Abstract
We review the main trends in the development of fluorescence probes to obtain information about the structure, dynamics, and interactions in biomembranes. These probes are efficient for studying the microscopic analogs of viscosity, polarity, and hydration, as well as the molecular order, environment relaxation, and electrostatic potentials at the sites of their location. Progress is being made in increasing the information content and spatial resolution of the probe responses. Multichannel environment-sensitive probes that can distinguish between different membrane physicochemical properties through multiple spectroscopic parameters show considerable promise.
Introduction
The biological functions of cell membranes are strongly coupled with their fundamental physicochemical properties. The membrane electrostatics, phase state, hydration, and dynamics of the constituting molecules determine the membrane structure and control the binding and transport of molecular and ionic species. They also determine the correct insertion, proper folding, and function of membrane proteins. Therefore, monitoring these properties in situ is an important task in membrane biophysics. The unique features of fluorescence techniques in comparison with other tools that are capable of monitoring these properties (notably NMR, FTIR, EPR, etc.) are their ultimate sensitivity up to a single-molecule level and their ability to operate in biological systems of varying complexity, up to the level of living cells and tissues.
In this work we review fluorescent probe approaches in lipid membranes with a particular focus on environment-sensitive fluorophores. These molecules provide information on the properties of their molecular environment directly by changing their fluorescence characteristics (wavelength maximum, fluorescence intensity, and/or fluorescence lifetime). However, many questions remain concerning fluorescence sensing of biomembranes with molecular probes. How well does the spectroscopic response of a particular probe reflect a studied membrane property? At which precise location in the membrane does the probe monitor a given parameter? Is it possible to obtain information about more than one membrane property by using the same probe? To address these questions, a careful analysis of the probe response as a function of its localization has to be performed, and multiparametric probes capable of simultaneously monitoring several parameters through different channels have to be developed.
Parameters targeted by fluorescence probing
First, we should define the membrane properties and understand how they are reflected in the spectroscopic response of the fluorescent probes. Microviscosity (the reciprocal to fluidity) is the measure of frictional resistance to rotational and translational motions of molecules. Membrane microviscosity can be estimated by fluorescence anisotropy of rod-shaped probes (typically diphenylhexatriene (DPH)) (1), which reflects their rotational motion in the membrane, or by fluorescence intensity of molecular rotors (2), which reflects intramolecular rotations of the probe segments. Because in lipid membranes the probe rotational mobility (of rods and rotors) is not isotropic but restricted in space, it also reflects the level of lipid order (or lipid packing) in the bilayer. Microviscosity and lipid order are the main parameters that discriminate different membrane phase states: gel (Lβ), liquid-ordered (Lo), and fluid (Lα and Ld). The gel phase and the liquid-ordered phase, which is believed to be responsible for the functional lipid domains (rafts) in cell membranes (3,4), present a higher level of lipid order and microviscosity compared to the fluid phase.
The polarity of lipid membranes is also frequently addressed with the use of fluorescent probes. In isotropic media, such as organic solvents, absorption and fluorescence spectra shift as a function of polarity, and this shift can be expressed in empirically established units (5) and analyzed on the basis of a well-developed physical theory (6). Its background takes into account the difference in the energies of the fluorophore ground and excited states, which can vary based on their dipole-dipole interactions with the environment. Polarity sensing in membranes is an attempt to transfer this concept to the highly anisotropic structure of bilayers, in which polarity gradients extend on the same length scale as the fluorophore sizes. Moreover, polarity sensing in membranes is complicated by the strong local electric fields produced by ordered charges and dipoles that generate spectroscopic effects similar to those of disordered dipoles in isotropic polar solvents. This makes the probe response in lipid membranes orientation dependent, and polarity sensing based on scaling in isotropic media, problematic.
The static electric fields in membranes are often classified as surface (Ψs), dipole (Ψd), and transmembrane (Ψt) potentials (7). These three potentials differ in origin and localization. The surface potential, which is generated by the charged headgroups of phospholipids and the adsorbed ions at the interface, spans between the membrane surface and the bulk water. This potential is strongly connected with the interfacial pH and therefore is commonly monitored by pH-sensitive surface-located probes (8). The dipole potential originates from the ordered dipoles of lipids (ester groups and dipolar headgroups) and ordered water molecules. It localizes between the membrane surface and the central hydrophobic part of the bilayer. Finally, the transmembrane potential is generated by the difference in the ion concentrations at the two sides of the bilayer and spans across all its length. Both Ψt and Ψd potentials can be detected by shifts in the position of absorption/emission bands of electric-field-sensitive (electrochromic) probes localized inside the membrane (9,10).
Hydration describes the highly depth-dependent distribution and dynamics of water molecules in the lipid membrane (11). Hydration is connected with membrane electrostatics, since the oriented water dipoles solvating the phosphate and carbonyl groups contribute to the electric fields in the bilayer (12). Because water is the most important dipolar and H-bonding component in lipid bilayers, sensing membrane hydration is based on wavelength shift (13) or quenching (14) resulting from dipolar and H-bonding interactions of water with the fluorophore. As in common polar solvents, fluorescent probes can detect the rate and extent of the structural relaxations of their surrounding (frequently called solvent relaxation (15)). These relaxations around the excited-state dipole describe mainly water dynamics (15). However, unlike neat water, where the relaxations are on a picosecond timescale, the relaxation time of water in lipid bilayers extends to the nanosecond scale due to the constraints imposed by the viscous lipid environment. Since the fluorescence lifetimes of the probes are also on the same timescale, fluorescence spectra may shift within the time of the probe emission and therefore the shifts in the steady-state fluorescence spectra will depend on the relaxation dynamics.
The structural anisotropy and the steep nanoscale gradients of all biophysical properties in membranes require new approaches with fluorescence probes. It is becoming clear that methodologies based on approximating the probe environment as an isotropic medium have reached the limits of their possibilities. Moreover, when measured in membranes, these properties become less clearly defined and are often interrelated. We believe that fluorescence probe approaches should be developed along the following lines:
-
1.
When probing a membrane, one should know the precise location and depth of the probe in the bilayer, and avoid the use of probes with a broad distribution of locations (16). The orientation of the fluorophore should also be well controlled, especially when the electrostatic properties (dipole and transmembrane potential) are being measured (9,17).
-
2.
A single fluorophore at a particular location should sense two or more independent membrane parameters that can be independently estimated. Two possibilities can be realized here. One is to apply the dyes that exist in two or several ground-state and/or excited-state forms, so that the fluorescence of these forms can be sensitive to different parameters. The other is to benefit from the ability to locate the same environment-sensitive fluorophore in different orientations in the membrane, which allows one to distinguish between vectorial (membrane potential) and scalar (hydration and fluidity) properties (18).
-
3.
There should be a switch from a continuous-medium to a molecular-scale interpretation of fluorescence probing data, which is a difficult task. Such a switch in the interpretation of protein fluorescence (19) showed that variations in positions of tryptophan emission bands that were interpreted earlier as polarity effects were in fact the effects of molecular-scale electric fields. This switch is more difficult to perform in biomembrane studies because of the lack of structural information with atomic-scale resolution. The development of molecular-dynamics simulations may offer solutions in the future.
Environment-sensitive probes
Among the environment-sensitive fluorophores currently used in membrane research, we should mention molecular rotors and solvatochromic and electrochromic dyes. The molecular rotors are fluorophores that exhibit strong variations in their fluorescence quantum yield depending on their intramolecular rotation, which is a function of the environment viscosity. Indeed, in more viscous media these rotations are slowed down, which increases the fluorescence intensity of the fluorophore. Being incorporated into lipid membranes, these fluorophores monitor the microviscosity of their surroundings at their sites of location (2). Typical examples of these probes are DCVJ, its carboxy analog, and some recently developed analogs of Prodan (Fig. 1).
Figure 1.
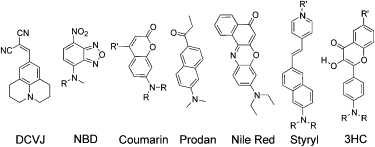
Examples of environment-sensitive fluorophores for studying the biophysical properties of lipid membranes. The fluorophores are denoted by their short names or abbreviations.
A more widely used class of environment-sensitive fluorophores consists of solvatochromic dyes that exhibit shifts in their emission spectra as a function of such properties as polarity and hydration of their environment. These dyes exhibit strong changes in dipole moments upon electronic excitation. Universal dipole-dipole and specific (H-bonding) interactions of these dyes with their surroundings change the energy of the probe electronic transitions and thus shift the maxima of their excitation and emission spectra. Typical examples of such dyes are NBD, Prodan, 7-(dialkylamino)coumarin, and Nile Red (Fig. 1). In these fluorophores, the dipole moment increases dramatically upon electronic excitation due to an intramolecular charge transfer (ICT) from the electron donor (dialkylamino group) to the electron acceptor (carbonyl group; Fig. 2). As a result, these dyes exhibit a red shift of their emission spectrum in response to an increase in solvent polarity and the relaxation rates of their surroundings (13,20). Moreover, an additional strong red shift of the Prodan emission is connected with H-bonding to H-bond donor molecules in the environment (21). Such effects are typical for environment-sensitive dyes that contain H-bond acceptor groups (such as carbonyl), which are of particular interest for studying membrane hydration.
Figure 2.
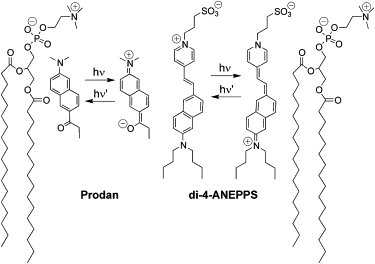
Schematic representation of the ground and excited states of the solvatochromic probe Prodan and the electrochromic probe di-4-ANEPPS in lipid bilayers; hν indicates the excitation, and hν′ indicates the emission. To indicate the probe locations, two PC molecules constituting the outer monolayer are shown.
Electrochromic dyes operate by the same photophysical principle as solvatochromic dyes, namely, a strong ICT upon electronic excitation. They are commonly rod-shaped molecules that bear electron donor and acceptor groups on the two opposite sides. A typical example is styryl pyridinium dyes (Fig. 2). The interaction of the ground- and excited-state dipoles with an external electric field results in electrochromic shifts of both absorption and emission bands (17,22). However, since the shifts in emission spectra are distorted by molecular relaxations, shifts in excitation spectra are commonly used (23). The excitation spectra are affected by the solvation of the highly polar ground states, which results in anomalous blue-shifted excitation spectra depending on the lipid composition, phase state, hydration, etc. (23–25). As a result, there is a strong demand for advanced environment-sensitive dyes capable of distinguishing solvation (and particularly hydration) from the electric potentials.
Advanced multiparametric probing
Measuring two or more membrane parameters by applying several probes is not always reliable, since even probes that are similar in structure can occupy different positions and interact with different partners. The ideal method would be to extract several types of information from a single probe. This can be achieved by establishing an equilibrium between several emissive states. Of the known excited-state reactions that generate several emissive species (26), excited-state intramolecular proton transfer (ESIPT) appears to be the most promising. Indeed, this reaction can generate emissive species with very different sensitivities to parameters of their environment (27).
Particularly interesting in this respect are 3-hydroxychromone (3HC) dyes. Due to a reversible ESIPT reaction, in addition to the commonly observed normal (N∗) excited state, they show a tautomer (T∗) excited state (Fig. 3), which is also highly emissive. The relative energies of these states control their relative intensities (28,29). Due to ICT from the 4′-dialkylamino to the 4-carbonyl group, the N∗ state attains a large dipole moment (29,30) similarly to classical solvatochromic dyes such as Prodan. In contrast, the ESIPT product T∗ state exhibits a much smaller dipole moment (30,31). Therefore, the position of the N∗ emission band, unlike the T∗ band, is an indicator of solvent polarity. Moreover, since the N∗ state possesses a much larger dipole moment, intermolecular interactions stabilize this state as compared to the T∗ state, resulting in a shift of the ESIPT equilibrium and redistribution of fluorescence intensity between the two bands. As a consequence, their intensity ratio (N∗/T∗) describes the environment polarity (29) and electrostatics (32).
Figure 3.
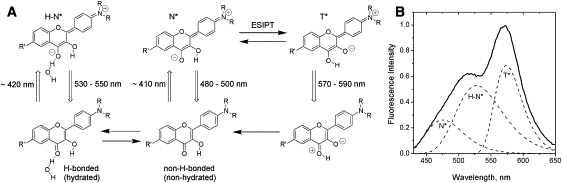
(A) Ground (below) and excited (above) states of 3HC derivatives in lipid membranes. Upward arrows represent excitation, and downward arrows represent emission. The correspondent band maxima (in nm) are indicated. (B) Fluorescence spectrum of 4-(dimethylamino)-3-hydroxyflavone in large unilamellar vesicles composed of egg yolk PC, and its deconvolution into the emission bands of the N∗, H-N∗, and T∗ forms.
In lipid bilayers, 3HC probes present two different ground-state forms: 1), the H-bonded form with water (hydrated) and 2), the non-H-bonded (nonhydrated) form (Fig. 3), which allows an additional parameter describing the membrane hydration to be obtained (16). These forms exhibit differences in the excitation and emission spectra. Whereas the nonhydrated form (blue-shifted in excitation) exhibits a dual emission (N∗ and T∗ bands) as in aprotic organic solvents, the H-bonded form exhibits a single emission band (H-N∗ band) similar to the spectra obtained in protic solvents (16,33). Deconvolution of their emission spectra into three bands (N∗, T∗, and H-N∗; Fig. 3 B) provides information about the relative contribution of the hydrated form of the probe through the so-called “hydration” parameter (H-parameter), defined as the ratio of intensities of the H-N∗ band to the sum of the N∗ and T∗ bands. Moreover, the N∗/T∗ ratio can be used as a polarity indicator.
Time-resolved studies of the 3HC probe F2N12S, in which the fluorophore is located at an interface (Fig. 4) and exhibits a significant H-N∗ emission, confirmed that ESIPT equilibrium between N∗ and T∗ forms is established on a timescale of 40–70 ps, and different relaxation kinetics involving the H-N∗ form are completed on a nanosecond timescale (34). These data suggest that the hydration H-parameter describes both the ground-state hydration and the water relaxation in the lipid environment, in analogy to the generalized polarization parameter previously introduced for Prodan and Laurdan dyes (13,16). In contrast, the N∗/T∗ ratio is controlled by the rapidly established N∗-T∗ equilibrium in the excited state, and therefore mainly describes the interactions with the static electric fields in the membrane. Thus, it is clear that the hydration H-parameter and the N∗/T∗ parameter are intrinsically independent and describe different membrane properties.
Figure 4.
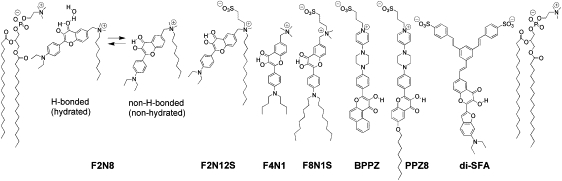
Probes of the 3HC family with different locations and orientations in lipid bilayers. For probe F2N8, the two ground state forms are shown. To indicate the probe locations, two PC molecules constituting the outer monolayer are shown.
Dye location in lipid bilayers
The gradients of all physical parameters across the bilayer are very steep even in comparison with the fluorophore sizes. As a consequence, knowledge of the precise probe location becomes crucial for evaluating their response. The most trivial approach is to apply polar fluorescent probes to study the polar membrane interface, and apolar probes to study its hydrophobic interior. However, this approach often fails because the probe molecule may exhibit several locations and orientations in the bilayer. In particular, small fluorophores of medium polarity can distribute in both polar and apolar regions. Moreover, ground-state hydration of a probe capable of forming H-bonds is an additional feature that causes this distribution. Steady-state and time-resolved fluorescence data on Nile Red (35), coumarins (36), and Prodan (37,38) demonstrate that these probes can be located simultaneously in both polar and apolar regions of the membrane. Similarly, two ground-state forms of the uncharged and nonsubstituted 3HC dye 4′-(dimethylamino)-3-hydroxyflavone (probe F)—one corresponding to the H-bonded form with a surface location, and the other to the non-H-bonded form located deeper—were found in lipid bilayers (16). Both forms show different emission profiles, reporting on different environments of the two locations.
Structural changes in membranes may cause relocation and reorientation of the probe. Recent data on giant unilamellar vesicles (GUVs) directly show that Prodan and Laurdan do not present any preferential orientation in fluid phase membranes, whereas in liquid-ordered and gel phase membranes they present a constrained vertical orientation (non-H-bonded form) (39). Moreover, hydrostatic pressure (38) and cholesterol (40) can cause Prodan relocation. Fluorophore relocation on modification of membrane properties was also proposed for NBD derivatives (41) and 3HC probe F (16). Relocation of a probe along the polarity gradient may also occur during the time of emission, when the excited state is much more polar than the ground state (42).
The polarity of the fluorophore itself is also an important factor in its ground-state location. For example, covalent attachment of the relatively polar NBD to flexible acyl chains does not allow its localization close to the bilayer center, because it loops back to the interface (43,44). This and other unsuccessful attempts suggest that instead of using modifications of lipids, amphiphilic constructions that can fix the fluorophore at a desired orientation and depth should be synthesized. This can be achieved by choosing a relatively low-polar fluorophore and applying a combination of charged and apolar substituents. Typical examples are Laurdan and Patman, which exhibit a more precise location in lipid bilayers than the parent Prodan (15). Recently developed fatty acid analogs of Prodan utilize similar design principles (45). A similar strategy was also applied to develop a series of 3HC probes (Fig. 4), in which the conjugation of the fluorophore to both an ionic headgroup and a long alkyl chain(s) provides a well-defined depth and orientation to the fluorophore in the lipid bilayer (33,46–48).
Hydration and relaxation dynamics
Two aspects of membrane hydration in lipid bilayers should be considered: 1), the distribution of water molecules across the bilayer; and 2), their dynamics. Both of these factors influence the spectral shifts and produce time-resolved effects (15,20). The relaxation dynamics of different probes in membranes, which includes relaxation of water molecules, is slower by 3–5 orders of magnitude than in neat water and comparable to the fluorescence lifetimes. These slow relaxation dynamics lead to characteristic dependencies of the positions of the fluorescence spectra on excitation wavelength (the red-edge effects (49,50)). A more retarded dynamics is observed at the level of the glycerol backbone and acyl chains than at the polar interface (42,51). Therefore, the relaxation dynamics in membranes should be studied with respect to a particular membrane site. To probe the relaxation dynamics on the level of the glycerol and the headgroup regions of the bilayer, a relatively polar fluorophore (such as NBD) can be attached to the polar headgroup of phospholipids (41,49). For instance, Laurdan (39) and Patman (36) allow the hydration and relaxation dynamics of the glycerol region of the bilayer to be probed.
Hydration in the glycerol region is connected with a variety of membrane parameters. It increases strongly with membrane curvature, as revealed by the relaxation dynamics of Prodan and Patman probes (36) and the spectral response of the F2N8 probe (33). In line with this curvature effect, mechanical stress (52) and external pressure (53) can also affect membrane hydration. Moreover, a strong increase in hydration is produced by an increase in temperature within the fluid phase, as reported by Prodan- (37) and NBD- (41) based probes, as well as the F2N8 probe (33).
Hydration is also connected to the presence of cholesterol and other sterols, which decrease the membrane's permeability to water (54). Cholesterol expels water from the fluid phase bilayers (13,33) and decreases the water relaxation dynamics in the fluid phase.
Hydration and lipid-order IN bilayers
The fluid phase of lipid bilayers, which is characterized by a low lipid order and high mobility of lipids, exhibits fast relaxation dynamics and a high level of hydration. In contrast, the gel phase, which is characterized by a high lipid order and slow lipid mobility, exhibits low hydration. Viscosity-sensitive probes, such as DPH derivatives and molecular rotors, clearly show the differences between these two phases (1,55). Moreover, the hydration evaluated from the lifetime measurements of the DPH-based probe (TMA-DPH) also correlates well with the phase state, so that the phase with higher lipid order (and higher microviscosity) presents a lower level of hydration (14).
In contrast, solvatochromic probes frequently show complicated behaviors with a change in the lipid order, particularly during the main phase transition. For instance, the spectroscopic response of acyl chain NBD-labeled lipid is opposite to the response of the corresponding headgroup-labeled lipids (41). On the gel to fluid phase transition, the NBD probe bound to the acyl chain relocates toward the membrane surface, and while bound to the headgroup, it undergoes a deeper embedding. A particularly strong spectroscopic response (red shift) during this transition is observed with Prodan and Laurdan, suggesting a dramatic increase in the hydration of the probe environment (56) and probably changes in the probe location/orientation. Indeed, while in the fluid phase, these probes show no preferential orientation in the bilayer, whereas in the gel phase they adopt a uniform vertical orientation (57), exhibiting poor accessibility to water.
The hydration (H-parameter), as measured by the 3HC probe F2N8 anchored at the glycerol region (Fig. 4), shows only a moderate increase on the gel-fluid phase transition (33). This small effect probably reflects the fact that this fluorophore is anchored by a charged group and thus is unable to relocate and/or attain vertical orientation in the gel phase. This speculation is supported by the larger changes in the apparent hydration for the unanchored 3HF derivative, probe F (16). The smaller dehydration effects observed with the F2N8 probe could also be due to partial expelling of the fluorophore by the ordered gel phase to a shallower bilayer region.
Therefore, when analyzing the response of environment-sensitive probes to phase transition, one should take into account the fact that changes in lipid packing may affect the probe location/orientation in the bilayer.
Raft domains
The liquid-ordered (Lo) phase of lipid bilayers, which consists of saturated lipids (either phosphatidylcholine (PC) or sphingomyelin (SM)) and cholesterol, is of particular interest since it is thought to be responsible for the so-called raft domains in cell membranes. Similarly to the gel phase, the Lo phase is also characterized by a high lipid order, as evidenced by both DPH (58) and molecular rotor probes (55). According to Prodan and Laurdan data, hydration is much lower in the Lo phase than in the fluid phase, being close to that of the gel phase (13). An environment-sensitive dye of the styrylpiridinium family, di-ANEPPQ, also shows a blue-shifted emission in the Lo phase with respect to the fluid phase (24), suggesting a decreased water relaxation. Moreover, in contrast to the fluid phase, the Lo phase shows a strong second harmonic generation signal, suggesting that the probe adopts a vertical orientation (24).
Using the 3HC F2N8 probe, we confirmed that hydration is lower in rafts than in the gel phase (59). This could be explained not only by exclusion of water by cholesterol in the glycerol region, but also by changes in the probe location and orientation in the bilayer. Observation of the probe fluorescence in GUVs under polarized excitation supports this idea (Fig. 5). Indeed, in the fluid phase, uniform fluorescence intensity was observed all over the GUVs, confirming that the probe exhibits a variety of orientations. In contrast, in the Lo phase, maximum fluorescence intensity is observed in the regions where the light polarization is perpendicular to the bilayer plane. This suggests that the high order of the Lo phase imposes a vertical orientation to the fluorophore, as previously shown with Laurdan and di-ANEPPQ.
Figure 5.

Probing raft domains using probe F2N12S. (A) Fluorescence spectra of F2N12S in lipid vesicles composed of DOPC (black solid curve), DOPC+35% cholesterol (green dashed curve), and SM + 35% cholesterol (magenta dotted curve). Fluorescence ratiometric images (520/580 nm) of GUVs composed of (B) DOPC, (C) SM + 35% cholesterol, and (D) the ternary mixture (DOPC/SM/ cholesterol in the molar ratios 1/1/0.7). Two-photon excitation (830 nm) was used. Arrows indicate the orientation of the light polarization. Image size: (B) 50×50 μm, (C) 15×15 μm, and (D) 45×45 μm.
The sensitivity of environment-sensitive probes to the membrane lipid phase has been used to visualize rafts in membranes (24,39,55,60). Since the Lo phase demonstrates high lipid order and slow dynamics, the corresponding domains can be visualized using the enhanced fluorescence intensity of a molecular rotor based on a Prodan analog (55). Because of their response to membrane hydration, Laurdan and di-ANEPPQ probes allow color imaging of the lipid domains (24,39). Remarkably, the latter approach is independent of the partition of the probe between the different phases, and provides a direct assignment of the phases according to their hydration. Recently, a Prodan-based fatty acid was introduced that has a higher specificity to cell plasma membranes and thus is probably more suitable than other Prodan derivatives for cellular studies of lipid domains (45). Similarly, a 3HC probe, F2N12S, partitions into both Lo and Ld phases, thus allowing a direct recognition of these phases by fluorescence ratiometric imaging (Fig. 5) (61).
Electrostatics: surface potential
The surface potential (Ψs) is the electrostatic potential at the membrane-water interface.
This parameter is difficult to measure directly with electrochromic dyes because such dyes are located at the interface and thus are easily quenched and/or strongly affected by water relaxation. Alternatively, sorption-desorption of charged dyes (62) can be used, as well as probes that respond to local proton concentrations at different distances from the bilayer interface (8). These limited approaches were recently complemented by the use of 3HC dyes (F2N8 and F2N12S) that are localized at the interface and thus are also sensitive to the surface potential (46,47).
To account for a possible contribution of hydration to the response of 3HC probes to the surface charge, we deconvolved their spectra into three bands (Fig. 3 B) and analyzed their intensity ratios. The N∗/T∗ ratio of these dyes is strongly connected to factors that affect the surface potential (e.g., the charge of the lipid headgroup), whereas the hydration H-parameter does not show any correlation with the surface charge (16). Moreover, the N∗/T∗ ratio remains insensitive to factors that strongly affect the H-parameter, namely, the membrane curvature, temperature, and phase state (16,33). Thus, the N∗/T∗ ratio of the interface-located 3HC probes is a parameter that is highly selective to the membrane surface potential. As shown further below, the N∗/T∗ parameter can be also used to evaluate the dipole and transmembrane potentials with other specially designed 3HC probes located deeper in the membrane.
An important application of the shallow-located 3HC dye F2N12S, which can simultaneously sense both hydration and surface charge, is to detect apoptosis, the biological process of programmed cell death. In its early stage, apoptosis results in the loss of transbilayer asymmetry, which is accompanied by changes in the surface charge (phosphatidylserine (PS) exposure), lipid order, and hydration. PS exposure is usually observed with annexin V-FITC, a fluorescently labeled Ca2+-dependent PS-binding protein (63). The F2N12S probe was designed to detect the exposure of anionic lipids at the outer leaflet of cell membranes. Cell apoptosis generated by actinomycin D resulted in significant changes in the dual emission of the probe, allowing ratiometric imaging as well as fluorescence flow cytometry to differentiate apoptotic from normal cells (Fig. 6). Analysis of the spectroscopic response of F2N12S suggests that it corresponds to a combination of two factors related to the loss of the transbilayer asymmetry: 1), the increase in the negative surface charge; and 2), the loss of the Lo phase of the outer leaflet of the plasma membrane.
Figure 6.
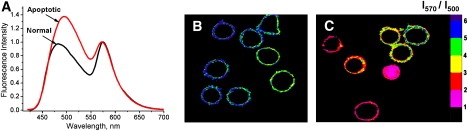
Apoptosis detection using probe F2N12S. (A) Fluorescence spectra of F2N12S in normal and apoptotic (treated with actinomycin D) CEM cells. Ratiometric images of (B) normal and (C) actinomycin D-treated cells stained with F2N12S. The size of the images is 60×70 μm.
Electrostatics: dipole potential and transmembrane potential
Unlike surface potential, dipole (Ψd) and transmembrane (Ψt) potentials are localized inside the lipid bilayer. Therefore, their detection requires an electrochromic fluorophore presenting a vertical orientation in the membrane. Because these two potentials apply at different levels in the lipid bilayer, probing Ψd requires a location of the fluorophore somewhere between the apolar bilayer center and the membrane surface, whereas probing Ψt requires a location in the apolar region close to the bilayer center.
To control the vertical orientation of a fluorophore within the bilayer, it appears more appropriate to design amphiphilic probe molecules rather than labeled lipids. These probes should contain a charged group on one end of the fluorophore to anchor it close to the bilayer headgroups, and also hydrophobic groups on the opposite end to ensure its deep insertion in the hydrophobic part of the bilayer. This was successfully realized with the use of styryl dyes (10,64) and their analogs (65). These probes, having a charged group and hydrophobic chains on the opposite sides of the rod-shaped fluorophore (Fig. 2), attain a vertical orientation in the bilayer and span between the headgroups and the alkyl chains of lipids. Therefore, they show a strong sensitivity of their excitation spectra to Ψd (9) and, to a lesser extent, Ψt (64). These probes respond to the insertion of dipolar molecules such as 6-ketocholestanol (6-KC) or phloretin into lipid bilayers, removal of the carbonyl groups from phospholipids (to obtain ether lipids), and modification of the lipid unsaturation degree, which significantly modify Ψd. Despite their sensitivity to ground-state interactions and excited-state relaxations, which are unrelated to membrane electrostatics, and their own dipole, which can modify the local Ψd itself (66), these styryl dyes are still the reference fluorescent probes for ψd measurements (9,23,25).
Further progress requires the development of probes that 1), do not possess a large ground-state dipole; 2), allow quantitative ratiometric recording of fluorescence emissions, with the major contribution from the electrostatic effects suppressing the side effects from the relaxation processes; and 3), allow the desired location and orientation of the fluorophore moiety. 3HC dyes meet all of these requirements well. By using a design similar to that of styryl dyes, we developed two probes (F4N1 and BBPZ) in which the fluorescent moieties and consequently the dipole moments are oriented vertically in lipid bilayers but inversely with respect to each other (Fig. 4). In both cases, the probes strongly respond to variation of Ψd, as evidenced by changes in the N∗/T∗ ratio of their two emission bands (18). Moreover, it was shown that these changes and the variations of the hydration H-parameter are independent (67). Two improved derivatives, F8N1S and PPZ8 (Fig. 4), which bind selectively to the cell plasma membranes, were applied to measure Ψd in living cells (Fig. 7) (68).
Figure 7.
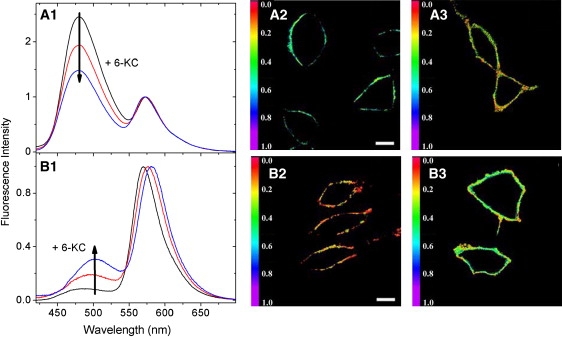
Monitoring the membrane dipole potential with F8N1S (A1–A3) and PPZ8 (B1–B3) probes in living cells. Fluorescence spectroscopic response of F8N1S (A1) and PPZ8 (B1) to variations of the dipole potential in THP-1 cell plasma membranes produced by addition of 6-KC. Confocal fluorescence images of cells stained with F8N1S (A2 and A3) and PPZ8 (B2 and B3) without (A2 and B2) and with (A3 and B3) 6-KC. Scale bar: 10 μm.
The transmembrane potential is a highly dynamic parameter that can change in living cells on the timescale of microseconds. Therefore, electrochromic probes, which are characterized by a fast response to Ψt, appear to be essential tools in this domain (69,70). As previously mentioned, styryl-based electrochromic dyes (such as di-4-ANEPPS; Fig. 2) and their analogs were originally proposed for Ψt detection (10,64). Recently improved analogs with near-infrared absorbance and higher solubility are particularly suited for in vivo Ψt imaging (71). However, their weak response in the excitation spectra (7% signal change per 100 mV) is frequently insufficient for measuring small Ψt variations. The response of these probes could be improved by the use of nonlinear fluorescence microscopy techniques, which are highly sensitive to probe orientation (72). However, since these measurements cannot be used with common microscopy instrumentation, their wide application in cellular research is limited, necessitating the search for new probes.
To improve the sensitivity of electrochromic dyes to the transmembrane potential, researchers developed much longer rigid analogs of styryl dyes (ANNINE probes) that span from the surface of the bilayer to almost the middle of the bilayer (65,73). This improved the sensitivity to Ψt more than twofold. However, as with their parent probes, only the excitation spectra could be used.
For this reason, we attempted to apply the 3HC fluorophore for Ψt detection. To that end, we selected an extended fluorophore to improve the two-wavelength electrochromic response at low polarities, and coupled it with two rigid arms bearing charged groups to impose a vertical orientation and a deep insertion in the lipid bilayer (probe di-SFA; Fig. 4). In model and cellular membranes, the obtained probe showed fluorescence spectra corresponding to a very low-polarity environment (ɛ ∼ 3–4), in line with a deep insertion (48). In cell membranes, the probe response of 12% per 100 mV was larger than the corresponding response of the styryl probes (64), but less than that of the new generation of ANNINE probes (65). Nevertheless, the key advantage of this 3HC probe lies in its ratiometric response in emission, which enables easy application in fluorescence microscopy by using a single excitation source and a two-color fluorescence detection setup.
Outlook
The field of biomembrane science is putting more and more stringent demands on organic fluorescent probes. Other fluorophores, such as quantum dots, metal nanoparticles, or conjugated polymers, being the size of the bilayer, are too large to provide the necessary site-selective response. Therefore, environment-sensitive fluorescent dyes remain the major tools for getting information about the structure and dynamics in biomembranes. The main developmental trends are to improve the probes' selectivity for a particular membrane property, to increase the information content of the probe response, and to address the biophysical properties at a particular membrane depth. In this respect, the development of multichannel environment-sensitive probes capable of distinguishing between different membrane physicochemical properties through additional spectroscopic parameters constitutes a substantial step forward. With the aid of these probes, essential cellular processes such as apoptosis can be followed at the level of plasma membranes.
References
- 1.Lentz B.R. Membrane ‘fluidity’ as detected by diphenylhexatriene probes. Chem. Phys. Lipids. 1989;50:171–190. [Google Scholar]
- 2.Haidekker M.A., Theodorakis E.A. Molecular rotors—fluorescent biosensors for viscosity and flow. Org. Biomol. Chem. 2007;5:1669–1678. doi: 10.1039/b618415d. [DOI] [PubMed] [Google Scholar]
- 3.Simons K., Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 4.Brown D.A., London E. Functions of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol. 1998;14:111–136. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- 5.Reichardt C. Solvatochromic dyes as solvent polarity indicators. Chem. Rev. 1994;94:2319–2358. [Google Scholar]
- 6.Lippert E.L. Laser-spectroscopic studies of reorientation and other relaxation processes in solution. In: Birks J.B., editor. Organic Molecular Photophysics. Wiley; New York.: 1975. pp. 1–31. [Google Scholar]
- 7.Cevc G. Membrane electrostatics. Biochim. Biophys. Acta. 1990;1031:311–382. doi: 10.1016/0304-4157(90)90015-5. [DOI] [PubMed] [Google Scholar]
- 8.Kraayenhof R., Sterk G.J., Wong Fong Sang H.W. Probing biomembrane interfacial potential and pH profiles with a new type of float-like fluorophores positioned at varying distance from the membrane surface. Biochemistry. 1993;32:10057–10066. doi: 10.1021/bi00089a022. [DOI] [PubMed] [Google Scholar]
- 9.Gross E., Bedlack R.S., Jr., Loew L.M. Dual-wavelength ratiometric fluorescence measurement of the membrane dipole potential. Biophys. J. 1994;67:208–216. doi: 10.1016/S0006-3495(94)80471-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fluhler E., Burnham V.G., Loew L.M. Spectra, membrane binding, and potentiometric reponses of new charge shift probes. Biochemistry. 1985;24:5749–5755. doi: 10.1021/bi00342a010. [DOI] [PubMed] [Google Scholar]
- 11.Disalvo E.A., Lairion F., Martini F., Tymczyszyn E., Frias M. Structural and functional properties of hydration and confined water in membrane interfaces. Biochim. Biophys. Acta. 2008;1778:2655–2670. doi: 10.1016/j.bbamem.2008.08.025. [DOI] [PubMed] [Google Scholar]
- 12.Gawrisch K., Ruston D., Zimmerberg J., Parsegian V.A., Rand R.P. Membrane dipole potentials, hydration forces, and the ordering of water at membrane surfaces. Biophys. J. 1992;61:1213–1223. doi: 10.1016/S0006-3495(92)81931-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parasassi T., Di Stefano M., Loiero M., Ravagnan G., Gratton E. Cholesterol modifies water concentration and dynamics in phospholipid bilayers: a fluorescence study using Laurdan probe. Biophys. J. 1994;66:763–768. doi: 10.1016/s0006-3495(94)80852-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ho C., Slater S.J., Stubbs C.D. Hydration and order in lipid bilayers. Biochemistry. 1995;34:6188–6195. doi: 10.1021/bi00018a023. [DOI] [PubMed] [Google Scholar]
- 15.Jurkiewicz P., Sýkora J., Olżyńska A., Humpoličkovà J., Hof M. Solvent relaxation in phospholipid bilayers: principles and recent applications. J. Fluoresc. 2005;15:883–894. doi: 10.1007/s10895-005-0013-4. [DOI] [PubMed] [Google Scholar]
- 16.Klymchenko A.S., Duportail G., Demchenko A.P., Mély Y. Bimodal distribution and fluorescence response of environment-sensitive probes in lipid bilayers. Biophys. J. 2004;86:2929–2941. doi: 10.1016/S0006-3495(04)74344-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loew L.M., Simpson L.L. Charge-shift probes of membrane potential. A probable electrochromic mechanism for p-aminostyrylpyridinium probes on a hemispherical lipid bilayer. Biophys. J. 1981;34:353–365. doi: 10.1016/S0006-3495(81)84854-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klymchenko A.S., Duportail G., Mély Y., Demchenko A.P. Ultrasensitive two-color fluorescence probes for dipole potential in phospholipid membranes. Proc. Natl. Acad. Sci. USA. 2003;100:11219–11224. doi: 10.1073/pnas.1934603100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vivian J.T., Callis P.R. Mechanisms of tryptophan fluorescence shifts in proteins. Biophys. J. 2001;80:2093–2109. doi: 10.1016/S0006-3495(01)76183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sýkora J., Kapusta P., Fidler V., Hof M. On what time scale does solvent relaxation in phospholipid bilayers happen? Langmuir. 2002;18:571–574. [Google Scholar]
- 21.Catalan J., Perez P., Laynez J., Blanco F.G. Analysis of the solvent effect on the photophysics properties of 6-propionyl-2-(dimethylamino)naphthalene (PRODAN) J. Fluoresc. 1991;1:215–223. doi: 10.1007/BF00865246. [DOI] [PubMed] [Google Scholar]
- 22.Bublitz G.U., Boxer S.G. Stark spectroscopy: applications in chemistry, biology, and materials science. Annu. Rev. Phys. Chem. 1997;48:213–242. doi: 10.1146/annurev.physchem.48.1.213. [DOI] [PubMed] [Google Scholar]
- 23.Clarke R.J., Kane D.J. Optical detection of membrane dipole potential: avoidance of fluidity and dye-induced effects. Biochim. Biophys. Acta. 1997;1323:223–239. doi: 10.1016/s0005-2736(96)00188-5. [DOI] [PubMed] [Google Scholar]
- 24.Jin L., Millard A.C., Wuskell J.P., Dong X., Wu D. Characterization and application of a new optical probe for membrane lipid domains. Biophys. J. 2006;90:2563–2575. doi: 10.1529/biophysj.105.072884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vitha M.F., Clarke R.J. Comparison of excitation and emission ratiometric fluorescence methods for quantifying the membrane dipole potential. Biochim. Biophys. Acta. 2007;1768:107–114. doi: 10.1016/j.bbamem.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 26.De Silva A.P., Gunaratne H.Q.N., Gunnlaugsson T., Huxley A.J.M., McCoy C.P. Signaling recognition events with fluorescent sensors and switches. Chem. Rev. 1997;97:1515–1566. doi: 10.1021/cr960386p. [DOI] [PubMed] [Google Scholar]
- 27.Demchenko A.P., Klymchenko A.S., Pivovarenko V.G., Ercelen S., Duportail G. Multiparametric color-changing fluorescence probes. J. Fluoresc. 2003;13:291–295. [Google Scholar]
- 28.Shynkar V.V., Mély Y., Duportail G., Piémont E., Klymchenko A.S. Picosecond time-resolved fluorescence studies are consistent with reversible excited-state intramolecular proton transfer in 4′-(dialkylamino)-3-hydroxyflavones. J. Phys. Chem. A. 2003;107:9522–9529. [Google Scholar]
- 29.Klymchenko A.S., Demchenko A.P. Multiparametric probing of intermolecular interactions with fluorescent dye exhibiting excited state intramolecular proton transfer. Phys. Chem. Chem. Phys. 2003;5:461–468. [Google Scholar]
- 30.Chou P.T., Pu S.C., Cheng Y.M., Yu W.S., Yu Y.C. Femtosecond dynamics on excited-state proton/ Charge-transfer reaction in 4′-N,N-diethylamino-3-hydroxyflavone. The role of dipolar vectors in constructing a rational mechanism. J. Phys. Chem. A. 2005;109:3777–3787. doi: 10.1021/jp044205w. [DOI] [PubMed] [Google Scholar]
- 31.Yesylevskyy S.O., Klymchenko A.S., Demchenko A.P. Semi-empirical study of two-color fluorescent dyes based on 3-hydroxychromone. J. Mol. Struct. Theochem. 2005;755:229–239. [Google Scholar]
- 32.Klymchenko A.S., Demchenko A.P. Electrochromic modulation of excited-state intramolecular proton transfer: the new principle in design of fluorescence sensors. J. Am. Chem. Soc. 2002;124:12372–12379. doi: 10.1021/ja027669l. [DOI] [PubMed] [Google Scholar]
- 33.Klymchenko A.S., Mély Y., Demchenko A.P., Duportail G. Simultaneous probing of hydration and polarity of lipid bilayers with 3-hydroxyflavone fluorescent dyes. Biochim. Biophys. Acta. 2004;1665:6–19. doi: 10.1016/j.bbamem.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 34.Das R., Klymchenko A.S., Duportail G., Mély Y. Excited state proton transfer and solvent relaxation of a 3-hydroxyflavone probe in lipid bilayers. J. Phys. Chem. B. 2008;112:11929–11935. doi: 10.1021/jp804956u. [DOI] [PubMed] [Google Scholar]
- 35.Krishna M.M.G. Excited-state kinetics of the hydrophobic probe Nile red in membranes and micelles. J. Phys. Chem. A. 1999;103:3589–3595. [Google Scholar]
- 36.Sýkora J., Jurkiewicz P., Epand R.M., Kraayenhof R., Langner M. Influence of the curvature on the water structure in the headgroup region of phospholipid bilayer studied by the solvent relaxation technique. Chem. Phys. Lipids. 2005;135:213–221. doi: 10.1016/j.chemphyslip.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 37.Jurkiewicz P., Olżyńska A., Langner M., Hof M. Headgroup hydration and mobility of DOTAP/DOPC bilayers: a fluorescence solvent relaxation study. Langmuir. 2006;22:8741–8749. doi: 10.1021/la061597k. [DOI] [PubMed] [Google Scholar]
- 38.Chong P.L.G. Effects of hydrostatic pressure on the location of PRODAN in lipid bilayers and cellular membranes. Biochemistry. 1988;27:399–404. doi: 10.1021/bi00401a060. [DOI] [PubMed] [Google Scholar]
- 39.Bagatolli L.A. To see or not to see: lateral organization of biological membranes and fluorescence microscopy. Biochim. Biophys. Acta. 2006;1758:1541–1556. doi: 10.1016/j.bbamem.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 40.Bondar O.P., Rowe E.S. Preferential interactions of fluorescent probe Prodan with cholesterol. Biophys. J. 1999;76:956–962. doi: 10.1016/S0006-3495(99)77259-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alakoskela J.M.I., Kinnunen P.K.J. Probing phospholipid main phase transition by fluorescence spectroscopy and a surface redox reaction. J. Phys. Chem. B. 2001;105:11294–11301. [Google Scholar]
- 42.Demchenko A.P., Shcherbatska N.V. Nanosecond dynamics of charged fluorescent probes at the polar interface of a membrane phospholipid bilayer. Biophys. Chem. 1985;22:131–143. doi: 10.1016/0301-4622(85)80035-1. [DOI] [PubMed] [Google Scholar]
- 43.Loura L.M., Ramalho J.P. Location and dynamics of acyl chain NBD-labeled phosphatidylcholine (NBD-PC) in DPPC bilayers. A molecular dynamics and time-resolved fluorescence anisotropy study. Biochim. Biophys. Acta. 2007;1768:467–478. doi: 10.1016/j.bbamem.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 44.Huster D., Muller P., Arnold K., Herrmann A. Dynamics of membrane penetration of the fluorescent 7-nitrobenz-2-oxa-1,3-diazol-4-yl (NBD) group attached to an acyl chain of phosphatidylcholine. Biophys. J. 2001;80:822–831. doi: 10.1016/S0006-3495(01)76061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim H.M., Choo H.J., Jung S.Y., Ko Y.G., Park W.H. A two-photon fluorescent probe for lipid raft imaging: C–Laurdan. ChemBioChem. 2007;8:553–559. doi: 10.1002/cbic.200700003. [DOI] [PubMed] [Google Scholar]
- 46.Klymchenko A.S., Duportail G., Oztürk T., Pivovarenko V.G., Mély Y. Novel two-band ratiometric fluorescence probes with different location and orientation in phospholipid membranes. Chem. Biol. 2002;9:1199–1208. doi: 10.1016/s1074-5521(02)00244-2. [DOI] [PubMed] [Google Scholar]
- 47.Shynkar V.V., Klymchenko A.S., Kunzelmann C., Duportail G., Muller C.D. Fluorescent biomembrane probe for ratiometric detection of apoptosis. J. Am. Chem. Soc. 2007;129:2187–2193. doi: 10.1021/ja068008h. [DOI] [PubMed] [Google Scholar]
- 48.Klymchenko A.S., Stoeckel H., Takeda K., Mély Y. Fluorescent probe based on intramolecular proton transfer for fast ratiometric measurement of cellular transmembrane potential. J. Phys. Chem. B. 2006;110:13624–13632. doi: 10.1021/jp062385z. [DOI] [PubMed] [Google Scholar]
- 49.Chattopadhyay A., Mukherjee S. Depth-dependent solvent relaxation in membranes: wavelength-selective fluorescence as a membrane dipstick. Langmuir. 1999;15:2142–2148. [Google Scholar]
- 50.Demchenko A.P. The red-edge effects: 30 years of exploration. Luminescence. 2002;17:19–42. doi: 10.1002/bio.671. [DOI] [PubMed] [Google Scholar]
- 51.Sýkora J., Slavicek P., Jungwirth P., Barucha J., Hof M. Time-dependent stokes shifts of fluorescent dyes in the hydrophobic backbone region of a phospholipid bilayer: combination of fluorescence spectroscopy and ab initio calculations. J. Phys. Chem. B. 2007;111:5869–5877. doi: 10.1021/jp0719255. [DOI] [PubMed] [Google Scholar]
- 52.Zhang Y.L., Frangos J.A., Chachisvilis M. Laurdan fluorescence senses mechanical strain in the lipid bilayer membrane. Biochem. Biophys. Res. Commun. 2006;347:838–841. doi: 10.1016/j.bbrc.2006.06.152. [DOI] [PubMed] [Google Scholar]
- 53.Nicolini C., Celli A., Gratton E., Winter R. Pressure tuning of the morphology of heterogeneous lipid vesicles: a two-photon-excitation fluorescence microscopy study. Biophys. J. 2006;91:2936–2942. doi: 10.1529/biophysj.106.088716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schuler I., Milon A., Nakatani Y., Ourisson G., Albrecht A.M. Differential effects of plant sterols on water permeability and on acyl chain ordering of soybean phosphatidylcholine bilayers. Proc. Natl. Acad. Sci. USA. 1991;88:6926–6930. doi: 10.1073/pnas.88.16.6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hwan M.K., Byeong H.J., Hyon J.Y., Myoung J.A., Mun S.S. Two-photon fluorescent turn-on probe for lipid rafts in live cell and tissue. J. Am. Chem. Soc. 2008;130:4246–4247. doi: 10.1021/ja711391f. [DOI] [PubMed] [Google Scholar]
- 56.Parasassi T., De Stasio G., Ravagnan G., Rusch R.M., Gratton E. Quantitation of lipid phases in phospholipid vesicles by the generalized polarization of Laurdan fluorescence. Biophys. J. 1991;60:179–189. doi: 10.1016/S0006-3495(91)82041-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bagatolli L.A., Gratton E. Two photon fluorescence microscopy of coexisting lipid domains in giant unilamellar vesicles of binary phospholipid mixtures. Biophys. J. 2000;78:290–305. doi: 10.1016/S0006-3495(00)76592-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gidwani A., Holowka D., Baird B. Fluorescence anisotropy measurements of lipid order in plasma membranes and lipid rafts from RBL-2H3 mast cells. Biochemistry. 2001;40:12422–12429. doi: 10.1021/bi010496c. [DOI] [PubMed] [Google Scholar]
- 59.M'Baye G., Mély Y., Duportail G., Klymchenko A.S. Liquid ordered and gel phases of lipid bilayers: fluorescent probes reveal close fluidity but different hydration. Biophys. J. 2008;95:1217–1225. doi: 10.1529/biophysj.107.127480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dietrich C., Bagatolli L.A., Volovyk Z.N., Thompson N.L., Levi M. Lipid rafts reconstituted in model membranes. Biophys. J. 2001;80:1417–1428. doi: 10.1016/S0006-3495(01)76114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Klymchenko A.S., Oncul S., Didier P., Schaub E., Bagatolli L. Visualization of lipid domains in giant unilamellar vesicles using an environment-sensitive membrane probe based on 3-hydroxyflavone. Biochim. Biophys. Acta. 2009;1788:495–499. doi: 10.1016/j.bbamem.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 62.Gibrat R., Romieu C., Grignon C. A procedure for estimating the surface potential of charged or neutral membranes with 8-anilino-1-naphthalenesulphonate probe. Adequacy of the Gouy-Chapman model. Biochim. Biophys. Acta. 1983;736:196–202. doi: 10.1016/0005-2736(83)90284-5. [DOI] [PubMed] [Google Scholar]
- 63.Vermes I., Haanen C., Steffens-Nakken H., Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J. Immunol. Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 64.Montana V., Farkas D.L., Loew L.M. Dual-wavelength ratiometric fluorescence measurements of membrane potential. Biochemistry. 1989;28:4536–4539. doi: 10.1021/bi00437a003. [DOI] [PubMed] [Google Scholar]
- 65.Kuhn B., Fromherz P. Anellated hemicyanine dyes in a neuron membrane: molecular Stark effect and optical voltage recording. J. Phys. Chem. B. 2003;107:7903–7913. [Google Scholar]
- 66.Malkov D.Y., Sokolov V.S. Fluorescent styryl dyes of the RH series affect a potential drop on the membrane/solution boundary. Biochim. Biophys. Acta. 1996;1278:197–204. doi: 10.1016/0005-2736(95)00197-2. [DOI] [PubMed] [Google Scholar]
- 67.M'Baye G., Shynkar V.V., Klymchenko A.S., Mély Y., Duportail G. Membrane dipole potential as measured by ratiometric 3-hydroxyflavone fluorescence probes. Accounting for the hydration effects. J. Fluoresc. 2006;7:1–8. doi: 10.1007/s10895-005-0022-3. [DOI] [PubMed] [Google Scholar]
- 68.Shynkar V.V., Klymchenko A.S., Duportail G., Demchenko A.P., Mély Y. Two-color fluorescent probes for imaging the dipole potential of cell plasma membranes. Biochim. Biophys. Acta. 2005;1712:128–136. doi: 10.1016/j.bbamem.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 69.Antic S., Major G., Zecevic D. Fast optical recordings of membrane potential changes from dendrites of pyramidal neurons. J. Neurophysiol. 1999;82:1615–1621. doi: 10.1152/jn.1999.82.3.1615. [DOI] [PubMed] [Google Scholar]
- 70.Bullen A., Saggau P. High-speed, random-access fluorescence microscopy: II. Fast quantitative measurements with voltage-sensitive dyes. Biophys. J. 1999;76:2272–2287. doi: 10.1016/S0006-3495(99)77383-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wuskell J.P., Boudreau D., Wei M.D., Jin L., Engl R. Synthesis, spectra, delivery and potentiometric responses of new styryl dyes with extended spectral ranges. J. Neurosci. Methods. 2006;151:200–215. doi: 10.1016/j.jneumeth.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 72.Millard A.C., Jin L., Wei M.D., Wuskell J.P., Lewis A. Sensitivity of second harmonic generation from styryl dyes to transmembrane potential. Biophys. J. 2004;86:1169–1176. doi: 10.1016/S0006-3495(04)74191-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fromherz P., Hübener G., Kuhn B., Hinner M.J. ANNINE-6plus, a voltage-sensitive dye with good solubility, strong membrane binding and high sensitivity. Eur. Biophys. J. 2008;37:509–514. doi: 10.1007/s00249-007-0210-y. [DOI] [PMC free article] [PubMed] [Google Scholar]


