Abstract
Background. Duchenne muscular dystrophy (DMD) patients used to die mainly from pulmonary problems. However, as advances in respiratory care increase life expectancy, mortality due to cardiomyopathy rises. Echocardiography remains the standard diagnostic modality for cardiomyopathy in DMD patients, but is hampered by scoliosis and poor echocardiographic acoustic windows in adult DMD patients. Multigated cardiac radionuclide ventriculography (MUGA) does not suffer from these limitations. N-terminal proBNP (NTproBNP) has shown to be a diagnostic factor for heart failure. We present our initial experience with plasma NT-proBNP measurement in the routine screening and diagnosis of cardiomyopathy in adult mechanically ventilated DMD patients.
Methods. Retrospective study, 13 patients. Echocardiography classified left ventricular (LV) function as preserved or depressed. NT-proBNP was determined using immunoassay. LV ejection fraction (LVEF) was determined using MUGA.
Results. Median (range) NT-proBNP was 73 (25 to 463) ng/l. Six patients had an NT-proBNP >125 ng/l. Seven patients showed an LVEF <45% on MUGA. DMD patients with depressed LV function (n=4) as assessed by echocardiography had significantly higher median NT-proBNP than those (n=9) with preserved LV function: 346 (266 to 463) ng/l versus 69 (25 to 257) ng/l (p=0.003). NT-proBNP significantly correlated with depressed LV function on echocardiogram and with LVEF determined by MUGA.
Conclusion. Although image quality of MUGA is superior to echocardiography, the combination of echocardiography and NT-proBNP achieves similar results in the evaluation of left ventricular function and is less time consuming and burdensome for our patients. We advise to add NT-proBNP to echocardiography in the routine cardiac assessment of DMD patients. (Neth Heart J 2009;17:232-7.)
Keywords: natriuretic peptides, echocardiography, multigated radionuclide ventriculography, cardiomyopathy, home mechanical ventilation, Duchenne muscular dystrophy
Duchenne muscular dystrophy (DMD) is an X-linked recessive muscular dystrophy caused by mutations in the dystrophin gene. The annual incidence is one per 3000 live male births. DMD patients used to die in their late teens or early twenties mainly from pulmonary problems. However, as advances in respiratory care increase life expectancy, morbidity and mortality due to cardiomyopathy rises. Preclinical cardiac involvement has been found in 25% of patients under 6 years of age, increasing to 60% between the ages of 6 and 10 years and then declining in incidence with age. Clinically apparent cardiomyopathy is first evident after 10 years of age and increases in incidence with age, being present in all patients over 18 years.1,2 Progressive cardiomyopathy is a common cause of death in Duchenne muscular dystrophy, presumably secondary to fibrosis of the myocardium. The posterobasal and left lateral free wall of the left ventricle are initial sites of myocardial fibrosis pathologically.3,4 Currently, 10 to 50% of DMD patients die of heart failure.5,6
A diagnosis of cardiomyopathy is not easily made in these patients. Interpretation of patient complaints, such as fatigue and decreased exercise tolerance, can be very difficult. Immobility, deformities, and pulmonary failure additionally obscure the clinical and radiographic signs and symptoms of heart failure. Because of the elusive nature of the disease additional tools for cardiac evaluation are often used in clinical work-up.
Echocardiography remains the standard diagnostic modality for cardiomyopathy in DMD patients and consensus meetings advise annual echocardiograms after the age of 10.1 However, scoliosis and poor echocardiographic acoustic windows in adult DMD patients hamper accurate assessment. Multigated cardiac radionuclide ventriculography (MUGA) is not affected by these problems and has been found to be sensitive in detecting subclinical DMD cardiomyopathy.7 However, MUGA is not easy to perform in DMD patients and, moreover, it is associated with radiation exposure, which is even more relevant in case of repeated investigations.
Taken together, there is a need for an easier approach to gathering information about cardiac function. The natriuretic peptides, brain natriuretic peptide (BNP) and N-terminal proBNP (NTproBNP), are useful as diagnostic factors for heart failure in patients presenting to an emergency department with acute dyspnoea and may provide prognostic information in patients with congestive heart failure.8,9 In a group of DMD patients that were not on mechanical ventilation, plasma levels of BNP were associated with systolic dysfunction.10
The goal of this retrospective study is to present our initial experience with plasma NT-xproBNP measurement in the routine screening and diagnosis of cardiomyopathy in home mechanically ventilated DMD patients attending our outpatient clinic, using MUGA and echocardiography.
Patients and methods
Patients
Between April 2005 and March 2006 all DMD patients attending the multidisciplinary outpatient clinic of our tertiary care hospital, who had a routine physical examination and cardiac work-ups including echocardiography, MUGA scanning and NT-proBNP assessment in one day, were included in this study report. The patients were older than 16 years of age and were on mechanical ventilatory support. Informed consent for the procedures was given by each patient. We obtained clinical follow-up information through chart review for all patients.
Measurements
Echocardiography was performed by skilled on duty technicians using a General Electric VIVID 7 system with a 2.5 mHz probe and consisted of twodimensional views in the parasternal long axis and short axis, and the apical view, according to standard procedures and definitions.11 LV systolic function was classified qualitatively as preserved or depressed. Plasma NT-proBNP was determined using the commercially available Elecsys proBNP immunoassay by Roche. The FDA approved cut-off value of 125 ng/l for NTproBNP was used to define systolic dysfunction.
MUGAs were standardised and performed in the left anterior oblique projection after the in vivo labelling of red blood cells with 500 MBq of 99mTc-pertechnetate to determine left ventricular ejection fraction (LVEF). A single-detector gamma camera (Orbiter, Siemens Medical Systems, Chicago, Illinois, USA) with a low energy, all purpose collimator was used. The camera head was positioned in the best septal left anterior oblique projection, typically with a caudal tilt of 5 to 10°. R-wave triggering was performed in a 20% beat acceptance window with 2/3 forward and 1/3 backward framing per cardiac cycle, for 20 frames per R-R interval for a total of six minutes. Data were acquired using 64×64 matrices in a 15% energy window centred on the 140 keV photopeak. Processing was performed on dedicated available computers (ICON, Siemens Medical Systems, Chicago, Illinois, USA). For each of the 20 frames a region of interest (ROI) was automatically drawn around the left ventricle using a validated, fully automated, operator independent, contour detection algorithm. Frames were automatically corrected for background activity. Background activity ROIs were generated automatically. All LVEF values were generated without decimals and are highly reproducible.12 The cut-off value of LVEF <45% was considered to indicate a significantly depressed systolic LV function.12 All measurements were carried out in one day.
Statistical analysis
With SPSS version 14 we used Spearmann correlation for continuous variables and Mann Whitney U test when appropriate to compare groups. The results are given as median (range) unless otherwise specified. P<0.05 was considered statistically significant.
Results
Patient characteristic1s
A total of 28 DMD patients attended our multidisciplinary outpatient clinic in this period. All were wheelchair dependent males with either non-invasive (via nose or facial mask) or invasive (via tracheostomy) assisted ventilation. Echocardiography, MUGA and measurement of NT-proBNP was performed for routine yearly cardiac assessment. We chose the patients who underwent all three investigations and all in one day in order to make a reliable comparison. Thirteen patients met these inclusion criteria; nine patients did not receive all three investigations in one day and six patients did not consent to all three investigations, because they expected that the investigations would be too burdensome.
Patient demographics and results of clinical assessment of the 13 included patients are summarised in table 1. Seven patients were taking cardiac medication: angiotensin-converting enzyme (ACE) inhibitor (6 patients) and β-blockers (2 patients). One patient had clear clinical signs and symptoms compatible with heart failure, as judged by the attending cardiologist.
Tabel 1 .
Baseline characteristics of study population.
| Patient characteristic (n=13) | Patients |
|---|---|
| Age, median (range) | 19 (16–36) |
| Wheelchair bound, n (%) | 13 (100%) |
| VC, litres (n=8), median (range) | 0.89 (0.36–2.5) |
| sO2 % (n=10), median (range) | 96 (90–98) |
| Minute volume, l/min (n=13), median (range) | 9.9 (7.0–14.7) |
| NIPPV, n (%) | 8 (61.5) |
| Invasive ventilation, n (%) | 5 (38.5) |
| Night-time ventilation, n (%) | 9 (69.2) |
| 24-hour ventilation, n (%) | 4 (30.8) |
| Duration of ventilation, years, (n=13) median (range) | 4 (0.5–13.5) |
| Heart rate, beats/min (n=13), median (range) | 88 (64–124) |
| Cardiac medication, (n=13) n (%)* | 7 (53.8) |
| Clinical heart failure, n (%) | 1 (7.7) |
FEV1 =forced expiratory volume in one second, VC=vital capacity, SpO2=saturation of peripheral oxygen, NIPPV=non-invasive positive pressure ventilation, *ACE-inhibitor (6), β-blocker (2).
Plasma NT-proBNP
The median NT-proBNP of 13 patients was 73 ng/l (range 25–463). Seven patients showed a normal NTproBNP level (<125 ng/l). No statistically significant relationship could be detected between the NTproBNP level and age, cardiac medication, type of ventilatory support, length of time since ventilator dependence or daily assisted ventilation requirement.
MUGA - NT-proBNP
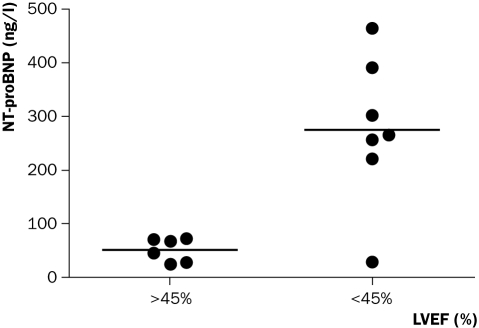
Figure 1 shows NT-proBNP levels of patients with preserved LV function (MUGA LVEF >45%) versus those with depressed LV function (MUGA LVEF <45%) (p=0.015 between groups). Mean (SD) LVEF in 13 patients was 45 (11). Seven patients showed an LVEF <45% and they had a higher median (range) of NT-proBNP 266 (30 to 463) ng/l than those patients with LVEF >45%: 58 (25 to 73) ng/l (p=0.014). There was an inverse relationship between NTproBNP and LVEF (r=−0.64, p=0.018).
Figure 1.
Left ventricular ejection fraction (%) measured by multigated cardiac radionuclide ventriculography (MUGA) grouped by preserved and depressed function and NT-proBNP levels in individual patients.
Echocardiography - NT-proBNP
Poor acoustic windows were mentioned in all patients. Due to the poor image quality no LVEF percentage was given in any of the patients. Preserved left ventricular (LV) function was found in nine patients and depressed LV function in four patients. DMD patients with depressed LV function (n=4) as assessed by echocardiography had higher median (range) NT-proBNP than those (n=9) with preserved LV function: 346(266 to 463) ng/l vs. 69(25 to 257) ng/l (p=0.003).
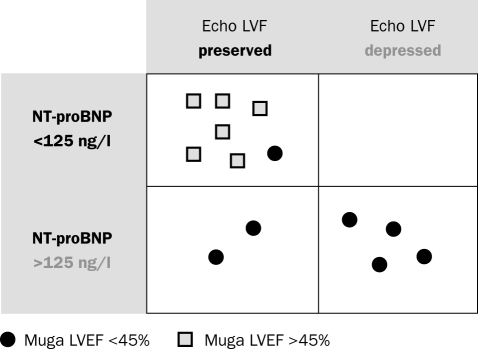
Echocardiography combined with NT-proBNP versus MUGA
Figure 2 shows the results of LV function assessment by echocardiography and plasma NT-proBNP compared with LVEF determined by MUGA. In all but one patient, the combined results of NT-proBNP and echocardiography are similar to those with LVEF determined by MUGA: all six patients with plasma NT-proBNP >125 ng/l and/or depressed LV function on echocardiography showed LVEF <45% on MUGA. Six out of seven patients with low NT-proBNP and preserved function on echocardiography showed LVEF >45% on MUGA. In one patient NT-proBNP was 30 ng/l and echocardiography showed preserved LV function, while LVEF on MUGA was 37%. Echocardiography results from previous years showed depressed left ventricular function. This 36-year-old patient did not receive cardiac medication and had been on long-term ventilatory support since 1992.
Figure 2 .
Left ventricular ejection fraction (%) measured by multigated cardiac radionuclide ventriculography (MUGA) versus left ventricular function (LVF) measured by echocardiography and NT-proBNP
Discussion
Decline in cardiac function in DMD occurs early in life. Detecting cardiomyopathy can be a particularly frustrating problem to patients and physicians due to the elusive and non-specific symptoms associated with the non-ambulatory state.2 In our population only one patient had overt clinical features of heart failure. In this retrospective study we evaluated the relationship between echocardiography, MUGA and NT-proBNP levels in DMD patients that were on home mechanical ventilated support. Combining echocardiography with NT-proBNP values in the assessment of left ventricular function, we found similar results compared with LVEF determined by MUGA: all patients with plasma NT-proBNP >125 ng/l and depressed LV function on echocardiography showed LVEF <45% on MUGA. In all but one patient low NT-proBNP and preserved function on echocardiography was associated with LVEF >45% on MUGA. In this patient NT-proBNP was 30 ng/l and echocardiography showed preserved LV function, while LVEF on MUGA was 37%.
We realise this is a small sample; nevertheless, there is no other literature comparing echocardiography, NT-proBNP and MUGA scan in an adult ventilated Duchenne patient group. Further studies are needed to confirm our conclusion.
In view of the prevalence of LV abnormalities, plasma NT-proBNP levels were relatively low in our patients. This is consistent with others that postulated it to be due to patients' physical inactivity limiting cardiac workload and therefore ventricular stress.10,13 An alternative hypothesis is that the heart of DMD patients is unable to make sufficient natriuretic peptides due to the presence of cardiac fibrosis.2,10 However, we consider this unlikely as we have seen high levels in cases of end-stage DMD (personal observation). Several studies similarly found high plasma BNP levels in deceased DMD patients.10,14 Another suggestion, as supported by Ishikawa, is that low levels are a consequence of cardioprotective medication.14 Our data did not clearly demonstrate a relationship between the use of cardiac medication and NT-proBNP levels. An alternative explanation would be obesity, which is a common finding in Duchenne patients. Unfortunately, weight was not recorded in our patients but we do recognise obesity as a potential cause of low natriuretic peptides, as has been seen in other patient groups.15
Studies using cardiac magnetic resonance techniques involving delayed enhancement techniques identify early regional myocardial fibrosis correlating with LV dysfunction.3,4 As found in earlier studies,10 there was relative sparing of the right ventricle. Furthermore, as LV dysfunction is also the main predictor of mortality in DMD patients,16 we primarily focussed on left ventricular function.
In a recent study echocardiography showed that young Duchenne patients have regional myocardial defects, not only systolic but also diastolic.17 Echocardiography can be helpful to detect early dysfunction in young Duchenne patients. Done properly, echocardiography is viewed as the gold standard to evaluate and quantify systolic and diastolic LV dysfunction. Several studies show that echocardiography is reliable and offers not only qualitative but also quantitative information in Duchenne patients.10,17,18 However, these studies have included mainly young, not ventilator-dependent, patients. Our adult population of ventilated DMD patients suffers from kyphoscoliosis and obesity and is basically tetraparetic. They need a patient lift to be positioned on an investigation table. Even then examination is severely hampered by poor acoustic windows. Echocardiography is generally performed in this patient group in a wheelchair which is not ideal but possible and practical. The duration of the examination is about 15 minutes.
MUGA scanning has been used to accurately assess left ventricular ejection fraction in all types of patients. Although it is an effective and sensitive technique for assessing LV function in DMD,7 it is much more time consuming. In total, this examination takes at least an hour as the patient has to be positioned in front of the camera and it is more laborious for the technician and accompanying parents or other helpers, as well as more burdensome for these severely disabled patients.
More advanced techniques, also currently used for determining cardiac function in DMD patients such as single photon emission computed tomography (SPECT) analysis and cardiac magnetic resonance (CMR), meet the same and perhaps even larger problems concerning burden, labour and time. Nevertheless there are interesting studies showing the ability to identify early fibrosis which seems to correlate with LV dysfunction.3,4 These are promising and suitable techniques for young and more mobile Duchenne boys as well as for female carriers.
In this study we did not focus on this last patient group of female carriers of DMD, although they are more frequently encountered and also at risk for cardiac disease. In a study among adult carriers echocardiographic examination was abnormal in 38% of the patients, including several patients with dilating cardiomyopathy.19 We can imagine that NT-proBNP is also useful in this patient group. In the literature there is some evidence by Adachi et al. that carriers had elevated brain natriuretic peptide, which correlated with indices of cardiac function.20
Further longitudinal follow-up studies are needed to determine whether early detection of subclinical cardiac dysfunction changes cardiac prognosis and whether early pharmaceutical interventions are useful in preventing progression of Duchenne muscular dystrophy.
Although limited data are available, growing evidence suggests that ACE inhibitors may prevent onset and progression of LV failure and that β-blockers may be cardioprotective in DMD.14,21,22 This carries important implications and implies that extensive routine cardiac assessment in addition to clinical evaluation is essential.
Conclusion
Important advances have improved our understanding of the nature of disease and life expectancy in DMD patients. Respiratory insufficiency is easily assessed and accurately treated with chronic mechanical ventilatory support. Despite these advantages, DMD continues to be associated with considerable morbidity and mortality, now often ascribed to the development of cardiomyopathy. We show that the combination of echocardiography and NT-proBNP achieves similar results to MUGA scanning in the evaluation of left ventricular function. This combination is less time consuming and burdensome for our patients. We advise adding NT-proBNP to echocardiography in the routine cardiac assessment of DMD patients. In case of normal echocardiographic findings (even if data quality is limited) in combination with non-elevated NT-proBNP, significant cardiomyopathy is rather unlikely. Further research is needed to determine whether tailoring medical therapy to NT-proBNP levels will allow early institution of appropriate therapies and improve outcome.
References
- 1.Bushby K, Muntoni F, Bourke JP. 107th ENMC international workshop: the management of cardiac involvement in muscular dystrophy and myotonic dystrophy. 7th–9th June 2002 Naarden, the Netherlands. Neuromuscul Disord 2003;13:166–72. [DOI] [PubMed] [Google Scholar]
- 2.Finsterer J, Stollberger C. The heart in human dystrophinopathies. Cardiology 2003;99:1–19. [DOI] [PubMed] [Google Scholar]
- 3.Silva MC, Meira ZM, Gurgel GJ, da Silva MM, Campos AF, Barbosa MM, et al. Myocardial delayed enhancement by magnetic resonance imaging in patients with muscular dystrophy. J Am Coll Cardiol 2007;49:1874–9. [DOI] [PubMed] [Google Scholar]
- 4.Puchalski MD, Williams RV, Askovich B, Sower CT, Hor KH, Su JT, et al. Late gadolinium enhancement: precursor to cardiomyopathy in Duchenne muscular dystrophy? Int J Cardiovasc Imaging 2009;25:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toussaint M, Steens M, Wasteels G, Soudon P. Diurnal ventilation via mouthpiece: survival in end-stage Duchenne patients. Eur Respir J 2006;28:549–55. [DOI] [PubMed] [Google Scholar]
- 6.Yasuma F, Konagaya M, Sakai M, Kuru S, Kawamura T. A new lease on life for patients with Duchenne muscular dystrophy in Japan. Am J Med 2004;117:363. [DOI] [PubMed] [Google Scholar]
- 7.Oguz D, Olgunturk R, Gucuyener K, Acikgoz GV, Tunaoglu FS. A comparison between MUGA and echocardiography in patients with muscular dystrophy in the early detection of cardiac involvement. Pediatr Cardiol 1998;19:150–4. [DOI] [PubMed] [Google Scholar]
- 8.Silver MA, Maisel A, Yancy CW, McCullough PA, Burnett JC Jr, Francis GS, et al. BNP Consensus Panel 2004: A clinical approach for the diagnostic, prognostic, screening, treatment monitoring, and therapeutic roles of natriuretic peptides in cardiovascular diseases. Congest Heart Fail 2004;10(5 Suppl 3):1–30. [DOI] [PubMed] [Google Scholar]
- 9.Maisel AS, Krishnaswamy P, Nowak RM, McCord J, Hollander JE, Duc P, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med 2002; 347:161–7. [DOI] [PubMed] [Google Scholar]
- 10.Mori K, Manabe T, Nii M, Hayabuchi Y, Kuroda Y, Tatara K. Plasma levels of natriuretic peptide and echocardiographic parameters in patients with Duchenne's progressive muscular dystrophy. Pediatr Cardiol 2002;23:160–6. [DOI] [PubMed] [Google Scholar]
- 11.Cheitlin MD, Armstrong WF, Aurigemma GP, Beller GA, Bierman FZ, Davis JL, et al. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography-summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/ AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography). J Am Coll Cardiol 2003;42:954–70. [DOI] [PubMed] [Google Scholar]
- 12.van der Vleuten PA, Slart RH, Tio RA, van der Horst IC, van Veldhuisen DJ, Dierckx RA et al. The feasibility of repeated left ventricular ejection fraction analysis with sequential single-dose radionuclide ventriculography. Nucl Med Commun 2005;26:711–5. [DOI] [PubMed] [Google Scholar]
- 13.Demachi J, Kagaya Y, Watanabe J, Sakuma M, Ikeda J, Kakuta Y et al. Characteristics of the increase in plasma brain natriuretic peptide level in left ventricular systolic dysfunction, associated with muscular dystrophy in comparison with idiopathic dilated cardiomyopathy. Neuromuscul Disord 2004;14:732–9. [DOI] [PubMed] [Google Scholar]
- 14.Ishikawa Y, Bach JR, Minami R. Cardioprotection for Duchenne's muscular dystrophy. Am Heart J 1999;137:895–902. [DOI] [PubMed] [Google Scholar]
- 15.Krauser DG, Lloyd-Jones DM, Chae CU, Cameron R, Anwaruddin S, Baggish AL, et al. Effect of body mass index on natriuretic peptide levels in patients with acute congestive heart failure: a ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) substudy. Am Heart J 2005;149:744–50. [DOI] [PubMed] [Google Scholar]
- 16.Corrado G, Lissoni A, Beretta S, Terenghi L, Tadeo G, Foglia-Manzillo G, et al. Prognostic value of electrocardiograms, ventricular late potentials, ventricular arrhythmias, and left ventricular systolic dysfunction in patients with Duchenne muscular dystrophy. Am J Cardiol 2002;89:838–41. [DOI] [PubMed] [Google Scholar]
- 17.Mertens L, Ganame J, Claus P, Goemans N, Thijs D, Eyskens B, et al. Early regional myocardial dysfunction in young patients with Duchenne muscular dystrophy. J Am Soc Echocardiogr 2008;21: 1049–54. [DOI] [PubMed] [Google Scholar]
- 18.Mohyuddin T, Jacobs IB, Bahler RC. B-type natriuretic peptide and cardiac dysfunction in Duchenne muscular dystrophy. Int J Cardiol 2007;119:389–91. [DOI] [PubMed] [Google Scholar]
- 19.Hoogerwaard EM, van der Wouw PA, Wilde AA, Bakker E, Ippel PF, Oosterwijk JC, et al. Cardiac involvement in carriers of Duchenne and Becker muscular dystrophy. Neuromuscul Disord 1999;9:347–51. [DOI] [PubMed] [Google Scholar]
- 20.Adachi K, Kawai H, Saito M, Naruo T, Kimura C, Mine H, et al. Plasma levels of brain natriuretic peptide as an index for evaluation of cardiac function in female gene carriers of Duchenne muscular dystrophy. Intern Med 1997;36:497–500. [DOI] [PubMed] [Google Scholar]
- 21.Duboc D, Meune C, Lerebours G, Devaux JY, Vaksmann G, Becane HM. Effect of perindopril on the onset and progression of left ventricular dysfunction in Duchenne muscular dystrophy. J Am Coll Cardiol 2005;45:855–7. [DOI] [PubMed] [Google Scholar]
- 22.Ramaciotti C, Scott WA, Lemler MS, Haverland C, Iannaccone ST. Assessment of cardiac function in adolescents with Duchenne muscular dystrophy: importance of neurohormones. J Child Neurol 2002;17:191–4. [DOI] [PubMed] [Google Scholar]