Abstract
One of the hallmark symptoms of patients with chronic heart failure (CHF) is exercise intolerance. Therefore, exercise testing has become an important tool for the evaluation and monitoring of heart failure. Whereas the maximal aerobic capacity (peak VO2) is a reliable indicator of the severity and prognosis of heart failure, submaximal exercise parameters may be more closely related to the ability to perform daily activities. As such, oxygen (O2) uptake kinetics, describing the rate change of O2 uptake during onset or recovery of submaximal constant-load exercise (O2 onset and recovery kinetics, respectively), have been shown to be useful parameters for objectively evaluating the functional capacity of CHF patients. However, their evaluation in this population is not a routine part of daily clinical practice. Possible reasons for this include a lack of standardisation of the assessment methodology and a limited number of studies evaluating the clinical use of O2 uptake kinetics in CHF patients. In addition, the pathophysiological mechanisms underlying the delay in O2 uptake kinetics in these patients are not completely understood. This review discusses the current literature on the clinical potency and physiological determinants of O2 uptake kinetics in CHF patients and provides directions for future research. (Neth Heart J 2009;17:238-44.Neth Heart J 2009;17:238–44.)
Keywords: chronic heart failure, exercise testing, oxygen uptake kinetics, time constant, mean response time
Chronic heart failure (CHF) can be defined as a clinical syndrome resulting from the inability of the heart to maintain sufficient cardiac output for adequate tissue oxygenation. As a consequence, CHF patients suffer from exercise intolerance. One of the main determinants of reduced exercise capacity in these patients is systolic and/or diastolic left ventricular dysfunction, which causes an impaired haemodynamic response to exercise.1 Other pathophysiological mechanisms include an impaired muscle blood flow caused by increased vasoconstriction2 and/or an impaired local vasodilatory capacity,3 muscle mitochondrial dysfunction,4 an exaggerated ventilatory response to exercise,5 and autonomic imbalance.6 Because resting indices of cardiac function7 and the level of perceived exercise intolerance8 correlate poorly with the exercise performance of these patients, exercise testing has become indispensable in the evaluation and monitoring of heart failure.
Exercise testing
Traditionally, maximal oxygen uptake ( VO2max) is considered the gold standard measure of aerobic fitness. In healthy individuals, VO2 max is usually defined as the point at which VO2 reaches a plateau despite a further increase in work rate during a symptom-limited exercise test. However, in CHF patients such a plateau in VO2 is rarely seen, suggesting that most of these patients do not attain a maximal exercise level during symptom-limited exercise testing. Therefore, the highest attainable VO2 in CHF patients is referred to as peak VO2, rather than VO2max. It has been demonstrated that peak VO2 is a reliable indicator of the severity of heart failure9 and a strong predictor of the prognosis in these patients.10 For the assessment of exercise performance in CHF patients, however, the use of peak VO2 has several limitations. First, the reliability of the assessment of this exercise parameter is hampered by the influence of the patients' motivation, the presence / absence of encouragement, and the criteria used to terminate the test. Second, as daily life mainly consists of repetitive submaximal activities, the maximal exercise capacity does not reflect the functional capacity of these patients very well. This may explain the relatively low sensitivity of peak VO2 for evaluating the efficacy of therapeutic interventions in heart failure.11,12 Therefore, there is a growing interest in submaximal exercise parameters to monitor changes in the functional capacity of CHF patients.
Frequently used submaximal exercise parameters are VO2 at the ventilatory threshold (VT) and the sixminute walking distance. The main limitation of the former is that it cannot be detected in a substantial number of CHF patients due to ventilatory irregularities. 13 The value is also dependent on the exercise protocol used, making it difficult to compare figures from different centres.14 The six-minute walk test is well tolerated and easy to perform in CHF patients, but the outcome is substantially influenced by motivation and encouragement. More importantly, the usefulness of the six-minute walking distance to assess changes in functional capacity is limited by a significant learning effect.15 Other submaximal exercise variables that have been used to assess the functional capacity in CHF patients include the ventilatory response to incremental exercise, expressed as the VE/ VCO2 slope or the oxygen uptake efficiency slope (OUES), and oxygen (O2) uptake kinetics during and after constant-load exercise with an intensity below the VT (O2 onset and O2 recovery kinetics, respectively). Both VE/VCO2 slope16 and OUES17 have been shown to be sensitive to the effects of physical training in CHF patients. However, as the assessment of these parameters requires exercise exceeding the anaerobic threshold, they should be considered more as parameters of maximal effort. O2 uptake kinetics during and after constant-load exercise below the VT represent a true measure of submaximal exercise capacity, but its clinical usefulness in CHF patients is not well established.
O2 uptake kinetics
O2 uptake kinetics describe the rate of change in VO2 during or after exercise. According to Fick's law (equation 1), VO2 is determined by cardiac output (Q) and systemic O2 extraction, which in turn is determined by arterial O2 content and O2 utilisation in the metabolising tissues. Therefore, O2 onset and recovery kinetics can be considered to reflect the interaction of the cardiovascular, pulmonary, and metabolic systems during and after exercise.
Equation 1
with VO2 = oxygen uptake (ml/min), Q = cardiac output (l/min), CaO2 = arterial oxygen content (ml/l) and CvO2 = mixed venous oxygen content (ml/l)
Assessment of O2 uptake kinetics
O2 onset kinetics
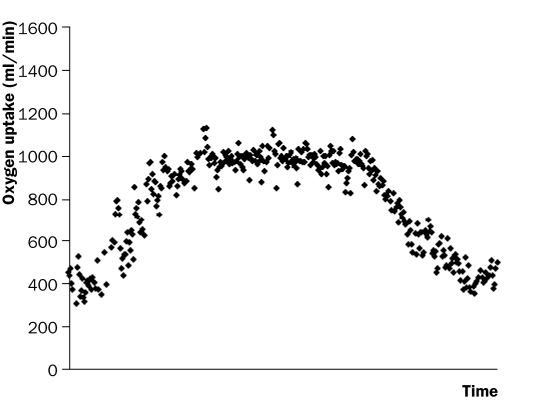
Figure 1Figure 1 shows an example of the time course of VO2 during and after constant-load exercise below the VT. Classically, O2 onset kinetics during submaximal constant-load exercise are considered to consist of three phases. Phase I, or the cardiodynamic phase, reflects a fast increase in VO2 of approximately 15 to 20 seconds as a consequence of an abrupt increase in pulmonary blood flow. Phase II reflects a mono-exponential increase in VO2, caused by an increase in cellular respiration at the skeletal muscle level. Phase III reflects a ‘steady state’ situation when exercise is performed below the VT, or a slow linear VO2 increase with exercise above the VT.18 Mathematically, O2 onset kinetics during exercise below the VT in healthy individuals are well described by a simple monoexponential model (equation 2), provided that phase I, which is functionally distinct from phase II, is omitted from the data.19
Figure 1 .
Breath-by-breath values of VO2 during and after a constant-load exercise test of six minutes at 50% of the maximal workload with a recovery period of five minutes in a patient with chronic heart failure.
Equation 2
with A = VO2-amplitude during exercise (ml/min), Td = time delay (sec) and τ = time constant (sec)
This model has several limitations when applied to CHF patients. First, as a consequence of a reduced ventilatory threshold, the exercise-related increase in VO2 is lower in CHF patients than in healthy subjects, and omitting phase I from the data leads to an even greater reduction of the VO2 amplitude. As pointed out by Lamarra et al., a relatively low VO2 amplitude results in a low accuracy of mono-exponential modelling of O2 onset kinetics, due to a relatively high noiseto-signal ratio.20 Second, the reliability of this method may be compromised by the typical ventilatory oscillations in CHF patients.21 Therefore, O2 onset kinetics have also been assessed by the mean response time in CHF patients, using an algebraic method. This method involves calculating the cumulative sum of VO2 in excess of resting VO2. Subsequently, the O2 deficit is calculated by subtracting this cumulative sum from the theoretical VO2 requirement (equation 3). The mean response time is calculated by dividing the O2 deficit by the theoretical VO2 requirement (equation 4).22
Equation 3
O2deficit = t * ΔVO,sub>2 - ΣVO2
with t = duration of constant-load test (min), ΔVO2 = VO2 steady state - VO2 baseline (ml/min) and ΣVO2 = cumulative sum of VO2 in excess of resting VO2 (ml)
Equation 4
Mean response time = O2deficit / ΔVO2
The accuracy and reproducibility of different methods to assess O2 onset kinetics in CHF patients are not well established. Although previous studies suggested an acceptable reproducibility of mono-exponential modeling23 and the algebraic method,22 intra-class correlations and limits of agreement were not mentioned. Therefore, we recently evaluated the accuracy and reproducibility of both methods in CHF patients.24 It was shown that the goodness-of-fit of monoexponential modelling was insufficient in a substantial number of patients. In addition, the reproducibility of both methods was too low to warrant their use for the assessment of effects of therapeutic interventions. Therefore, future studies should consider strategies to improve the reproducibility of O2 onset kinetics through a reduction of the noise-to-signal ratio. This may be achieved by increasing the VO2 amplitude, e.g. by starting exercise from rest instead of unloaded cycling, or by averaging the VO2 responses from multiple exercise tests.20 However, the latter approach is complicated and time-consuming, and therefore only suitable for research purposes, but not for daily clinical practice.
O2 recovery kinetics
O2 recovery kinetics after submaximal exercise are generally considered to follow a mono-exponential course (Equation 5) in both healthy subjects19 and CHF patients.25,26
Equation 5
with B = VO2-amplitude during exercise recovery (ml/min), Td = time delay (sec)
and τ = time constant (sec)
In a recent study, we showed that that the assessment of O2 recovery kinetics following submaximal exercise by a mono-exponential model is feasible and reproducible in CHF patients.24 By using the most optimal sampling interval (5 breaths) to calculate the time constant (τ), we showed that a change of at least 13 seconds in τ is needed to exceed the normal test-totest variations.
Possible explanations for the higher reproducibility of O2 recovery kinetics as compared with onset kinetics include fewer motion artefacts and a more stable breathing pattern during exercise recovery. Also, as ventilatory oscillations in CHF patients are most pronounced during the transition from rest to exercise,27 they may have a greater influence on the reliability of the assessment of O2 onset kinetics than O2 recovery kinetics. Another explanation may be a lesser influence of a cardiodynamic phase (phase I) during exercise recovery, resulting in a higher VO2amplitude that can be used for analysis of the data.
Clinical applications of O2 uptake kinetics
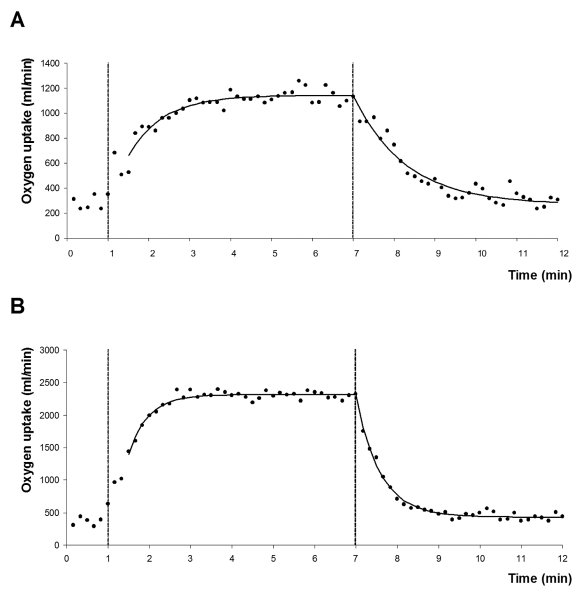
In 1927 Meakins et al.28 observed slower O2 onset and recovery kinetics in a patient with circulatory failure as compared with a healthy subject. It was not until the 1990s that O2 uptake kinetics were further evaluated as a potential instrument for objectively assessing the functional capacity of CHF patients. Most of these studies clearly demonstrated that O2 onset kinetics during constant-load exercise below the VT are delayed in CHF patients, with slower O2 uptake kinetics being associated with more fatigue due to a greater reliance on the anaerobic metabolism.22,23,25,29 Although less attention has been paid to exercise recovery, several studies showed that O2 recovery kinetics after submaximal exercise22,25,26,30 are also prolonged in CHF patients, with the degree of the delay correlating with the functional impairment in these patients. An example of O2 uptake kinetics in a CHF patient and a healthy subject of comparable age and body mass index is shown in figure 2.
Figure 2 .
O2 uptake kinetics during and after a constant-load exercise test of six minutes at 50% of the maximal workload with a recovery period of five minutes in a patient with chronic heart failure (A) and a healthy subject of comparable age and body mass index (B). VO2 data were averaged in ten-second intervals. The curved line represents the mono-exponential model fit. The first vertical line indicates onset of exercise and the second vertical line the end of exercise.
Whether O2 uptake kinetics are sensitive to the effects of therapeutic interventions in CHF patients is not well established. So far, preliminary studies with small sample sizes showed that O2 onset kinetics may be useful in CHF patients to evaluate the effects of β-blocking agents,31 physical training,32 and heart transplantation.33 To our knowledge, no studies have evaluated the clinical utility of O2 recovery kinetics after submaximal exercise to assess the effect of therapeutic interventions in CHF patients.
In addition to grading functional impairments, O2 uptake kinetics may also be used for risk stratification in CHF. O2 onset kinetics during exercise below the VT were shown to be an independent predictor of mortality in CHF.34,35 The prognostic value of O2 onset kinetics may even be superior to peak VO2.35 However, studies with large numbers of patients are needed to confirm this finding. The prognostic value of O2 recovery kinetics after submaximal exercise has not yet been evaluated.
In all of the above-mentioned studies, standardisation is lacking because different exercise protocols and calculation methods were used to assess O2 uptake kinetics in CHF patients. Therefore, before using O2 uptake kinetics in clinical practice, it is of crucial importance to establish uniform assessment methods. To achieve this, more data are needed on the accuracy of the modelling techniques and exercise protocols currently being used in CHF patients. Furthermore, kinetic parameters should be investigated to discover which is best suited to serve various clinical purposes, such as assessment of prognosis or quantifying or predicting effects of therapeutic interventions (e.g. medication, physical training, cardiac resynchronisation therapy).
Physiological determinants of O2 uptake kinetics
As mentioned before, O2 uptake kinetics provide objective information on the ability of CHF patients to perform daily activities. Therefore, more knowledge of the physiological determinants of O2 uptake kinetics may lead to a better understanding of the pathophysiological mechanisms causing functional impairments in these patients. This may eventually aid in the development of therapeutic approaches to improve the exercise capacity in CHF patients. Assessment of the physiological determinants of O2 uptake kinetics may also be used to classify CHF patients better, allowing a more appropriate treatment selection. For example, patients who are mainly limited by peripheral derangements may benefit from physical training,36 while patients with more pronounced circulatory dysfunction during exercise may be better candidates for treatments to improve the central haemodynamics, such as CRT37 or heart transplantation.38
Theoretically, changes in during or after exercise are determined by tissue oxygenation (O2 delivery) and the speed at which O2 can be used for oxidative metabolism (O2 utilisation). O2 delivery depends on O2 transport and diffusion in the lungs, O2 content of the blood, cardiac function, peripheral vasoconstriction, local vasodilatory capacity, capillary density, and diffusion of O2 from the blood to the tissues. O2 utilisation is determined by the number of mitochondria, which is influenced by muscle fibre type distribution in skeletal muscles, and mitochondrial enzyme activity. Several mechanisms contribute to a reduction in O2 delivery in CHF: cardiac insufficiency, elevated vasoconstriction due to increased sympathetic activity,39 elevated plasma angiotensin levels,2 impaired nitric oxide-mediated vasodilatation,3 and a blunted redistribution of blood flow from the non-exercising tissues to the working skeletal muscles.40 O2 utilisation may be limited by mitochondrial dysfunction and / or a reduction in the number of mitochondria due to muscle atrophy or a shift in muscle fibre type distribution to type II fibres.41
O2 onset kinetics
Whereas impairments in both O2 delivery and O2 utilisation may be present in CHF, the relative contribution of these factors to the delay in O2 onset kinetics is not well established. Animal studies have previously demonstrated a delayed increase in muscle capillary blood flow42 and a more rapid decrease in muscle microvascular O2 pressure during submaximal exercise in rats with heart failure,43 suggesting a limitation of O2 onset kinetics by O2 delivery. This notion is supported by human studies showing that the delay in O2 onset kinetics during submaximal exercise ergometry in CHF patients is associated with a delayed increase in cardiac output.44,45 Yet, studies evaluating the physiological determinants of O2 uptake kinetics at a local muscle level showed that abnormalities in muscle metabolism are not associated with a reduced muscle blood flow during submaximal exercise in CHF patients.4,46 The exercise protocols applied in these studies, however, involved small-muscle groups (calf and forearm muscles respectively) and, therefore, the results of these studies may not be representative for whole body exercise.
O2 recovery kinetics
Data are even scarcer about the pathophysiological mechanisms underlying the delay in O2 recovery kinetics in CHF. In an animal study, CHF was associated with a slower recovery of microvascular O2 pressure following submaximal exercise than in healthy controls,47 suggesting that submaximal exercise recovery in CHF is limited by O2 delivery. In another study with rats, this delayed recovery of microvascular O2 pressure was shown to be associated with a diminished vascular NO availability,48 suggesting an important role for endothelial function in the delay of metabolic recovery in CHF. The results from human studies are conflicting. Toussaint et al. showed that a prolonged skeletal muscle metabolic recovery after submaximal exercise was associated with a reduction of reactive hyperaemic blood flow and postulated that local circulatory dysfunction is an important contributor to the prolonged metabolic recovery in these patients.49 Yet, Hanada et al. found that muscle metabolic recovery following submaximal exercise was more delayed than muscle tissue re-oxygenation in CHF patients and concluded that metabolic recovery in these patients is mainly limited by O2 utilisation.50 Measurements of muscle metabolism and muscle perfusion / oxygenation were not performed simultaneously in either of the studies.
Conclusions
O2 uptake kinetics at submaximal exercise are potentially useful to objectively assess the functional capacity of CHF patients. As O2 recovery kinetics can be assessed more reliably than O2 onset kinetics in CHF patients, they may be particularly valuable in clinical practice. Future studies should therefore evaluate the usefulness of these exercise parameters for clinical purposes such as assessment of prognosis and quantifying effects of therapeutic interventions aiming at an improvement of the exercise capacity of these patients (e.g. physical training and cardiac resynchronisation therapy).
Considering the pathophysiological background of the delay in O2 uptake kinetics in CHF patients, more knowledge on this issue may be useful for the development of treatments and an improved classification of these patients, allowing for a more appropriate treatment selection. Whereas the results from animal studies suggest that the delay in O2 onset and recovery kinetics in CHF patients is primarily caused by impairments in O2 delivery to skeletal muscles, data from human studies are scarce and sometimes contradictory. Therefore, additional research is needed, preferably by performing simultaneous measurements of O2 delivery and O2 utilisation.
References
- 1.Sullivan MJ, Knight JD, Higginbotham MB, Cobb FR. Relation between central and peripheral hemodynamics during exercise in patients with chronic heart failure. Muscle blood flow is reduced with maintenance of arterial perfusion pressure. Circulation 1989;80:769–81. [DOI] [PubMed] [Google Scholar]
- 2.Kato M, Kinugawa T, Omodani H, Osaki S, Ahmmed GU, Ogino K, et al. Responses of plasma norepinephrine and reninangiotensin-aldosterone system to dynamic exercise in patients with congestive heart failure. J Card Fail 1996;2:103–10. [DOI] [PubMed] [Google Scholar]
- 3.Katz SD, Biasucci L, Sabba C, Strom JA, Jondeau G, Galvao M, et al. Impaired endothelium-mediated vasodilation in the peripheral vasculature of patients with congestive heart failure. J Am Coll Cardiol 1992;19:918–25. [DOI] [PubMed] [Google Scholar]
- 4.Wiener DH, Fink LI, Maris J, Jones RA, Chance B, Wilson JR. Abnormal skeletal muscle bioenergetics during exercise in patients with heart failure: role of reduced muscle blood flow. Circulation 1986;73:1127–36. [DOI] [PubMed] [Google Scholar]
- 5.Ponikowski P, Francis DP, Piepoli MF, Davies LC, Chua TP, Davos CH, et al. Enhanced ventilatory response to exercise in patients with chronic heart failure and preserved exercise tolerance: marker of abnormal cardiorespiratory reflex control and predictor of poor prognosis. Circulation 2001;103:967–72. [DOI] [PubMed] [Google Scholar]
- 6.Meredith IT, Eisenhofer G, Lambert GW, Dewar EM, Jennings GL, Esler MD. Cardiac sympathetic nervous activity in congestive heart failure. Evidence for increased neuronal norepinephrine release and preserved neuronal uptake. Circulation 1993;88:136–45. [DOI] [PubMed] [Google Scholar]
- 7.Faggiano P, D'Aloia A, Gualeni A, Giordano A. Relative contribution of resting haemodynamic profile and lung function to exercise tolerance in male patients with chronic heart failure. Heart 2001;85:179–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson JR, Rayos G, Yeoh TK, Gothard P, Bak K. Dissociation between exertional symptoms and circulatory function in patients with heart failure. Circulation 1995;92:47–53. [DOI] [PubMed] [Google Scholar]
- 9.Weber KT, Kinasewitz GT, Janicki JS, Fishman AP. Oxygen utilization and ventilation during exercise in patients with chronic cardiac failure. Circulation 1982;65:1213–23. [DOI] [PubMed] [Google Scholar]
- 10.Mancini DM, Eisen H, Kussmaul W, Mull R, Edmunds LH Jr, Wilson JR. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation 1991;83:778–86. [DOI] [PubMed] [Google Scholar]
- 11.Larsen AI, Aarsland T, Kristiansen M, Haugland A, Dickstein K. Assessing the effect of exercise training in men with heart failure; comparison of maximal, submaximal and endurance exercise protocols. Eur Heart J 2001;22:684–92. [DOI] [PubMed] [Google Scholar]
- 12.Metra M, Nodari S, Raccagni D, Garbellini M, Boldi E, Bontempi L, et al. Maximal and submaximal exercise testing in heart failure. J Cardiovasc Pharmacol 1998;32(Suppl 1):S36–S45. [DOI] [PubMed] [Google Scholar]
- 13.Meyer K, Hajric R, Westbrook S, Samek L, Lehmann M, Schwaibold M, et al. Ventilatory and lactate threshold determinations in healthy normals and cardiac patients: methodological problems. Eur J Appl Physiol Occup Physiol 1996;72:387–93. [DOI] [PubMed] [Google Scholar]
- 14.Meyer K, Stengele E, Westbrook S, Beneke R, Schwaibold M, Gornandt L, et al. Influence of different exercise protocols on functional capacity and symptoms in patients with chronic heart failure. Med Sci Sports Exerc 1996;28:1081–6. [DOI] [PubMed] [Google Scholar]
- 15.Wu G, Sanderson B, Bittner V. The 6-minute walk test: how important is the learning effect? Am Heart J 2003;146:129–33. [DOI] [PubMed] [Google Scholar]
- 16.European Heart Failure Training Group. Experience from controlled trials of physical training in chronic heart failure. Protocol and patient factors in effectiveness in the improvement in exercise tolerance. Eur Heart J 1998;19:466–75. [DOI] [PubMed] [Google Scholar]
- 17.Gademan MG, Swenne CA, Verwey HF, van de Vooren H, Haest JC, van Exel HJ, et al. Exercise training increases oxygen uptake efficiency slope in chronic heart failure. Eur J Cardiovasc Prev Rehabil 2008;15:140–4. [DOI] [PubMed] [Google Scholar]
- 18.Wasserman K, Hansen JE, Sue D, et al. Physiology of exercise. In: Weinberg R, editor. Principles of exercise testing and interpretation. Baltimore: Lippincott Williams & Wilkins, 1999: 10–61. [Google Scholar]
- 19.Ozyener F, Rossiter HB, Ward SA, Whipp BJ. Influence of exercise intensity on the on- and off-transient kinetics of pulmonary oxygen uptake in humans. J Physiol 2001;533:891–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lamarra N, Whipp BJ, Ward SA, Wasserman K. Effect of interbreath fluctuations on characterizing exercise gas exchange kinetics. J Appl Physiol 1987;62:2003–12. [DOI] [PubMed] [Google Scholar]
- 21.Francis DP, Davies LC, Willson K, Wensel R, Ponikowski P, Coats AJ, et al. Impact of periodic breathing on measurement of oxygen uptake and respiratory exchange ratio during cardiopulmonary exercise testing. Clin Sci (Lond) 2002;103:543–52. [DOI] [PubMed] [Google Scholar]
- 22.Sietsema KE, Ben Dov I, Zhang YY, Sullivan C, Wasserman K. Dynamics of oxygen uptake for submaximal exercise and recovery in patients with chronic heart failure. Chest 1994;105:1693–700. [DOI] [PubMed] [Google Scholar]
- 23.Belardinelli R, Zhang YY, Wasserman K, Purcaro A, Agostoni PG. A four-minute submaximal constant work rate exercise test to assess cardiovascular functional class in chronic heart failure. Am J Cardiol 1998;81:1210–4. [DOI] [PubMed] [Google Scholar]
- 24.Kemps HM, de Vries WR, Hoogeveen AR, Zonderland ML, Thijssen EJ, Schep G. Reproducibility of onset and recovery oxygen uptake kinetics in moderately impaired patients with chronic heart failure. Eur J Appl Physiol 2007;100:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koike A, Yajima T, Adachi H, Shimizu N, Kano H, Sugimoto K, et al. Evaluation of exercise capacity using submaximal exercise at a constant work rate in patients with cardiovascular disease. Circulation 1995;91:1719–24. [DOI] [PubMed] [Google Scholar]
- 26.Picozzi NM, Clark AL, Lindsay KA, McCann GP, Hillis WS. Responses to constant work exercise in patients with chronic heart failure. Heart 1999;82:482–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kremser CB, O'Toole MF, Leff AR. Oscillatory hyperventilation in severe congestive heart failure secondary to idiopathic dilated cardiomyopathy or to ischemic cardiomyopathy. Am J Cardiol 1987;59:900–5. [DOI] [PubMed] [Google Scholar]
- 28.Meakins J, Long CN. Oxygen consumption, oxygen debt and lactic acid in circulatory failure. J Clin Invest 1927;4:273–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chelimsky-Fallick C, Stevenson LW, Lem V, Whipp BJ. Excessive oxygen deficit during low-level exercise in heart failure. Am J Cardiol 1995;76:799–802. [DOI] [PubMed] [Google Scholar]
- 30.Belardinelli R, Barstow TJ, Nguyen P, Wasserman K. Skeletal muscle oxygenation and oxygen uptake kinetics following constant work rate exercise in chronic congestive heart failure. Am J Cardiol 1997;80:1319–24. [DOI] [PubMed] [Google Scholar]
- 31.Taniguchi Y, Ueshima K, Chiba I, Segawa I, Kobayashi N, Saito M, et al. A new method using pulmonary gas-exchange kinetics to evaluate efficacy of beta-blocking agents in patients with dilated cardiomyopathy. Chest 2003;124:954–61. [DOI] [PubMed] [Google Scholar]
- 32.Roditis P, Dimopoulos S, Sakellariou D, Sarafoglou S, Kaldara E, Venetsanakos J, et al. The effects of exercise training on the kinetics of oxygen uptake in patients with chronic heart failure. Eur J Cardiovasc Prev Rehabil 2007;14:304–11. [DOI] [PubMed] [Google Scholar]
- 33.Borrelli E, Pogliaghi S, Molinello A, Diciolla F, Maccherini M, Grassi B. Serial assessment of peak VO2 and VO2 kinetics early after heart transplantation. Med Sci Sports Exerc 2003;35:1798–804. [DOI] [PubMed] [Google Scholar]
- 34.Brunner-La Rocca HP, Weilenmann D, Schalcher C, Schlumpf M, Follath F, Candinas R, et al. Prognostic significance of oxygen uptake kinetics during low level exercise in patients with heart failure. Am J Cardiol 1999;84:741–4, A9. [DOI] [PubMed] [Google Scholar]
- 35.Schalcher C, Rickli H, Brehm M, Weilenmann D, Oechslin E, Kiowski W, et al. Prolonged Oxygen Uptake Kinetics During Low-Intensity Exercise Are Related to Poor Prognosis in Patients With Mild-to-Moderate Congestive Heart Failure. Chest 2003;124: 580–6. [DOI] [PubMed] [Google Scholar]
- 36.Wilson JR, Groves J, Rayos G. Circulatory status and response to cardiac rehabilitation in patients with heart failure. Circulation 1996;94:1567–72. [DOI] [PubMed] [Google Scholar]
- 37.Lafitte S, Bordachar P, Lafitte M, Garrigue S, Reuter S, Reant P, et al. Dynamic ventricular dyssynchrony: an exercise-echocardiography study. J Am Coll Cardiol 2006;47:2253–9. [DOI] [PubMed] [Google Scholar]
- 38.Chomsky DB, Lang CC, Rayos GH, Shyr Y, Yeoh TK, Pierson RN III, et al. Hemodynamic exercise testing. A valuable tool in the selection of cardiac transplantation candidates. Circulation 1996;94:3176–83. [DOI] [PubMed] [Google Scholar]
- 39.Francis GS, Goldsmith SR, Ziesche SM, Cohn JN. Response of plasma norepinephrine and epinephrine to dynamic exercise in patients with congestive heart failure. Am J Cardiol 1982;49:1152–6. [DOI] [PubMed] [Google Scholar]
- 40.Chiba Y, Maehara K, Yaoita H, Yoshihisa A, Izumida J, Maruyama Y. Vasoconstrictive response in the vascular beds of the non-exercising forearm during leg exercise in patients with mild chronic heart failure. Circ J 2007;71:922–8. [DOI] [PubMed] [Google Scholar]
- 41.Drexler H, Riede U, Munzel T, Konig H, Funke E, Just H. Alterations of skeletal muscle in chronic heart failure. Circulation 1992;85:1751–9. [DOI] [PubMed] [Google Scholar]
- 42.Richardson TE, Kindig CA, Musch TI, Poole DC. Effects of chronic heart failure on skeletal muscle capillary hemodynamics at rest and during contractions. J Appl Physiol 2003;95:1055–62. [DOI] [PubMed] [Google Scholar]
- 43.Diederich ER, Behnke BJ, McDonough P, Kindig CA, Barstow TJ, Poole DC, et al. Dynamics of microvascular oxygen partial pressure in contracting skeletal muscle of rats with chronic heart failure. Cardiovasc Res 2002;56:479–86. [DOI] [PubMed] [Google Scholar]
- 44.Koike A, Hiroe M, Adachi H, Yajima T, Yamauchi Y, Nogami A, et al. Oxygen uptake kinetics are determined by cardiac function at onset of exercise rather than peak exercise in patients with prior myocardial infarction. Circulation 1994;90:2324–32. [DOI] [PubMed] [Google Scholar]
- 45.Matsumoto A, Itoh H, Yokoyama I, Aoyagi T, Sugiura S, Hirata Y, et al. Kinetics of oxygen uptake at onset of exercise related to cardiac output, but not to arteriovenous oxygen difference in patients with chronic heart failure. Am J Cardiol 1999;83:1573–6, A8. [DOI] [PubMed] [Google Scholar]
- 46.Mancini DM, Wilson JR, Bolinger L, Li H, Kendrick K, Chance B, et al. In vivo magnetic resonance spectroscopy measurement of deoxymyoglobin during exercise in patients with heart failure. Demonstration of abnormal muscle metabolism despite adequate oxygenation. Circulation 1994;90:500–8. [DOI] [PubMed] [Google Scholar]
- 47.McDonough P, Behnke BJ, Musch TI, Poole DC. Effects of chronic heart failure in rats on the recovery of microvascular PO2 after contractions in muscles of opposing fibre type. Exp Physiol 2004;89:473–85. [DOI] [PubMed] [Google Scholar]
- 48.Ferreira LF, Hageman KS, Hahn SA, Williams J, Padilla DJ, Poole DC, et al. Muscle microvascular oxygenation in chronic heart failure: role of nitric oxide availability. Acta Physiol (Oxf) 2006; 188:3–13. [DOI] [PubMed] [Google Scholar]
- 49.Toussaint JF, Koelling TM, Schmidt CJ, Kwong KK, LaRaia PJ, Kantor HL. Local relation between oxidative metabolism and perfusion in leg muscles of patients with heart failure studied by magnetic resonance imaging and spectroscopy. J Heart Lung Transplant 1998;17:892–900. [PubMed] [Google Scholar]
- 50.Hanada A, Okita K, Yonezawa K, Ohtsubo M, Kohya T, Murakami T, et al. Dissociation between muscle metabolism and oxygen kinetics during recovery from exercise in patients with chronic heart failure. Heart 2000;83:161–6. [DOI] [PMC free article] [PubMed] [Google Scholar]