Abstract
Cerebral embolic infarction is the most common neurologic complication of cardiac myxoma (CM). Development of cerebral aneurysms in CM is very rare. We present a 64-year-old woman with acute cerebral infarction and multiple cerebral aneurysms complicated by CM. The aneurysms were multiple, fusiform-shaped, and located in distal branch of major cerebral arteries. The serum interleukin (IL)-6 was highly elevated, which was normalized after surgical resection of CM. There was no regression of aneurysms on follow-up neuroimaging. Multiple cerebral aneurysms in CM are rare condition. Highly elevated serum IL-6 may be associated with increased risk of cerebral aneurysmal formation.
Keywords: Myxoma, Aneurysm, Interleukin-6
INTRODUCTION
Cardiac myxoma (CM) is the most common primary tumor of the heart6). It frequently develops from the third to the sixth decades of life and has a female predominance. The characteristic symptoms of CM are cardiac (dyspnea, syncope), constitutional (fever, weight loss) and neurological manifestation. Systemic embolism has been reported in 30-50% of patients, half of which had cerebral infarction7). The CM is a major cardioembolic source of ischemic stroke. However, formation of cerebral aneurysm has been rarely reported in patients with CM.
We present a case with embolic infarction and multiple fusiform-cerebral aneurysms complicated by CM, and discuss the possible mechanism.
CASE REPORT
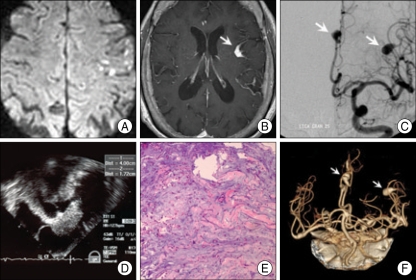
A 64-year-old woman was admitted for dysarthria, generalized weakness, and gait disturbance for 1 day. She had no medical problem except hypertension for 8 years. She complained of fatigability, malaise and weight loss for several months. There were no abnormalities on physical examination including respiratory and cardiac function. On neurological examination, her mental status was alert and cognitive function was normal. There were no abnormalities on cranial nerve examination. She had dysarthria and motor weakness on both arms (motor strength grade G4+/G4+). Sensory function was not remarkable. There was clumsiness and dysmetria on both hands, and bilateral sway on tandem gait. On admission, non-contrast computed tomography (CT) of brain was normal. Brain magnetic resonance image (MRI) on 4 days after admission showed acute multifocal embolic infarctions at bilateral cerebral hemisphere and cerebellum on (Fig. 1A). Additionally, multiple, dilated intracranial vessels at left frontal, parasagittal and left temporal cortex was observed on T1-weighted image after gadolinium injection, (Fig. 1B). Digital subtraction angiography showed multiple fusiform-cerebral aneurysms at distal branches of anterior cerebral arteries (ACA) and middle cerebral arteries (MCA) (Fig. 1C). On transthoracic echocardiography, there was a mass in left atrium. The ejection fraction was 54% and functions of mitral valve were normal on routine echocardiography. Transesophageal echocardiography (TEE) demonstrated a huge, irregular shaped mass (4.0×1.7 cm) with heterogeneous echogeneity in left atrium, attaching to inter-atrial septum (Fig. 1D). There was no intracardiac thrombus in left atrium. The result of routine chemistry was not remarkable but highly elevated serum interleukin (IL)-6 level (120 pg/mL, normal range : <8 pg/mL) was noted.
Fig. 1.
A : Multiple embolic infarctions at bilateral cerebral hemispheres on diffusion-weighted image. B : Enlarged enhanced vessel at surface of left frontal cortex (arrow) on T1-weighted image after gadolinium injection. C : Multiple fusiform aneurysms (arrows) at distal branches of anterior cerebral arteries and middle cerebral arteries on conventional cerebral angiography. D : A huge irregular shaped mass (4.0×1.7 cm) in left atrium on transesophageal echocardiograpgy. E : Prominent acid-mucopolysaccharides with interspersed round, plump and stellate-appearing mesenchymal cells on histology (H&E×100). F : No change of aneurysms (arrows) in size and number on brain computed tomographic angiography after 6 months.
Treatments with intravenous (IV) heparin and aspirin (Astrix® 100 mg, once daily) were started. The target range of activated partial thromboplastin time (aPTT) was from 60 to 80 sec during heparinization. Seven days after admission, IV heparin was switched to oral anticoagulation. The motor weakness and cerebellar ataxia was gradually recovered but mild dysarthria still persisted (modified Rankin scale=1). The mass was surgically removed 3 weeks after admission. On pathology, the tumor consisted of prominent acid-mucopolysaccharides with interspersed round, plump and stellate-appearing mesenchymal cells, sometimes in a cord-like arrangement, which was compatible with myxoma (Fig. 1E).
She regained full health without neurologic sequelae (modified Rankin's score=0). The oral anticoagulation was maintained with adjustment of international normalized ratio of prothrombin time (2.0-2.4). The serum IL-6 level one month after admission was normalized (<8 pg/mL), and continued to be within normal range at 6 months. After 6 months after admission, there was neither recurrence of CM on TEE, nor change of cerebral aneurysm in size or number on CT angiography (Fig. 1F).
DISCUSSION
The present case developed acute cerebral infarction and multiple fusiform-cerebral aneurysms complicated by CM. Multiple fusiform-cerebral aneurysms are rarely found in choriocarcinoma, infective endocarditis or cardiac myxoma. The true incidence of cerebral aneurysm in CM is still uncertain because of the rarity of disease. Recent study reported that 12% of total 74 cases with CM had neurological complication, all of which had cerebral embolic infarction2). Only one case (1.4%) had concurrent cerebral aneurysm in addition to embolic infarction, indicating that development of cerebral aneurysms in CM a rare. In meta-analysis of 34 reported cases with cerebral aneurysm associated with CM,7) cerebral aneurysms were usually multiple (median number of aneurysm=3), were of fusiformshaped (91%), and were located in distal branches of both sides of middle cerebral artery (74%). Cardiac and constitutional manifestation was found in 40% and 25% of cases, respectively. Although the pathogenesis of aneurysm formation in CM is not fully understood, radiological characteristics of cerebral aneurysms associated with CM are similar to those of emboli in choriocarcinoma or infective endocarditis, suggesting that aneurismal formation in CM is not caused by intracerebral hemodynamic change, but direct myxomatous cell invasion into the vessel wall2). Embolization of the myxomatous cell to the intracranial vasculature occurred, with tumor cells lodging in the distal intracranial arteries10). With the ability of mitotic and proliferating activity within the vessel, myxomatous cells can penetrate the vessel wall and infiltrate the internal elastic lamina, subsequently leading to weakening of the subintimal wall. The weakened vessel results in aneurysmal formation1). This hypothesis is supported by pathological study showing penetration of a vessel wall and entering the surrounding neural tissue by myxomatous cells6).
Recent studies suggest that autocrine production of IL-6 by myxoma plays a pivotal role in distal embolization of myxomatous cell8). IL-6 is a cytokine that produces differentiation and proliferation of cells, and induces acute phase responses. The IL-6 was highly expressed in the myxomatous cells in an immunohistochemical study3). Serum IL-6 level is usually elevated in patients with CM, and is normalized immediately after surgical removal of CM5). Mendoza, et al.3) demonstrated that there was a positive correlation between serum IL-6 level and size of CM, and that overproduction of IL-6 by myxomatous cell was associated with tumor recurrence or distal embolization3). IL-6 induces overexpression of several proteolytic enzymes, such as metalloproteinase, that degrade the extracellular matrix (ECM), then promoting fragmentation of tumor and acceleration of the embolic event4). Our patient showed very high level of serum IL-6, and was also rapidly normalized after tumor removal.
Formation of cerebral aneurysm may be also associated with overproduction of IL-6 by tumor emboli that induce degradation of ECM in cerebral vessels. Yaguchi, et al.9) found that IL-6 level in cerebrospinal fluid (CSF) as well as in serum was elevated in a patient with cerebral aneurysm associated with CM. The author concluded that high level of IL-6 in CSF represented overproduction of IL-6 by myxomatous cell in the intracranial vessel. This finding provides the critical role of IL-6 produced by intracerebral myxomatous emboli for cerebral aneurysm formation. Although further studies are needed to clarify this hypothesis, we suggest that IL-6 influences cerebral aneurysmal formation directly (promoting tumor invasion in to the intracranial artery) or indirectly (increasing the chance of distant embolization of CM).
Although cardiac surgery is the first choice of treatment for prevention of distant embolization of tumor in CM, there has been no recommendation for treatment of cerebral aneurysm in CM. Because of rarity of reports about sequential angiographic findings of aneurysm, it is still unclear whether cerebral aneurysms regress or progress after cardiac surgery. Sequential angiographic study in some patients revealed that the time course of aneurysm formation and growth is highly variable. No further growth of aneurysms after cardiac surgery was reported in several cases including our case, but persistent growth of aneurysm despite successful removal of CM was also reported in a few cases7). This suggests that surgical resection of primary CM cannot completely abolish the risk of delayed cerebral aneurysm formation in some cases, because of preceding intracranial metastasis of myxomatous cell before surgery. Therefore, sequential monitoring of aneurysm is needed, especially in patients with persistent growth of aneurysm. Although further investigation are warranted to clarify the clinical relevance of serum IL-6 levels, it may be a helpful biomarker for the diagnosis or monitoring of the time course of cerebral aneurysms.
CONCLUSION
We present a case with multiple fusiform-cerebral aneurysms complicated by CM. Cerebral aneurysm is a very rare condition in CM. Highly elevated serum IL-6 may be associated with increased risk of cerebral aneurismal formation in CM. The serum IL-6 level may be applied as a surrogate marker for distant embolization of tumor or cerebral aneurysm in CM.
References
- 1.Furuya K, Sasaki T, Yoshimoto Y, Okada Y, Fujimaki T, Kirino T. Histologically verified cerebral aneurysm formation secondary to embolism from cardiac myxoma. Case report. J Neurosurg. 1995;83:170–173. doi: 10.3171/jns.1995.83.1.0170. [DOI] [PubMed] [Google Scholar]
- 2.Lee VH, Connolly HM, Brown RD., Jr Central nervous system manifestations of cardiac myxoma. Arch Neurol. 2007;64:1115–1120. doi: 10.1001/archneur.64.8.1115. [DOI] [PubMed] [Google Scholar]
- 3.Mendoza CE, Rosado MF, Bernal L. The role of interleukin-6 in cases of cardiac myxoma. Clinical features, immunologic abnormalities, and a possible role in recurrence. Tex Heart Inst J. 2001;28:3–7. [PMC free article] [PubMed] [Google Scholar]
- 4.Orlandi A, Ciucci A, Ferlosio A, Pellegrino A, Chiariello L, Spagnoli LG. Increased expression and activity of matrix metalloproteinases characterize embolic cardiac myxomas. Am J Pathol. 2005;166:1619–1628. doi: 10.1016/S0002-9440(10)62472-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parissis JT, Mentzikof D, Georgopoulou M, Gikopoulos M, Kanapitsas A, Merkouris K, et al. Correlation of interleukin-6 gene expression to immunologic features in patients with cardiac myxomas. J Interferon Cytokine Res. 1996;16:589–593. doi: 10.1089/jir.1996.16.589. [DOI] [PubMed] [Google Scholar]
- 6.Pinede L, Duhaut P, Loire R. Clinical presentation of left atrial cardiac myxoma. A series of 112 consecutive cases. Medicine (Baltimore) 2001;80:159–172. doi: 10.1097/00005792-200105000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Sabolek M, Bachus-Banaschak K, Bachus R, Arnold G, Storch A. Multiple cerebral aneurysms as delayed complication of left cardiac myxoma : a case report and review. Acta Neurol Scand. 2005;111:345–350. doi: 10.1111/j.1600-0404.2005.00413.x. [DOI] [PubMed] [Google Scholar]
- 8.Wada A, Kanda T, Hayashi R, Imai S, Suzuki T, Murata K. Cardiac myxoma metastasized to the brain : potential role of endogenous interleukin-6. Cardiology. 1993;83:208–211. doi: 10.1159/000175971. [DOI] [PubMed] [Google Scholar]
- 9.Yaguchi H, Murakami Y, Sengoku R, Sato H, Inoue K. [A case of cardiac myxoma presenting with multiple cerebellar hemorrhages and elevation of interleukin-6 in the cerebrospinal fluid] Rinsho Shinkeigaku. 2004;44:677–681. [PubMed] [Google Scholar]
- 10.Yilmaz MB, Akin Y, Güray U, Kisacik HL, Korkmaz S. Late recurrence of left atrial myxoma with multiple intracranial aneurysms. Int J Cardiol. 2003;87:303–305. doi: 10.1016/s0167-5273(02)00348-0. [DOI] [PubMed] [Google Scholar]