of blood, the absence of leukocytosis, AST (not increased), and the absence of hyperglycemia. It was also observed that surgically treated group (a hematoma volume >60 mL and/or GCS score at admission of ≤8) had a lower 90-day FR rate than those treated conservatively (16.1% versus 37.8%) (p=0.000) (Table 3).
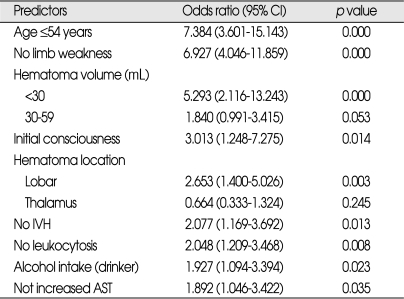
Multivariate logistic regression analysis showed that the following 9 admission variables were independently associated with FR at 90 days post-PICH (Table 5); an age of ≤54 years, absence of limb weakness, a hematoma volume of <30 ml, maintained initial consciousness, hematoma location (lobar), the absence of ventricular extension of blood, the absence of leukocytosis, alcohol consumption (drinkers), and a non-elevated AST. GCS score was found to be a highly significant predictor of 90 day FR by univariate analysis, but for the reasons mentioned above was not included in multivariate analysis.
Table 5.
Multivariate analysis and the predictors of 90-day functional recovery after PICH
PICH : primary intracerebral hemorrhag, IVH : ventricular extension of blood, CI : confidence interval
DISCUSSION
Previous surveys, have found that 30-day mortality rates range from 35% in Australia1), 44% in the United States7), and up to 51% in France15), England3), and Finland13). Surprisingly, a recent study conducted by Inagawa et al.19) in Japan returned a 30-day mortality rate after PICH of only 14%. Our overall 30-day mortality rate of 15.9% is similar to that reported by Inagawa et al., and is substantially lower than those reported in western countries17,39). The reasons for this low mortality rate are not clear, although it has been suggested that the hospital-based approach used in the present study meant that subjects who died before hospital admission were not included. Initial early withdrawal of support in the emergency room, especially for elderly and poor grade patients, and the exclusion of infratentorial PICH from data set, because infratentorial PICH is known to have a poorer outcome than supratentorial PICH, might also explain our low 30-day mortality rate.
Old age, disturbed consciousness, a low GCS score, hematoma volume, intraventricular and subarachnoid spread of blood, midline shift, narrow pulse pressure, high pulse pressure, and high blood glucose levels have all been identified as independent predictors of early death in other studies9,11,14,34,40). In the present hospital-based study, an initially disturbed consciousness and bilateral nonreactive pupils were found to be independent predictors of 30-day mortality. In particular, level of consciousness on admission has been reported to be one of the most important independent predictors of 30-day36), 1-year31), and all in hospital mortalities4). Other studies12,40) have used GCS to assess level of consciousness. In the present study, we found a similar relation between GCS and level of consciousness graded simply as conscious or unconscious, as described in the RLS11,37). In addition to initial unconsciousness, bilateral nonreactive pupils emerged as an independent predictor of 30-day mortality. However, a pupillary abnormality per se was not found to be an independent predictor of 90-day FR by multivariate logistic regression analysis.
Ventricular extension of blood (IVH) was not found to be an independent predictor of 30-day mortality, but did show borderline significance (p=0.068, OR=1.713). The literature is inconsistent regarding the predictive value of IVH, that is, some authors have demonstrated a clear association between IVH and mortality rate using multivariate analysis11,27,34,48), whereas others have failed to do so14,19,20). Nevertheless, in the present study, IVH was found to have significant role in predicting the 90-day FR. No generally accepted explanation for the adverse effects of IVH has been proposed, though it may be related to the development of obstructive hydrocephalus42) or the direct mass effect of ventricular blood on periventricular structures and associated global hypoperfusion of the overlying cortex30).
Although age was not found to independently influence mortality at 30 days, an age of <55 was found to be a strong independent predictor of FR at 90 days. A similar correlation between age and long-term (1 or 2 years), but not short-term mortality rates, has been previously reported11,14,18). Elderly people may sustain more serious neurological injuries after PICH, and in one randomized controlled trial, in which surgery was compared with medical management, it was found that the benefit of surgery in terms of quality of life is limited to patients aged less than 602). However, it is also possible that the withdrawal of care may have influenced prognosis in elderly patients5,16).
Hematoma volume was not found to be an independent predictor of 30-day mortality in the present study, whereas it has been identified as a powerful predictor of mortality in most recent studies40,48). On the other hand, hematoma volume was found to be an important independent predictor of FR in the present study, which is in-line with previous studies14,31,36). Increased intracranial pressure and cerebral edema associated with initial hematoma volume may influence outcome. However, direct comparisons between the present and previous studies are difficult, because the specific volume cut-off points used (30 mL36), 40 mL14), and 60 mL31)) and the methods of measurement varied. In the present study, lateral shift of midline structures in supratentorial hematomas was found to be closely correlated with hematoma volume, but it was not found to have any independent predictive value with respect to outcome.
It is known that alcohol intake is associated with an impaired outcome after hemorrhagic stroke8,20,47). However, unexpectedly, in the present study, patients who consumed alcohol (drinkers) were found to have a lower mortality rate (p=0.049) and a higher FR rate (p=0.001) by univariate analysis. Furthermore, alcohol consumption was found to be an independent predictor of FR by multivariate logistic regression analysis. Ikehara et al.18) recently reported that heavy alcohol consumption (>46 g of alcohol per day) is associated with increased mortality for all strokes (particularly hemorrhagic stroke), for total cardiovascular disease in men, and for coronary heart disease in women, but added that light to moderate drinking (<23 g of alcohol per day) may be associated with reduced mortality from cardiovascular disease in both sexes. Direct comparisons between our results, which were obtained in a Korean population, and Japanese or western results are difficult. We suggest that a further evaluation of the relation between alcohol intake and PICH outcome is warranted.
In the present study, multivariate analysis showed that initial consciousness is a powerful predictor of 30-day mortality and 90-day FR. In fact, 39.8% of patients that were presented in an unconscious state died within 30 days of PICH, and only 5.8% achieved FR after 90 days. Furthermore, it has also been reported on several occasions that level of consciousness is consistently associated with outcome after PICH32,44,45).
Limb paresis was not found to be an independent predictor of 30-day mortality, but was found to powerfully predict 90-day FR (p=0.000, OR=6.927). This finding has been reported previously11) but the literature is inconsistent on the topic. We believe that limb paresis is an important factor, not only because of its statistical significance, but also because of its importance as a clinical marker of tissue destruction. Furthermore, a small hematoma located in an eloquent parenchymal area obviously has the potential to cause severe hemiparesis and sustained disability.
Hematoma location was also identified as an independent predictor of 90-day FR after PICH, and patients with lobar hemorrhage (including subcortical hemorrhage) had the highest rate of FR (38.7%). However, relatively few studies have demonstrated that hematoma location is an independent prognostic factor by multivariate analysis20), though one study found that subcortical hemorrhages are associated with a better prognosis than ganglionic or thalamic hemorrhages31).
In our series, univariate analysis showed that those who underwent surgery had a significantly lower rate of 90-day FR and a higher 30-day mortality rate than those who received initial conservative treatment. However, we do not consider that these results mean that initial conservative treatment is a better treatment option than surgery in patients with PICH, because significantly more of those treated surgically had clinical signs of herniation with neurological deterioration, which is likely to have affected outcome, and because those with a lower preoperative GCS score and with large PICH amount tended to be treated surgically. It is also clear that in this retrospective study, we cannot compare the operated and non-operated groups because, by definition, there are biases of selection. For example, the choices of operation depended on the neurosurgeons' preference and early withdrawal of care was not uncommon. Nevertheless, the debate regarding surgical versus medical management is likely to persist6,29,44). However, if very early surgery can remove most of the original hemorrhage with minimal additional tissue damage, edema development and the mass effect can be reduced, white matter injury prevented, and clinical outcome improved in some patients2,41).
In the present study, initial WBCs count and serum AST were found to be independent predictors of 90-day FR by multivariate logistic regression analysis. Clark et al.10) reported that indicators of an acute phase response (interleukin-6, fibrinogen, WBC, and serum albumin) in stroke patients free of apparent infections are highly correlated with stroke recovery. Furthermore, liver enzymes are known to be associated with several cardiovascular risk factors22,25,26). Kim et al.21) reported that PICH risk in the Korean population is more closely related with aminotransferase level than with alcohol consumption. However, no reports have been issued that claim clear relationships between liver enzyme and prognosis in PICH. Accordingly, the prognostic value of WBC counts and liver enzyme levels in PICH patients evidently require further evaluation.
It was somewhat surprising to find that age, limb weakness, and hematoma volume were substantially more powerful at predicting FR (p=0.000, OR>5.0) than the other independent prognostic variables examined, despite the fact that they were not found to be independent predictors of 30-day mortality. The relationships identified during the present study could be used to answer management-oriented questions regarding PICH. During the acute stage, every effort should be made to salvage those that present in an unconsciousness state or with nonreactive pupils, regardless of age, hematoma volume, and other factors. Furthermore, for those that survive the acute stage, intensive management of the complications associated with a poor general condition and early rehabilitation are required to induce rapid functional independence. It has been suggested that early death is most directly related to hemorrhage severity, e.g., initial level of consciousness or hematoma volume, whereas long-term morbidity and mortality rates are determined more by general condition, immobilization-associated complications, and increasing age31).
It is hoped that taking into account the predictors for mortality and functional recovery identified in the present study, individual patient management can be better optimized and outcomes improved.
CONCLUSION
In this hospital-based study of Korean PICH patients, it was found that age, limb weakness, and hematoma volume much more powerfully predict FR than the other independent predictors examined, and that initial consciousness and a pupillary abnormality are independent predictors of early mortality. WBC counts and serum AST levels were also found to be independent predictors of FR after PICH, though further evaluation of these relations is needed. We hope that the predictors of mortality and FR after PICH in the Korean population identified in this study will assist during clinical decision-making, when advising patients or family members regarding prognosis, and during the implementation of intervention trials in PICH.
References
- 1.Anderson CS, Chakera TM, Stewart-Wynne EG, Jamrozik KD. Spectrum of primary intracerebral haemorrhage in Perth, Western Australia, 1989-90 : incidence and outcome. J Neurol Neurosurg Psychiatry. 1994;57:936–940. doi: 10.1136/jnnp.57.8.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Auer LM, Deinsberger W, Niederkorn K, Gell G, Kleinert R, Schneider G, et al. Endoscopic surgery versus medical treatment for spontaneous intracerebral hematoma : a randomized study. J Neurosurg. 1989;70:530–535. doi: 10.3171/jns.1989.70.4.0530. [DOI] [PubMed] [Google Scholar]
- 3.Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. A prospective study of acute cerebrovascular disease in the community : the Oxfordshire Community Stroke Project-1981-86. 2. Incidence, case fatality rates and overall outcome at one year of cerebral infarction, primary intracerebral and subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry. 1990;53:16–22. doi: 10.1136/jnnp.53.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becker KJ, Baxter AB, Bybee HM, Tirschwell DL, Abouelsaad T, Cohen WA. Extravasation of radiographic contrast is an independent predictor of death in primary intracerebral hemorrhage. Stroke. 1999;30:2025–2032. doi: 10.1161/01.str.30.10.2025. [DOI] [PubMed] [Google Scholar]
- 5.Becker KJ, Baxter AB, Cohen WA, Bybee HM, Tirschwell DL, Newell DW, et al. Withdrawal of support in intracerebral hemorrhage may lead to self-fulfilling prophecies. Neurology. 2001;56:766–772. doi: 10.1212/wnl.56.6.766. [DOI] [PubMed] [Google Scholar]
- 6.Broderick JP, Adams HP, Jr, Barsan W, Feinberg W, Feldmann E, Grotta J, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 1999;30:905–915. doi: 10.1161/01.str.30.4.905. [DOI] [PubMed] [Google Scholar]
- 7.Broderick JP, Brott T, Tomsick T, Miller R, Huster G. Intracerebral hemorrhage more than twice as common as subarachnoid hemorrhage. J Neurosurg. 1993;78:188–191. doi: 10.3171/jns.1993.78.2.0188. [DOI] [PubMed] [Google Scholar]
- 8.Charness ME, Simon RP, Greenberg DA. Ethanol and the nervous system. N Engl J Med. 1989;321:442–454. doi: 10.1056/NEJM198908173210706. [DOI] [PubMed] [Google Scholar]
- 9.Cheung RT, Zou LY. Use of the original, modified, or new intracerebral hemorrhage score to predict mortality and morbidity after intracerebral hemorrhage. Stroke. 2003;34:1717–1722. doi: 10.1161/01.STR.0000078657.22835.B9. [DOI] [PubMed] [Google Scholar]
- 10.Clark WM, Beamer NB, Wynn M, Coull BM. The initial acute phase response predicts long-term stroke recovery. J Stroke Cerebrovasc Dis. 1998;7:128–131. doi: 10.1016/s1052-3057(98)80139-0. [DOI] [PubMed] [Google Scholar]
- 11.Daverat P, Castel JP, Dartigues JF, Orgogozo JM. Death and functional outcome after spontaneous intracerebral hemorrhage. A prospective study of 166 cases using multivariate analysis. Stroke. 1991;22:1–6. doi: 10.1161/01.str.22.1.1. [DOI] [PubMed] [Google Scholar]
- 12.Dixon AA, Holness RO, Howes WJ, Garner JB. Spontaneous intracerebral haemorrhage : an analysis of factors affecting prognosis. Can J Neurol Sci. 1985;12:267–271. doi: 10.1017/s0317167100047144. [DOI] [PubMed] [Google Scholar]
- 13.Fogelholm R, Nuutila M, Vuorela AL. Primary intracerebral haemorrhage in the Jyväskylä region, central Finland, 1985-89 : incidence, case fatality rate, and functional outcome. J Neurol Neurosurg Psychiatry. 1992;55:546–552. doi: 10.1136/jnnp.55.7.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franke CL, van Swieten JC, Algra A, van Gijn J. Prognostic factors in patients with intracerebral haematoma. J Neurol Neurosurg Psychiatry. 1992;55:653–657. doi: 10.1136/jnnp.55.8.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giroud M, Gras P, Chadan N, Beuriat P, Milan C, Arveux P, et al. Cerebral haemorrhage in a French prospective population study. J Neurol Neurosurg Psychiatry. 1991;54:595–598. doi: 10.1136/jnnp.54.7.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hemphill JC, 3rd, Newman J, Zhao S, Johnston SC. Hospital usage of early do-not-resuscitate orders and outcome after intracerebral hemorrhage. Stroke. 2004;35:1130–1134. doi: 10.1161/01.STR.0000125858.71051.ca. [DOI] [PubMed] [Google Scholar]
- 17.Hollander M, Koudstaal PJ, Bots ML, Grobbee DE, Hofman A, Breteler MM. Incidence, risk, and case fatality of first ever stroke in the elderly population. The Rotterdam Study. J Neurol Neurosurg Psychiatry. 2003;74:317–321. doi: 10.1136/jnnp.74.3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ikehara S, Iso H, Toyoshima H, Date C, Yamamoto A, Kikuchi S, et al. Alcohol consumption and mortality from stroke and coronary heart disease among Japanese men and women : the japan collaborative cohort study. Stroke. 2008;39:2936–2942. doi: 10.1161/STROKEAHA.108.520288. [DOI] [PubMed] [Google Scholar]
- 19.Inagawa T, Shibukawa M, Inokuchi F, Tokuda Y, Okada Y, Okada K. Primary intracerebral and aneurysmal subarachnoid hemorrhage in Izumo City, Japan. Part II: management and surgical outcome. J Neurosurg. 2000;93:967–975. doi: 10.3171/jns.2000.93.6.0967. [DOI] [PubMed] [Google Scholar]
- 20.Juvela S. Risk factors for impaired outcome after spontaneous intracerebral hemorrhage. Arch Neurol. 1995;52:1193–1200. doi: 10.1001/archneur.1995.00540360071018. [DOI] [PubMed] [Google Scholar]
- 21.Kim HC, Kang DR, Nam CM, Hur NW, Shim JS, Jee SH, et al. Elevated serum aminotransferase level as a predictor of intracerebral hemorrhage : Korea medical insurance corporation study. Stroke. 2005;36:1642–1647. doi: 10.1161/01.STR.0000173404.37692.9b. [DOI] [PubMed] [Google Scholar]
- 22.Kim HC, Nam CM, Jee SH, Han KH, Oh DK, Suh I. Normal serum aminotransferase concentration and risk of mortality from liver diseases : prospective cohort study. BMJ. 2004;328:983. doi: 10.1136/bmj.38050.593634.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korean Neurological Association. Korean Neurological Association Epidemiology of cerebrovascular disease in Korea :a collaborative study, 1989-1990. J Korean Med Sci. 1993;8:281–289. doi: 10.3346/jkms.1993.8.4.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kothari RU, Brott T, Broderick JP, Barsan WG, Sauerbeck LR, Zuccarello M, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996;27:1304–1305. doi: 10.1161/01.str.27.8.1304. [DOI] [PubMed] [Google Scholar]
- 25.Lee DH, Ha MH, Christiani DC. Body weight, alcohol consumption and liver enzyme activity--a 4-year follow-up study. Int J Epidemiol. 2001;30:766–770. doi: 10.1093/ije/30.4.766. [DOI] [PubMed] [Google Scholar]
- 26.Lee DH, Jacobs DR, Jr, Gross M, Kiefe CI, Roseman J, Lewis CE, et al. Gamma-glutamyltransferase is a predictor of incident diabetes and hypertension : the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Clin Chem. 2003;49:1358–1366. doi: 10.1373/49.8.1358. [DOI] [PubMed] [Google Scholar]
- 27.Lisk DR, Pasteur W, Rhoades H, Putnam RD, Grotta JC. Early presentation of hemispheric intracerebral hemorrhage : prediction of outcome and guidelines for treatment allocation. Neurology. 1994;44:133–139. doi: 10.1212/wnl.44.1.133. [DOI] [PubMed] [Google Scholar]
- 28.MacWalter RS, Ersoy Y, Wolfson DR. Cerebral Haemorrhage. Parenchymal intracranial haemorrhage. Gerontology. 2001;47:119–130. doi: 10.1159/000052785. [DOI] [PubMed] [Google Scholar]
- 29.Manno EM, Atkinson JL, Fulgham JR, Wijdicks EF. Emerging medical and surgical management stratiegies in the evaluation and treatment of intracerebral hemorrhage. Mayo Clin Proc. 2005;80:420–433. doi: 10.4065/80.3.420. [DOI] [PubMed] [Google Scholar]
- 30.Mayer SA, Kessler DB, Van Heertum RL, Thomas CE, Fink ME, Brannigan T. Effect of intraventricular blood on global cortical perfusion in acute intracerebral hemorrhage : a single-photon emission computed tomographic study. Ann Neurol. 1995;38:288. [Google Scholar]
- 31.Nilsson OG, Lindgren A, Brandt L, Säveland H. Prediction of death in patients with primary intracerebral hemorrhage : a prospective study of a defined population. J Neurosurg. 2002;97:531–536. doi: 10.3171/jns.2002.97.3.0531. [DOI] [PubMed] [Google Scholar]
- 32.Paillas JE, Alliez B. Surgical treatment of spontaneous intracerebral hemorrhage. Immediate and long-term results in 250 cases. J Neurosurg. 1973;39:145–151. doi: 10.3171/jns.1973.39.2.0145. [DOI] [PubMed] [Google Scholar]
- 33.Park HS, Kang MJ, Huh JT. Recent epidemiological trends of stroke. J Korean Neurosurg Soc. 2008;43:16–20. doi: 10.3340/jkns.2008.43.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Portenoy RK, Lipton RB, Berger AR, Lesser ML, Lantos G. Intracerebral haemorrhage : a model for the prediction of outcome. J Neurol Neurosurg Psychiatry. 1987;50:976–979. doi: 10.1136/jnnp.50.8.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF. Spontaneous intracerebral hemorrhage. N Engl J Med. 2001;344:1450–1460. doi: 10.1056/NEJM200105103441907. [DOI] [PubMed] [Google Scholar]
- 36.Roquer J, Rodríguez Campello A, Gomis M, Ois A, Puente V, Munteis E. Previous antiplatelet therapy is an independent predictor of 30-day mortality after spontaneous supratentorial intracerebral hemorrhage. J Neurol. 2005;252:412–416. doi: 10.1007/s00415-005-0659-5. [DOI] [PubMed] [Google Scholar]
- 37.Starmark JE, Stålhammar D, Holmgren E. The Reaction Level Scale (RLS85). Manual and guidelines. Acta Neurochir (Wine) 1988;91:12–20. doi: 10.1007/BF01400521. [DOI] [PubMed] [Google Scholar]
- 38.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 39.Thrift AG, Dewey HM, Macdonell RA, McNeil JJ, Donnan GA. Incidence of the major stroke subtypes : initial findings from the North East Melbourne stroke incidence study (NEMESIS) Stroke. 2001;32:1732–1738. doi: 10.1161/01.str.32.8.1732. [DOI] [PubMed] [Google Scholar]
- 40.Tuhrim S, Dambrosia JM, Price TR, Mohr JP, Wolf PA, Heyman A, et al. Prediction of intracerebral hemorrhage survival. Ann Neurol. 1988;24:258–263. doi: 10.1002/ana.410240213. [DOI] [PubMed] [Google Scholar]
- 41.Tuhrim S, Dambrosia JM, Price TR, Mohr JP, Wolf PA, Hier DB, et al. Intracerebral hemorrhage : external validation and extension of a model for prediction of 30-day survival. Ann Neurol. 1991;29:658–663. doi: 10.1002/ana.410290614. [DOI] [PubMed] [Google Scholar]
- 42.Tuhrim S, Horowitz DR, Sacher M, Godbold JH. Volume of ventricular blood is an important determinant of outcome in supratentorial intracerebral hemorrhage. Crit Care Med. 1999;27:617–621. doi: 10.1097/00003246-199903000-00045. [DOI] [PubMed] [Google Scholar]
- 43.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–607. doi: 10.1161/01.str.19.5.604. [DOI] [PubMed] [Google Scholar]
- 44.Volpin L, Cervellini P, Colombo F, Zanusso M, Benedetti A. Spontaneous intracerebral hematomas : a new proposal about the usefulness and limits of surgical treatment. Neurosurgery. 1984;15:663–666. doi: 10.1227/00006123-198411000-00004. [DOI] [PubMed] [Google Scholar]
- 45.Waga S, Yamamoto Y. Hypertensive putaminal hemorrhage : treatment and results. Is surgical treatment syperior to conservative one? Stroke. 1983;14:480–485. doi: 10.1161/01.str.14.4.480. [DOI] [PubMed] [Google Scholar]
- 46.Willmot M, Leonardi-Bee J, Bath PM. High blood pressure in acute stroke and subsequent outcome: A systematic review. Hypertension. 2004;43:18–24. doi: 10.1161/01.HYP.0000105052.65787.35. [DOI] [PubMed] [Google Scholar]
- 47.Yang GY, Betz AL, Chenevert TL, Brunberg JA, Hoff JT. Experimental intracerebral hemorrhage: relationship between brain edema, blood flow, and blood-brain barrier permeability in rats. J Neurosurg. 1994;81:93–102. doi: 10.3171/jns.1994.81.1.0093. [DOI] [PubMed] [Google Scholar]
- 48.Young WB, Lee KP, Pessin MS, Kwan ES, Rand WM, Caplan LR. Prognostic significance of ventricular blood in supratentorial hemorrhage : a volumetric study. Neurology. 1990;40:616–619. doi: 10.1212/wnl.40.4.616. [DOI] [PubMed] [Google Scholar]