Abstract
In this study, Ca2+ release due to spontaneous Ca2+ waves was measured both from inside the sarcoplasmic reticulum (SR) and from the cytosol of rabbit cardiomyocytes. These measurements utilized Fluo5N-AM for intra-SR Ca2+ from intact cells and Fluo5F in the cytosol of permeabilized cells. Restricted subcellular volumes were resolved with the use of laser-scanning confocal microscopy. Local Ca2+ signals during spontaneous Ca2+ release were compared with those induced by rapid caffeine application. The free cytoplasmic [Ca2+] increase during a Ca2+ wave was 98.1% ± 0.3% of that observed during caffeine application. Conversion to total Ca2+ release suggested that Ca2+ release from a Ca2+ wave was not significantly different from that released during caffeine application (104% ± 6%). In contrast, the maximum decrease in intra-SR Fluo-5N fluorescence during a Ca2+ wave was 82.5% ± 2.6% of that observed during caffeine application. Assuming a maximum free [Ca2+] of 1.1 mM, this translates to a 96.2% ± 0.8% change in intra-SR free [Ca2+] and a 91.7% ± 1.6% depletion of the total Ca2+. This equates to a minimum intra-SR free Ca2+ of 46 ± 7 μM during a Ca2+ wave. Reduction of RyR2 Ca2+ sensitivity by tetracaine (50 μM) reduced the spontaneous Ca2+ release frequency while increasing the Ca2+ wave amplitude. This did not significantly change the total depletion of the SR (94.5% ± 1.1%). The calculated minimum [Ca2+] during these Ca2+ waves (87 ± 19 μM) was significantly higher than control (p < 0.05). A computational model incorporating this level of Ca2+ depletion during a Ca2+ wave mimicked the transient and sustained effects of tetracaine on spontaneous Ca2+ release. In conclusion, spontaneous Ca2+ release results in substantial but not complete local Ca2+ depletion of the SR. Furthermore, measurements suggest that Ca2+ release terminates when luminal [Ca2+] reaches ∼50 μM.
Introduction
Under conditions of increased cellular Ca2+ load, ventricular cardiomyocytes exhibit spontaneous transient increases of cytoplasmic [Ca2+] as a result of Ca2+ release from the sarcoplasmic reticulum (SR). The spontaneous increase of intracellular [Ca2+] starts in a discrete area of the cardiomyocyte as a result of the opening of a cluster of SR Ca2+ release channels (type-2 ryanodine receptors (RyR2)). The release then propagates along the length of the myocyte as a Ca2+ wave. The mechanism for propagation is thought to involve a “fire-diffuse-fire” system (1), i.e., Ca2+ release from one cluster of RyR2s, followed by diffusion of cytosolic Ca2+ to a neighboring region of the SR, which causes firing of the adjacent RyR2 cluster via a process of Ca2+-induced Ca2+ release. This mechanism contrasts with normal excitation-contraction (E-C) coupling, where Ca2+ is simultaneously released from the majority of RyR2 clusters throughout the cardiomyocyte after the synchronous influx of Ca2+ via L-type Ca2+ channels (2,3).
Although the mechanism of Ca2+-induced activation of SR Ca2+ release is well characterized, the processes controlling the termination of the release process are less clear. Recent work suggests that reduction of the intra-SR [Ca2+] acts to reduce the open probability of RyR2, thereby terminating release via regulatory proteins bound to the luminal surface of RyR2 (4–8). This is supported by recent work in which luminal Ca2+ was measured during Ca2+ release events (9–11). However, a number of issues remain uncertain. Primarily, it remains to be determined at what luminal [Ca2+] the release process is terminated. Furthermore, luminal Ca2+ measurements in stimulated cardiomyocytes suggest a minimum free [Ca2+] of ∼0.5 mM (11). Estimates of depletion during spontaneous SR Ca2+ release in both intact and permeabilized cardiomyocytes range from 40% to 100% of the total SR content (12–15). This fraction is thought to change in the presence of agents that modulate the Ca2+ sensitivity of the RyR2, such as tetracaine or caffeine (15–17). In the study presented here, cytosolic and luminal Ca2+ measurements were used to determine the degree of Ca2+ depletion from the SR during a spontaneous Ca2+ wave.
Materials and Methods
Cell isolation and permeabilization
New Zealand White rabbits (2–2.5 kg) were euthanized by administration of an intravenous injection of 500 IU heparin, together with an overdose of sodium pentobarbitone (100 mg · kg−1). The hearts were removed in accordance with the United Kingdom Animals (Scientific Procedures) Act 1986. Ventricular myocytes were isolated from Langendorff-perfused rabbit hearts using a previously described enzymatic digestion method (18). Isolated cells were maintained in a modified Krebs solution (zero Ca2+) at a concentration of ∼104 cells/mL until use. The cells were allowed to settle onto the coverslip at the base of a cell perfusion bath. A mock intracellular solution (see below) containing β-escin (Sigma-Aldrich, Dorset, UK) was perfused at 0.1 mg · mL−1 for 0.5–1 min. When spontaneous oscillations were observed, the β-escin was removed.
Solutions
Permeabilized cells were perfused with a mock intracellular solution with the following composition (mM): 100 KCl, 5 K2ATP, 5 Na2PCr, 5.5 MgCl2, 25 HEPES, 0.05 K2EGTA, pH 7.0 (20–21°C). The [Ca2+] in the perfusing solution was varied by the addition of known amounts of 1 M CaCl2 stock solution (VWR, Leicestershire, UK). Free Ca2+ was confirmed using the intracellular calibration method (19) with solutions containing 10 mM EGTA. Free [Ca2+] was calculated by means of a computer program (React; G.L. Smith, University of Glasgow, Glasgow, UK) with known affinity constants for H+, Ca2+, and Mg2+ for EGTA (20) and ATP and PCr (21). These chemicals were supplied by Sigma (Dorset, UK).
Measurements of cytoplasmic and SR [Ca2+]
To monitor cytosolic [Ca2+] within permeabilized cardiomyocytes, cells were perfused with a mock intracellular solution containing 10 μM Fluo-5F (Invitrogen). The methods associated with calibration and conversion of the Fluo-5F fluorescence signal to cytoplasmic [Ca2+] were previously described in detail (14). Briefly, at the end of the experiment, cardiomyocytes were perfused with calibration solutions containing a range of [Ca2+] buffered with 10 mM EGTA. The resulting intracellular fluorescence was used to calculate intracellular [Ca2+] from the preceding fluorescence signal, assuming that the affinity of Ca2+ for Fluo-5F was 1.04 μM (22).
Intra-SR Ca2+ measurements were made by Sigma-Aldrich incubating cardiomyocytes with 10 μM Fluo-5N AM (Invitrogen, Paisley, UK) for 3–6 h at 37°C. Cytosolic dye was removed upon permeabilization, leaving the remaining dye trapped within intracellular organelles, including the SR. The SR-related component was assessed at the end of an experimental procedure by perfusing cardiomyocytes with 10 mM caffeine. Under these conditions, luminal [Ca2+] was considered to be equilibrated across the SR membrane. Separate measurements established that the degree of chemical quenching of the Fluo-5N fluorescence by caffeine was minimal and had no significant consequences on the calculated values of intra-SR Ca2+. Previous findings suggest that the maximum free [Ca2+] achieved within the lumen of the SR is within the range of 0.7–1.5 mM (23). The recorded Fluo-5N fluorescence signals were converted to values of free luminal [Ca2+] using the following assumptions: 1), the maximal fluorescence observed between Ca2+ waves corresponds to a free [Ca2+] of 1.1 mM (taken as Fo); 2), the minimum fluorescence observed after perfusion with 10 mM caffeine is equal to the extracellular [Ca2+]; and 3), the affinity of Fluo-5N for Ca2+ is 400 μM (11). The mean ΔFcs/Fo was taken as the caffeine-sensitive change in fluorescence relative to Fo. Where total Ca2+ was calculated, it was expressed per liter cell cytosol, given an SR volume of 3.5% and a total cytosolic volume that was 65% of the cell (24). The Kd and Bmax of intra-SR buffering were taken as 630 μM and 140 μmol/L cytosol, respectively (25). Cytosolic buffering was accounted for as previously described (14,25).
Confocal microscopy
Fluorescence confocal linescan images were recorded using a BioRad Radiance 2000. The Fluo-5F in the perfusing solution or the Fluo-5N in the SR was excited at 488 nm using a Kr/Ar laser and measured above 515 nm using the epifluorescence optics of a Nikon Eclipse inverted microscope with a Fluor 60× water objective lens (NA 1.2). The iris diameter was set at 1.9, providing an axial (z) resolution of ∼0.9 μm and x-y resolution of ∼0.5 μm based on full width at half-maximum amplitude measurements of images of 0.1 μm fluorescent beads (Invitrogen). Data were acquired in linescan mode at 2 ms/line, and the pixel dimension was 0.3 μm (512 pixels/scan; zoom = 1.4). The scanning laser line was oriented parallel with the long axis of the cell and placed approximately equidistant between the outer edge of the cell and the nucleus/nuclei to ensure that the nuclear area was not included in the scan line. To avoid movement artifacts, contraction was suppressed by inclusion of 10 μM cytochalasin D in the perfusing solution.
Image analysis
All image analysis was carried out using programs written in the Borland Delphi language, with the Pixelook library (http://plsoft.users.btopenworld.com/). Temporal offsetting of Ca2+ wave linescan images was only carried out on wavefronts with homogeneous velocity over at least 40 μm. To acquire accurate measurements of the time course and amplitude of the fluorescence changes that occurred during the Ca2+ waves, >100 pixelwide sections (∼1/3 of the cell) with homogeneous velocity were averaged. Because of the nonsynchronous nature of the propagated release, velocity was corrected for by offsetting each pixel by the amount required to produce a new image with the linescan realigned to the same time for the individual spontaneous release event (Figs. 1 A and 2 A). This was accomplished by means of the autocorrelation method used by Cheng et al. (26). This realignment allowed accurate resolution of the amplitude and time course of the Ca2+ wave, minimizing temporal smearing of the mean fluorescence signal from the selected region. This technique was applied to signals derived from the cytoplasmic Ca2+ indicator (Fluo-5F; see Fig. 1 A) and intra-SR Ca2+ indicator (Fluo-5N; see Fig. 2 A).
Figure 1.
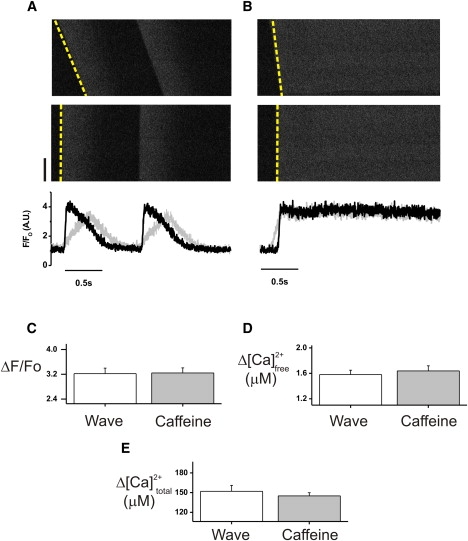
Measurement of cytosolic Ca2+ during spontaneous Ca2+ waves and caffeine-induced Ca2+ release. (A) Upper: Typical confocal linescan image of two Ca2+ waves from a single permeabilized cardiomyocyte perfused with 600 nM Ca2+ (in 50 μM EGTA, 10 μM Fluo-5F). The image is taken from approximately half the length of the cell (black bar indicates 20 μm). Middle: Image from the upper panel after temporal realignment to allow for analysis of the propagation velocity (∼160 μms−1). Lower: Normalized fluorescence traces corresponding to the original (black) and aligned (gray) images. (B) The same cell during a caffeine-induced Ca2+ release, with the original (upper) and aligned (middle) images and their corresponding fluorescence traces (lower). Bar graphs show a comparison of the amplitude of spontaneous Ca2+ wave versus caffeine in terms of F/Fo (C), free cytosolic [Ca2+] (D), and total cytosolic [Ca2+] (E).
Figure 2.
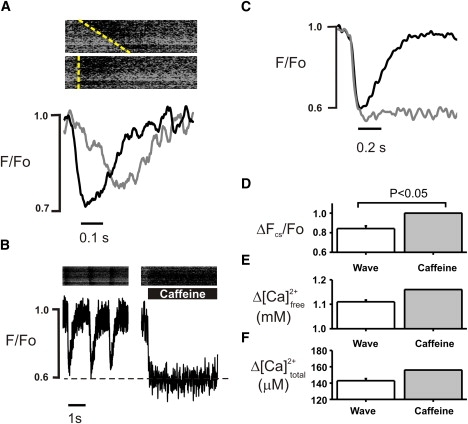
Measurement of luminal Ca2+ in spontaneous Ca2+ waves and caffeine-induced Ca2+ release. (A) Confocal linescan image of cardiomyocyte loaded with fluo-5N-AM before (upper) and after (middle) correction for propagation. Lower: Resultant fluorescence trace from the original (gray line) and corrected (black line) images. Fluorescence was normalized to F/Fo, where Fo is the fluorescence recorded just before release occurred. (B) Temporally realigned fluorescence signal indicating changes in SR content during spontaneous and caffeine-induced release. (C) Superimposed signals from caffeine (gray) and spontaneous release (black). Bar graphs of Fcs/Fo amplitude (D), free [Ca2+]SR (E), and total [Ca2+]SR (F).
Computational modeling
A previously published (14) three-compartment model consisting of 1), the SR; 2), the cytosol; and 3), the extracellular compartments of the cell allowed reconstruction of the changes in Ca2+ observed (see inset in Fig. 5 for diagram of fluxes). Estimates of the magnitude and time course of the various Ca2+ flux pathways were derived from experimental measurements in permeabilized cardiac myocytes. Cytoplasmic [Ca2+] from a resolution-limited volume of a cardiac myocyte during the Ca2+ wave was calculated from confocal Fluo-5F fluorescence signals. These values, in conjunction with estimates for intracellular buffering and the diffusion rate constant, were used to calculate the underlying Ca2+ fluxes. This approach was based on the assumption that once initiated, the Ca2+ wave in cardiac muscle is a one-dimensional wave, i.e., effectively, the Ca2+ wave occurs simultaneously throughout the depth and width of the cell. SR Ca2+ release was initiated when luminal [Ca2+] reached a threshold of 1.1 mM. The magnitude and time course of the change in RyR2 permeability during a Ca2+ wave was based on experimental measurements (14). The application of tetracaine was modeled by increasing the luminal [Ca2+] necessary to trigger release by 40%. This increase approximated experimentally observed values (see Fig. 3).
Figure 5.
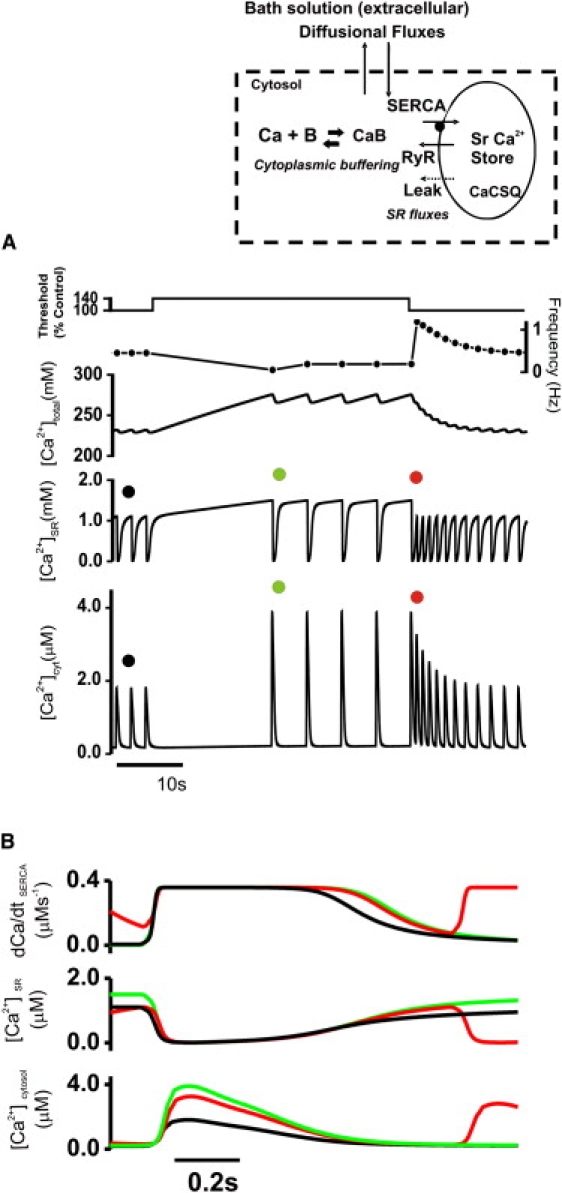
Data from a three-compartment simulation of spontaneous Ca2+ release, showing the effects of an increased SR release threshold. (Inset) Three-compartment model diagram outlining the Ca2+ fluxes and buffering involved in the extracellular, cytosolic, and SR compartments. (A) Traces showing the threshold for release, Ca2+ wave frequency, total cell [Ca2+], SR [Ca2+], and cytosolic Ca2+. Circles denote the starting points of traces of corresponding colors in B. (B) Rescaled trace of data in A.
Figure 3.
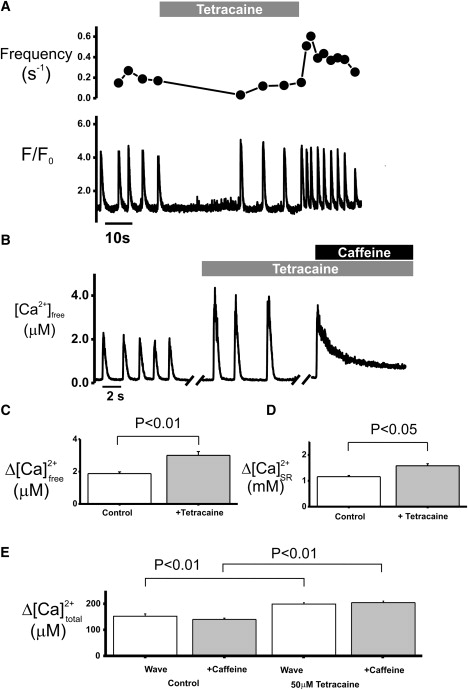
Effect of 50 μM tetracaine on spontaneous Ca2+ release in permeabilized cardiomyocytes. (A) Traces show spontaneous Ca2+ wave frequency (upper) and corresponding F/Fo (lower), showing the quiescent period, steady state, and rapid burst of releases on removal of tetracaine. (B) Traces from linescan recordings from a different cell after conversion to free Ca2+ from a typical cell in control conditions (600 nM Ca2+, Fluo-5F). After steady state is achieved, larger, less frequent spontaneous releases occur. The amplitude of caffeine-induced release in the presence of tetracaine is similar to that of spontaneous release. Waves were corrected for temporal smearing. Bar graphs showing mean data ± SE comparing values for free [Ca2+] (C), SR [Ca2+] (D), and total [Ca2+] (E), without (control) and in the presence of 50 μM tetracaine (n = 6).
Statistics
Data are shown as means ± standard error (SE) and were compared with the use of Students' t-test in all cases, with significance accepted at p < 0.05.
Results
Comparison of cytoplasmic Ca2+ signals during a Ca2+ wave and caffeine-induced Ca2+ release
During a spontaneous wave, cellular Ca2+ rises to ∼1.5 μM as a result of release from the SR. This then falls, mostly due to uptake via sarco/endoplasmic reticulum Ca2+-ATPase (SERCA), but also as a result of a loss via diffusion across the permeabilized membrane. It was previously shown that the diffusional loss is recovered as SERCA lowers the cellular Ca2+ below that of the bulk extracellular perfusate (to reach a minimum of ∼150 nM), and that over a period of three to five waves, the mean extra- and intracellular [Ca2+] levels are balanced. Fig. 1 A shows a linescan image from a permeabilized rabbit cardiomyocyte. The perfusing solution contained ∼600 nM Ca2+; under these conditions, Ca2+ waves occurred spontaneously at a rate of ∼0.4 Hz. The Ca2+ waves shown propagate along the length of the cell at a constant velocity (173.3 ± 9.7 μms−1, n = 7). The slow rate of rise of the mean fluorescence signal is due to the smearing of a more rapid upstroke by the nonsynchronous nature of the Ca2+ wave. This is evident from Fig. 1 A (middle), where the linescan image was corrected for a constant propagation velocity. The mean fluorescence from the corrected fluorescence signal shows the rapid upstroke associated with the Ca2+ wave. Fig. 1 B (upper) shows the linescan image from the same permeabilized cardiomyocyte upon rapid application of 10 mM caffeine. The rise of fluorescence is almost synchronous along the length of the cell (velocity ∼800 μms−1). The linescan image was corrected for the small delay in Ca2+ release that occurred along the length of the cell (Fig. 1 B, middle). After correction, the amplitude of the fluorescence transient was comparable to that of the corrected Ca2+ wave signals, indicating that equivalent amounts of Ca2+ were released from the SR during both events. These signals can be converted to free [Ca2+] changes with the use of calibration solutions applied at the end of the protocol (14). The free cytoplasmic [Ca2+] was subsequently converted to total cytoplasmic [Ca2+] using published values of the cytoplasmic buffering (27). The mean values for Ca2+ wave amplitude and the subsequent caffeine-induced Ca2+ release are shown in Fig. 1 C. On average, the amplitude of change in cytoplasmic [Ca2+] during a Ca2+ wave was 98.1% ± 0.3% (n = 7) of that induced by caffeine. When the total cytoplasmic Ca2+ signals were compared, the increase in total [Ca2+] during a Ca2+ wave was 104% ± 6% (n = 7) of that from a caffeine-induced release. The larger error in the latter measurement is due to the variation in initial values of free [Ca2+] used to calculate the change in total cytoplasmic [Ca2+]. These data indicate that the total Ca2+ released from the SR during a Ca2+ wave was indistinguishable from that released by rapid application of caffeine, and suggests complete depletion of the SR during a Ca2+ wave. The rise in total cytoplasmic [Ca2+] as a result of rapid caffeine application was 164 ± 6 μM (Fig. 1 E), which translates to a total [Ca2+] within the SR before release of 2.83 ± 0.11 mM and a free [Ca2+] of 1.12 mM using previously published values of SR volume and buffering (25).
Comparison of luminal Ca2+ signals during a Ca2+ wave and caffeine-induced Ca2+ release
Fig. 2 shows Ca2+ waves and caffeine-induced release examined from the perspective of intra-SR Ca2+ by loading the lumen of the SR with the low-affinity dye Fluo-5N. After permeabilization and perfusion with a mock intracellular solution, the staining of the cardiac cell showed a marked striated pattern comparable to that observed in previous studies (10,28). Perfusion with a mock intracellular solution containing 600 nM Ca2+ caused spontaneous Ca2+ release to occur at a frequency of ∼0.4 Hz. Linescan images indicated transient decreases in fluorescence that propagated along the length of the cell with a velocity similar to that observed with signals derived from the cytoplasmic indicator Fluo-5F (172 ± 6 μms−1, n = 9). Rapid application of caffeine caused a rapid decrease in fluorescence uniformly along the length of the cardiomyocyte. The fluorescence signals derived from both spontaneous Ca2+ waves and rapid caffeine application were corrected for their associated propagation velocity by using the same realignment process applied to the cytoplasmic signals (see Fig. 2 A). Fig. 2 B shows three sequential waves of approximately equal velocity realigned to show the relative fluorescence change compared to the caffeine response in the same cell. These waves show an ∼80% reduction in relative fluorescence compared to the caffeine response. The mean cellular fluorescence derived from the corrected signal in Fig. 2 B is shown in Fig. 2 C. The rapid decrease in fluorescence associated with a Ca2+ wave was routinely smaller in amplitude than that observed during rapid caffeine application, with a mean decrease in the fluorescence signal associated with a Ca2+ wave 82.5% ± 2.6% (n = 7) of that observed on rapid application of caffeine.
Using the previously published value of the affinity constant for Ca-Fluo5N (29), and assuming a maximum intra-SR [Ca2+] before release of 1.1 mM, the decrease in free intra-SR [Ca2+] during a Ca2+ wave was 96.2% ± 0.7% (n = 7) of that observed upon rapid addition of caffeine. The calculations suggest that during a Ca2+ wave, the minimum intra-SR free Ca2+ was 46 ± 7 μM (n = 7). These values were converted to changes in total Ca2+ within the SR using previously published values of the intra-SR Ca2+ buffering power (27). The results predicted that the total SR [Ca2+] would decrease by 2.46 ± 0.04 mM and the total cytoplasmic Ca2+ would increase by 143 ± 2.5 μM (n = 7) (Fig. 2 F). The measurements indicate that the total SR [Ca2+] during a Ca2+ wave decreased by 91.7% ± 1.6% of that observed during rapid caffeine administration. This value is significantly different from 100% (p < 0.05) and indicates that despite the significant amount of Ca2+ remaining within the SR during a Ca2+ wave (46 μM free, 142 μM total), the total Ca2+ released was ∼92% of that within the SR. The mean values of fluorescence change, change in free Ca2+, and total Ca2+ released during both a Ca2+ wave and caffeine application are given in Fig. 2, D–F. Note that the increase in total cellular [Ca2+] for a caffeine-induced release is ∼160 μM, and this value is within 5% of the value calculated from cytosolic signals (Fig. 1 E). These observations predict that SR depletion is close to complete during a Ca2+ wave.
Effects of tetracaine on spontaneous SR Ca2+ release and caffeine-induced Ca2+ release
The effects of tetracaine (50 μM) on spontaneous Ca2+ release are shown in Fig. 3. Previous studies of permeabilized (17) and intact rat cardiomyocytes (16,30) showed that addition of tetracaine transiently suppressed the spontaneous Ca2+ release. When SR Ca2+ release returned, the frequency of spontaneous release was lower than control values, whereas the amplitude of the Ca2+ wave was increased. Removal of tetracaine caused a transient burst of higher-frequency spontaneous Ca2+ waves before amplitude and frequency returned to control values. The changes in the spontaneous Ca2+ wave frequency are shown in detail in Fig. 3 A (upper). Fig. 3 B shows typical Ca2+ signals derived from velocity-corrected linescan signals before and during application of tetracaine. The larger transient amplitudes recorded in tetracaine were of comparable amplitude to the Ca2+ release caused by rapid caffeine application. The mean values of the Ca2+ signals from a number of experiments are shown in Fig. 3, C–E. These data show that addition of tetracaine caused a significant increase in Ca2+ wave amplitude and reduced Ca2+ wave frequency (Fig. 3, C and D). Quantification of the Ca2+ released from the SR during Ca2+ waves indicated that, as seen in the absence of tetracaine, the SR Ca2+ release could not be distinguished from caffeine-induced release (97.3% ± 0.4%). The increased amplitude of both release events indicated an increased SR Ca2+ content in tetracaine of ∼30%. Using previously published values of intra-SR buffering, this would predict a maximum free [Ca2+] within the SR of 1.58 ± 0.08 mM in tetracaine before release, a value significantly higher than that calculated under control conditions (p < 0.01).
Comparison of luminal Ca2+ signals during a Ca2+ wave and caffeine-induced Ca2+ release after tetracaine addition
Fig. 4 shows the effect of tetracaine on spontaneous Ca2+ release using intra-SR signals derived from luminal Fluo-5N. On application of tetracaine, there was a modest elevation of the fluorescence signal before release, indicating an increased luminal [Ca2+]. An increased luminal [Ca2+] was observed in ∼25% of the cells. The lack of a consistent increase despite a ∼30% increase in SR Ca2+ release observed in cytoplasmic signals can be explained by the poor sensitivity of the luminal indicator in this range of Ca2+ (29). Although the dye has a relatively low Ca2+ affinity, the conversion of Fluo-5N fluorescence to SR [Ca2+] will be associated with a 2.5-fold higher error at 1.6 mM compared to 1.1 mM (see Fig S1 in the Supporting Material). The decrease in Ca2+ wave frequency when tetracaine was applied indicated an effect comparable to those observed using the cytoplasmic indicator. Fig. 4 A (upper) shows fluorescence signals during a Ca2+ wave and caffeine application in the absence and presence of tetracaine. Fig. 4 A (lower) shows a typical signal from spontaneous and caffeine-induced Ca2+ release in the presence of tetracaine. Fig. 4, B–D, show the mean values of SR depletion during a Ca2+ wave and caffeine application. Assuming a maximum free [Ca2+] of 1.6 mM, a Ca2+ wave in tetracaine caused a change in free [Ca2+] of 94.5% ± 1.1% (n = 6), a change in total [Ca2+] of 88.1% ± 2.2% and a minimum [Ca2+] of 87 ± 19 μM. These data suggest that inhibition of RyR2 by tetracaine does not dramatically modify the fractional release of Ca2+ from the SR; the larger amount of Ca2+ release is achieved by an increased threshold [Ca2+] for spontaneous release.
Figure 4.
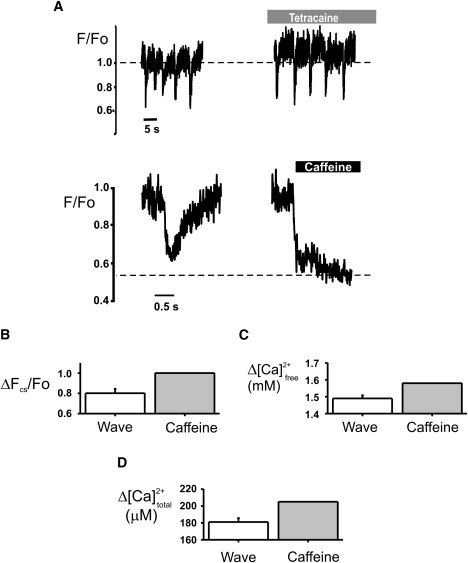
Effect of 50 μM tetracaine on luminal Ca2+ in spontaneous Ca2+ waves. (A) Upper: Fluo-5N fluorescence signal before and after application of 50 μM tetracaine, demonstrating a decrease in spontaneous Ca2+ release frequency. Lower: An averaged Ca2+ wave signal from the last four transients and the subsequent caffeine application (20 mM) (black bar). Bar graphs show mean ΔFcs/Fo (B), ΔCa2+ free (C), and ΔCa2+ total (D) (n = 6) during wave- and caffeine-induced releases. No significant differences were found.
Computational modeling of the response of SR to tetracaine addition
In previous work, we established a computational model of spontaneous Ca2+ release with values of diffusion coefficient, SR Ca2+ uptake, and release fluxes that were based on measurements from permeabilized rabbit cardiomyocytes (14). This model was used to test whether completely depleting the SR was capable of reproducing the behavior of intact and permeabilized cardiac myocytes. As demonstrated above, lowering the Ca2+-sensitivity of RyR2 using tetracaine increased the apparent SR Ca2+ threshold for spontaneous Ca2+ release. These changes were imposed on a computational model of SR Ca2+ release as shown in Fig. 5 A. The threshold for release was raised from an SR free [Ca2+] of 1.16 mM to 1.58 mM. This resulted in an inhibition of spontaneous release as the Ca2+ content of the SR was filled to the new higher threshold value. Spontaneous Ca2+ release was then reestablished with a new steady state at a lower rate of oscillation. Restoration of the control threshold for SR Ca2+ release (i.e., removal of tetracaine) induced a burst of high-frequency spontaneous Ca2+ releases very similar to those observed experimentally (Fig. 3 A). Fig. 5 B shows a detailed time course of the individual Ca2+ release events from selected parts of the trace. In addition to free cytoplasmic [Ca2+] and free luminal [Ca2+], the calculated rate of SERCA-mediated Ca2+ uptake is also shown. The higher rate of spontaneous release on removal of tetracaine can be explained by the extended phase of maximal SR Ca2+ uptake after release. This is retained for several seconds after the normal threshold for SR Ca2+ release is restored. The higher rate of SR Ca2+ uptake is the result of a higher cytoplasmic [Ca2+] during the decay phase of the spontaneous Ca2+ release. This phase is prolonged because the minimum diastolic [Ca2+] remains increased for several seconds after the removal of tetracaine. During this phase, total cellular Ca2+ will gradually return to control levels (Fig. 5 A, after red circle), with a net loss of Ca2+ due to diffusion across the permeabilized surface membrane.
Discussion
Spontaneous Ca2+ release in cardiac cells is thought to be caused by an elevated SR [Ca2+] that sensitizes clusters of RyR2s to cytosolic Ca2+, resulting in channel opening. Similarly, termination of release is thought to occur when SR Ca2+ falls to a level that desensitizes the cytosolic Ca2+ release site to close the RyR2. Currently, there is no consensus about the level of SR depletion required to terminate spontaneous Ca2+ release.
In this study, SR Ca2+ release during spontaneous Ca2+ waves in permeabilized ventricular myocytes was used as a tool to elucidate the mechanisms underlying RyR2 function. Cytoplasmic Ca2+ measurements indicated that the amount of Ca2+ released from the SR during a Ca2+ wave was indistinguishable from that produced by rapid caffeine application. Therefore, these measurements cannot resolve whether any intra-SR Ca2+ remains after Ca2+ release. In contrast, measurements of intra-SR free [Ca2+] indicated that depletion of the SR during a Ca2+ wave was significantly less than that observed during caffeine application. This apparent contradiction was resolved when the intra-SR [Ca2+] signals were converted to changes in total [Ca2+]; under these circumstances, the Ca2+ release during a Ca2+ wave resulted in a degree of SR depletion that was ∼96% of the total available Ca2+, a value well within the error of the measurements from cytoplasmic [Ca2+] signals.
Assessment of SR depletion using cytoplasmic Ca2+ sensitive indicators
This study shows that when the cytoplasmic Ca2+ indicator Fluo-5F is used, the amplitude of the Ca2+ transients caused by a spontaneous Ca2+ wave is only ∼3% smaller than that caused by rapid caffeine application. This result contrasts with previous work on whole cell Ca2+ and shortening, which indicated that spontaneous Ca2+ release caused a much smaller rise of intracellular Ca2+ or cell shortening compared to caffeine-induced release (15). The explanation lies in the temporal smearing of the propagated Ca2+ wave. Normally, a Ca2+ wave takes ∼1 s to propagate along the length of the cell at room temperature. As demonstrated in Fig. 1, at any one time over this period only ∼30% of the volume of the cardiomyocyte experiences an increased cytoplasmic [Ca2+], whereas ∼70% of the volume is either awaiting the arrival of the Ca2+ wave or is recovering after the release phase. In contrast, caffeine releases Ca2+ synchronously throughout the cardiomyocytes; therefore, local and whole cell signals are comparable.
Allowing for differences in initial cytoplasmic [Ca2+] values while calculating the increase in total [Ca2+] during a Ca2+ wave and from rapid caffeine application did not reveal significantly different values between these two Ca2+ release events, as the variability prevented differences of <10% from being distinguished. The higher variability arises from the uncertainties over the resting (minimum) free [Ca2+] and peak (maximum) free [Ca2+], which translates to much larger variations in total cellular [Ca2+]. The use of an alternative Ca2+-sensitive dye would not reduce these errors, because although an indicator with a lower Ca2+ affinity than Fluo-5N may provide better resolution of the peak Ca2+ signal, estimation of the minimum Ca2+ values would be less precise.
Assessment of SR depletion using luminal Ca2+ indicators
The staining pattern of Fluo-5N AM-loaded cardiomyocytes observed in this study is similar to that published in previous studies (10,28) and consistent with the majority of the dye accumulating in the terminal cisternae of the SR. When velocity-correction techniques were applied to the linescan images of Fluo-5N fluorescence signals, the amplitude of the fluorescence change associated with a Ca2+ wave was only 82% of that observed during a rapid caffeine application. This value was similar to the approximate value reported in an earlier study (10). Assuming that caffeine allows equilibration of the [Ca2+] between intra-SR and cytoplasmic compartments, the fluorescence signal at the peak of the Ca2+ wave translates to a minimum intra-SR [Ca2+] of ∼47 μM. With the additional assumption that the maximum free intra-SR [Ca2+] signal between Ca2+ waves is 1.1 mM, the change in free [Ca2+] during a Ca2+ wave is ∼96% of that during caffeine exposure. Converting these values to total Ca2+ values suggests that the total Ca2+ released from the SR during a wave is ∼92% of that released during caffeine. These values are comparable to the estimates provided by the cytoplasmic Ca2+ signals and suggest that although SR Ca2+ release terminates before complete SR depletion, the total Ca2+ released is >90% of the total SR content. As shown in Fig. 6, this estimate of the degree of depletion was relatively insensitive to the assumed maximal free [Ca2+] within the SR, indicating that this was not a crucial parameter in this calculation. These measurements were made at room temperature (20°C) and should be repeated at 37°C. An increased temperature will alter the Kd and Vmax of SERCA, and may change the affinity of cytoplasmic and intra-SR buffers. None of these changes will alter the fractional SR Ca2+-release. However, the temperature dependence of RyR2 in terms of Ca2+ sensitivity and cooperative function within the cluster is unclear; both of these factors will determine the degree of depletion at 37°C.
Figure 6.
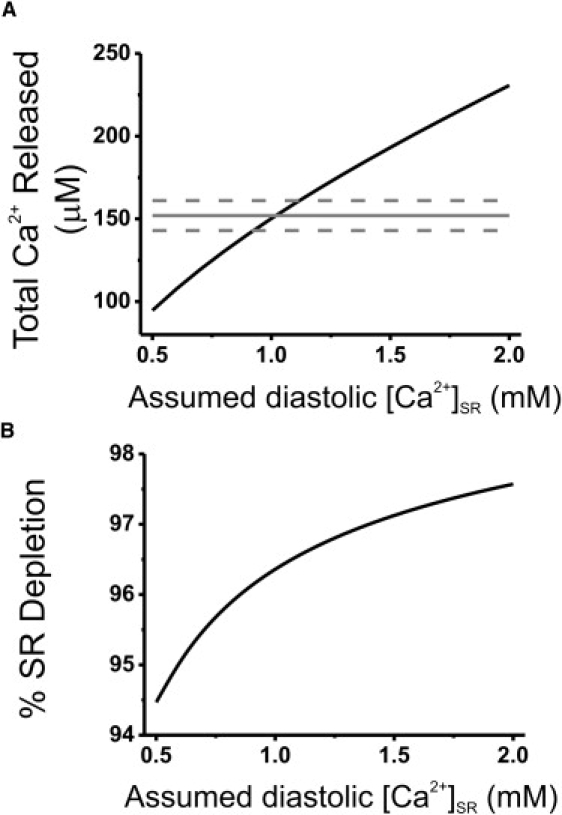
Calculated relationship between assumed maximal diastolic [Ca2+] and % SR depletion based on a range of “assumed” resting SR [Ca2+] values. (A) The solid black line is the relationship between assumed diastolic Ca2+ and the calculated total Ca2+ released during a Ca2+ wave. Gray lines indicate measured values of total Ca2+ released from the SR during a Ca2+ wave (based on cytosolic Ca2+ measurements). Dotted lines indicate ±10%. (B) Calculated % depletion of the SR during a Ca2+ wave, assuming a minimum [Ca2+]SR of 50 μM and a range of values of maximal diastolic [Ca2+]SR.
Future studies will also determine whether the level of intra-SR Ca2+ remaining after a Ca2+ wave is similar when Ca2+ release is activated via E-C coupling mechanisms. Previous measurements of SR depletion on whole intact cardiomyocytes indicated a minimum SR Ca2+ of 0.5 mM across a range of inotropic levels (11). This is considerably higher than the ∼50 μM reported in this study on permeabilized cells, and implies a large reserve remaining within the SR. Further studies are required to investigate the reasons for this discrepancy, which may lie in the efficiency of E-C coupling and/or in the difference between local and global depletion measurements.
Implications for the control of Ca2+ release from the SR
The data presented in this study can be used to describe a simple model for the control of Ca2+ release from the SR by both cytoplasmic and intra-SR control processes. As illustrated in Fig. 7, before Ca2+ release, cytoplasmic [Ca2+] adjacent to the cluster of RyR2 is ∼100 nM and the intra-SR [Ca2+] approaches 1.1 mM due to SERCA-mediated Ca2+ uptake. As intra-SR [Ca2+] increases, the open probability (Po) of RyR2 increases due to the action of Ca2+on luminal regulatory proteins (junctin/triadin/calsequestrin). When intra-SR free [Ca2+] approaches 1 mM, the Po of RyR2 increases dramatically and triggers Ca2+ efflux from the RyR2 cluster, thereby depleting intra-SR [Ca2+] and increasing the [Ca2+] on the cytoplasmic surface of the RyR2 cluster. The raised cytoplasmic [Ca2+] will further activate the RyR2 cluster via the Ca2+-induced Ca2+-release process. The sustained increase in cytoplasmic [Ca2+] would maintain a high RyR2 permeability, effectively shifting the intra-SR Ca2+ dependence of RyR2 activity to the left (see Fig. 7). Under these circumstances, intra-SR Ca2+ would continue to decrease during the release phase until levels reach ∼50 μM. At this point, intra-SR regulatory processes would reduce the RyR2 Po sufficiently to terminate release, thereby allowing the cytoplasmic Ca2+ to start to decrease. This process would occur on a timescale of <50 ms.
Figure 7.

Proposed cytosolic and intra-SR RyR Ca2+ sensitivity during a Ca2+ wave. (A) Time course of cytosolic (upper) and intra-SR (lower) [Ca2+] during a simulated Ca2+ wave. Open and solid circles indicate points on curves in B and C. (B) A series of curves showing the sensitivity of RyR. Increasing cytosolic Ca2+ shifts the curve to the left. (C) Series of sensitivity curves of RyR; reducing luminal Ca2+ shifts the curve to the left.
In theory, the mechanism proposed in Fig. 7 could be employed to explain the graded nature of fractional SR Ca2+ release observed during physiological E-C coupling (11). The graded release can be explained in terms of the interplay of the Ca2+-sensing sites on the luminal and cytoplasmic faces of the RyR2 cluster. To explore one example, the relative depletion of SR Ca2+ during E-C coupling is lower when the SR Ca2+ content is low (11). This result can be explained in terms of a reduced number of release clusters activated and partial depletion of the active release sites. Low luminal [Ca2+] will reduce the Ca2+ sensitivity of the cytoplasmic sensors to Ca2+. This will reduce the probability of the cluster firing, thereby reducing the number of active clusters recruited by the L-type Ca2+ current trigger. The active clusters will then experience a reduced dyadic [Ca2+] due to the lower [Ca2+] gradient across the SR membrane. This will limit the activation of RyR2 and therefore encourage the premature termination of the release event.
Effects of altered RyR2 threshold: comparison of experimental and computational results
Pharmacological agents can be used to raise or lower RyR2 sensitivity, thus effectively reducing or increasing the effective threshold [Ca2+] within the SR for spontaneous Ca2+ release. In this study, tetracaine was used to investigate whether reduced RyR2 sensitivity can alter the fractional depletion of the SR during a Ca2+ wave. Under the conditions of this study, tetracaine (50 μM) resulted in an increased SR Ca2+ content of ∼30%, but there was no dramatic change in the fractional depletion of the SR during a Ca2+ wave. However, the minimum [Ca2+] remaining within the SR was ∼2 times higher than in the absence of RyR inhibition. This suggests that tetracaine's action on RyR2 includes the desensitization of the processes that control termination of the release process. This can be envisaged as a parallel rightward shift in the Ca2+-sensitivity curves for both the luminal and cytoplasmic control sites (Fig. 7). This proposal is supported by data on isolated RyR2 channels indicating that tetracaine would increase the [Ca2+] required to reaching a minimal Po value (31). Alternatively, a similar relationship would result from a simple blocking action of tetracaine on RyR2. Activation would require a higher fraction of the RyR2 channels in a cluster to achieve SR release, and to increase the effectiveness of stochastic attrition when SR Ca2+ release occurs.
The larger total Ca2+ release observed in tetracaine was simply the result of the higher maximal free [Ca2+]. As reported in previous studies (16,17), removal of tetracaine caused a burst of high-frequency Ca2+ waves, which returned to normal amplitude and frequency after 3–6 s. Previous studies suggested that this characteristic burst is due to a rapid increase of the sensitivity of RyR2, but Ca2+ waves do not return to normal until the SR content has been restored (16,17). This explanation was based on the measurements showing that a Ca2+ wave depleted the SR by only ∼30%, and therefore it took several release events to return SR content to normal. However, this explanation is not supported by the data provided here, which indicate that SR Ca2+ release in a single Ca2+ wave effectively empties the SR. To investigate the phenomenon further, a computational model was used to simulate the effects of the addition of tetracaine on the local Ca2+ release events, assuming that Ca2+ waves transiently deplete the SR of Ca2+. Based on the measurements in this study, addition of tetracaine was simulated by a rapid increase in the threshold for SR Ca2+ release from 1.1 mM to 1.6 mM. This resulted in a reduction in the frequency but an increase in amplitude of Ca2+ waves. Upon removal of tetracaine, the computational model predicted a burst of high-frequency Ca2+ waves. Examination of the underlying Ca2+ fluxes showed that the burst resulted from a chronically high cytoplasmic Ca2+ that stimulated Ca2+ uptake by SERCA and reduced the time taken for the SR to reach the threshold for Ca2+ release. Thus, the cause of the burst of Ca2+ waves on removal of tetracaine was the result of increased cytoplasmic Ca2+, and not increased intra-SR Ca2+ as previously thought.
Conclusions
In conclusion, the results of this study show that spontaneous Ca2+ waves lead to substantial but not complete depletion of the SR, a feature that is more easily resolved using the intra-SR signal derived from Fluo-5N rather than cytosolic Fluo-5F. A proportional level of depletion was also seen when RyR2 sensitivity was lowered with tetracaine. The complex behavior seen upon application and removal of tetracaine can be explained by the combined actions of the cytosolic and luminal Ca2+-sensing sites of RyR2. This persistent high level of depletion leads to the hypothesis that luminal control of the termination of Ca2+ release occurs when the SR is almost completely depleted of Ca2+. Thus, global SR Ca2+ release may be controlled by the number of release sites and not the degree of depletion at each local release site. Further work is necessary to test this hypothesis.
Acknowledgments
This work was supported by a grant from the British Heart Foundation.
Supporting Material
References
- 1.Keizer J., Smith G.D. Spark-to-wave transition: saltatory transmission of calcium waves in cardiac myocytes. Biophys. Chem. 1998;72:87–100. doi: 10.1016/s0301-4622(98)00125-2. [DOI] [PubMed] [Google Scholar]
- 2.Fabiato A. Simulated calcium current can both cause calcium loading in and trigger calcium release from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J. Gen. Physiol. 1985;85:291–320. doi: 10.1085/jgp.85.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wier W.G., Egan T.M., Lopez-Lopez J.R., Balke C.W. Local control of excitation-contraction coupling in rat heart cells. J. Physiol. 1994;474:463–471. doi: 10.1113/jphysiol.1994.sp020037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gyorke I., Gyorke S. Regulation of the cardiac ryanodine receptor channel by luminal Ca2+ involves luminal Ca2+ sensing sites. Biophys. J. 1998;75:2801–2810. doi: 10.1016/S0006-3495(98)77723-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lukyanenko V., Wiesner T.F., Gyorke S. Termination of Ca2+ release during Ca2+ sparks in rat ventricular myocytes. J. Physiol. 1998;507:667–677. doi: 10.1111/j.1469-7793.1998.667bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Terentyev D., Viatchenko-Karpinski S., Valdivia H.H., Escobar A.L., Gyorke S. Luminal Ca2+ controls termination and refractory behavior of Ca2+- induced Ca2+ release in cardiac myocytes. Circ. Res. 2002;91:414–420. doi: 10.1161/01.res.0000032490.04207.bd. [DOI] [PubMed] [Google Scholar]
- 7.Ching L.L., Williams A.J., Sitsapesan R. Evidence for Ca(2+) activation and inactivation sites on the luminal side of the cardiac ryanodine receptor complex. Circ. Res. 2000;87:201–206. doi: 10.1161/01.res.87.3.201. [DOI] [PubMed] [Google Scholar]
- 8.Sitsapesan R., Williams A.J. Regulation of the gating of the sheep cardiac sarcoplasmic reticulum Ca(2+)-release channel by luminal Ca2+ J. Membr. Biol. 1994;137:215–226. doi: 10.1007/BF00232590. [DOI] [PubMed] [Google Scholar]
- 9.Brochet D.X., Yang D., Maio A.D., Lederer W.J., Franzini-Armstrong C. Ca2+ blinks: rapid nanoscopic store calcium signaling. Proc. Natl. Acad. Sci. USA. 2005;102:3099–3104. doi: 10.1073/pnas.0500059102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kubalova Z., Gyorke I., Terentyeva R., Viatchenko-Karpinski S., Terentyev D. Modulation of cytosolic and intra-sarcoplasmic reticulum calcium waves by calsequestrin in rat cardiac myocytes. J. Physiol. 2004;561:515–524. doi: 10.1113/jphysiol.2004.073940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shannon T.R., Guo T., Bers D.M. Ca2+ scraps: local depletions of free [Ca2+] in cardiac sarcoplasmic reticulum during contractions leave substantial Ca2+ reserve. Circ. Res. 2003;93:40–45. doi: 10.1161/01.RES.0000079967.11815.19. [DOI] [PubMed] [Google Scholar]
- 12.Kubalova Z., Terentyev D., Viatchenko-Karpinski S., Nishijima Y., Gyorke I. Abnormal intrastore calcium signaling in chronic heart failure. Proc. Natl. Acad. Sci. USA. 2005;102:14104–14109. doi: 10.1073/pnas.0504298102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Terentyev D., Nori A., Santoro M., Viatchenko-Karpinski S., Kubalova Z. Abnormal interactions of calsequestrin with the ryanodine receptor calcium release channel complex linked to exercise-induced sudden cardiac death. Circ. Res. 2006;98:1151–1158. doi: 10.1161/01.RES.0000220647.93982.08. [DOI] [PubMed] [Google Scholar]
- 14.MacQuaide N., Dempster J., Smith G.L. Measurement and modeling of Ca2+ waves in isolated rabbit ventricular cardiomyocytes. Biophys. J. 2007;93:2581–2595. doi: 10.1529/biophysj.106.102293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trafford A.W., Sibbring G.C., Diaz M.E., Eisner D.A. The effects of low concentrations of caffeine on spontaneous Ca release in isolated rat ventricular myocytes. Cell Calcium. 2000;28:269–276. doi: 10.1054/ceca.2000.0156. [DOI] [PubMed] [Google Scholar]
- 16.Overend C.L., Eisner D.A., O'Neill S.C. The effect of tetracaine on spontaneous Ca2+ release and sarcoplasmic reticulum calcium content in rat ventricular myocytes. J. Physiol. 1997;502:471–479. doi: 10.1111/j.1469-7793.1997.471bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith G.L., O'Neill S.C. A comparison of the effects of ATP and tetracaine on spontaneous Ca(2+) release from rat permeabilised cardiac myocytes. J. Physiol. 2001;534:37–47. doi: 10.1111/j.1469-7793.2001.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McIntosh M.A., Cobbe S.M., Smith G.L. Heterogeneous changes in action potential and intracellular Ca2+ in left ventricular myocyte sub-types from rabbits with heart failure. Cardiovasc. Res. 2000;45:397–409. doi: 10.1016/s0008-6363(99)00360-0. [DOI] [PubMed] [Google Scholar]
- 19.Loughrey C.M., MacEachern K.E., Cooper J., Smith G.L. Measurement of the dissociation constant of Fluo-3 for Ca2+ in isolated rabbit cardiomyocytes using Ca2+ wave characteristics. Cell Calcium. 2003;34:1–9. doi: 10.1016/s0143-4160(03)00012-5. [DOI] [PubMed] [Google Scholar]
- 20.Smith G.L., Miller D.J. Potentiometric measurements of stoichiometric and apparent affinity constants of EGTA for protons and divalent ions including calcium. Biochim. Biophys. Acta. 1985;839:287–299. doi: 10.1016/0304-4165(85)90011-x. [DOI] [PubMed] [Google Scholar]
- 21.Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J. Physiol. (Paris) 1979;75:463–505. [PubMed] [Google Scholar]
- 22.Loughrey C.M., MacEachern K.E., Neary P., Smith G.L. The relationship between intracellular [Ca(2+)] and Ca(2+) wave characteristics in permeabilised cardiomyocytes from the rabbit. J. Physiol. 2002;543:859–870. doi: 10.1113/jphysiol.2002.021519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shannon T.R., Bers D.M. Assessment of intra-SR free [Ca] and buffering in rat heart. Biophys. J. 1997;73:1524–1531. doi: 10.1016/S0006-3495(97)78184-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Page E., McCallister L.P., Power B. Sterological measurements of cardiac ultrastructures implicated in excitation-contraction coupling. Proc. Natl. Acad. Sci. USA. 1971;68:1465–1466. doi: 10.1073/pnas.68.7.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shannon T.R., Ginsburg K.S., Bers D.M. Reverse mode of the sarcoplasmic reticulum calcium pump and load- dependent cytosolic calcium decline in voltage-clamped cardiac ventricular myocytes. Biophys. J. 2000;78:322–333. doi: 10.1016/S0006-3495(00)76595-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng H., Lederer M.R., Lederer W.J., Cannell M.B. Calcium sparks and [Ca2+]i waves in cardiac myocytes. Am. J. Physiol. 1996;270:C148–C159. doi: 10.1152/ajpcell.1996.270.1.C148. [DOI] [PubMed] [Google Scholar]
- 27.Hove-Madsen L., Bers D.M. Passive Ca buffering and SR Ca uptake in permeabilized rabbit ventricular myocytes. Am. J. Physiol. 1993;264:C677–C686. doi: 10.1152/ajpcell.1993.264.3.C677. [DOI] [PubMed] [Google Scholar]
- 28.Wu X., Bers D.M. Sarcoplasmic reticulum and nuclear envelope are one highly interconnected Ca2+ store throughout cardiac myocyte. Circ. Res. 2006;99:283–291. doi: 10.1161/01.RES.0000233386.02708.72. [DOI] [PubMed] [Google Scholar]
- 29.Guo T., Ai X., Shannon T.R., Pogwizd S.M., Bers D.M. Intra-sarcoplasmic reticulum free [Ca2+] and buffering in arrhythmogenic failing rabbit heart. Circ. Res. 2007;101:802–810. doi: 10.1161/CIRCRESAHA.107.152140. [DOI] [PubMed] [Google Scholar]
- 30.Venetucci L.A., Trafford A.W., Diaz M.E., O'Neill S.C., Eisner D.A. Reducing ryanodine receptor open probability as a means to abolish spontaneous Ca2+ release and increase Ca2+ transient amplitude in adult ventricular myocytes. Circ. Res. 2006;98:1299–1305. doi: 10.1161/01.RES.0000222000.35500.65. [DOI] [PubMed] [Google Scholar]
- 31.Gyorke S., Lukyanenko V., Gyorke I. Dual effects of tetracaine on spontaneous calcium release in rat ventricular myocytes. J. Physiol. 1997;500:297–309. doi: 10.1113/jphysiol.1997.sp022021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


