Abstract
Objective
It has been suggested that elevated cardiac troponin T (cTnT) level is a marker of increased risk of mortality in acute ischemic stroke and subarachnoid hemorrhage (SAH). However, the association of serum cTnT level and prognosis of intracerebral hemorrhage (ICH) has been sparsely investigated. The aim of this study was to identify the relationship between cTnT level and the outcome in patients with spontaneous ICH.
Methods
We retrospectively investigated 253 patients identified by a database search from records of patients admitted in our department for ICH between January 1, 2003 and December 31, 2007. The patients were divided into 2 groups; the patients in group 1 (n=225) with serum cTnT values of 0.01 ng/mL or less, and those in group 2 (n=28) with serum cTnT values greater than 0.01 ng/mL.
Results
The serum cTnT level was elevated in 28 patients. There were significant differences in sex, hypertension, creatine kinase-myocardial band, midline shift, side of hematoma, and presence of intraventricular hemorrhage between the 2 groups. Logistic regression analysis identified the level of consciousness on admission, cTnT and midline shift as independent predictors of hospital mortality.
Conclusion
Theses results suggest that increased serum cTnT level at admission is associated with in-hospital mortality and the addition of a serum cTnT assay to routine admission testing should be considered in patients with ICH.
Keywords: Cardiac troponin T, Intracerebral hemorrhage, Outcome
INTRODUCTION
Intracerebral hemorrhage (ICH) is associated with considerable morbidity and mortality, with estimates of 30-day mortality ranging from 10 to 52%2,24). It accounts for approximately 10-20% of all strokes6).
Cardiac troponin T (cTnT) and cardiac troponin I (cTnI) are currently the tests of choice for the detection of myocardial injury1). Elevated cTnT level and cardiac injury in patients with subarachnoid hemorrhage (SAH) has been well recognized8,16,28,30,31) and more recently they have been associated with a stunned myocardium, resulting in pump failure and congestive heart failure21). The presence of elevated values of cardiac troponin in patients with acute neurologic diseases has been associated with an adverse prognosis. In a study of a heterogeneous group of patients with strokes, head injuries, and seizures, elevated cTnI levels were observed in 19% of the patients and correlated with poor prognosis10). It has been suggested that elevated troponin may be a marker of increased mortality in patients with acute ischemic stroke9,17), although some investigators reported no relationship between cTnT and prognosis4,12). However, only a few studies have examined the relation between cTnT and prognosis of ICH, and results were largely inconclusive15,22).
In this study, we sought to identify the relationship between cTnT and outcome in patients with spontaneous ICH.
MATERIALS AND METHODS
Patient identification and exclusion
Patients were retrospectively identified by a search of neurosurgical records for ICH between January 1, 2003 and December 31, 2007. The inclusion criteria for the study were as following : 1) spontaneous ICH, 2) measurement of serum cTnT level and electrocardiography were performed within 24 hours of insult, 3) patients older than 18 years old, and 4) availability of medical records including at least one Computed Tomography (CT) scan. The exclusion criteria were infratentorial ICH, drug abuse and ICH secondary to trauma, neoplasm, vascular malformation or aneurysm, hemorrhagic transformation of infarction, and use of thrombolytic agents. Other exclusion criteria were any ischemic heart disease, including previous myocardiac infarction, symptoms suggestive of acute myocardiac infarction or unstable angina before admission, as well as previous coronary angioplasty or bypass surgery.
Clinical data collection
Clinical data, including age, sex, significant comorbid conditions, creatine kinase myocardial band isoform (CK-MB) and admission Glasgow coma scale (GCS) score were obtained by chart review. Hypertension, diabetes mellitus, and smoking history were considered significant comorbid conditions. A standard 12-lead ECG was performed at admission, and additional ECGs were obtained if there were abnormalities in rhythm or morphology. Cardiologists interpreted all of the ECGs. Outcomes were assessed at the time of death or 1 month post-ICH using the five-point GOS.
ICH evaluation
Initial cranial CT scans were evaluated in order to determine the location and volume of the hemorrhage, midline shift, and the presence of intraventricular hemorrhage (IVH). Supratentorial ICH was classified as deep (originating in the thalamus, basal ganglia, or external capsules) or lobar. The volume of the hemorrhage was estimated using the formula (ABC)/2 where A, B, and C represent the respective diameters5). The midline shift was measured as the distance from either the septum pellucidum or pineal gland to the line connecting the anterior and posterior reflections of the falx cerebri.
Troponin assay
Initial cTnT within 24 admission hours was recorded for each patient. Serum cTnT was measured from venous blood samples using the Elecsys 2010 platform (Roche Diagnostics, Mannheim, Germany). A serum cTnT value ≥0.01 ng/mL was set as the cut-off value in our laboratory.
Statistical analysis
A spreadsheet with statistical functions (Microsoft Excel; Microsoft Corp., Redmond, WA) was used for all preliminary calculations. All other analyses were performed using a statistical software package (SPSS for Windows version 10.0 : SPSS INC., Chicago, IL). Comparisons of the categorical variables were made using the Chi-square test or Fisher's exact test. Continuous variables were compared among groups using the Student's t-test. Logistic regression analyses were performed with adjustments for demographic, clinical and radiographic predictors of mortality in ICH to determine whether elevated cTnT is an independent predictor of mortality.
RESULTS
Patient characteristics
A total of 253 patients were enrolled in this study. The patients ranged in age from 23 to 88 years (mean age, 59.8 years), and 60.5% were male. The location of the supratentorial ICH was ganglionic in 159 patients, thalamic in 52, external capsular in 2, and lobar in 40. Right-sided supratentorial ICH occurred in 117 patients, intraventricular extension of ICH was found in 85 patients, and subarachnoid extension was observed in 18 patients. A cTnT assay was performed in all patients. The serum cTnT level was elevated in 28 patients.
Comparison between the 2 groups and predictors of hospital mortality
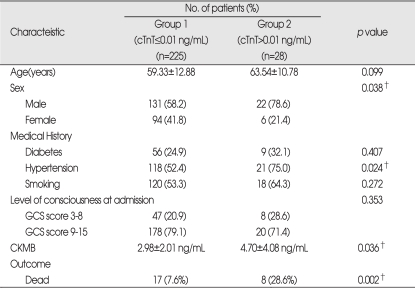
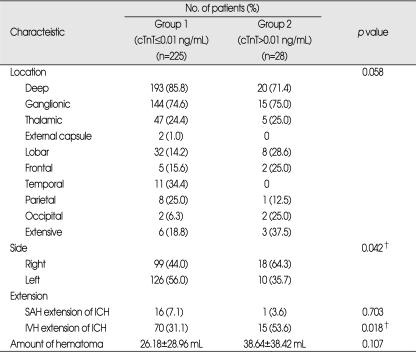
Serum cTnT levels on admission were elevated in 28 (11.1%) of the 253 patients. The patients were divided into 2 groups : the patients in group 1 had serum cTnT values of 0.01 ng/mL or less, and those in group 2 had cTnT values greater than 0.01 ng/mL. Although there was no significant difference in age, diabetes mellitus, smoking history, and level of consciousness between 2 groups, there were significant differences in sex, history of hypertension, CK-MB level. Their p values were 0.038, 0.024, and 0.036 respectively. Mortality was 7.6% in group 1 and 28.6% in group 2. Outcome differed considerably between 2 groups (Table 1). In radiologic features, there were significant differences in midline shift (p=0.041), side of hemorrhage (p=0.042), and presence of IVH (p=0.018). Amount of hematoma was 26.18±28.96 mL in group 1 and 38.64±38.42 mL in group 2. Location of hemorrhage, SAH extension, and amount did not differ between groups (Table 2).
Table 1.
Demographic and clinical characteristics of patients
*Data are presented as n (%) or mean±standard deviations, †Statistically significant difference between groups (p<0.05). cTnT=cardiac troponin, T CK-MB : creatine kinase-myocardial band isoform, GCS : Glasgow Coma Scale
Table 2.
Radiologic characteristics of patients
*Data are presented as n (%) or mean±standard deviations, †Statistically significant difference between groups (p<0.05). cTnT : cardiac troponin T, ICH : intracerebral hemorrhage, IVH : intraventricular hemorrhage, SAH : subarachnoid hemorrhage
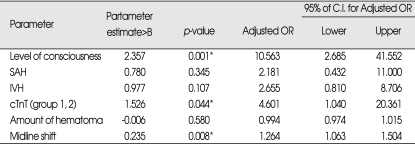
Logistic regression analysis by use of enter method, identified level of consciousness on admission, midline shift and cardiac troponin level ≥0.01 ng/mL as independent predictors of hospital mortality. In final model, dependent variables included level of consciousness on admission, SAH, IVH, cTnT, hematoma amount and midline shift and other variables were excluded because p-value was not statistically significant (p-value>0.05). When the model was applied to the data set, 92.1% of the patients were correctly classified. The parameter estimates, standard errors, odds ratios, and p-values for variables are summarized in Table 3.
Table 3.
Multivariate analysis of risk of in-hospital mortality
*Statistically significant difference between groups (p<0.05). cTnT : cardiac troponin T, IVH : intraventricular hemorrhage, OR : Odds Ratio, SAH : subarachnoid hemorrhage.
ECG changes
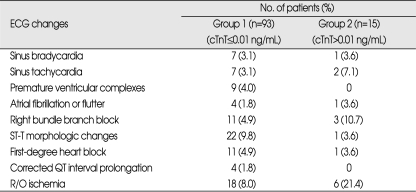
Changes in ECGs were seen in 108 patients. The common rhythm abnormalities were first-degree heart block, right bundle branch block, and premature ventricular complex in group 1 and right bundle branch block and sinus tachycardia in group 2 (Table 4). There was no significant difference in ECG changes between 2 groups, but ischemic ECG change was suspected in 6 patients of group 2.
Table 4.
Types of ECG changes
cTnT : cardiac troponin T
DISCUSSION
It is widely accepted that cTnT is a more specific marker for myocardial damage than CK-MB in patient with coronary artery disease. cTnT is one of the troponin group and a thin filament protein which takes part in muscle contraction. The troponin group consists of troponin C, troponin I and troponin T. The troponin complex on the actin filaments regulates the force and the velocity of muscle contraction. Troponin C functions as a calciumreceptor while troponin I prevents the adenosine triphosphatase activity when bound to actin. Troponin T fixes the troponin group to tropomyosin29). After myocardial cell damage, troponins are released from the myocytes. The cTnT levels are detectable in 3-12 hours after the myocardial injury, and the concentration is in direct proportion to the extent of myocardial injury. Mean time to peak cTnT level is about 12-48 hours. The concentration returns to normal range after 5-14 days. The cTnT is very cardiac specific, and it is not present in the serum following nonmyocardial muscle or other tissue damage18).
Cardiac troponin elevations have been reported in numerous conditions such as sepsis, pulmonary embolism, pericarditis, chronic renal failure, major trauma, stroke and SAH3,14,19). The presumed cause for this elevation in acute neurologic disease is related to an increase in systemic cathecolamines4). This association has not been fully described in ICH. This study shows that elevated cTnT level is associated with poor outcome in patients with spontaneous ICH.
The mechanism of cardiac dysfunction after aneurysmal SAH is not fully understood. Autopsy studies have reported that SAH results in vascular damage to hypothalamus11). Most studies implicate the hypothalamus as the primary center of dysfunction13,23). Stimulating the posterior hypothalamus and midbrain reticular formation result in ECG changes similar to those following SAH25). The assertion that cardiac alterations are mediated by catecholamine is supported by the fact that disrupting the sympathetic chain at the cervical level stops the arrhythmias, whereas a vagotomy does not20,30). These changes can be inhibited by catecholamine blocking agents7).
Another hypothesis suggests that after ischemic stroke, increased intracranial pressure or insular disinhibition leads to marked release of catecholamine by activation of central autonomic network, which can induce tachycardia, coronary vasospasm, coronary and peripheral vasoconstriction, and direct myocardial toxicity due to increased intracellular calcium22). The insula is the site for the integration of sensory, autonomic, limbic functions through its reciprocal connections with principal sensory areas, paralimbic areas in the orbital, temporopolar, and cingulate cortices, and hypothalamus26). Right hemispheric strokes are more arrhythmogenic than left-sided strokes, involvement of the insular cortex predisposes patients to sudden cardiac death1,27,32).
There are a few reports about relationship between elevated cTnT level and outcome in patients with ICH. Hays et al.15) has reported that elevated cTnI values occur frequently in ICH and are independently associated with higher in-hospital mortality but Maramattom et al.22) has demonstrated contrary result that elevation of cTnT levels do not influence the early outcome of patients with ICH. In this study we report on the relationship between cTnT and outcomes in patients with ICH. An elevated cTnT group was associated with a higher mortality compared with a normal cTnT group (28.6% vs. 7.6%, p=0.002). Level of consciousness on admission, cTnT, and midline shift were independently associated with ICH mortality in this study.
There are several limitations to our study. The most important limitations are its retrospective nature and small sample size. A future prospective trial would be useful in order to definitively ascertain whether cardiac injury plays a role in morbidity and mortality resulting from ICH. Second, this study may have included patients with occult coronary artery disease who would be more likely to have cTnT elevations during the acute phase, which would have assisted in establishing the relationship to mortality. Third, because we could not test consecutive serum cTnT levels in all patients, our study relied on single baseline serum level. A consecutive assay could have provided additional information on the development and evolution of myocardial damage in patients with ICH.
CONCLUSION
This study shows that elevated cTnT levels in patients with ICH are related to poor outcomes. We suggest that the addition of a serum cTnT assay to routine admission testing should be considered in patients with ICH. We also suggest that serial serum cTnT assessment and long-term clinical outcome data from a large prospective study would clarify the clinical implications of elevated serum cTnT levels in patients with ICH.
References
- 1.Adams JE, 3rd, Bodor GS, Dávilá-Roman VG, Delmez JA, Apple FS, Ladenson JH, et al. Cardiac troponin I. A marker with high specificity for cardiac injury. Circulation. 1993;88:101–106. doi: 10.1161/01.cir.88.1.101. [DOI] [PubMed] [Google Scholar]
- 2.Ahn CS, Lee SK, Kim HS, Kong MH, Song KY, Kang DS. Surgical outcome of spontaneous intracerebral hemorrhage in less than stuporous mental status. J Korean Neurosurg Soc. 2004;35:290–296. [Google Scholar]
- 3.Ammann P, Maggiorini M, Bertel O, Haenseler E, Joller-Jemelka HI, Oechslin E, et al. Troponin as a risk factor for mortality in critically ill patients without acute coronary syndromes. J Am Coll Cardiol. 2003;41:2004–2009. doi: 10.1016/s0735-1097(03)00421-2. [DOI] [PubMed] [Google Scholar]
- 4.Barber M, Morton JJ, Macfarlane PW, Barlow N, Roditi G, Stott DJ. Elevated troponin levels are associated with sympathoadrenal activation in acute ischemic stroke. Cerebrovasc Dis. 2007;23:260–266. doi: 10.1159/000098325. [DOI] [PubMed] [Google Scholar]
- 5.Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral heamorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke. 1993;24:987–993. doi: 10.1161/01.str.24.7.987. [DOI] [PubMed] [Google Scholar]
- 6.Caplan LR. Intracerebral hemorrhage. Lancet. 1992;339:656–658. doi: 10.1016/0140-6736(92)90804-c. [DOI] [PubMed] [Google Scholar]
- 7.Cruickshank JM, Neil-Dwyer G, Lane J. The effect of oral propranolol upon the ECG changes occurring in subarachnoid haemorrhage. Cardiovasc Res. 1975;9:236–245. doi: 10.1093/cvr/9.2.236. [DOI] [PubMed] [Google Scholar]
- 8.Deibert E, Barzilai B, Braverman AC, Edwards DF, Aiyagari V, Dacey R, et al. Clinical significance of elevated troponin I levels in patients with nontraumatic subarachnoid hemorrhage. J Neurosurg. 2003;98:741–746. doi: 10.3171/jns.2003.98.4.0741. [DOI] [PubMed] [Google Scholar]
- 9.Di Angelantonio E, Fiorelli M, Toni D, Sacchetti ML, Lorenzano S, Falcou A, et al. Prognostic significance of admission levels of troponin I in patients with acute ischaemic stroke. J Neurol Neurosurg Psychiartry. 2005;76:76–81. doi: 10.1136/jnnp.2004.041491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dixit S, Castle M, Velu RP, Swisher L, Hodge C, Jaffe AS. Cardiac involvement in patients with acute neurologic disease : confirmation with cardiac troponin I. Arch Intern Med. 2000;160:3153–3158. doi: 10.1001/archinte.160.20.3153. [DOI] [PubMed] [Google Scholar]
- 11.Doshi R, Neil-Dwyer G. A clinicopathological study of patients following a subarachnoid hemorrhage. J Neurosurg. 1980;52:295–301. doi: 10.3171/jns.1980.52.3.0295. [DOI] [PubMed] [Google Scholar]
- 12.Etgen T, Baum H, Sander K, Sander D. Cardiac troponins and N-terminal pro-brain natriuretic peptide in acute ischemic stroke do not relate to clinical prognosis. Stroke. 2005;36:270–275. doi: 10.1161/01.STR.0000151364.19066.a1. [DOI] [PubMed] [Google Scholar]
- 13.Goldfien A, Ganong WF. Adrenal medullary and adrenal cortical response to stimulation of diencephalon. Am J Physiol. 1962;202:205–211. doi: 10.1152/ajplegacy.1962.202.2.205. [DOI] [PubMed] [Google Scholar]
- 14.Hamm CW, Giannitsis E, Katus HA. Cardiac troponin elevations in patients without acute coronary syndrome. Circulation. 2002;106:2871–2872. doi: 10.1161/01.cir.0000044342.50593.63. [DOI] [PubMed] [Google Scholar]
- 15.Hays A, Diringer MN. Elevated troponin levels are associated with higher mortality following intracerebral hemorrhage. Neurology. 2006;66:1330–1334. doi: 10.1212/01.wnl.0000210523.22944.9b. [DOI] [PubMed] [Google Scholar]
- 16.Horowitz MB, Willet D, Keffer J. The use of cardiac troponin-I (cTnI) to determine the incidence of myocardial ischemia and injury in patients with aneurysmal and presumed aneurysmal subarachnoid hemorrhage. Acta Neurochir (Wien) 1998;140:87–93. doi: 10.1007/s007010050063. [DOI] [PubMed] [Google Scholar]
- 17.James P, Ellis CJ, Whitlock RM, McNeil AR, Henley J, Anderson NE. Relation between troponin T concentration and mortality in patients presenting with an acute stroke : observational study. BMJ. 2000;320:1502–1504. doi: 10.1136/bmj.320.7248.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karim MA, Shinn MS, Oskarsson H, Windle J, Deligonul U. Significance of cardiac troponin T release after percutaneous transluminal coronary angioplasty. Am J Cardiol. 1995;76:521–523. doi: 10.1016/s0002-9149(99)80144-1. [DOI] [PubMed] [Google Scholar]
- 19.King DA, Codish S, Novack V, Barski L, Almog Y. The role of cardiac troponin I as a prognosticator in critically ill medical patients : a prospective observational cohort study. Crit Care. 2005;9:R390–R395. doi: 10.1186/cc3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klouda MA, Brynjolfsson G. Cardiotoxic effects of electrical stimulation of the stellate ganglia. Ann N Y Acad Sci. 1969;156:271–280. doi: 10.1111/j.1749-6632.1969.tb16733.x. [DOI] [PubMed] [Google Scholar]
- 21.Kono T, Morita H, Kuroiwa T, Onaka H, Takatsuka H, Fujiwara A. Left ventricular wall motion abnormalities in patients with subarachnoid hemorrhage : neurogenic stunned myocardium. J Am Coll Cardiol. 1994;24:636–640. doi: 10.1016/0735-1097(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 22.Maramattom BV, Manno EM, Fulgham JR, Jaffe AS, Wijdicks EF. Clinical importance of cardiac troponin release and cardiac abnormalities in patients with supratentorial cerebral hemorrhages. Mayo Clin Proc. 2006;81:192–196. doi: 10.4065/81.2.192. [DOI] [PubMed] [Google Scholar]
- 23.Marion DW, Segal R, Thompson ME. Subarachnoid hemorrhage and the heart. Neurosurgery. 1986;18:101–106. doi: 10.1227/00006123-198601000-00019. [DOI] [PubMed] [Google Scholar]
- 24.Martí-Fábregas J, Belvís R, Guardia E, Cocho D, Muñoz J, Marruecos L, et al. Prognostic value of Pulsatility index in acute intra-cerebral hemorrhage. Neurology. 2003;61:1051–1056. doi: 10.1212/01.wnl.0000090520.67254.14. [DOI] [PubMed] [Google Scholar]
- 25.Melville KI, Blum B, Shister HE, Silver MD. Cardiac ischemic changes and arrhythmias induced by hypothalamic stimulation. Am J Cardiol. 1963;12:781–791. doi: 10.1016/0002-9149(63)90281-9. [DOI] [PubMed] [Google Scholar]
- 26.Mesulam MM, Mufson EJ. Insula of the old world monkey. III : Efferent cortical output and comments on function. J Comp Neurol. 1982;212:38–52. doi: 10.1002/cne.902120104. [DOI] [PubMed] [Google Scholar]
- 27.Oppenheimer SM, Gelb A, Girvin JP, Hachinski VC. Cardiovascular effects of human insular cortex stimulation. Neurology. 1992;42:1727–1732. doi: 10.1212/wnl.42.9.1727. [DOI] [PubMed] [Google Scholar]
- 28.Parekh N, Venkatesh B, Cross D, Leditschke A, Atherton J, Miles W, et al. Cardiac troponin I predicts myocardial dysfunction in aneurysmal subarachnoid hemorrhage. J Am Coll Cardiol. 2000;36:1328–1335. doi: 10.1016/s0735-1097(00)00857-3. [DOI] [PubMed] [Google Scholar]
- 29.Saba Z, Nassar R, Ungerleider RM, Oakeley AE, Anderson PA. Cardiac troponin T isoform expression correlates with pathophysiological descriptors in patients who underwent ocrrective surgery for congenital heart disease. Circulation. 1996;94:472–476. doi: 10.1161/01.cir.94.3.472. [DOI] [PubMed] [Google Scholar]
- 30.Sato K, Masuda T, Izumi T. Subarachnoid hemorrhage and myocardial damage clinical and experimental studies. Jpn Heart J. 1999;40:683–701. doi: 10.1536/jhj.40.683. [DOI] [PubMed] [Google Scholar]
- 31.Tung P, Kopelnik A, Banki N, Ong K, Ko N, Lawton MT, et al. Predictors of neurocardiogenic injury after subarachnoid hemorrhage. Stroke. 2004;35:548–551. doi: 10.1161/01.STR.0000114874.96688.54. [DOI] [PubMed] [Google Scholar]
- 32.Yasui Y, Breder CD, Saper CB, Cechetto DF. Autonomic responses and efferent pathways from the insular cortex in the rat. J Comp Neurol. 1991;303:355–374. doi: 10.1002/cne.903030303. [DOI] [PubMed] [Google Scholar]