Abstract
Percutaneous coronary intervention (PCI) has become an effective therapy to treat coronary artery diseases. However, one of the major drawbacks of PCI is the occurrence of restenosis in 8 to 40% of all treated patients. The GENetic Determinants of Restenosis (GENDER) project was designed to study the association between genetic polymorphisims and clinical restenosis. The discovery of genetic variants associated to the occurrence of restenosis after PCI may provide a more tailored therapy and may serve as rationale for new antirestenotic therapies. So far, several candidate gene approaches had already been performed in the GENDER samples but a Genome Wide Association Scan (GWAS) was still lacking. Here, we present preliminary results from the GWAS we are currently carrying out in the GENDER population. (Neth Heart J 2009;17:262-4.)
Keywords: Percutaneous coronary intervention, restenosis, SNP, genome wide association scan
Atherosclerotic diseases are the leading cause of mortality worldwide.1 According to the World Health Organization (WHO), 5.5 million people die from stroke and 7.2 million people die from coronary heart disease each year. The therapy to treat atherosclerosis depends on the severity of the coronary event. The least invasive treatment is drug therapy to reduce plasma lipid levels. However, when this treatment is insufficient, the treatment of choice has become percutaneous coronary intervention (PCI). Initially, PCI was performed only with balloon catheters but technical advances made it possible to improve patient outcome by using bare metal stents (BMS) or drug-eluting stents (DES) at the site of blockage.2,3
Nevertheless, one of the main drawbacks of PCI is the occurrence of restenosis.4 Clinical restenosis is defined as the re-narrowing of the lumen of the blood vessel to greater than 50% occlusion after PCI usually within three to six months. It occurs in 8 to 40% of all treated persons, depending on patient characteristics and the techniques used. Initially and when only balloon catheters were used, restenosis occurred in around 40% of treated patients. However, the stent placement has reduced the incidence of restenosis to 8 to 25%.5,6
It is believed that restenosis is the response of the body's immune system to the injury induced by PCI. This damage causes segmental thrombus formation and subsequent invasion of macrophages and polymorphonuclear leucocytes, followed by expression and release of numerous growth factors and cytokines from blood cells and stretched smooth muscle cells, leading to proliferation of smooth muscle cells.7 However; it is not well-understood why this process occurs in some individuals but not in others. Several factors have been suggested for increasing the risk of having restenosis. Biological factors such as advanced age, diabetes, hypertension or the total occlusion or the artery before undergoing PCI have been associated with an elevated risk of restenosis after PCI.8,9 However, these biological factors have not been consistently replicated in different studies. Nevertheless, it has been suggested that genetic factors could play a role in the development of the disease.10,11 The discovery of these genetic differences may provide a more tailored therapy for patients with identified increased risk of restenosis after PCI, and may serve as rationale for new antirestenotic therapies.
The GENetic DEterminants of Restenosis (GENDER) project was designed to study the association between various gene polymorphisms and clinical restenosis. It is a multicentre prospective follow-up study with both clinical and angiographic restenosis as clinical endpoint, supported by, among others, the Interuniversity Cardiology Institute of the Netherlands. Inclusion of patients started in March 1999. In June 2001 the last patient was included in the project. In total 3104 consecutive patients were treated successfully by PCI for an acute coronary in four referral centres for interventional cardiology in the Netherlands: Academic Medical Center in Amsterdam, University Medical Center Groningen, Leiden University Medical Center and University Hospital Maastricht. Only patients treated for acute ST-elevation myocardial infarction (MI) were excluded. After having obtained written informed consent, blood was sampled for DNA isolation and future analysis. Clinical and procedural data were gathered prospectively. Clinical restenosis was established during a nine-month follow-up for death, myocardial infarction and target vessel revascularisation. A repeat angiographic study was performed in a subpopulation after six months. An independent endpoint committee evaluated all potential endpoints. Patients were followed for more than nine months and 346 of the patients developed clinical restenosis.
In pilot studies already performed in the GENDER population, several genes have already been identified that may predispose to restenosis, such as the genes for inflammation (e.g. IL-1, CD14, TNF-α), proliferation (CSF) and remodelling (MMP-3), thereby indicating the importance of genetic variation in several candidate genes.11–17 Nevertheless, this candidate gene approach only covers a small proportion of the genetic variation present in the human genome. This drawback can be circumvented by applying new genotyping technologies which can be used to study the genetic variation of millions of genetic markers spread all over the human genome. Very recently we applied these technologies to the GENDER population. Initially, 321 cases and 620 controls were selected to carry on the genetic epidemiology study. Cases and controls were matched by age, gender and other clinical factors such as diabetes and current smoking that have been previously associated to restenosis (table 1).
Table 1.
Matched cases and controls used for the Genome Wide Association scan in the GENDER study.
| Controls (630) | Cases (325) | |||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Sex (Male) | 0.74 | 0.44 | 0.72 | 0.45 |
| Age | 62.01 | 11.09 | 62.44 | 10.57 |
| Stenting | 0.67 | 0.47 | 0.68 | 0.47 |
| Total occlusion | 0.18 | 0.38 | 0.18 | 0.39 |
| Diabetes | 0.21 | 0.41 | 0.2 | 0.4 |
| Current smoking | 0.27 | 0.44 | 0.22 | 0.41 |
| Residual stenosis | 0.11 | 0.33 | 0.16 | 0.37 |
In the first stage, we conducted the genome-wide association analysis using the Illumina Human 610-Quad Beadchips following the manufacturer's instructions. These beadchips contain 620,901 single nucleotide polymorphism (SNP) and copy number variants (CNV) probes. CNV represent a new type of common structural genomic variation, comprising insertions and deletions ranging from kilo bases to mega bases.18 Several studies have already reported associations between CNV and risk of several complex diseases (e.g. autism, psoriasis, schizophrenia).10,19–21
The median spacing between markers is 2.7 kb, therefore offering a dense coverage of the human genome both of SNPs and CNVs and ensuring a good power for detecting these genetic variants associated to the phenotype.
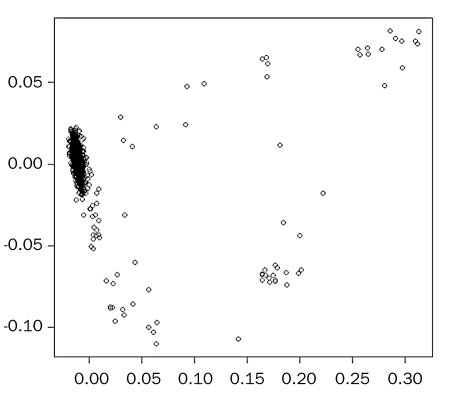
After genotyping, samples and genetic markers were subjected to a stringent quality control protocol. Fourteen samples gave a genotyping Gen Call (GC) lower than 99% and were discarded for further analysis. The mean GC after eliminating the poorly performing samples was 99.9%. Markers with a GC lower than 95% and with minor allele frequency (MAF) lower than 1% across individuals were not considered for further analyses. As a preliminary analysis, we checked for the presence of population substructure in the GENDER study, which could introduce spurious associations when performing the case-control association test. This was conducted by means of a statistical multivariate technique called MultiDimensional Scaling (MDS). This technique reduces the amount of variables (dimensions) needed to explain a matrix of genetic distances between pairs of individuals and plots them in a bi-dimensional plot. The closer the individuals are, the more genetically related they are. So far, we see that the majority of the individuals fall into the same cluster (that is, they are genetically homogeneous) but there are some that are clearly genetic outliers (n=67) and they have been excluded from further analysis at this point in time (figure 1). Finally, one sample was eliminated because it showed an IBS value higher than 0.95, therefore suggesting a close relationship with another sample of the GENDER study. In total, 571 controls, 295 cases and 561,346 SNP markers fulfilled all criteria.
Figure 1 .
Multidimensional scaling plot.
We are currently performing the allelic association test for each SNP and CNV probe. Preliminary results point to interesting loci that might be related to the development of restenosis after PCI. In a second stage and to reduce the chance of false-positive results, we plan to replicate our findings in another European population or populations.
References
- 1.Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet 1997;349: 1498–504. [DOI] [PubMed] [Google Scholar]
- 2.Moses JW, Kipshidze N, Leon MB. Perspectives of drug-eluting stents: the next revolution. Am J Cardiovasc Drugs 2002;2:163–72. [DOI] [PubMed] [Google Scholar]
- 3.Sigwart U, Puel J, Mirkovitch V, Joffre F, Kappenberger L. Intravascular stents to prevent occlusion and restenosis after transluminal angioplasty. N Engl J Med 1987;316:701–6. [DOI] [PubMed] [Google Scholar]
- 4.Lee MS, David EM, Makkar RR, Wilentz JR. Molecular and cellular basis of restenosis after percutaneous coronary intervention: the intertwining roles of platelets, leukocytes, and the coagulation-fibrinolysis system. J Pathol 2004;203:861–70. [DOI] [PubMed] [Google Scholar]
- 5.Agema WR, Monraats PS, Zwinderman AH, de Winter RJ, Tio RA, Doevendans PA, et al. Current PTCA practice and clinical outcomes in The Netherlands: the real world in the pre-drug-eluting stent era. Eur Heart J 2004;25:1163–70. [DOI] [PubMed] [Google Scholar]
- 6.Pache J, Kastrati A, Mehilli J, Schuhlen H, Dotzer F, Hausleiter J, et al. Intracoronary stenting and angiographic results: strut thickness effect on restenosis outcome (ISAR-STEREO-2) trial. J Am Coll Cardiol 2003;41: 1283–8. [DOI] [PubMed] [Google Scholar]
- 7.Nobuyoshi M, Kimura T, Nosaka H, Mioka S, Ueno K, Yokoi H, et al. Restenosis after successful percutaneous transluminal coronary angioplasty: serial angiographic follow-up of 229 patients. J Am Coll Cardiol 1988;12: 616–23. [DOI] [PubMed] [Google Scholar]
- 8.Bourassa MG, Lesperance J, Eastwood C, Schwartz L, Cote G, Kazim F, et al. Clinical, physiologic, anatomic and procedural factors predictive of restenosis after percutaneous transluminal coronary angioplasty. J Am Coll Cardiol 1991;18:368–76. [DOI] [PubMed] [Google Scholar]
- 9.Sobel BE. Acceleration of restenosis by diabetes: pathogenetic implications. Circulation 2001;103:1185–7. [DOI] [PubMed] [Google Scholar]
- 10.Kastrati A, Schomig A, Elezi S, Schuhlen H, Wilhelm M, Dirschinger J. Interlesion dependence of the risk for restenosis in patients with coronary stent placement in multiple lesions. Circulation 1998;97:2396–401. [DOI] [PubMed] [Google Scholar]
- 11.Monraats PS, Agema RP, Jukema JW. Genetic predictive factors in restenosis. Pathol Biol (Paris) 2004;52:186–95. [DOI] [PubMed] [Google Scholar]
- 12.Monraats PS, Pires NM, Schepers A, Agema WR, Boesten LS, De Vries MR, et al. Tumor necrosis factor-alpha plays an important role in restenosis development. FASEB J 2005;19:1998–2004. [DOI] [PubMed] [Google Scholar]
- 13.Monraats PS, Pires NM, Agema WR, Zwinderman AH, Schepers A, de Maat MP, et al. Genetic inflammatory factors predict restenosis after percutaneous coronary interventions. Circulation 2005;112:2417–25. [DOI] [PubMed] [Google Scholar]
- 14.Monraats PS, Rana JS, Nierman MC, Pires NM, Zwinderman AH, Kastelein JJ, et al. Lipoprotein lipase gene polymorphisms and the risk of target vessel revascularization after percutaneous coronary intervention. J Am Coll Cardiol 2005;46:1093–100. [DOI] [PubMed] [Google Scholar]
- 15.Monraats PS, Rana JS, Zwinderman AH, de Maat MP, Kastelein JP, Agema WR, et al. -455G/A polymorphism and preprocedural plasma levels of fibrinogen show no association with the risk of clinical restenosis in patients with coronary stent placement. Thromb Haemost 2005;93:564–9. [DOI] [PubMed] [Google Scholar]
- 16.Monraats PS, de Vries F, de Jong LW, Pons D, Sewgobind VD, Zwinderman AH, et al. Inflammation and apoptosis genes and the risk of restenosis after percutaneous coronary intervention. Pharmacogenet Genomics 2006;16:747–54. [DOI] [PubMed] [Google Scholar]
- 17.Monraats PS, Kurreeman FA, Pons D, Sewgobind VD, de Vries FR, Zwinderman AH, et al. Interleukin 10: a new risk marker for the development of restenosis after percutaneous coronary intervention. Genes Immun 2007;8:44–50. [DOI] [PubMed] [Google Scholar]
- 18.Scherer SW, Lee C, Birney E, Altshuler DM, Eichler EE, Carter NP, et al. Challenges and standards in integrating surveys of structural variation. Nat Genet 2007;39(7 Suppl):S7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Cid R, Riveira-Munoz E, Zeeuwen PL, Robarge J, Liao W, Dannhauser EN, et al. Deletion of the late cornified envelope LCE3B and LCE3C genes as a susceptibility factor for psoriasis. Nat Genet 2009;41:211–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marshall CR, Noor A, Vincent JB, Lionel AC, Feuk L, Skaug J, et al. Structural variation of chromosomes in autism spectrum disorder. Am J Hum Genet 2008;82:477–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stefansson H, Rujescu D, Cichon S, Pietilainen OP, Ingason A, Steinberg S, et al. Large recurrent microdeletions associated with schizophrenia. Nature 2008;455:232–6. [DOI] [PMC free article] [PubMed] [Google Scholar]