Abstract
Background. The results of acute type A dissection (AAD) surgery in the Netherlands are largely unknown, as was recently stated in a report by the Health Council of the Netherlands. In order to gain more insight into the Dutch situation we investigated predictors of in-hospital mortality of surgically treated AAD patients and assessed threeyear survival.
Methods. 104 consecutive patients undergoing surgery for AAD in a 16-year period (1990–2006) were evaluated. Preoperative and intraoperative variables were analysed to identify predictors of early mortality.
Results. Preoperative malperfusion (limb ischaemia or mesenteric ischaemia) was present in 15.4%, shock in 18.3%, and 6.7% were operated under cardiac massage. Marfan syndrome was present in four patients and four patients had a bicuspid aortic valve. In-hospital mortality was 22.1%. Seven patients died intraoperatively; other causes of inhospital mortality were major brain damage in ten patients, multiple organ failure in three patients, low cardiac output in two patients and sudden cardiac death in one patient. Multivariate logistic regression revealed preoperative malperfusion (p=0.004) to be the only independent predictor of in-hospital mortality. Three-year survival was 68.8±4.7% (including hospital mortality). Hospital survivors had a three-year survival of 88.3±3.9%.
Conclusion. In-hospital mortality of our patients (22.1%) is comparable with the results of larger case series published in the literature. Prognosis after successful surgical treatment is relatively good with a three-year survival of 88.3% in our series. (Neth Heart J 2009;17:226-31.)
Keywords: acute type A aortic dissection, in-hospital mortality
International studies report an in-hospital mortality between 10 to 25% for surgically treated acute type A dissection (AAD).1–3 The results of AAD surgery in the Netherlands, however, are largely unknown. In a report in January 2007, the Health Council of the Netherlands stated that there is insufficient insight into the results of aortic surgery in the Netherlands, which includes the surgical treatment of AAD.4 The St Antonius Hospital in Nieuwegein constitutes an exception as they have frequently published about their surgical experience with AAD patients and report an in-hospital mortality of approximately 20%.2,5 We sought to contribute to a better understanding of the situation in the Netherlands by investigating predictors of in-hospital mortality and assessing threeyear survival of surgically treated AAD patients in our institution.
Patients and methods
All patients who were surgically treated for AAD (Stanford classification type A) at the Department of Cardiothoracic Surgery of the University Medical Center Groningen (UMCG), Groningen, the Netherlands, between January 1990 and December 2006, were included (n=104). If chest pain or related symptoms were present less then 14 days before operation, aortic dissection was defined as acute. All subacute and chronic dissections were excluded. Information regarding presentation, treatment and follow-up was systematically obtained by retrospective review of hospital records. In addition information regarding follow-up was also obtained via the attending cardiologist or telephone contact with general physicians, patients and/or relatives of patients.
Operative technique
During the 16-year period in which the patients underwent surgery, different surgical techniques were used. The general operative approach that we used was as follows.
After establishing the diagnosis, patients underwent median sternotomy followed by cannulation of the femoral artery and the right atrium. Other arterial cannulation sites used were the iliac artery, ascending aorta, aortic arch and the subclavian artery, depending on patient characteristics and preference of the surgeon. Cooling was started immediately after cardiopulmonary bypass was instituted. The aorta was crossclamped just proximal of the innominate artery following induction of ventricular fibrillation. The left ventricle was vented through the right superior pulmonary vein. The ascending aorta was opened longitudinally and the intimal tear located. If the intimal tear extended through the aortic clamp, no intimal tear could be located in the ascending aorta, the quality of the aorta at the site of the distal anastomosis was unfavourable or the surgeon preferred to perform an open distal anastomosis, the patient was further cooled down. In the meantime the proximal aorta was reconstructed. In the early 1990s felt was used to reinforce the fragile tissue of the proximal aorta; later in the series also glue (GFR® or Bioglue®) was used. When the dissection process had caused aortic valve regurgitation we attempted to preserve the valve by re-suspension of the commissures but the valve was replaced if the cusps showed severe morphological alteration. In selected cases a modified Bentall procedure or a valve sparing root replacement was applied according to techniques described by Tirone David.6
Until the year 2000 we used deep hypothermic circulatory arrest (DHCA) or in a few cases a circulatory arrest combined with retrograde selective cerebral perfusion (RSCP) during inspection of the aortic arch. Since 2000 we have used antegrade selective cerebral perfusion (ASCP) during the circulatory arrest in combination with transcranial Doppler (TCD) to monitor and, if necessary, adjust blood flow to the cerebrum. When an intimal tear was present in the arch, it was partially or completely replaced by a prosthetic aortic tube graft, depending on the location and extension of the intimal tear. After completion of the distal anastomosis cardiopulmonary bypass was re-instituted.
Follow-up
After the operation and discharge from hospital, patients were routinely referred to a cardiologist, either at our institution or a local hospital. Medical treatment and follow-up was left to the discretion of the attending cardiologist. Of note, in case of a high index of suspicion (e.g. habitus) the patient was also referred to the clinical geneticist for analysis of possible Marfan syndrome.
Statistical analysis
Statistical analysis was performed using SPSS 12.0.1. Continuous data are presented as mean ± standard deviation and categorical data as percentages. Inhospital mortality was defined as death occurring within 30 days of primary surgery or during the initial hospitalisation. For discrete variables the Χ2 test or Fisher's exact test were used to identify univariate risk factors of in-hospital mortality. For continuous variables the unpaired Student's t-test was used (all tests twosided). A p-value <0.05 was considered statistically significant. In order to assess the influence of the operating surgeon on in-hospital mortality we created a new variable (surgeon). Surgeons were divided into two groups: group I constituted surgeons who operated on <10 cases and group II surgeons who operated on >10 cases. Variables that were statistically significant in univariate analysis were analysed by multivariate logistic regression to determine independent predictors of in-hospital mortality. In addition one- and threeyear survival rates were estimated and survival curves were plotted using the Kaplan-Meier method.
Results
Clinical characteristics and operative data
Clinical characteristics of the patients at presentation are summarised in table 1. Diagnosis of AAD was usually made by echocardiography (58.7%). Other imaging techniques used to establish the diagnosis were CT scan (35.6%), aortography (3.8%) and MRI (1.9%). In one patient preoperative imaging did not reveal an aortic dissection; only an aneurysmatic thoracic aorta was discovered. Because laboratory investigations showed that the patient was losing blood and the patient suffered from severe thoracic pain, acute surgery was performed and an aortic dissection was discovered. Operative data are summarised in table 2.
Table 1 .
Clinical characteristics (n=104).
| Characteristic | |
|---|---|
| Age (years) | 60±12 (23–87) |
| Gender | |
| - Male | 77 (74.0) |
| - Female | 27 (26.0 |
| Hypertension | 54 (51.9) |
| Diabetes | 2 (1.9) |
| Hypercholesterolaemia | 4 (3.8) |
| Marfan syndrome (medical history or newly diagnosed) | 4 (3.8) |
| Turner syndrome | 1 (1.0) |
| Previous cardiac surgery | 2 (1.9) |
| Pre-existing cardiac disease | |
| - Atrial fibrillation | 4 (3.8) |
| - Valvular heart disease | 4 (3.8) |
| - Myocardial infarction | 3 (2.9) |
| - Ischaemic heart disease | 2 (1.9) |
| - Chronic heart failure | 1 (1.0) |
| Peripheral arterial disease | 16 (15.4) |
| History of type B aortic dissection | 2 (1.9) |
| COPD | 12 (11.5) |
| Aortic valve insufficiency grade I-IV (new) | 64 (61.5) |
| Cardiac tamponade | 16 (15.4) |
| Malperfusion | |
| - Mesenteric | 1 (1.0) |
| - Limbs | 15 (14.4) |
| Neurological deficit | |
| - Hemipareses | 3 (2.9) |
| - Comatose | 1 (1.0) |
| - Spinal cord lesion | 2 (1.9) |
| Shock (systolic tension <90 mmHg) | 19 (18.3) |
| Cardiopulmonary resuscitation | 7 (6.7) |
| Bicuspid aortic valve | 4 (3.8) |
| Iatrogenic dissection (due to coronary angiography) | 1 (1.0) |
Figures are numbers of patients with percentages in brackets.
Table 2 .
Operative data.
| n (%) | |
|---|---|
| Arterial cannulation | |
| - Femoral artery | 78 (74.0) |
| - Iliac artery | 17 (16.3) |
| - Ascending aorta | 1 (1.0) |
| - Aortic arch | 5 (4.8) |
| - Innominate artery | 1 (1.0) |
| - Subclavian artery | 3 (2.9) |
| Venous cannulation | |
| - Right atrium | 89 (85.6) |
| - Left atrium | 1 (1.0) |
| - Bicaval | 6 (5.8) |
| - Inferior venal cava | 8 (7.7) |
| Perfusion technique | |
| - Extracorporeal circulation (ECC) | 58 (55.8) |
| - ECC + DHCA | 18 (17.3) |
| - ECC + circulatory arrest + ASCP* | 23 (21.9) |
| - ECC + circulatory arrest + RSCP† | 5 (4.8) |
| Operative procedures | |
| - Ascending aorta replacement (AAR) | 55 (52.9) |
| - AAR + hemiarch replacement | 9 (8.7) |
| - AAR + arch replacement | 5 (4.8) |
| - AAR + resuspension aortic valve | 15 (14.4) |
| - AAR + hemiarch replacement + resuspension aortic valve | 5 (4.8) |
| - AAR + David procedure‡ | 2 (1.9) |
| - AAR + hemiarch replacement + David‡ | 1 (1.0) |
| - AAR + Bentall¥ | 9 (8.7) |
| - Bentall¥ + arch replacement + elephant trunk institution| | 1 (1.0) |
| - AAR + aortic valve replacement (AVR) | 1 (1.0) |
| - AAR + AVR + Cabrol shunt¶ | 1 (1.0) |
| Extracorporeal circulation (ECC) time (min) | 197.9±101.9 |
| - Range | 69–745 |
| Cross-clamp time (min) | 103.8±42.5 |
| - Range | 43–290 |
| ASCP* time (min) | 35.9±19.1 |
| - Range | 15–90 |
| RSCP† time (min) | 37±22.9 |
| - Range | 20–70 |
* Antegrade selective cerebral perfusion, † retrograde selective cerebral perfusion, ‡ valve sparing aortic root replacement, ¥ aortic root replacement with prosthetic valved conduit, | technique in which a tube prosthesis is employed in an antegrade manner with a free end downstream in the descending aorta, anticipating and facilitating future thoracic descending aorta surgery, ¶ a shunt from the space between the aorta prosthesis and the original aorta to the right atrium in order to control bleeding from the reconstruction, this technique can be used when the original ascending aorta is wrapped around the aortic prosthesis.
In-hospital mortality
In-hospital mortality was 22.1% (23/104). Seven patients died intraoperatively, of whom three could not be weaned from cardiopulmonary bypass, one patient had a large haematoma in the right ventricular wall which ruptured beyond repair, one patient had an irreparable cardiac disintegration due to an invading haematoma, in one patient no haemostatic aorta-prosthesis-anastomosis could be created and in one patient friable aortic tissue prevented the formation of an adequate proximal aortoprosthesis-anastomosis. Other causes of in-hospital mortality were major brain damage in ten patients, multiple organ failure in three patients, low cardiac output in two patients and sudden cardiac death in one patient.
Table 3 shows the results of univariate analysis of predictors of in-hospital mortality.
Table 3 .
Univariate analysis of predictors of in-hospital mortality.
| Risk factor | P value | Odds ratio | 95% CI |
|---|---|---|---|
| Preoperative creatinine >115 μmol/l | 0.030 | 2.95 | 1.09–8.00 |
| Preoperative malperfusion | 0.007 | 4.87 | 1.58–15.01 |
| ECC* time >310 min | 0.037 | 4.35 | 1.13–16.74 |
| RSCP time (min) | 0.008 | 16.84 | 1.78–159.42 |
| Preoperative neurological deficit | NS | ||
| Preoperative CPR | NS | ||
| Preoperative cardiac tamponade | NS | ||
| Preoperative shock | NS | ||
| Surgeon | NS | ||
| ASCP time | NS |
NS=non significant, ECC=extracorporeal circulation, RSCP=retrograde selective cerebral perfusion, CPR=cardiopulmonary resuscitation, ASCP=antegrade selective cerebral perfusion.
Univariate predictors of mortality were preoperative creatinine >115 μmol/l, preoperative malperfusion, extracorporeal circulation time >310 minutes and retrograde selective cerebral perfusion.
Multivariate logistic regression revealed preoperative malperfusion (p=0.004) to be the only independent predictor of in-hospital mortality.
Midterm outcome
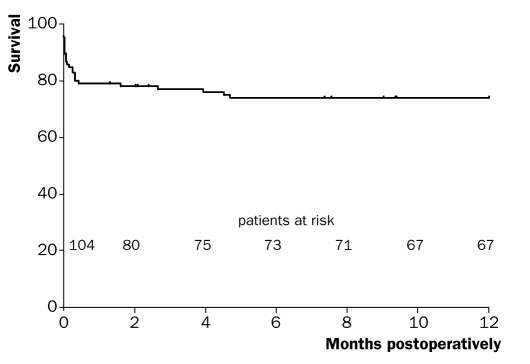
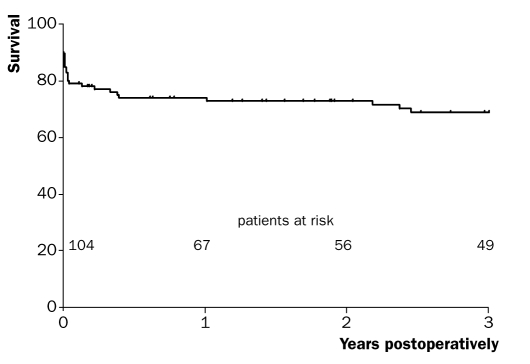
One- and three-year survival rates (including hospital mortality) were 73.8±4.3% and 68.8±4.7%, respectively. Figures 1 and 2 show the one- and three-year survival curves. One- and three-year survival rates of hospital survivors were 94.9±2.5% and 88.3±3.9%, respectively. Follow-up was complete.
Figure 1 .
Kaplan-Meier curve of one-year survival (including hospital mortality).
Figure 2 .
Kaplan-Meier curve of three-year survival (including hospital mortality).
Discussion
Previous studies have identified many predictors of inhospital mortality of surgically treated AAD patients, including shock, malperfusion, cardiac tamponade, neurological deficit, CPR, myocardial ischaemia, preexisting cardiac disease, old age and previous cardiac surgery.3,7–13 In our study preoperative malperfusion was the only independent predictor of hospital death. Patients with preoperative malperfusion usually presented with a compromised limb circulation. Simultaneously with a deficient limb perfusion, internal organ blood supply can also be obstructed by the same intimal flap or haematoma leading to severe hypoxic injury and thereby putting these patients at increased risk for early death.14
The number of patients operated on by individual surgeons did not influence in-hospital mortality in our population. Surgeons who operated on >10 patients had similar results to surgeons who operated on <10 patients (21.1 vs. 22.7% p=0.84). However, patient selection might bias these results against surgeons operating on >10 cases, as surgeons with more experience in aortic surgery will often be asked to operate on ‘difficult’ cases. To our knowledge, no studies have ever investigated the influence of the operating surgeon on in-hospital mortality in AAD surgery. One study did mention that the operating surgeon was the most important risk factor for morbid events in AAD surgery in their institution.15 In CABG surgery the influence of the surgeon on hospital mortality has been well recognised; the more patients a surgeon operates on, the better the outcome.16–18 This is probably also true for aortic surgery and aortic dissection surgery in particular as this is technically more challenging. However, it should be reminded that AAD requires emergency surgery and the most experienced surgeons are not always on call.
We believe our in-hospital mortality of 22.1% is comparable with previous (international) studies which report an in-hospital mortality between 10 to 25%,1–3 although it is difficult to compare results of different cardiothoracic centres, patient populations vary significantly, a range of surgical and perfusion techniques are being used and experience of cardiothoracic centres with aortic surgery may influence results. With respect to the Dutch situation, we can only compare our results with the St Antonius Hospital in Nieuwegein. They report an in-hospital mortality between 20 to 25% which is essentially the same as we found in our institution.2,5 It should, however, be realised that their studies comprised larger patient groups and also included patients undergoing surgery before 1990 when surgical and perfusion techniques were less advanced.
Surgical and perfusion techniques
Currently antegrade arterial perfusion and an opendistal-anastomosis technique are being promoted for the surgical treatment of AAD. We support the use of antegrade arterial perfusion since this technique prevents possible complications of retrograde arterial perfusion, such as organ malperfusion, as a consequence of intimal flap elevation, expansion of the ‘false’ lumen and retrograde embolisation of atherosclerotic debris.19,20
We do not apply a standard open-distal-anastomosis technique. Instead, we prefer a stepwise approach in which we initially cross-clamp the aorta. We only use a circulatory arrest (since the year 2000 in combination with antegrade selective cerebral perfusion) to resect the remaining intimal tear and to inspect the aortic arch for tears when the intimal tear expands through the aortic cross-clamp or if no intimal tear is located in the ascending aorta. Our conservative approach might predispose to reoperation on the remaining aorta as unresected intimal tears have been identified as a risk for late re-operation on the distal aorta.21 However, most intimal tears are located in the ascending aorta or proximal aortic arch and therefore we believe not to leave many unresected intimal tears behind predisposing to reoperation.22 This approach is supported by the fact that only one of our patients required a reoperation for a post-dissection aneurysm of the distal aorta.
Mid-term survival
From the survival curves (figures 1 and 2) it becomes clear that if patients survive the initial hospitalisation, prognosis is relatively good. One- and three-year survival rates of hospital survivors were 94.9 and 88.3%, respectively and within the range of previously reported survival rates (90.7 to 96.4%24–26 one-year survival and 90%26 three-year survival).
Conclusion
Acute type A dissection is a catastrophic event in the aorta and without treatment accompanied by high mortality rates making rapid surgical treatment crucial for survival. In-hospital mortality of our patients (22.1%) is comparable with the results of larger case series published in the literature. Prognosis after successful surgical treatment is relatively good, with a three-year survival of 88.3% in our series.
References
- 1.Bavaria JE, Brinster DR, Gorman RC, Woo YJ, Gleason T, Pochettino A. Advances in the treatment of acute type A dissection: an integrated approach. Ann Thorac Surg 2002;74:S1848–52. [DOI] [PubMed] [Google Scholar]
- 2.Chiappini B, Tan ME, Morshuis W, Kelder H, Dossche K, Schepens M. Surgery for acute type A aortic dissection: is advanced age a contraindiction? Ann Thorac Surg 2004;78:585–90. [DOI] [PubMed] [Google Scholar]
- 3.Trimarchi S, Nienaber CA, Rampoldi V, Myrmel T, Suzuki T, Mehta RH, et al. Contemporary results of surgery in acute type A aortic dissection: the IRAD experience. J Thorac Cardiovasc Surg 2005;129:112–22. [DOI] [PubMed] [Google Scholar]
- 4.Health Council of the Netherlands. Cardiac interventions: a 2007 update. The Hague: Health Council of the Netherlands, 2007; publication no. 2007/01. [Google Scholar]
- 5.Tan ME, Dossche KM, Morshuis WJ, Knaepen PJ, Defauw JJ, van Swieten HA, et al. Operative risk factors of type A aortic dissection: analysis of 252 consecutive patients. Cardiovasc Surg 2003;11:277–85. [DOI] [PubMed] [Google Scholar]
- 6.David TE, Feindel CM. An aortic valve-sparing operation for patients with aortic incompetence and aneurysm of the ascending aorta. J Thorac Cardiovasc Surg 1992;103:617–22. [PubMed] [Google Scholar]
- 7.Mehta RH, Suzuki T, Hagan PG, Bossone E, Gilon D, Llovet A, et al. Predicting death in patients with acute type A aortic dissection. Circulation 2002;105:200–6. [DOI] [PubMed] [Google Scholar]
- 8.Santini F, Montalbano G, Casali G, Messina A, Iafrancesco M, Luciani GB, et al. Clinical presentation is the main predictor of in-hospital death for patients with acute type A aortic dissection admitted for surgical treatment: a 25 years experience. Int J Cardiol 2007;115:305–11. [DOI] [PubMed] [Google Scholar]
- 9.Apaydin AZ, Buket S, Posacioglu H, Islamoglu F, Calkavur T, Yagdi T, et al. Perioperative risk factors for mortality in patients with acute type A aortic dissecton. Ann Thorac Surg 2002;74:2034–9. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka H, Okada K, Yamashita T, Morimoto Y, Kawanishi Y, Okita Y. Surgical results of acute aortic dissection complicated with cerebral malperfusion. Ann Thorac Surg 2005;80:72–6. [DOI] [PubMed] [Google Scholar]
- 11.Chiappini B, Schepens M, Tan E, Dell'Amore A, Morshuis W, Dossche K, et al. Early and late outcomes of acute type A aortic dissection: analysis of risk factors in 487 consecutive patients. Eur Heart J 2005;26:180–6. [DOI] [PubMed] [Google Scholar]
- 12.Mehta RH, O'Gara PT, Bossone E, Nienaber CA, Myrmel T, Cooper JV, et al. Acute type A aortic dissection in the elderly: clinical characteristics, management, and outcomes in the current era. J Am Coll Cardiol 2002;40:685–92. [DOI] [PubMed] [Google Scholar]
- 13.Collins JS, Evangelista A, Nienaber CA, Bossone E, Fang J, Cooper JV, et al. Differences in clinical presentation management, and outcomes of acute type A aortic dissection in patients with and without previous cardiac surgery. Circulation 2004;110:SII237–42. [DOI] [PubMed] [Google Scholar]
- 14.Bossone E, Rampoldi V, Nienaber CA, Trimarchi S, Ballota A, Cooper JV, et al. Usefulness of pulse deficit to predict in-hospital complications and mortality in patients with acute type A aortic dissection. Am J Cardiol 2002;89:851–5. [DOI] [PubMed] [Google Scholar]
- 15.Westaby S, Saito S, Katsumata T. Acute type A dissection: conservative methods provide consistently low mortality. Ann Thorac Surg 2002;73:707–13. [DOI] [PubMed] [Google Scholar]
- 16.Hannan EL, Kilburn H Jr, Bernard H, O'Donnell JF, Lukacik G, Shields EP. Coronary artery bypass surgery: the relationship between inhospital mortality rate and surgical volume after controlling for clinical risk factors. Med Care 1991;29:1094–107. [PubMed] [Google Scholar]
- 17.Hannan EL, Siu AL, Kumar D, Kilburn H Jr, Chassin MR. The decline in coronary artery bypass graft surgery mortality in New York State. The role of surgeon volume. JAMA 1995;273:209–13. [PubMed] [Google Scholar]
- 18.Wen HC, Tang CH, Lin HC, Tsai CS, Chen CS, Li CY. Association between surgeon and hospital volume in coronary bypass graft surgery outcomes: a population-based study. Ann Thorac Surg 2006;81:835–42. [DOI] [PubMed] [Google Scholar]
- 19.Sabik JF, Lytle BW, McCarthy PM, Cosgrove DM. Axillary artery: an alternative site of arterial cannulation for patients with extensive aortic and peripheral vascular disease. J Thorac Cardiovasc Surg 1995;109:885–90. [DOI] [PubMed] [Google Scholar]
- 20.Van Arsdell GS, David TE, Butany J. Autopsies in acute type A aortic dissection. Surgical Implications. Circulation 1998;98:SII299–302. [PubMed] [Google Scholar]
- 21.Moon MR, Sundt III TM, Pasque MK, Barner HB, Huddleston CB, Damiano RJ Jr, et al. Does the extent of proximal or distal resection influence outcome for type A dissections? Ann Thorac Surg 2001;71:1244–9 [DOI] [PubMed] [Google Scholar]
- 22.Ehrlich MP, Ergin MA, McCullough JM, Lansman SL, Galla JD, Bodian CA, et al. Results of immediate surgical treatment of all acute type A dissections. Circulation 2000;102:SIII248–52. [DOI] [PubMed] [Google Scholar]
- 23.Borst HG, Heinemann MK, Stone CD. Indications for Surgery. In: Allan Ross, editor. Surgical treatment of aortic dissection. 1st ed. New York: Churchill Livingstone Inc; 1996. p. 103–7. [Google Scholar]
- 24.Halstead JC, Meier M, Etz C, Spielvogel D, Bodian C, Wurm M, et al. The fate of the distal aorta after repair of acute type A aortic dissection. J Thorac Cardiovascu Surg 2007;133:127–35. [DOI] [PubMed] [Google Scholar]
- 25.Tan ME, Morshuis WJ, Dossche KM, Kelder JC, Waanders FG, Schepens MA. Long-term results after 27 years of surgical treatment of acute type A aortic dissection. Ann Thorac Surg 2005;80:523–9. [DOI] [PubMed] [Google Scholar]
- 26.Tsai TT, Evangelista A, Nienaber CA, Trimarchi S, Sechtem U, Fattori R, et al. Long-term survival in patients presenting with type A acute aortic dissection: insights from the International Registry of Acute Aortic Dissection (IRAD). Circulation 2006; 114:SI350–6. [DOI] [PubMed] [Google Scholar]