Abstract
The degree of helical order of the thick filament of mammalian skeletal muscle is highly dependent on temperature and the nature of the ligand. Previously, we showed that there was a close correlation between the conformation of the myosin heads on the surface of the thick filaments and the extent of their helical order. Helical order required the heads to be in the closed conformation. In addition, we showed that, with the same ligand bound at the active site, three conformations of myosin coexisted in equilibrium. Hitherto, however, there was no detectable helical order as measured by x-ray diffraction under the temperatures studied for myosin with MgADP and the nucleotide-free myosin, raising the possibility that the concept of multiple conformations has limited validity. In this study, blebbistatin was used to stabilize the closed conformation of myosin. The degree of helical order is substantially improved with MgATP at low temperature or with MgADP or in the absence of nucleotide. The thermodynamic parameters of the disorder↔order transition and the characteristics of the ordered array were not significantly altered by binding blebbistatin. The simplest explanation is that the binding of blebbistatin increases the proportion of myosin in the closed conformation from being negligible to substantial. These results provide further evidence for the coexistence of multiple conformations of myosin under a wide range of conditions and for the closed conformation being directly coupled to helical order.
Introduction
In mammalian muscle fibers, the degree to which the myosin heads are helically ordered in the thick filament is highly dependent on temperature and the ligand bound at the active site. Schlichting and Wray (1) suggested that the ordering required the ATP hydrolysis products, ADP and Pi, be bound at the active site of myosin. A subsequent quantitative study comparing the equilibrium constant for the ATP hydrolysis step in solution and for the disorder↔order equilibrium in the thick filaments in muscle gave support to this hypothesis (2). However, further experiments with a range of ligands indicated that ordering in the thick filament did not require hydrolysis per se (3), since some nonhydrolyzable ligands, such as ADP.BeFx, could produce partial ordering. By correlating the temperature and ligand dependence of the disorder↔order equilibrium with the conformations of S1 in solution as indicated by tryptophan fluorescence (4), it was concluded that the formation of helical order required the myosin heads to be in the closed conformation, while the open conformation produced disorder. (In this article, definitions of closed and open conformations of myosin follow those adopted by Geeves and Holmes (5–7): namely, closed conformation consists of the switch-2 element of S1 being in a position that interacts with the γ-phosphate-binding pocket; open conformation, the switch-2 loop being rotated away ∼4 Å from the γ-phosphate-binding pocket, facilitating phosphate release. Myosin in the closed conformation is thought to be in the putative pre-powerstroke state.) The temperature-dependence of helical order could thus be explained because the equilibrium constant of hydrolysis is strongly temperature-dependent, and the M.ADP.Pi state strongly favors the closed conformation. Detailed analysis showed that a second disordered conformation coexisted with the open and closed conformations although its identity is, as yet, unclear. Low temperature favors the disordered open conformation, while high temperature favors the ordered closed conformation together with the second disordered conformation. The requirement of the closed conformation for helical order was confirmed by electron microscopic studies (8).
Our results provided evidence that the same ligand could induce multiple conformations of myosin coexisting in equilibrium, whose relative proportions are influenced both by temperature and the nature of the bound ligand (3). It has been long established that myosin can adopt several conformations (5,6,9,10). It was previously thought that one conformation could convert to another, but only if the ligand changes. In addition, the crystal structures of myosin have largely been interpreted to mean that the conformation of myosin is absolutely determined by the nature of the ligand. Our results, in contrast, indicated that different forms of myosin could coexist with the same ligand bound. This concept was supported by a recent multifrequency electron paramagnetic resonance study of myosin (11).
However, in the presence of MgADP or under nucleotide-free conditions, we observed that the thick filaments remained disordered even at higher temperatures (2). It appeared possible that myosin could not be shifted from the open to the closed conformation under those conditions, at least to levels where helical ordering could be detected.
One of the goals of this study was to determine whether the concept of coexisting multiple conformations of myosin is valid when MgADP was bound or under nucleotide-free conditions. Our approach was to attempt to shift the equilibrium favoring the closed conformation by using blebbistatin, a potent small-molecule inhibitor of actomyosin-II-dependent cellular functions (12). The crystal structure of S1 with bound blebbistatin and M.ADP.vanadate revealed that blebbistatin is bound in the actin-binding cleft, close to the γ-phosphate binding pocket (13). It is thought that blebbistatin inhibits actomyosin ATPase activity primarily by stabilizing the closed conformation and inhibiting the release of phosphate (14–16). It seemed plausible that addition of blebbistatin could sufficiently displace the equilibrium between multiple conformations in favor of the closed conformation to increase helical order.
It was found that blebbistatin indeed enhanced the ordering of the thick filaments not only in the presence of MgATP at low temperature but also in the presence of MgADP or indeed even in the absence of nucleotide. Thus, the disorder↔order equilibrium can be shifted by manipulating the conformation of myosin, providing further evidence for the coexistence of multiple conformations of myosin in the thick filaments and for the importance of the closed conformation in determining helical order.
Preliminary results have been reported previously (17,18).
Materials and Methods
Muscle preparation
Single bundles, fasciculi, from chemically skinned rabbit psoas muscle, ∼0.3 mm × 0.6 mm in cross section, were used. To minimize possible complications due to actin-myosin interactions, all experiments were performed at a sarcomere length of ∼4.2 μm where there is no overlap between thick and thin filaments, unless mentioned otherwise.
The sarcomere length was constantly monitored by laser light diffraction during stretch. Only those stretched bundles giving a sharp laser pattern and without a single broken fiber were used. The integrity of the fibers was further checked under the microscope immediately before the experiments (for details, see (3)). Some x-ray diffraction patterns were taken of bundles with a sarcomere length of 2.4 μm under relaxed and rigor conditions for the purpose of calibration.
Solutions
-
1.
The relaxing (MgATP) solution contained 2 mM MgATP, 2 mM MgCl2, 2 mM ethylene glycol tetraacetic acid (EGTA), 5 mM dithiothreitol (DTT), 10 mM imidazole, 10 mM creatine phosphate, and 133 mM potassium propionate. pH = 7.0; ionic strength = 170 mM. To complete the ATP-backup system, 109 units/mL of creatine kinase was included.
-
2.
The rigor solution contained 2.5 mM EGTA, 2.5 mM ethylenediaminetetraacetic acid (EDTA), 10 mM imidazole, 5 mM DTT, and 150 mM potassium propionate. pH = 7.0; ionic strength = 170 mM.
-
3.
The nucleotide-free solution contained 2 mM EGTA, 4 mM MgCl2, 10 mM imidazole, 50 mM glucose, 2 units/mL hexokinase, 147 mM potassium propionate, 0.25 mM Ap5A, and 5 mM DTT. pH = 7.0; ionic strength = 170 mM.
-
4.
The ADP-containing solution contained 2 mM MgADP, 2 mM MgCl2, 2 mM EGTA, 5 mM DTT, 10 mM imidazole, 50 mM glucose, 2 mM units/hexokinase, 143 mM potassium propionate, and 0.25 mM Ap5A. pH = 7.0, μ = 170 mM. ADP solution with “phosphate mop” was made by adding 0.01 unit/mL purine nucleoside phosphorylase and 0.1 mM 7-methylguanosine to the ADP solution (19).
-
5.
Blebbistatin treatment: Half-maximal inhibition occurred at 1.4 μM blebbistatin for the basal ATPase activity, or at 0.4 μM blebbistatin for the actin-activated ATPase activity. Kd for blebbistatin binding to S1 at 25°C is KM.D.P = 3.1 μM, KM.D = 24 μM, and Kapo = 25 μM (16). To ensure saturation, 100 μM blebbistatin was added to drive the equilibrium toward the closed conformation. The (s)-(-)-blebbistatin was purchased from Toronto Research Chemicals (Ontario, Canada).
Before the rigor solution or nucleotide-free solution was applied, the bundles were rinsed several times with a quick-rinse solution containing 5 mM EGTA, 15 mM EDTA and 20 mM imidazole (pH 7.0, ionic strength = 70 mM) (20). The temperature of the bathing solution in the chamber with the bundles was maintained at the preset temperatures ±1°C by two thermal electric devices controlled by a feedback circuit by Cambion (Cambridge, MA). The temperature ranged between 5 and 35°C.
X-ray source, camera, and detector system
X-ray diffraction patterns from chemically skinned muscle fibers were obtained at beamline X27C (Advanced Polymer PRT) at the National Synchrotron Light Source, Brookhaven National Laboratory, Upton, NY. The specimen-to-detector distance was 1500 mm and the beam size at the specimen was ∼0.4 mm in diameter. A MAR charge-coupled device x-ray detector system (Marresearch, Hamburg, Germany) was used for collecting the x-ray data. The spacing of all reflections was calibrated by the 1/144.3 Å−1 meridional reflection from skinned rabbit psoas muscle in rigor at ionic strength = 170 mM and 25°C (21).
Data reduction and analysis
The data in the four quadrants of the x-ray diffraction patterns were first rotated, folded, and averaged. Intensities summed within slices parallel either to the meridian or to the equator of the diffraction patterns provided one-dimensional intensity profiles. For the distribution of intensity along layer lines, the widths of the slices were chosen so as to include the entire widths of the layer lines.
To compare directly the intensities obtained under different conditions with minimum error, diffraction patterns were always recorded from the same bundle for all the conditions of interest (e.g., the temperature change from 5°C to 35°C). To compare quantitatively the data from different-sized bundles, the integrated intensity of the first myosin layer line from each muscle sample obtained with the relaxing solution (ionic strength = 170 mM) at 25°C was used for normalization. For analysis, contributions from the thick filament backbone and the thin filament were excluded by taking difference patterns: the patterns obtained in the absence of nucleotide were subtracted from those in the presence of ligands with or without blebbistatin. Integrated intensities of the layer lines in the difference patterns were obtained with the program PCA (Oxford Instrument, Oakridge, TN).
Equilibrium constant of disorder↔order in the thick filament
The layer lines in the difference patterns indexed on a 43-nm repeat, and therefore, arise from the myosin filaments alone. The higher orders of the myosin layer lines in most cases (e.g., in ATP at low temperatures) are weak and it is therefore difficult to obtain reliable intensity measurements above the background. As a first approximation, the intensities of the myosin layer lines were taken to be directly proportional to the square of the mass of the myosin heads in the helical array scattering coherently. Hence, the amplitudes, square-root of the intensities, are proportional to the mass. The equilibrium constant of the disorder↔order distribution is defined as
| (1) |
where a is the fraction of the heads in the ordered state. The value a is determined in the following manner. The fraction of heads in the ordered state in ATP at 25°C was determined previously to be 0.93 (2). Amplitudes obtained under other conditions were first normalized by the amplitude obtained from the same fiber bundle in the presence of ATP at 25°C and multiplied by 0.93.
The temperature dependence of the equilibrium constants for the disorder↔order distribution, and for the conformational change in myosin, can be expressed by the van' t Hoff equation,
| (2) |
where K is the equilibrium constant, T the absolute temperature in Kelvin units, R the gas constant, ΔH° the enthalpy change of the transition, and ΔS° the entropy change. The dependence on temperature of these equilibrium constants were fitted using the nonlinear least-squares fitting routines in the software package Scientist (Micromath, Ogden, Utah) (For details, see (2)).
Results
Two-dimensional x-ray diffraction patterns were obtained from overstretched muscle fibers in solutions containing MgATP, MgADP, or in nucleotide-free solutions with or without blebbistatin. In general, the binding of blebbistatin to the myosin head significantly enhanced the intensity of the first and other myosin layer lines.
Effects of blebbistatin on the myosin layer line intensities in the presence of MgATP
The effects of blebbistatin on the layer line intensities in the presence of MgATP at 5°C are illustrated in Fig. 1. At this temperature, in the absence of blebbistatin, the myosin layer lines are weak (Fig. 1 A), and the first myosin layer line had an intensity of only ∼20% that at 25°C. However, in the presence of blebbistatin, the myosin layer lines are readily observable (Fig. 1 B). The disorder↔order equilibrium constant increased from 0.8 to 3.0. The meridional reflections M1, M2, M3, and M6 are also enhanced.
Figure 1.
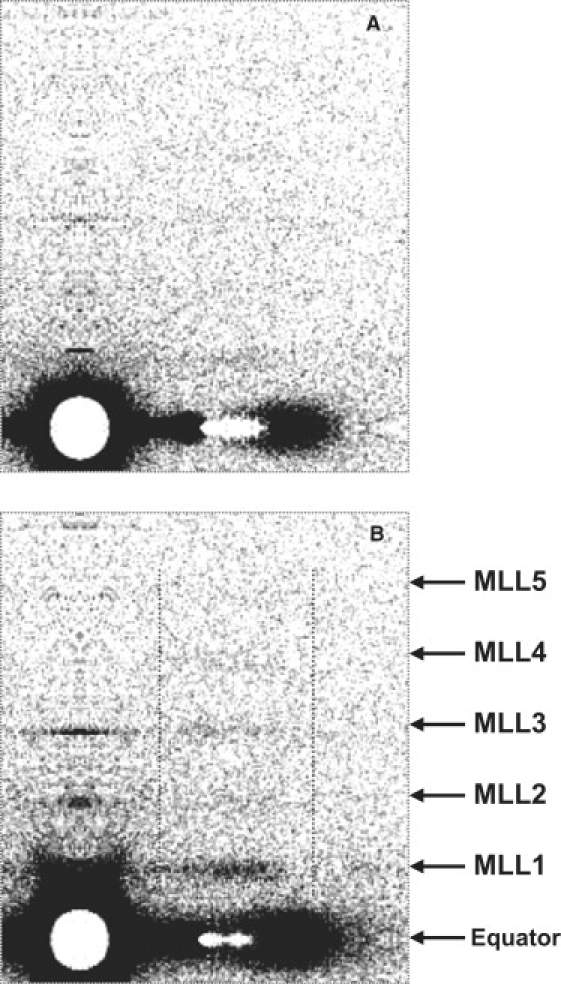
Enhancement of myosin layer line intensities in MgATP solution by blebbistatin at 5°C. (A) Without and (B) with 0.1 mM blebbistatin. Ionic strength = 170 mM. The dotted lines indicate the area of integration of the vertical slices shown in Fig. 2. For clarity, the patterns were first subtracted by the nucleotide-free patterns, so that contributions from the thin filaments were removed.
Intensity profiles of slices cut across the first myosin layer line at 5°C demonstrate that the intensity of this layer line is more than doubled by blebbistatin (Fig. 2 A). The increase is not limited to the first myosin layer line. The higher orders also increased in intensities (Fig. 2 B), but the signals are very weak in the absence of blebbistatin and quantitative determination of the increase would be rather unreliable. At 25°C, only a slight enhancement of the first myosin layer line was observed (Fig. 2 A) ( ∼ 13 and 23, without and with blebbistatin, respectively). At 35°C, the ordered fraction decreased slightly ( = 18 and 10).
Figure 2.
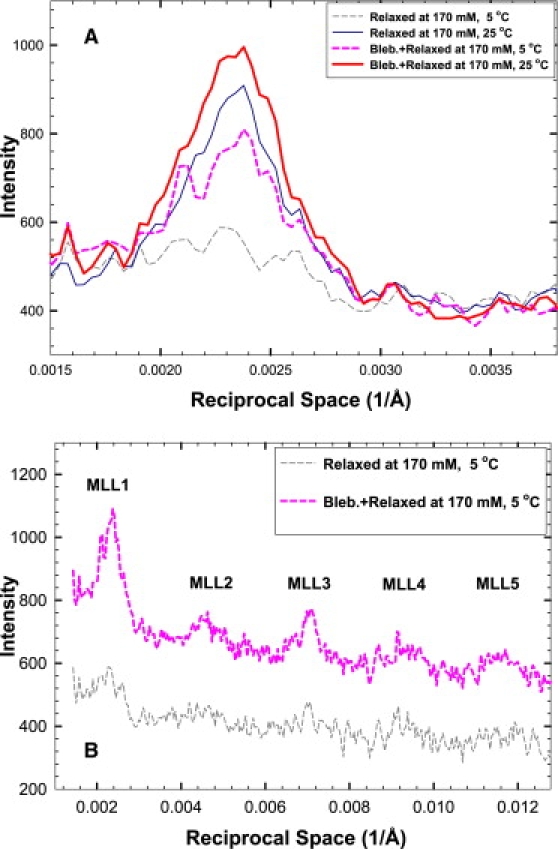
Integrated intensities across the first myosin layer line in slices parallel to the meridian from 0.002631 to 0.008040 Å−1 of the patterns obtained from fibers in MgATP solution: (A) Only the first myosin layer lines are shown; (i) at 5°C (silver dashed line); (ii) at 5°C with added blebbistatin (magenta dashed line); (iii) at 25°C (thin blue line); and (iv) at 25°C with added blebbistatin (thick red line). Note that (i) and (ii) are based on the data shown in Fig. 1; (iii) and (iv) are from the same bundle preparation. (B) Same conditions as in (i) and (ii) of panel A, but higher orders of MLL are shown. The intensity profiles were displaced vertically to reveal the changes more clearly.
Effects of blebbistatin on the myosin layer line in the presence of MgADP
In the presence of MgADP, myosin layer lines were not detectable throughout the temperature range studied (2), indicating that under these conditions, the myosin heads are mostly disordered (Fig. 3 A). However, when blebbistatin was added, reasonably strong myosin layer lines are detected (Fig. 3 B and Fig. 4) throughout the temperature range (5–35°C) studied, although these were not as strong as those seen in the presence of MgATP at the same temperatures (Fig. 4–6).
Figure 3.
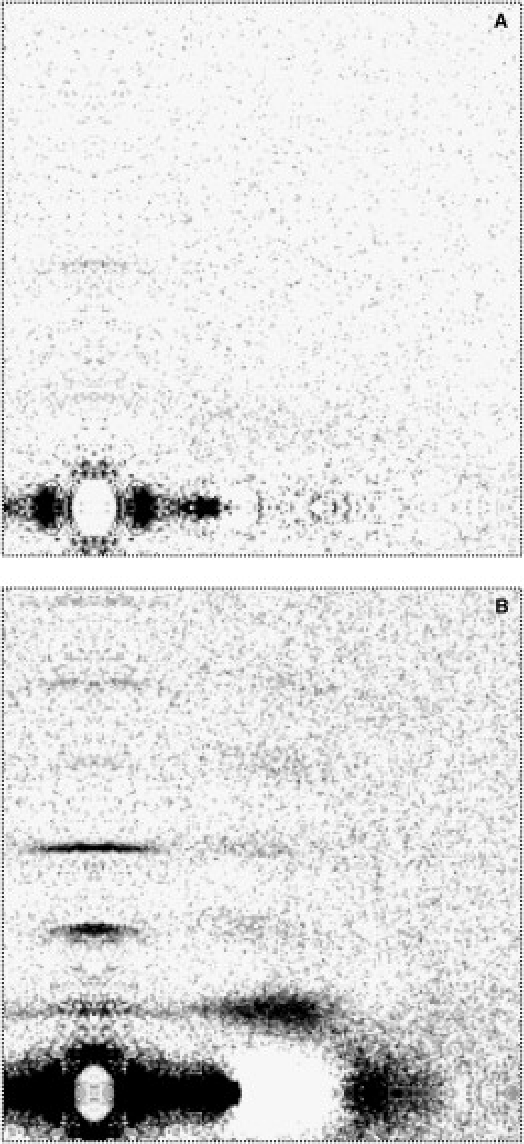
Effect of blebbistatin on the thick filament in MgADP-containing solutions. Diffraction patterns shown here were all obtained from the same fiber bundle. For clarity, the patterns were first subtracted by the NTP-free patterns, so that contributions from the thin filaments were removed. (A) In MgADP solutions. (B) In the same solution as in panel A with 0.1 mM blebbistatin added. Ionic Strength = 170 mM. Temperature = 25°C.
Figure 4.
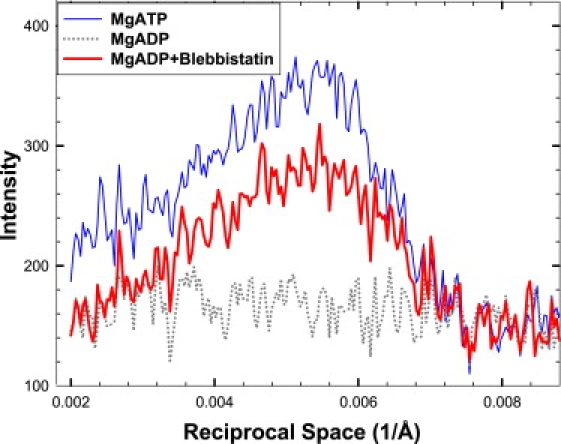
Intensity profiles from slices along the first myosin layer line shown in Fig. 3: MgADP (dotted line) and MgADP+blebbistatin (thick solid line). For comparison, profile from the same muscle bundle at the same temperature of 25°C in the presence of MgATP (thin line) is included. Data have been subtracted by the pattern obtained in the absence of nucleotide.
Figure 5.
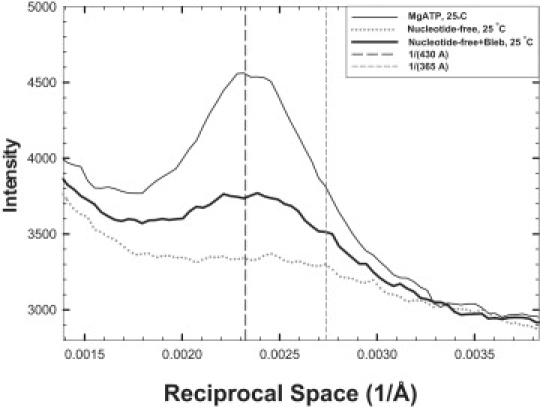
Vertical profiles across the first myosin layer line and the first actin layer line. (Dotted line) obtained under nucleotide-free condition, showing no myosin component but a weak actin component at 1/(365A); (thick line) obtained under nucleotide-free plus blebbistatin condition, showing a strong myosin component at 1/(430A). For comparison, the profile (thin line) obtained under MgATP is included. Note that the profiles are based on the original record, not on difference patterns. The vertical long dashed line indicates the location of 1/(430A) and the short dashed line, 1/(365A). Temperature = 25°C.
Figure 6.
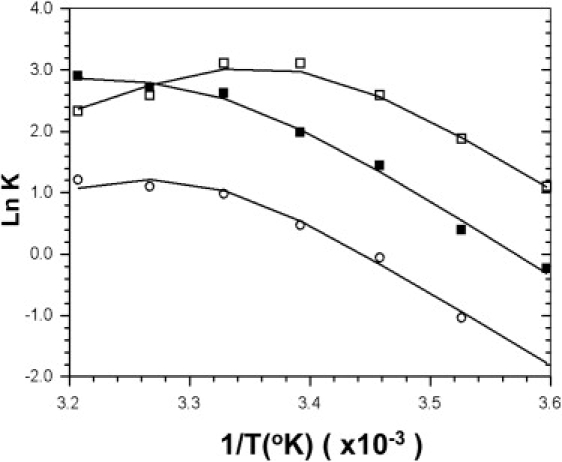
Van' t Hoff plots showing the effects of temperature on the disorder↔order equilibrium in the thick filament. Values of were obtained in the presence of MgATP (solid squares), MgATP+blebbistatin (open squares), and MgADP+blebbistatin (open circles). The lines are results of curve-fitting to the van' t Hoff equations (see Eq. 2). For MgATP and in the absence of blebbistatin, the enthalpy changes were determined to be ΔHAB = −144 kJ/mol, and ΔHBC = 116 kJ/mol. These values are similar to those previously obtained with a series of nucleotide ligands (3). For conditions that contained blebbistatin, ΔHAB = −181 kJ/mol and ΔHBC = 111 kJ/mol. The best fit values for ΔSAB and ΔSBC are shown in Table 1.
Appearance of helical order with nucleotide-free myosin in the presence of blebbistatin
It has been shown that in the absence of nucleotide, thick filaments are disordered (2,22). It was generally thought that without any nucleotide bound at the active site in myosin II (the apo state), switch-2 is open (23). An ordered array in the thick filament therefore may not be formed. Remarkably, in the presence of blebbistatin, myosin layer lines appear at 25°C (Fig. 5), demonstrating the effectiveness of blebbistatin at enhancing helical order. The intensities are substantial, with the equilibrium constant ∼ 2, although at 5°C the intensities remain below detectable level.
Fig. 6 compares van' t Hoff plots for the temperature dependence of for MgATP with and without blebbistatin, and for MgADP in the presence of blebbistatin. The nonlinearity of these van' t Hoff plots indicates that there are more than two conformations in equilibrium, their relative proportions depending on temperature. We previously interpreted these plots in terms of temperature affecting the relative proportions of three conformations A, B, and C of myosin in equilibrium (i.e., see Scheme 1, below).
Scheme 1.
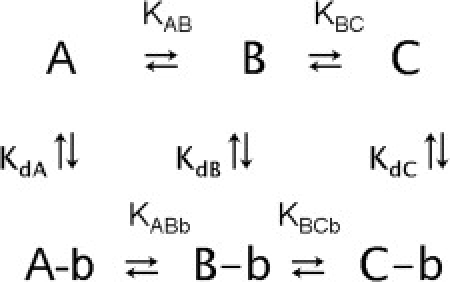
Only C produced helical order and was identified with the closed conformation; B produced disorder in the filament and was supposed to be the open conformation; the nature of A was not clear but it was identified as a second disordered conformation (3). At low temperatures, the low-enthalpy conformation, B, dominated. As the temperature increases the proportion of the higher enthalpy states, A and C, increases. The slope of the van' t Hoff plots at low temperature is determined by ΔHBC, while at high temperature the slope is determined by ΔHAC (see Eq. 2).
The experimental data set of obtained for ATP, ATP+blebbistatin, and ADP+blebbistatin conditions (Fig. 6) were curve-fitted according to van't Hoff plots for Scheme 1 in the absence (upper line) or presence (lower line) of blebbistatin. For ATP, ΔHAB = −144 kJ/mol and ΔHBC = 116 kJ/mol. For ATP+blebbistatin and ADP+blebbistatin conditions, the calculated enthalpy change ΔHAB was −181 kJ/mol while ΔHBC was 111 kJ/mol, so that ΔHAC was −70 kJ/mol. At 25°C in the presence of ATP, KBC was 18 and KAC = 33 (Table 1) such that the closed conformation C is overwhelmingly favored. With the addition of blebbistatin, there is an enhancement of helical order in the thick filament and the closed conformation is favored. The increase in the ordered fraction is particularly prominent at low temperature. For instance, at 5°C, the fraction a increases from 0.37 to 0.73, while at 25°C, the increase is slight and within experimental error, 0.92 to 0.95. At 35°C, there is a slight decrease, from 0.94 to 0.92. The slight decrease appears to suggest that the population of the A conformation increases at higher temperature, and that blebbistatin stabilizes the A conformation even more than the closed C conformation. However, it should be pointed out that since (Eq. 1), the magnitude of becomes highly sensitive to a when a exceeds 0.90. The accuracy of intensity measurements is limited to ∼±5% for strong layer lines such as MLL1. Therefore, as approaches 10, differences found between ATP+blebbistatin and ATP could be attributed to experimental error.
Table 1.
Thermodynamic parameters best fitted for the disorder↔order equilibrium in the thick filaments with various ligands and the resultant equilibrium constants at 25°C
| Ligand | ΔHAB (kJ mol−1) | ΔSAB (kJ mol−1 K−1) | ΔGAB (kJ mol−1)at 25°C | KAB at 25°C | ΔHBC (kJ mol−1) | ΔSBC (kJ mol−1 K−1) | ΔGBC (kJ mol−1) at 25°C | KBC at 25°C | KAC at 25°C |
|---|---|---|---|---|---|---|---|---|---|
| ATP | −144 | −0.479 | −1.3 | 1.7 | 116 | 0.413 | −7.2 | 18 | 30 |
| ATP + blebbistatin | −181 | −0.614 | 2.0 | 0.45 | 110 | 0.404 | −10.4 | 66 | 29 |
| ADP + blebbistatin | −181 | −0.600 | −2.2 | 2.4 | 110 | 0.38 | −3.2 | 3.7 | 9.1 |
The plot in the presence of MgADP with added blebbistatin was fitted with the same two enthalpy changes obtained for the case of MgATP+blebbistatin (ΔHAB = −181 kJ/mol, ΔHBC = 111 kJ/mol). Entropy changes, ΔS, were used as fitting parameters (Table 1). In the presence of MgADP alone, there is no detectable helical order at all temperatures studied, but with blebbistatin the ordered fraction a is ∼0.75 at 25°C. Distributions of the three conformations at various temperatures are illustrated in Fig. 7.
Figure 7.
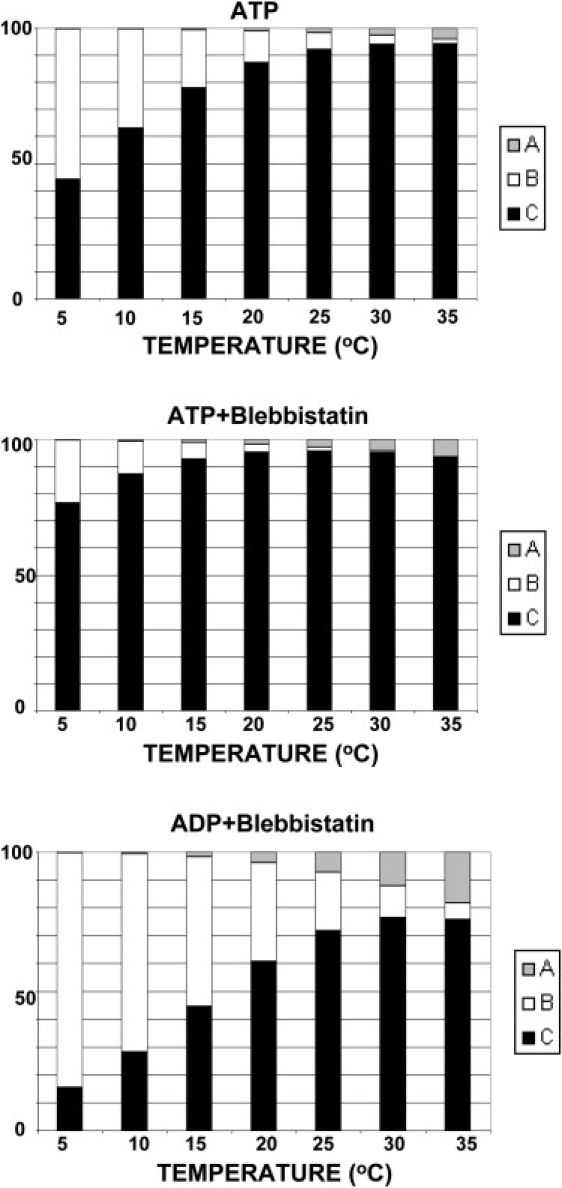
Calculated distributions of conformations A (shaded), B (open), and C (solid) at various temperatures in the presence of MgATP, MgATP+blebbistatin, and MgADP+blebbistatin. Calculations are based on parameters obtained by curve-fitting. Parameters and equilibrium constants at 25°C are shown in Table 1.
The upward shift to higher when blebbistatin is bound can be explained in a qualitative way by a simple scheme. We assume that blebbistatin binds to all three conformations A,B,C without altering conformations but with various affinities, and that only [C] and [C.bleb] produce helical order in the thick filament. Since
| (3) |
it can be shown that
| (4) |
where KdA, KdB, and KdC are the dissociation constants for blebbistatin from its complexes with A, B, and C, respectively, while KAB, KBC, and KAC are the equilibrium constants for the A↔B, B↔C, and A↔C transitions. One may derive an estimate on the improvement in the helical order in the presence of 100 μM blebbistatin. As an example, consider the case of MgATP without blebbistatin. At low temperature, KAC >> 1 (Fig. 7). For the sake of illustration, we assume that myosin·MgADP has the open conformation B and that the dissociation constants for blebbistatin reported by Kovacs et al. (16) for Kd(M.D.P) = 3.1 μM and Kd(M.D) = 24 μM may be equated to KdC and KdB, respectively. It follows that in the absence of blebbistatin = KBC, but in 100 μM of blebbistatin, = 6.4 KBC. As shown in Fig. 6, the experimental data at 5°C show that = 0.6 and 2.8 in the absence and in the presence of 100 μM blebbistatin, respectively. Considering the difficulties in measurements, the enhancement of helical order by blebbistatin we observed by x-ray diffraction is in reasonable agreement with theoretical considerations.
At high temperature, KBC >> 1, and
| (5) |
If blebbistatin binds more strongly to the A conformation than to the C conformation, i.e., KdC > KdA, could be lowered by the presence of blebbistatin. Thus, the slight decrease in the observed for MgATP+blebbistatin could be due to blebbistatin binding more strongly to A than to C at high temperature.
Radial distributions of intensity along the first myosin layer line
The question we now address is whether the helically ordered array induced by blebbistatin in the presence of MgADP is identical to the physiological structure in MgATP without blebbistatin. By comparing the radial distributions of the intensities along the myosin layer lines, differences in the radius of the center-of-mass of the myosin heads in the helical array can be determined. Fig. 4 demonstrates that the profiles obtained in MgATP and in MgADP + blebbistatin solutions are closely matched in the location of the centroid and in the shape of the distribution. The results indicate that the myosin heads under these two conditions are distributed with the same radius of the center of mass in the thick filament.
Discussion
The intensification of the myosin layer lines by blebbistatin indicates that it induces more mass, i.e., a larger fraction of the myosin heads, to become organized in the helical array of the thick filament. Since the spacing of the layer lines and the intensity profiles along the first myosin layer line are identical to those in the absence of blebbistatin, the helical pitch, and the radius of the center of mass of the myosin heads in the thick filaments are the same as those in the native state. By implication, the distribution of the myosin heads in the newly formed helical track is the same as those native heads without blebbistatin. However, because of the limited resolution of the x-ray diffraction patterns, the data do not reveal conformational changes at the atomic level.
The crystal structure of the complex of S1 with blebbistatin (13) indicated that the binding of blebbistatin does not alter the closed conformation of S1. Furthermore, the disorder↔order equilibrium in the presence of blebbistatin follows a temperature-dependence (Fig. 6) very similar to that reported for other ligands bound to S1 (3), although there is a slight difference in the enthalpy changes (Table 1) likely due to a nonzero enthalpy of binding of blebbistatin. Therefore, the disorder↔order transition with blebbistatin reflects the same underlying processes as those without blebbistatin.
The main effect of blebbistatin appears to be the stabilization of the closed C conformation (Scheme 1), bringing about a shift in the equilibrium distribution among the three conformations A, B, and C of myosin in the thick filament (3). The ratio of change in the equilibrium constants between the conformations is proportional to the ratio of blebbistatin binding constants to various ligand states. Similar effects of improved helical order have also been observed by electron microscopy of myosin filaments (24). The slight decrease in at temperatures >25°C, in the case of MgATP+blebbistatin suggests that there might be an increase in the fraction of the A conformation, coupled with a slight decrease in the closed C conformation. A likely explanation is that blebbistatin binds stronger to A than to C, and hence the A conformation is stabilized more than the closed C conformation.
It might be argued that the disorder↔order transition might involve cooperative interaction between the myosin heads in the thick filament. However, such interaction among the myosin heads is unlikely. The hydrolysis of ATP by S1 in solution was matched closely with increases in the intensities of the myosin layer lines as a function of temperature (2). The movement of the switch-2 in the motor domain of S1 as monitored by tryptophan fluorescence from a Dictyostelium construct is described by the same enthalpy changes as for the intensity changes we have observed, signifying that the same reaction occurs in both systems. Furthermore, similar correlation has been found when a variety of ligands is bound at the active site (3). Cooperativity among S1 could not exist in solution. Hence, cooperativity among myosin heads in a thick filament is unlikely.
The superb electron microscopic studies (25,26) on the vertebrate muscle myosin filaments under relaxing conditions revealed head-to-head interaction within each myosin molecule. The elegant and detailed x-ray diffraction study and modeling of the intact vertebrate striated muscle (27) suggested a model of close proximity between individual heads from separate myosins in neighboring crown levels. We believe, however, that their observations, i.e., head-head interactions/contacts, regardless of whether they are within the same molecules or otherwise, cannot constitute the main requirement for the disorder↔order equilibrium. One may consider quantitatively the consequences if the ordered structure were to require interactions between myosin heads (in the closed conformation). Assume, for simplicity, the myosin heads exist only in two conformations, say, open and closed, with an equilibrium constant Kconf for the open to closed transition, i.e.,
| (6) |
Assume in addition that only the heads in the closed conformation can associate head-to-head to form an ordered structure with an equilibrium constant Kassoc given by
| (7) |
The overall equilibrium constant Kord for disorder↔order transition is given by
| (8) |
From Eqs. 6 and 7,
| (9) |
Note that if Kconf << 1, i.e., the heads are mostly in the open conformation, Kord = Kconf ∗ Kassoc. If Kconf >> 1, i.e., the heads are mostly in the closed conformation, Kord = Kassoc. If the closed conformation gives helical order without requiring head-head association, Kord is straightforwardly Kconf. The critical basis of our interpretation of changes in the myosin layer lines is the close match between the x-ray data and the solution data of ATP hydrolysis and the movement of switch-2 in S1 (2,3). A modulation by as shown in Eq. 9 would yield results inconsistent with our findings. Therefore, the simplest explanation appears to be that the formation of helical order is a direct consequence of the closed conformation.
The greater ordering could be a consequence of a more rigid atomic structure of the myosin heads found in the closed conformation (28,29), where the four subdomains of the myosin head closely interact; in contrast, in the open conformation, the subdomains are loosely coupled, particularly in the relay-converter interface. With a loosely linked structure, the time-averaged distribution of mass would be smeared (or disordered as defined in this article) leading to a loss of intensities. With a rigid structure, on the other hand, the spatial fluctuation of the heads would be reduced, giving rise to strong layer line intensities.
Our conclusion is consistent with the inhibitory role of blebbistatin on tension. By trapping myosin heads in the closed conformation, the heads are limited to the low affinity state for actin and unable to enter the high affinity states (16). Preliminary data indicate that for muscle fibers in full overlap, even when the thin filament is activated by calcium in the presence of blebbistatin, the thick filament remains ordered and the affinity between actin and myosin is not significantly affected (S. Xu and L. C. Yu, unpublished data).
The significance of our results is that shifting the open↔closed equilibrium by blebbistatin has provided further evidence for the coexistence of multiple conformations of myosin under a wide range of conditions (3) and further proof that the closed conformation is required for helical order. Myosin·MgADP normally does not occur in the closed conformation in any detectable fraction. However, by binding blebbistatin, the closed conformation is favored sufficiently for detectable helical order to occur in the thick filament and the results reveal an equilibrium between the same three conformations as reported previously (3). Thus, the distribution of coexisting myosin conformations can be manipulated by temperature and various ligands and possibly by other factors not yet studied.
Acknowledgments
We thank the scientific staff of beamline X27C at the National Synchrotron Light Source at the Brookhaven National Laboratory, NY for their expert assistance. We thank G. Melvin of the Office of Science and Technology of the National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health, for technical support. We also thank Mihály Kovács of Eötvös University, Hungary for helpful discussions.
This research was supported in part by the Intramural Research Program of the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health and in part by National Institutes of Health grants (No. EB00209 and HL040864 to H.D.W.).
References
- 1.Schlichting I., Wray J. Behavior of crossbridges in non-overlap frog muscle in the presence and absence of ATP. J. Muscle Res. Cell Motil. 1986;7:79–80. [Google Scholar]
- 2.Xu S., Gu J., Rhodes T., Belknap B., Rosenbaum G. The M.ADP.Pi state is required for helical order in the thick filaments of skeletal muscle. Biophys. J. 1999;77:2665–2676. doi: 10.1016/s0006-3495(99)77101-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu S., Offer G., Gu J., White H.D., Yu L.C. Temperature and ligand dependence of conformation and helical order in myosin filaments. Biochemistry. 2003;42:390–401. doi: 10.1021/bi026085t. [DOI] [PubMed] [Google Scholar]
- 4.Malnasi-Csizmadia A., Woolley R.J., Bagshaw C.R. Resolution of conformational states of Dictyostelium myosin II motor domain using tryptophan (W501) mutants: implications for the open-closed transition identified by crystallography. Biochemistry. 2000;39:16135–16146. doi: 10.1021/bi001125j. [DOI] [PubMed] [Google Scholar]
- 5.Geeves M.A., Holmes K.C. Structural mechanism of muscle contraction. Annu. Rev. Biochem. 1999;68:687–728. doi: 10.1146/annurev.biochem.68.1.687. [DOI] [PubMed] [Google Scholar]
- 6.Smith C.A., Rayment I. X-ray structure of the magnesium(II).ADP.vanadate complex of the Dictyostelium discoideum myosin motor domain to 1.9 Å resolution. Biochemistry. 1996;35:5404–5417. doi: 10.1021/bi952633+. [DOI] [PubMed] [Google Scholar]
- 7.Fisher A.J., Smith C.A., Thoden J.B., Smith R., Sutoh K. X-ray structures of the myosin motor domain of Dictyostelium discoideum complexed with MgADP.BeFx and MgADP.AlF4. Biochemistry. 1995;34:8960–8972. doi: 10.1021/bi00028a004. [DOI] [PubMed] [Google Scholar]
- 8.Zoghbi M.E., Woodhead J.L., Craig R., Padron R. Helical order in tarantula thick filaments requires the “closed” conformation of the myosin head. J. Mol. Biol. 2004;342:1223–1236. doi: 10.1016/j.jmb.2004.07.037. [DOI] [PubMed] [Google Scholar]
- 9.Bagshaw C.R., Eccleston J.F., Eckstein F., Goody R.S., Gutfreund H. The magnesium ion-dependent adenosine triphosphatase of myosin. Two-step processes of adenosine triphosphate association and adenosine diphosphate dissociation. Biochem. J. 1974;141:351–364. doi: 10.1042/bj1410351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor E.W. Transient phase of adenosine triphosphate hydrolysis by myosin, heavy meromyosin, and subfragment 1. Biochemistry. 1977;16:732–739. doi: 10.1021/bi00623a027. [DOI] [PubMed] [Google Scholar]
- 11.Nesmelov Y.E., Agafonov R.V., Burr A.R., Weber R.T., Thomas D.D. Structure and dynamics of the force-generating domain of myosin probed by multifrequency electron paramagnetic resonance. Biophys. J. 2008;95:247–256. doi: 10.1529/biophysj.107.124305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Straight A.F., Cheung A., Limouze J., Chen I., Westwood N.J. Dissecting temporal and spatial control of cytokinesis with a myosin II Inhibitor. Science (Wash. DC) 2003;299:1743–1747. doi: 10.1126/science.1081412. [DOI] [PubMed] [Google Scholar]
- 13.Allingham J.S., Smith R., Rayment I. The structural basis of blebbistatin inhibition and specificity for myosin II. Nat. Struct. Mol. Biol. 2005;12:378–379. doi: 10.1038/nsmb908. [DOI] [PubMed] [Google Scholar]
- 14.Limouze J., Straight A.F., Mitchison T., Sellers J.R. Specificity of blebbistatin, an inhibitor of myosin II. J. Muscle Res. Cell Motil. 2004;25:337–341. doi: 10.1007/s10974-004-6060-7. [DOI] [PubMed] [Google Scholar]
- 15.Ramamurthy B., Yengo C.M., Straight A.F., Mitchison T.J., Sweeney H.L. Kinetic mechanism of blebbistatin inhibition of nonmuscle myosin IIb. Biochemistry. 2004;43:14832–14839. doi: 10.1021/bi0490284. [DOI] [PubMed] [Google Scholar]
- 16.Kovacs M., Toth J., Hetenyi C., Malnasi-Csizmadia A., Sellers J.R. Mechanism of blebbistatin inhibition of myosin II. J. Biol. Chem. 2004;279:35557–35563. doi: 10.1074/jbc.M405319200. [DOI] [PubMed] [Google Scholar]
- 17.Xu S., Gu J., White H.D., Offer G.W., Yu L.C. Structural effects on myosin of three ATPase inhibitors. 2005 Biophysical Society Meeting Abstract. Biophys. J. 2005;88:124. [Google Scholar]
- 18.Xu S., White H.D., Offer G.W., Yu L.C. Stabilization of the helical order of myosin filaments by blebbistatin. 2008 Biophysical Society Meeting Abstract. Biophys. J. 2008;94:629. [Google Scholar]
- 19.Heeley D.H., Belknap B., White H.D. Mechanism of regulation of phosphate dissociation from actomyosin-ADP-Pi by thin filament proteins. Proc. Natl. Acad. Sci. USA. 2002;99:16731–16736. doi: 10.1073/pnas.252236399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brenner B., Yu L.C., Chalovich J.M. Parallel inhibition of active force and relaxed fiber stiffness in skeletal muscle by caldesmon: implications for the pathway to force generation. Proc. Natl. Acad. Sci. USA. 1991;88:5739–5743. doi: 10.1073/pnas.88.13.5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu S., Gu J., Melvin G., Yu L.C. Structural characterization of weakly attached cross-bridges in the A∗M∗ATP state in permeabilized rabbit psoas muscle. Biophys. J. 2002;82:2111–2122. doi: 10.1016/S0006-3495(02)75558-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Padron R., Craig R. Disorder induced in nonoverlap myosin cross-bridges by loss of adenosine triphosphate. Biophys. J. 1989;56:927–933. doi: 10.1016/S0006-3495(89)82738-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeng W., Conibear P.B., Dickens J.L., Cowie R.A., Wakelin S. Dynamics of actomyosin interactions in relation to the cross-bridge cycle. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2004;359:1843–1855. doi: 10.1098/rstb.2004.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao F.Q., Padron R., Craig R. Blebbistatin stabilizes the helical order of myosin filaments by promoting the switch-2 closed state. Biophys. J. 2008;95:3322–3329. doi: 10.1529/biophysj.108.137067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.al-Khayat H.A., Morris E.P., Kensler R.W., Squire J.M. 3D structure of relaxed fish muscle myosin filaments by single particle analysis. J. Struct. Biol. 2006;155:202–217. doi: 10.1016/j.jsb.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 26.Zoghbi M.E., Woodhead J.L., Moss R.L., Craig R. Three-dimensional structure of vertebrate cardiac muscle myosin filaments. Proc. Natl. Acad. Sci. USA. 2008;105:2386–2390. doi: 10.1073/pnas.0708912105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oshima K., Takezawa Y., Sugimoto Y., Kobayashi T., Irving T.C. Axial dispositions and conformations of myosin crossbridges along thick filaments in relaxed and contracting states of vertebrate striated muscles by x-ray fiber diffraction. J. Mol. Biol. 2007;367:275–301. doi: 10.1016/j.jmb.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 28.Houdusse A., Szent-Gyorgyi A.G., Cohen C. Three conformational states of scallop myosin S1. Proc. Natl. Acad. Sci. USA. 2000;97:11238–11243. doi: 10.1073/pnas.200376897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Himmel D.M., Gourinath S., Reshetnikova L., Shen Y., Szent-Gyorgyi A.G. Crystallographic findings on the internally uncoupled and near-rigor states of myosin: further insights into the mechanics of the motor. Proc. Natl. Acad. Sci. USA. 2002;99:12645–12650. doi: 10.1073/pnas.202476799. [DOI] [PMC free article] [PubMed] [Google Scholar]


