Abstract
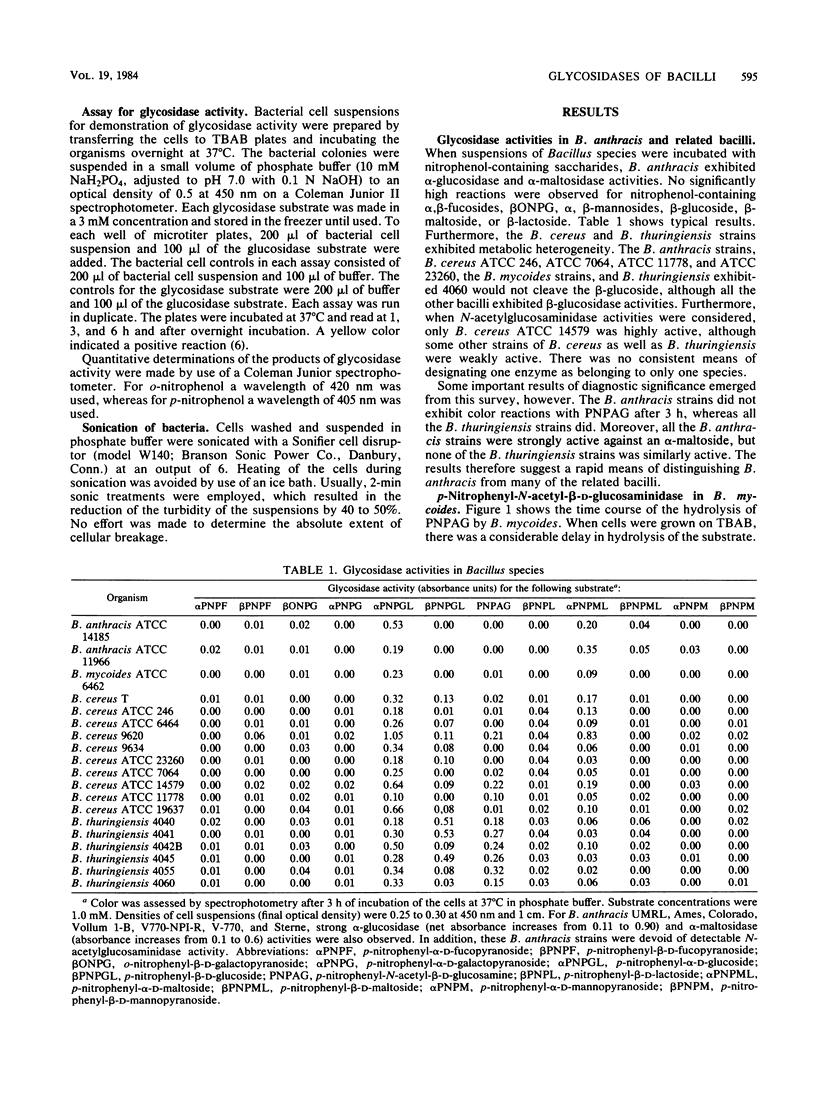
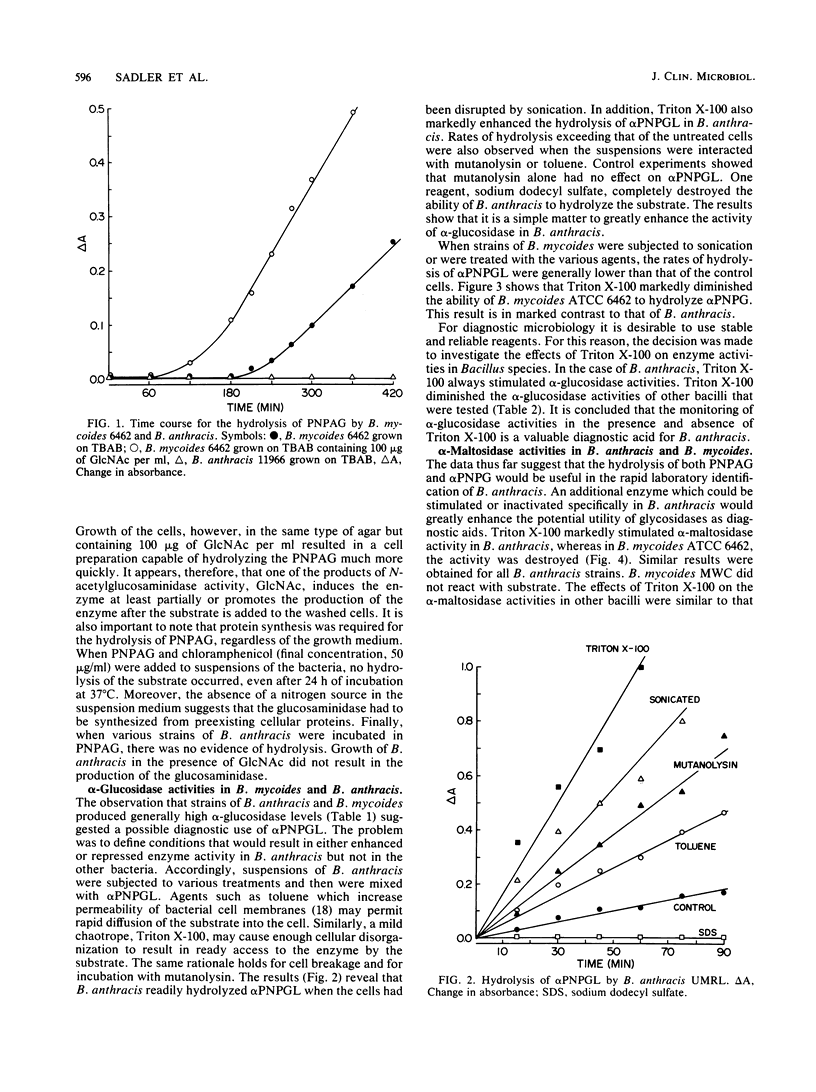
Bacillus anthracis could be distinguished from the taxonomically related species B. cereus, B. mycoides, and B. thuringiensis by a comparison of glycosidase activities. All the bacilli tested possessed alpha-glucosidase activity, as evidenced by the hydrolysis of p-nitrophenyl-alpha-D-glucoside. In B. anthracis, the glucosidase activity could be enhanced by the addition of agents which damage cellular surface structures. Treatment of B. anthracis strains with toluene. Triton X-100, or mutanolysin or cellular disruption by sonication resulted in higher rates of alpha-glucoside hydrolysis than were accomplished by cells suspended in buffer. It is suggested that intact B. anthracis cells have a limited permeability to the glucosidase substrate. In contrast to the results obtained for B. anthracis, Triton X-100 markedly diminished the enzymatic hydrolysis of p-nitrophenyl-alpha-D-glucoside by strains of B. cereus, B. mycoides, and B. thuringiensis. Triton X-100 also enhanced the alpha-maltosidase activity of B. anthracis but not that of the other bacilli. B. mycoides possessed an apparently inducible N-acetylglucosaminidase although the enzyme was absent in B. anthracis. The glucosaminidase was inducible in the presence of p-nitrophenyl-N-acetylglucosamine in the absence of conventional nitrogen sources. Chloramphenicol prevented the induction of the glucosaminidase in B. mycoides. In several B. cereus and all B. thuringiensis strains, the glucosaminidase was constitutive. The results suggest a means for the rapid laboratory differentiation of B. anthracis from other closely related bacilli. Assays for alpha-glucosidase and alpha-maltosidase, in the presence and absence of Triton X-100, can be used to distinguish B. anthracis from B. cereus, B. mycoides, and B. thuringiensis. Similarly, the hydrolysis of p-nitrophenyl-beta-N-acetylglucosamine induced by B. mycoides but not by B. anthracis provides an additional means for differentiating these similar bacilli.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BATES C. J., PASTERNAK C. A. FURTHER STUDIES ON THE REGULATION OF AMINO SUGAR METABOLISM IN BACILLUS SUBTILIS. Biochem J. 1965 Jul;96:147–154. doi: 10.1042/bj0960147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BATES C. J., PASTERNAK C. A. THE INCORPORATION OF LABELLED AMINO SUGARS BY BACILLUS SUBTILIS. Biochem J. 1965 Jul;96:155–158. doi: 10.1042/bj0960155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkeley R. C., Brewer S. J., Ortiz J. M., Gillespie J. B. An exo- -N-acetylglucosaminidase from Bacillus subtilis B; characterization. Biochim Biophys Acta. 1973 May 5;309(1):157–168. doi: 10.1016/0005-2744(73)90327-6. [DOI] [PubMed] [Google Scholar]
- Brown W. C., Young F. E. Dynamic interactions between cell wall polymers, extracellular proteases and autolytic enzymes. Biochem Biophys Res Commun. 1970 Feb 20;38(4):564–568. doi: 10.1016/0006-291x(70)90618-2. [DOI] [PubMed] [Google Scholar]
- Cole H. B., Ezzell J. W., Jr, Keller K. F., Doyle R. J. Differentiation of Bacillus anthracis and other Bacillus species by lectins. J Clin Microbiol. 1984 Jan;19(1):48–53. doi: 10.1128/jcm.19.1.48-53.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda T. Fatty acids in the genus Bacillus. II. Similarity in the fatty acid compositions of Bacillus thuringiensis, Bacillus anthracis, and Bacillus cereus. J Bacteriol. 1968 Jun;95(6):2210–2216. doi: 10.1128/jb.95.6.2210-2216.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko T., Nozaki R., Aizawa K. Deoxyribonucleic acid relatedness between Bacillus anthracis, Bacillus cereus and Bacillus thuringiensis. Microbiol Immunol. 1978;22(10):639–641. doi: 10.1111/j.1348-0421.1978.tb00414.x. [DOI] [PubMed] [Google Scholar]
- Levina E. N., Kats L. N. Izuchenie antigenov V. anthracis i B. cereus s pomoshch'iu liuminestsentno-serologicheskogo i tsitokhimicheskikh metodov issledovaniia. Zh Mikrobiol Epidemiol Immunobiol. 1966 Apr;43(4):98–103. [PubMed] [Google Scholar]
- Muftic M., Schröder E. Peptidasegram in the differentiation of bacilli species. Pathol Microbiol (Basel) 1966;29(2):252–256. doi: 10.1159/000161907. [DOI] [PubMed] [Google Scholar]
- Nath K., Koch A. L. Protein degradation in Escherichia coli. II. Strain differences in the degradation of protein and nucleic acid resulting from starvation. J Biol Chem. 1971 Nov 25;246(22):6956–6967. [PubMed] [Google Scholar]
- Phillips A. P., Martin K. L., Broster M. G. Differentiation between spores of Bacillus anthracis and Bacillus cereus by a quantitative immunofluorescence technique. J Clin Microbiol. 1983 Jan;17(1):41–47. doi: 10.1128/jcm.17.1.41-47.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priest F. G. Extracellular enzyme synthesis in the genus Bacillus. Bacteriol Rev. 1977 Sep;41(3):711–753. doi: 10.1128/br.41.3.711-753.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taku A., Gardner H. L., Fan D. P. Reconstitution of cell wall synthesis in toluene- and LiCl-treated Bacillus megaterium cells by addition of a soluble protein extract. J Biol Chem. 1975 May 10;250(9):3375–3380. [PubMed] [Google Scholar]
- Yokogawa K., Kawata S., Nishimura S., Ikeda Y., Yoshimura Y. Mutanolysin, bacteriolytic agent for cariogenic Streptococci: partial purification and properties. Antimicrob Agents Chemother. 1974 Aug;6(2):156–165. doi: 10.1128/aac.6.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]


