Abstract
To clarify the significance of expression of system L amino acid transporter 1 (LAT1) and 4F2 heavy chain (4F2hc) in the developing intestine, immunohistochemical investigation and molecular analysis were performed in the human embryonic and/or fetal intestines, ranging from 28–30 days to 34–35 weeks gestation. The molecular analysis for the expression of LAT1 and 4F2hc mRNAs was done in the pure epithelial cell samples prepared after laser assisted microdissection. The immunoreactivities against LAT1 and 4F2hc were detected along the basolateral cell membrane of the primitive gut epithelium at 28–30 days gestation. According to advance in gestational age of up to 24–25 weeks gestation, the immunoreactivity of LAT1 was predominantly observed in the supranuclear cytplasmic localization with a granular or dot-like staining pattern. Up to 8–9 weeks gestation, the immunoreactivity of 4F2hc showed almost the same as that of LAT1. However, after the age of 12–13 weeks gestation, the immunoreactivity of 4F2hc was predominantly localized along the cell membrane of apical surface of the epithelial cells. No apical and linear membranous localization of LAT1 was observed until nearly 20 weeks gestation. In the late gestational stage, both the immunoreactivities against LAT1 and 4F2hc were localized along the apical surface of the epithelial cells. In conclusion, the expression of LAT1 and 4F2hc in early developing intestine suggests they have a more important role in cell proliferation rather than functional differentiation. The predominant cytoplasmic localization of LAT1 during mid-fetal life seems to be largely inactive as amino acid transporter. On the other hand, the apical and linear membranous co-localization of LAT1 and 4F2hc in the late fetal life suggests that these molecules may play a role as a functional amino acid transporter in the fetal intestinal epithelium.
Keywords: immunohistochemistry, human intestine, system L amino acid transporter, LAT-1, 4F2hc
I. Introduction
It has been demonstrated that the uptake of amino acids into the cells is essential to cell growth in both physiological and neoplastic conditions [3, 11]. In addition to DNA and protein synthesis, amino acid uptake is quite important to cell differentiation [12, 24]. Amino acid uptake is mediated by several types of amino acid transporters [3, 8]. System L amino acid transporter 1 (LAT1), a Na+-independent amino acid transporter, is a membrane protein with 12 putative membrane-spanning domains and consisting of 512 amino acids, that was originally cloned by Kanai et al. [10]. In adult tissue, LAT1 expression is very low, except for brain, testis and placenta [10, 25]. On the other hand, its expression is at a relatively high level in tumor tissues and also in the tissues showing high proliferating activity [9, 10, 15, 17], Therefore, it has been suggested that LAT1 is one of the oncofetal antigens [10, 25]. For its functional expression as an amino acid transporter, LAT1 requires the presence of 4F2hc (CD98), a heavy chain of the cell surface antigen, forming a heterodimer (LAT1/4F2hc) on the cell membrane surface [10]. The expression of LAT1 and LAT2 was examined extensively in the different species including human and also in the cell lines [2, 4, 5, 10, 14, 18–20, 25]. According to previous studies using northern blotting, no expression of LAT1 was demonstrated in adult human intestine [18, 25]. In addition, there is no systematic investigation of the expression of LAT1 and 4F2hc in human fetal intestine. In this study, we examined the expression of LAT1 and 4F2hc proteins and their mRNAs, and discuss the significance of their expression in the human developing intestine.
II. Materials and Method
Human intestines
Twelve embryonic and/or fetal intestines, ranging from the estimated gestational age of 28–30 days (Streeter’s horizon XIV) to 34–35 weeks, were obtained from the surgical specimens of spontaneous abortus and/or autopsy cases of stillbirths and premature births under informed consent. All the tissue samples were handled according to the Ethical Guidelines for Clinical Studies (July 30, 2003, amended December 28, 2004, Ministry of Health, Labour and Welfare). The intestines were fixed in 20% formalin, and embedded in paraffin. Four micrometer thick sections were processed in the usual manner, and stained with hematoxylin and eosin.
Immunohistochemistry for LAT1 and 4F2hc
Immunohistochemistry was done on formalin-fixed, paraffin embedded tissue sections using labeled streptavidin biotin; LSAB method (Histofine SAB-PO (R) kit, Nichirei Biosciences Inc). Deparaffinized sections were quenched for endogenous peroxidase activity by immersing the sections into 0.3% hydrogen peroxide solution for 20 min at room temperature, and washing several times in phosphate buffer saline (PBS), pH 7.2. Prior to the incubation with primary antibodies (rabbit anti-LAT1 and anti-4F2hc polyclonal, described elsewhere) [25], the sections were incubated with 5% skimmed milk in PBS for blocking nonspecific binding. The sections were then incubated with primary antibodies for 1 hr at room temperature. After washing in PBS several times, biotinylated anti-goat antibody was applied for 30 min at room temperature. After washing in the same way, the tissue sections were incubated with horseradish peroxidase (HRP) labeled streptavidin for 30 min at room temperature. The tissue-bound HRP activity was visualized by immersing the sections in 0.005% 3,3'-diaminobenzidine tetrahydrochloride (DAB) in PBS containing hydrogen peroxide (10 µl/150 ml DAB solution). After the completion of the immunohistochemical process, the sections were stained lightly with hematoxylin, processed in the usual manner and mounted.
Laser microdissection for tissue sections
Eight µm thick paraffin sections of the intestine were mounted on membrane film-coated slide glasses. After dewax with xylene, the sections were stained lightly with toluidine blue, then the target cells were microdissected by ultraviolet laser beam under a light microscope. For laser microdissection, we used PALM MBIII-N (Zeiss). The microdissected target cells were retrieved precisely into an Eppendorf lid with mineral oil.
RNA extraction and reverse transcription
RAN extraction and reverse transcription were done in accordance with our method described previously [16]. With the microdissected cells mounted lids, the Eppendorf’s tubes with 200 µl of denaturing buffer, containing 10 mM Tris-HCl (pH 8.0), 25 mM EDTA (pH 8.0), 0.5% SDS and 100 mM NaCl and proteinase K were carefully covered and incubated at 55°C overnight. Their total RNA was purified with 20 µl 2M sodium acetate (pH 4.0), 220 µl citrate saturated phenol (pH 4.3), and 60 µl chloroform-isoamyl alcohol. They were centrifuged for 15 min at 15,000 rpm and transferred to the upper aqueous layer into a new tube. The aqueous layer in the new tube was added to 200 µl isopropanol, 2 µl glycogen as a carrier and allowed to stand at −80°C for more than 30 min. They were then recentrifuged for 30 min at 14,000 rpm, and their supernatant was decanted. The pellets were washed with 70% ethanol and air dried on ice. They were then dissolved with 10 µl diethyl pyrocarbonate (DEPC) treated water, and store at −80°C until use. To assess the concentration of the total RNA, one µl of it was directly analyzed on a Nanodrop 1000 (Thermo Fisher Scientific, Newark, DE, USA). The RNA extracted from the microdissected cells was reverse transcribed in a final volume of 20 µl using a Quantitect reverse transcription kit (QIAGEN, Tokyo, Japan) according to the manufacture’s instructions.
Reverse transcriptase polymerase chain reaction (RT-PCR)
The quantity of LAT1 and 4F2hc mRNAs was measured by quantitative RT-PCR technique using the ABI Prism 7000 Sequence Detection System (Applied Biosystems Japan, Tokyo Japan). As an internal control, mRNA of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was measured. The RT-PCR primers used in this study are shown in Table 1. The PCR amplification was performed with a final volume of 20 µl of reaction mixture containing 900 µmol/l of each primer and 1×SYBR Green PCR Master Mix (PE Applied Biosystems, Foster City, CA, USA). The reaction was followed by 50 cycles at 95°C for 15 sec and 60°C for 1 min. The quantities of LAT1 and 4F2hc mRNAs were standardized relative to the mRNA of GAPDH using the standard curve method. The RT-PCR products were electrophoresed through 2% agar gel and visualized with SYBR Green I.
Table 1.
RT-PCR primer sequences of LAT1 and 4F2hc, and product sizes
| Target | Forward primer | Reverse primer | Annealing temperature (°C) | Product size (bp) |
|---|---|---|---|---|
| hLAT1 | 5'-CAT CCT GCT GGG CTT CGT-3' | 5'-AGT TTG GTG CCT TCA AAT GAG AA-3' | 60 | 81 |
| h4F2hc | 5'-CTC AGG CAA GGC TCC TGA CT-3' | 5'-GGC AGG GTG AAG AGC ATC A-3' | 60 | 76 |
| GAPDH | 5'-GAA GGT GAA GGT CGG AGT C-3' | 5'-GAA GAT GGT GAT GGG ATT TC-3' | 60 | 226 |
III. Results
Morphology of the primitive gut and fetal intestine
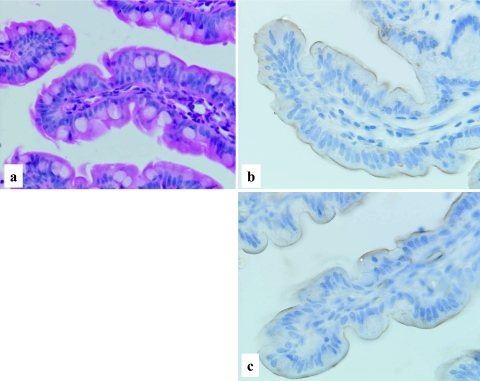
The primitive gut at the age of 28–30 days gestation (Streeter’s horizon XIV) [21] is characterized by a simple hollow tubular structure lined by stratified immature epithelial cells and embedded in dense primitive mesenchymal cells. No cellular differentiation is evident in the epithelial cells (Fig. 1a). At the age of 34–36 days gestation (Streeter’s horizon XVII) [22], the histology of the gut is still primitive, and similar to that of 28–30 days gestation. However, the subnuclear clear cytoplasm has become more obvious. On the other hand, cellular differentiation can be seen in the mesenchymal cells surrounding the primitive gut. The mesenchymal cells tend to encircle the primitive gut epithelial cells, suggesting immature muscular differentiation (Fig. 2a, b). At the age of 9–10 weeks gestation, cellular differentiation is noted in the gut epithelial cells and the occurrence of goblet cells is easily identified. After the age of 14–15 weeks gestation, throughout the mid to late gestational stage (34–36 weeks), the morphological appearance of the fetal intestines is almost same, though the intestinal villi appear to be lengthen and goblet cells are much more increased in number (Fig. 3a, 4a, 5a). In the late gestational stage (34–36 weeks), the formation of the brush border is obviously demonstrated in the epithelial cells of intestinal villi (Fig. 5a).
Fig. 1.
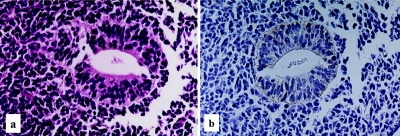
Human developing intestine, 28–30 days gestation. Primitive gut, characterized by a hollow tubular structure made up of multilayered immature epithelial cells, embedding in a dense mesenchymal background (a), H-E stain ×400 and a distinct basolateral membranous staining of LAT1 (b), LSAB method, ×400.
Fig. 2.
Human developing intestine, 34–36 days gestation. Primitive gut, characterized by multilayered immature epithelial cells with clear subnuclear appearance (a), (b), H-E stain ×200, ×400. Intense basolateral membranous staining of LAT1 (c) and 4F2hc (d), LASB method, ×400.
Fig. 3.
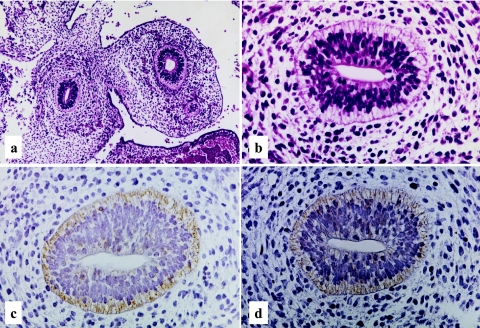
Human developing intestine, 15–16 weeks gestation. Well developed intestinal villi with occasional goblet cells, H-E stain ×200 (a). Predominant cytoplasmic staining of LAT1 with a granular or dot-like pattern (b), and mainly linear staining of 4F2hc along the apical surface of the epithelial cells (c), LASB method, ×400.
Fig. 4.
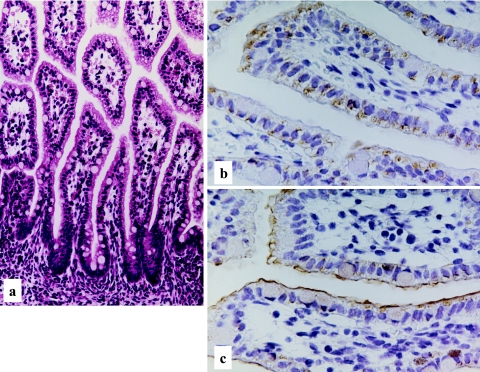
Human developing intestine, 20–21 weeks gestation. Well developed and elongated intestinal villi with occasional goblet cells (a), H-E stain ×200. Predominant cytoplasmic, but occasional membranous staining patterns of LAT1 (b), and chiefly membranous staining of 4F2hc (c), LSAB method, ×400.
Fig. 5.
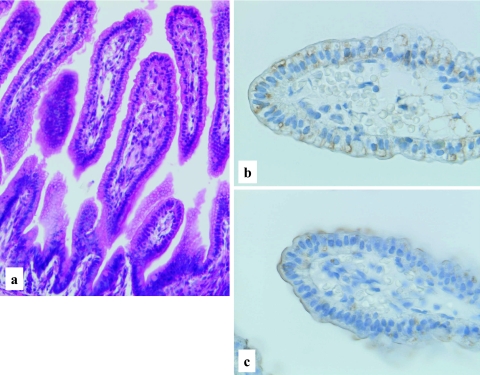
Human developing intestine, 34–36 weeks gestation. Well developed intestinal villi with increased goblet cells and distinct brush borders (a), H-E stain, ×400. Distinct membranous staining of LAT1 (b) and 4F2hc (c) in the intestinal villi along the apical surface, LSAB method, ×400.
Immunohistochemistry for LAT1 and 4F2hc
Both the LAT1 and 4F2hc immunoreactivities are demonstrated in the primitive gut epithelial cells at the age of 28–30 days gestation. Rather weak but distinct immunoreactivity of LAT1 was observed along the basolateral membrane of epithelial cells (Fig. 1b). No immunoreactivity was observed in the surrounding primitive mesenchymal cells. At the age of 34–36 days gestation, more intense immunoreactivities against LAT1 and 4F2hc were observed in the primitive gut epithelial cells, mainly along the basolateral membrane (Fig. 2c, d). However, the immunoreactivity against LAT1 is also observed in the cytoplasm, particularly on the subnuclear basal side (Fig. 2c). An intense immunoreactivity against LAT1 was also observed in the primitive muscle cells, vascular endothelial cells, immature chondrocytes, lining of embryonic coelomic cavity, generally covering the embryo or primitive epidermis (data not shown). According to the advance of the intestinal development, at the age of 15–16 weeks gestation, the immunoreactivities against LAT1 and 4F2hc showed different localization patterns. The immunoreactivity against LAT1 localized chiefly in the cytoplasm, particularly in supranuclear localization with a granular or dot-like staining pattern (Fig. 3b). On the other hand, the immunoreactivity against 4F2hc predominantly showed an intense linear localization along the cell membrane of the apical surface of the epithelial cells and also in the cytoplasm (Fig. 3c). During the mid gestational stage, the immunolocalization of LAT1 and 4F2hc was nearly the same as those of 15–16 weeks gestation, though the basolateral membranous staining was also observed (Fig. 4b, c). In the late gestational stage (34–36 weeks), the immunoreactivities against LAT1 and 4F2hc showed rather intense linear staining along the apical membrane of the villous epithelial cells (Fig. 5b, c). Results of the morphological development and immunohistochemical expression of LAT1 and 4F2hc in human developing intestines are summarized in Table 2.
Table 2.
Results of morphological development and immunohistochemical expression of LAT1 and 4F2hc in the human developing intestines
| Cases | Gestational ages | Morphology | LAT1 | 4F2hc |
|---|---|---|---|---|
| 1 | 28–30 days | No villi or cellular differentiation | +basolateral, membranous | +basolateral, membranous |
| 2 | 34–36 days | No villi or cellular differentiation | 2+basolateral, membranous | +basolateral, membranous |
| 3 | 44–46 days | No villi or cellular differentiation | 2+basolateral, membranous | +basolateral, membranous |
| 4 | 8–9 weeks | No villi or cellular differentiation | 2+basolateral, membranous | +basolateral, membranous |
| 5 | 9–10 weeks | Villi (+), brush border (−/+), goblet cell (+) | 2+supra-/sub-nuclear, granular focally membranous | +supra-/sub-nuclear, granular focally apical and linear |
| 6 | 14–15 weeks | Villi (+), brush border (−), goblet cell (+) | 2+predominantly supranuclear, granular focally subnuclear and membranous | 2+predominantly apical and linear, focally membranous |
| 7 | 15–16 weeks | Villi (+), brush border (−), goblet cell (2+) | 2+predominantly supranuclear and granular, occasionally subnuclear | +/2+predominantly apical and linear, focally membranous |
| 8 | 16–17 weeks | Villi (+), brush border (−), goblet cell (2+) | 2+predominantly supranuclear and granular, occasionally subnuclear | +/2+predominantly apical and linear, focally membranous |
| 9 | 18–19 weeks | Villi (+), brush border (−/+), goblet cell (2+) | 2+predominantly supranuclear and granular, occasionally subnuclear, focally apical and linear | 2+predominantly apical and linear, focally supranuclear and subnuclear |
| 10 | 20–21 weeks | Villi (+), brush border (−/+), goblet cell (2+) | 2+predominantly supranuclear and granular, occasionally subnuclear, focally apical and linear | 2+predominantly apical and linear, focally supranuclear and subnuclear |
| 11 | 24–25 weeks | Villi (+), brush border (+), goblet cell (2+) | 2+predominantly supranuclear and granular, occasionally subnuclear, focally apical and linear | +supranuclear, and apical and linear |
| 12 | 34–35 weeks | Villi (+), brush border (2+), goblet cell (3+) | +/2+apical and linear | +/2+apical and linear, focally subnuclear |
(−): not observed, (−/+): focal and incompletely developed, (+): occasionally observed, (2+): frequently observed. +: weakly positive, +/2+: positive, 2+: intensely positive
Expression of LAT1 and 4F2h mRNAs in developing intestine by RT-PCR
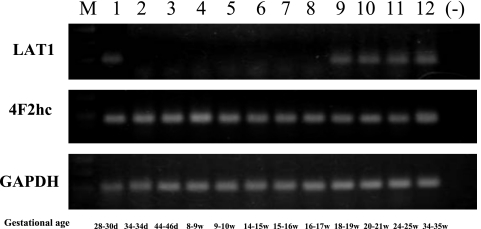
In early developmental stage (Streeter’s horizon XIV, 28–30 days), the expression of LAT1 and 4F2hc mRNAs was clearly observed in the cell sample obtained from the primitive gut by laser microdissection. The PCR products of 4F2hc were constantly and intensely observed throughout the developmental stages. On the other hand, the PCR products of LAT1 were usually barely observed compared with those of 4F2hc. In some samples, the level of LAT1 mRNA expression seemed to be below the range of detection (Fig. 6).
Fig. 6.
Expression of LAT1 and 4F2hc mRNAs in the human developing intestine. The PCR products of LAT1 and 4F2hc are both identified in the early developing intestine (28–30 days gestation). The products of 4F2hc are constantly identified in the developing intestines throughout the gestational stages. On the other hand, the products of LAT1 are usually barely observed. GAPDH: positive control. M: marker; (−): No sample (negative control).
IV. Discussion
To clarify the significance of expression of amino acid transporter in the intestinal development and functional differentiation, we investigated the expression of system L amino acid transporter 1 (LAT1) and its cofactor 4F2 heavy chain (4F2hc) in the various stages of human developing intestines by immunohistochemistry and molecular analysis using RT-PCR method. According to our previous study, the development of intestinal villi and cellular differentiation begins around at the age of 9–10 weeks gestation [7]. Before this developmental stage (28–30 days to 34–36 days gestation), the primitive gut is characterized by a straight hollow tubular structure, embedded in rather dense undifferentiated mesenchymal cells. At this developmental stage, of course, intestinal epithelial cells are also immature and show marked stratification without any identifiable cellular differentiation. This histological appearance is a hallmark of embryonic gut, and the stratification of immature epithelial cells may reflect the marked proliferating activity. In the embryonic gut epithelial cells, the immunoreactivities against LAT1 and 4F2hc were satisfactorily demonstrated in the cell membrane, particularly along the basolateral membrane. This finding seems to be quite reasonable, because the immature epithelial cells uptake nutrients and receive some growth signals from the basal side via capillaries and/or paracrine modes.
LAT1 is an inducible type cell membrane protein, which can be induced by physiologically activated substances such as hormones and growth factors, and the expression of LAT1 is considered to be up-regulated in association with the degree of cellular protein synthesis requirement [3, 19, 20]. For example, in the process of tissue regeneration, in other words, in the repairing process, the up-regulated LAT1 expression has been demonstrated in the cells of experimentally induced rat gastric ulcer edge [13]. The up-regulated LAT1 expression at the ulcer edge in the repairing process can be explained by the fact that LAT1 enhances amino acid transport to both the mesenchymal cells in the granulation tissue, and epithelial cells in the process of ulcer healing. On the other hand, in the neoplastic condition, tumor cells require increased amino acid uptake for DNA and/or protein synthesis [6, 23]. The cells contributing to the repairing or neoplastic condition require increased uptake of amino acids for cell proliferation [1, 18].
In the early developmental stage, LAT1 and 4F2hc are co-localized along the basolateral plasma membrane which suggests the formation of LAT1/4F2hc heterodimer on the cell surface. At this stage, the LAT1/4F2hc heterodimer plays a role as a functional amino acid transporter contributing to the cell proliferation.
According to the advance of the gestational age, LAT1 is expressed predominantly in the cytoplasm, particularly as supranuclear localization. This expression pattern of LAT1 continued throughout the end of mid-fetal life (24–25 weeks gestation), and such cytoplasmic localization seems to be functionally inactive. On the other hand, 4F2hc is expressed constantly throughout the whole embryonic and/or fetal life. During the mid to late fetal life, 4F2hc expressed predominantly membranous staining in the apical surface of the gut epithelial cells. Such a discrepant localization of LAT1 and 4F2hc in the mid-fetal life suggests that 4F2hc may form a heterodimer with other light chain, such as LAT2.
In this study, we did not confirm the subcellular immunolocalization of LAT1 in the intestinal epithelial cells. However, the granular or dot-like immunoreactivity of LAT1 in supranuclear location may suggest that LAT1 exists as inappropriately folded and aggregated form at the level of endoplasmic reticulum or Golgi apparatus during the mid-fetal life. We do not have any reliable evidence for the explanation of LAT1 localization changed from cytoplasmic to apical and linear pattern in the end of mid-fetal to the late fetal life. Possible explanation of such apical and linear expression of both LAT1and 4F2hc seems to be physiological need, because the substrate of LAT1/4F2hc comes form the ingested amniotic fluid at this stage. And such a drastic change may be induced by time spatial activation of molecular chaperon of the transporter proteins.
RT-PCR examination showed rather intense and constant expression of LAT2 mRNA in the gut epithelial cell samples throughout the embryonic and/or fetal life (data not shown). The constant and intense expression of 4F2hc mRNA in the gut epithelial cells seems to be reasonable, because 4F2hc expression has been demonstrated in almost all cells.
Although the result of RT-PCR failed to visualize the expression of LAT1 mRNA in some intestinal samples, we obtained very low expression of LAT1 mRNA by the results of relative to reference genes. The expression level of LAT1 mRNA showed roughly 1×10−3 and 1×10−3 lower than that of LAT2 and 4F2hc, respectively (data not shown).
We observed co-localization of LAT1 and 4F2hc in the epithelial cells of intestinal villi with a membranous and linear staining pattern, at the late gestational stage (34–35 weeks gestation). Such a co-localization of LAT1 and 4F2hc may play as a functional amino acid transporter at the intestinal villi. It has been shown that intestinal epithelial cells and renal tubular epithelial cells have developed original transport system in the basolateral and luminal sides. There is no report about the expression of LAT1/4F2hc in the fetal intestine, and also in the adult ones. However, we thought that the expression of functional amino acid transporter, LAT1/4F2hc in the fetal intestine is possibly time-spatial. It is necessary to investigate the expression of LAT1/4F2hc in the intestine with circumstances of milk-feeding.
In this study, we disclosed that the expression of LAT1 and 4F2hc in the human embryonic and/or fetal intestines, by immunohistochemistry and RT-PCR method. To our knowledge, this is the first report to demonstrate the co-expression of LAT1 and 4F2hc as an active molecule of amino acid transporter in the human developing intestines.
In conclusion, the expression of LAT1 and 4F2hc in early developing intestine suggests more important role in cell proliferation rather than functional differentiation. The predominant cytoplasmic localization of LAT1 during the mid-fetal life seems to be largely inactive as amino acid transporter. On the other hand, the apical and linear membranous co-localization of LAT1 and 4F2hc in the late fetal life suggests that these molecules may play as a functional amino acid transporter in the fetal intestinal epithelium.
V. Acknowledgments
This authors wish to thank Ms. Yukari Obana for her excellent technical assistance and also to the staff of Pathology Division, Nihon University Itabashi Hospital. This work was partly supported by a grant from the Ministry of Education, Science and Culture of Japan (18780259).
VI. References
- 1.Babu E., Kanai Y., Chairoungdua A., Kim D. K., Iribe Y., Tangtrongsup S., Jutabha P., Li Y., Ahmed N., Sakamoto S., Anzai N., Nagamori S., Endou H. Identification of a novel system L amino acid transporter structurally distinct from heterodimeric amino acid transporters. J. Biol. Chem. 2003;278:43838–43845. doi: 10.1074/jbc.M305221200. [DOI] [PubMed] [Google Scholar]
- 2.Boado R. J., Li Y. J., Nagoya M., Zhang C., Pardridge W. M. Selective expression of the large neutral amino acid transporter at the blood-brain barrier. Proc. Natl. Sci. U S A. 1999;96:12079–12084. doi: 10.1073/pnas.96.21.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christensen H. N. Role of amino acid transport and countertransport in nutrition and metabolism. Physiol. Rev. 1990;70:43–77. doi: 10.1152/physrev.1990.70.1.43. [DOI] [PubMed] [Google Scholar]
- 4.Dave M. H., Schulz N., Zecevic M., Wagner C. A., Verrey F. Expression of heteromeric amino acid transporters along the murine intestine. J. Physiol. 2004;558:597–610. doi: 10.1113/jphysiol.2004.065037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fraga S., Pinho M. J., Soarer da-Silva P. Expression of LAT1 and LAT2 amino acid transporters in human and rat intestinal epithelial cells. Amino Acids. 2005;29:229–233. doi: 10.1007/s00726-005-0221-x. [DOI] [PubMed] [Google Scholar]
- 6.Fuchs B. C., Bode B. P. Amino acid transporters ASCT2 and LAT1 in cancer: Partners in crime? Semin. Cancer Biol. 2005;15:254–266. doi: 10.1016/j.semcancer.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Haga T., Lu W., Nemoto N. Expression of extracellular matrix components (fibronectin and tenascin) and morphological development of human small intestine. Nihon Univ. J. Med. 2000;42:1–15. [Google Scholar]
- 8.Hediger M. A., Romero M. F., Peng J. B., Rolfs A., Takanuga H., Bruford E. A. The ABCs of solute carriers: physiological, pathological and therapeutic implications of human membrane transport protein induction. Pflugers. Arch. 2004;447:465–468. doi: 10.1007/s00424-003-1192-y. [DOI] [PubMed] [Google Scholar]
- 9.Kaira K., Oriuchi N., Imai H., Shimizu K., Yanagitani N., Sunaga N., Hisada T., Tanaka S., Ishizuka T., Kanai Y., Endou H., Nakajima T., Mori M. Prognostic significance of L-type amino acid transporter 1 expression in resectable stage I–III nonsmall cell lung cancer. Br. J. Cancer. 2008;98:742–748. doi: 10.1038/sj.bjc.6604235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanai Y., Segawa H., Miyamoto K., Uchino H., Takeda E., Endou H. Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy change of 4F2 antigen (CD98) J. Biol. Chem. 1998;273:23629–23632. doi: 10.1074/jbc.273.37.23629. [DOI] [PubMed] [Google Scholar]
- 11.Kanai Y., Endou H. Heterodimeric amio acid transporters: molecular biology and pathological and pharmacological relevance. Curr. Drug Metab. 2001;2:339–354. doi: 10.2174/1389200013338324. [DOI] [PubMed] [Google Scholar]
- 12.Medina M. A., Sanchez-Jimenesz F., Marquez J., Quesada A. R., de Castro I. N. Relevance of glutamine metabolism to tumor cell growth. Mol. Cell Biochem. 1992;113:1–15. doi: 10.1007/BF00230880. [DOI] [PubMed] [Google Scholar]
- 13.Miyamoto S., Kato K., Ishii Y., Mizuno S., Asai S., Jike T., Iwasaki A., Nemoto N., Kanai Y., Endou H., Arakawa Y. Increased expression of the amino acid transporter LAT1 during healing of acetic acid-induced chronic gastric ulcer in rat. Nihon Univ. J. Med. 2006;48:19–29. [Google Scholar]
- 14.Nakamura E., Sato M., Yang H., Miyagawa F., Haraqsaki M., Tomia K., Matsuoka S., Noma A., Iwai K., Minato N. 4F2 (CD98) heavy chain is associated covalently with an amino acid transporter and controls intracellular trafficking and membrane topology of 4F2 heterodimer. J. Biol. Chem. 1999;274:3009–3016. doi: 10.1074/jbc.274.5.3009. [DOI] [PubMed] [Google Scholar]
- 15.Nakanishi K., Matsuo H., Kanai Y., Endou H., Hiroi S., Tominaga S., Mukai M., Ikeda E., Ozeki Y., Aida S., Kawai T. LAT1 expression in normal lung and in atypical adenomatous hyperplasia and adenocarcinoma of the lung. Virch. Arch. 2006;448:142–150. doi: 10.1007/s00428-005-0063-7. [DOI] [PubMed] [Google Scholar]
- 16.Nakanishi Y., Mizutani G., Sano M., Oinuma T., Nemoto N. Comparison of HER2 mRNA amplification with immunohistochemistry in human breast cancer using laser assisted microdissection technique. Acta Histochem. Cytochem. 2004;37:73–79. [Google Scholar]
- 17.Ohkame H., Masuda H., Ishii Y., Kanai Y. Expression of L-type amino acid transporter 1 (LAT1) and 4F2 heavy chain (4F2hc) in liver tumor lesions of rat models. J. Surg. Oncol. 2001;78:265–272. doi: 10.1002/jso.1165. [DOI] [PubMed] [Google Scholar]
- 18.Prasad P. D., Wang H., Huang W., Kekuda R., Rajan D. P., Leibach F. H., Ganapathy V. Human LAT1, a subunit of system L amino acid transporter: molecular cloning and transport function. Biochem. Biophys. Res. Commun. 1999;255:283–288. doi: 10.1006/bbrc.1999.0206. [DOI] [PubMed] [Google Scholar]
- 19.Rossier G., Meier C., Bauch C., Summa V., Sordat B., Verrey F., Kuhn L. C. LAT2, a new basolateral 4F2hc/CD98-associated amino acid transporter of kidney and intestine. J. Biol. Chem. 1999;274:34948–34954. doi: 10.1074/jbc.274.49.34948. [DOI] [PubMed] [Google Scholar]
- 20.Segawa H., Fukasawa Y., Miyamoto K., Takeda E., Endou H., Kanai Y. Identification and functional characterization of a Na+-independent neutral amino acid transporter with broad substrate selectivity. J. Biol. Chem. 1999;274:19745–19751. doi: 10.1074/jbc.274.28.19745. [DOI] [PubMed] [Google Scholar]
- 21.Streeter G. L. Development horizons in human embryos. Description of age groups XIII, embryos about 4 or 5 millimeters long, and age group XIV, period of indentation of the lens vesicle. Contrib. Embryol. 1945;31:27–63. [Google Scholar]
- 22.Streeter G. L. Developmental horizon in human embryos: Description of age groups XV, XVI, XVII, XVIII. Contrib. Embryol. 1948;32:133–203. [Google Scholar]
- 23.Tamai S., Masuda H., Ishii Y., Suzuki S., Kanai Y., Endou H. Expression of L-type amino acid transporter 1 in a rat model of liver metastasis: Positive correlation with tumor size. Cancer Detec. Prevent. 2001;25:439–445. [PubMed] [Google Scholar]
- 24.Wasa M., Bode B. P., Abcouwer S. F., Collins C., Tanabe K., Souba W. W. Glutamine as a regulator of DNA and protein biosynthesis in human solid tumor cell lines. Ann. Surg. 1996;224:189–197. doi: 10.1097/00000658-199608000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yanagida O., Kanai Y., Chairoungdua A., Kim D. K., Segawa H., Nii T., Cha S. H., Matsuo H., Fukushima J., Fukasawa Y., Tani Y., Taketani Y., Uchino H., Kim J. Y., Inatomi J., Okayasu I., Miyamoto K., Takeda E., Goya T., Endou H. Human L-type amino acid transporter 1 (LAT1): characterization of function and expression in tumor cell lines. Biochim. Biophys. Acta. 2001;1514:291–302. doi: 10.1016/s0005-2736(01)00384-4. [DOI] [PubMed] [Google Scholar]