Abstract
Detection of periodontal or peri-implant sites exhibiting progressing disease or those at risk of deterioration has proven difficult. Pyridinoline cross-linked carboxyterminal telopeptide of type I collagen (ICTP), a marker specific for bone degradation found in gingival crevicular fluid (GCF), has been associated with both bone and attachment loss in periodontitis and may be useful for predicting disease activity. The aim of this cross-sectional study was to examine the relationship between ICTP levels and subgingival species around implants and teeth from 20 partially and 2 fully edentulous patients. GCF and plaque samples were collected from the mesiobuccal site of each implant and tooth. Radioimmunoassay techniques were utilized to determine GCF ICTP levels. Plaque samples were analyzed utilizing checkerboard DNA-DNA hybridization. Traditional clinical parameters were assessed. Seventy-one implants and 370 teeth from 22 subjects were examined. ICTP levels and subgingival plaque composition were not significantly different between implants and teeth. Implant sites colonized by Pre-votella intermedia, Capnocytophaga gingivalis, Fusobacterium nucleatum ss vincentii, and Streptococcus gordonii exhibited odds ratios of 12.4, 9.3, 8.1, and 6.7, respectively of detecting ICTP. These results suggest a relationship between elevated ICTP levels at implant sites and some species associated with disease progression. Longitudinal studies are necessary to determine whether elevated ICTP levels may predict the development of peri-implant bone loss.
Keywords: pyridinoline cross-links, periodontal pathogens, diagnosis, gingival crevicular fluid, dental implants, periodontal disease
The treatment of edentulous and partially edentulous individuals with endosseous implants has been demonstrated to be a predictable treatment option for tooth replacement (Fiorellini et al. 1998). However, a small percentage of individuals experience peri-implantitis, an inflammatory condition characterized by both connective tissue and bone destruction which can result in failure of the implant and/or prosthesis. Current methods used to assess peri-implant and periodontal health include probing and radiography which present a historical perspective, but provide no information regarding current disease activity (Goodson 1992). A potential new diagnostic involves the direct measurement of a bone-specific molecule called pyridinoline cross-linked carboxyterminal telopeptide of Type I collagen (ICTP) (Risteli et al. 1993). Several human studies have demonstrated a relationship between ICTP and high/low bone turnover diseases including post-menopausal osteoporosis (Eriksen et al. 1993; Hassager et al. 1994). ICTP has also been associated with bone and attachment loss in periodontitis (Talonpoika & Hämäläinen, 1994; Giannobile et al. 1996; Giannobile et al. 1997). In an experimental periodontitis model, ICTP was found to be highly sensitive and predictive for future radiographic bone loss as measured by computer-assisted digital radiography (Giannobile et al. 1995). During the active phases of periodontal bone destruction, ICTP also correlated strongly with osseous metabolic activity as measured by the bone-seeking radiopharmaceutical uptake of 99mTc-MDP (Giannobile et al. 1995).
More recently, it has been demonstrated that ICTP is elevated in human periodontitis subjects exhibiting high levels of putative periodontal pathogens including Bacteroides forsythus, Porphyromonas gingivalis, Fusobacterium nucleatum subspecies, and Treponema denticola (Palys et al. 1998). Preliminary longitudinal data suggest that elevated ICTP levels may identify sites undergoing active attachment loss (Giannobile et al. 1997). Finally, treatment with a matrix metalloproteinase (MMP) inhibitor significantly reduced ICTP levels in patients exhibiting active periodontal disease (Golub et al. 1997). The purpose of the present cross-sectional study was to examine the relationship between ICTP levels and subgingival species around endosseous oral implants and natural teeth in a periodontal maintenance population.
Material and methods
Patient population
The subjects examined in this study were obtained by referral from dentists treating individuals with endosseous dental implants at the Harvard School of Dental Medicine. These patients were currently in the maintenance phase of periodontal therapy. This study was approved by the Institutional Review Board at the Harvard Medical School, and informed written consent was obtained from each subject prior to study commencement. Information collected at enrollment included: the patient’s age; gender; time since last prophylaxis; and time since implant loading (Table 1)
Table 1.
Characteristics of subject group (mean and range)
| Age (years) | %Female | Last prophylaxis (weeks) | Prosthetic loading time (months) | Implants per patient |
|---|---|---|---|---|
| 55.8 | 36% | 22.7(2–72) | 17.5(6–48) | 3.2(1–7) |
| (38.8–76.9) |
The subjects were partially or totally edentulous with one or more implants supporting fixed or removable prostheses. Therefore, 20 of the 22 subjects possessed both teeth and implants while 2 subjects were completely edentulous (Table 1). To be eligible for this study, dental implants must have been in place a minimum of 1 year and in function a minimum of 6 months prior to study inclusion. Dental implant systems included in this study were ITI (Strauman Institute, Waldenburg, Switzerland), Brånemark (Nobel Biocare AB, Göteborg, Sweden), or IMZ (Friatec AG, Mannheim, Germany). Patients with the following characteristics were excluded from the study: pregnancy or lactation; treatment with antibiotics within 6 weeks prior to inclusion; treatment with medications known to affect periodontal status such as chronic NSAID therapy; diabetes; presence of any condition necessitating antibiotic prophylaxis; history of significant cardiovascular, pulmonary, renal, hepatic, gastrointestinal, or hematological disease which may influence patient compliance; and patients with known metabolic bone diseases.
Clinical measurements
Clinical measurements were recorded at 6 sites per tooth and implant in each subject (excluding third molars). The clinical parameters assessed included: probing pocket depth, relative attachment level, and a dichotomous evaluation of bleeding on probing, plaque accumulation, gingival redness, and suppuration. Probing pocket depth and attachment level measurements were recorded to the nearest millimeter using a calibrated PCP-UNC 15 periodontal probe (Hu-Friedy Manufacturing Company, Chicago, IL, USA). The attachment level measurement was recorded from either the top of the abutment or the crown margin at the implant sites and from the cemento–enamel junction of natural teeth. GCF and subgingival plaque samples were collected, as described later, following measurement of plaque accumulation and gingival redness and prior to measurement of probing pocket depth, attachment level, and bleeding on probing.
Clinical measurements were performed by 2 examiners. To assess intra-examiner reliability, the standard deviation of the differences between duplicate sets of measurements was calculated for probing depth and attachment level measurements for both clinical examiners. The 2 clinical examiners were also compared to a previously calibrated third examiner (gold standard) to confirm adequate inter-examiner reliability utilizing an unpaired t-test. The standard deviation for probing depth and attachment level measurements for examiner 1 were 0.51 mm and 0.71 mm, respectively, and for examiner 2 were 0.56 mm and 0.75 mm, respectively. No significant differences were noted in the probing depth and attachment level measurements recorded by the gold standard examiner compared to the 2 clinical examiners participating in the study (P>O.05).
Biochemical monitoring (ICTP)
The area around each mesiobuccal site sampled was dried with gauze and supragingival plaque removed. Gingival crevicular fluid was collected for 10 s using methylcellulose strips (Pro Flow, Inc., Amityville, NY, USA). Each strip was placed gently into the gingival crevice until slight resistance was felt and volume determined with a Periotron 6000 (Harco Electronics, Tustin, CA). Following collection and volume determination, the samples were kept on ice for transport to the laboratory and stored at − 20 ° C. The frozen samples were thawed at room temperature and proteins eluted by centrifugation with 20 ml phosphate buffered saline (PBS) 5X at 1500 × g for 5 min. The PBS solution contained 15 nM aprotenin (Sigma Chemical Company, St Louis, MO, USA), 1 mM phenylmethylsulfonylfluoride (PMSF) (Sigma Chemical Company, St Louis, MO, USA), and 0.1% human serum albumin (HSA). ICTP levels were determined by radioimmunoassay as described previously (Risteli et al. 1993). The extraction procedure showed >90% efficiency in ICTP recovery when tested in trial experiments (Giannobile et al. 1995). The assay has a sensitivity of detecting 34 pg of ICTP (Incstar Corporation, Stillwater, MN).
Microbiological monitoring
Sampling
Subgingival plaque samples were collected from the mesiobuccal site of each implant or tooth using sterile graphite or steel curettes. Samples were placed into individual tubes containing 0.15 ml TE (10 mM Tris-HCl, 1 mM EDTA, pH 7.6). Then 0.15 ml of 0.5 M NaOH was added to each tube at the end of each examination. The checkerboard DNA-DNA hybridization technique was utilized to analyze plaque samples semi-quantitatively for the presence of 40 subgingival species (Table 2). Each sample was boiled for 5 min and neutralized using 0.8 ml 5 M ammonium acetate. The released DNA was placed into the extended slots of a Minislot apparatus (Immunetics, Cambridge, MA) and then concentrated onto a nylon membrane (Boehringer Mannheim) by vacuum and fixed to the membrane by exposure to ultraviolet light followed by baking at 120°C for 20 min. The Minislot device permitted the deposition of up to 28 plaque samples in individual “lanes” on a single 15 × 15 cm nylon membrane as well as 2 control lanes containing 105 and 106 cells of each test species. The membrane with fixed DNA was placed in a Miniblotter 45 with the “lanes” of DNA at 90° to the channels of the device. A 30×45 “checkerboard” pattern was produced. Each channel was used as a hybridizing chamber for separate DNA probes. Signals were detected by chemiluminescence. The sensitivity of the assay for bacterial species was optimized to detect 104 cells of a given species.
Table 2.
DNA probes used to examine subgingival plaque samples
| Actinobacillus actinomycetemcomitans a | Fusobacterium nucleatum ss nucleatum |
| Actinobacillus actinomycetemcomitans b | Fusobacterium nucleatum ss polymorphum |
| Actinomyces naeslundii 2 | Fusobacterium nucleatum ss vincentii |
| Actinomyces odontolyticus | Fusobacterium periodonticum |
| Bacteroides forsythus | Peptostreptococcus micros |
| Bacteroides fragilis | Porphyromonas endodontalis |
| Bacteroides ureolyticus | Porphyromonas gingivalis |
| Campylobacter concisus | Prevotella intermedia |
| Campylobacter curvus | Prevotella nigrescens |
| Campylobacter gracilis | Selenomonas noxia |
| Campylobacter rectus | Streptococcus constellatus |
| Campylobacter showae | Streptococcus gordonii |
| Campylobacter sputorum ss bubulus | Streptococcus intermedius |
| Campylobacter sputorum ss sputorum | Streptococcus mitis |
| Capnocytophaga gingivalis | Streptococcus oralis |
| Capnocytophaga ochracea | Streptococcus sanguis |
| Capnocytophaga sputigena | Streptococcus sp. |
| Capnocytophaga sp. | Treponema denticola |
| Eikenella corrodens | Veillonella parvula |
| Eubacterium nodatum | Wolinella succinogenes |
DNA-DNA Hybridization
The membranes were prehybridized at 42°C for 1 h in 50% formamide, 5X SSC (IX SSC=150 mM NaCl, 15 mM Na citrate, pH 7.0), 1% casein (Sigma Chemical Company, St Louis, MO), 5X Denhardt’s reagent, 25 mM sodium phosphate (pH 6.5) and 0.5 mg/ml yeast RNA (Boehringer Mannheim). The membrane was placed back into a Miniblotter 45 (Immunetics, Cambridge, MA). Digoxigenin-labeled, whole chromosomal DNA probes were prepared using a random primer technique (Feinberg & Vogelstein, 1983). The probes and hybridization buffer were placed in individual lanes of the Miniblotter and the whole apparatus placed in a sealed plastic bag. Membranes were hybridized overnight at 42°C in a hybridizing solution containing 45% formamide, 5X SSC, IX Denhardt’s reagent, 20 mM sodium phosphate (pH 6.5), 0.2 mg/ml yeast RNA, 20 ng/ml of labeled probe, 10% dextran sulfate and 1% casein. Membranes were washed at low stringency to remove loosely bound probe and then at high stringency (68°C, 1% SDS, 20 min, twice) in a Disk Wisk apparatus (Schleicher and Schuell, Keene, NH).
Detection and enumeration
To detect hybrids, membranes were blocked and then incubated with a 1:25,000 dilution of anti-digoxigenin antibody conjugated with alkaline phosphatase (Engler-Blum et al. 1993). After washing, the membranes were incubated in Lumiphos 530 (Lumigen, Southfield, MI) for 45 min at 37°C, placed in a film cassette with Reflection NEF film (Dupont, Boston, MA) for 1 h at 37°C and then developed. Two lanes in each run had standards at different concentrations. The sensitivity of this assay was adjusted to permit detection of 104 cells of a given species by adjusting the concentration of each DNA probe. This procedure was carried out in order to provide the same sensitivity of detection for each species, Failure to detect a signal was recorded as zero, although counts in the 1 to 1000 range could have been present. Signal intensities were evaluated by visual comparison of the signal with known concentrations of each test species (standards) run in the same lane. The thresholds were defined as 0, not detected; 1, <105 cells; 2, ≈ 105; 3, 105 to 106; 4, ≈ 106; and 5, >106 cells.
Data analysis
Mean values for clinical parameters, ICTP levels (pg/site/10 s), ICTP concentrations (ng/ml), and subgingival species levels were calculated for each subject, and pooled across subjects based upon 4 categories: tooth, implant, ICTP positive sites, and ICTP negative sites. The mean values were compared utilizing an unpaired t-test. The relationship of ICTP levels with clinical parameters and subgingival species was examined by regression analysis using both subject and site data. Sensitivities, specificities, odds ratios, and adjusted odds ratios (Mantel-Haenszel) were calculated to determine the likelihood of detecting ICTP given the presence of a particular subgingival species.
Results
Patient demographics
A total of 71 implants and 370 teeth from 22 subjects were examined. Of the 22 subjects, 20 were partially edentulous and 2 were totally edentulous. The 71 implants were comprised of 39 ITI (Strauman Institute, Waldenburg, Switzerland), 27 Brånemark (Nobel Biocare AB, Göteborg, Sweden), and 5 IMZ (Friatec AG, Mannheim, Germany) fixtures. Implants were prosthetically loaded on the average of 17.5 months (6–48 months). The mean time interval since last prophylaxis was 22.7 weeks (2–72 weeks).
Clinical measurements, ICTP levels, and subgingival species levels
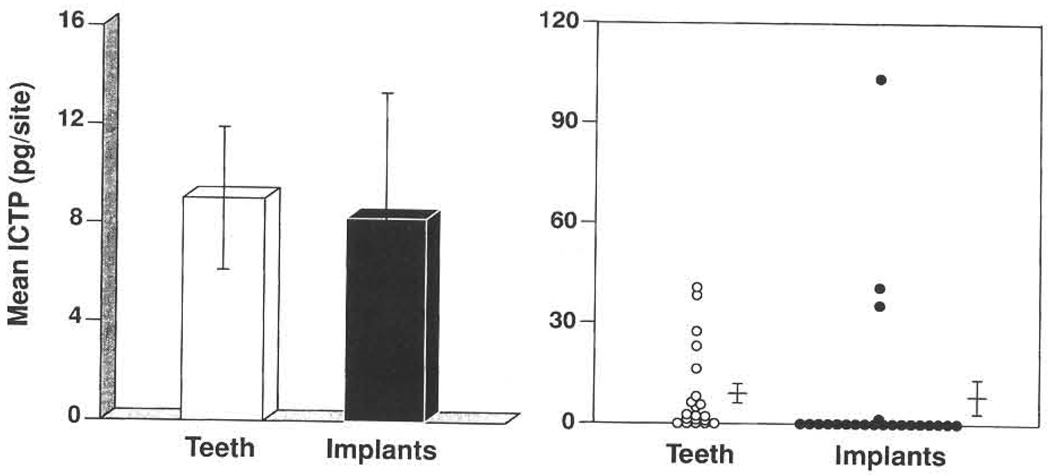
Mean values (± SD) for clinical parameters and GCF ICTP levels around implants and teeth are presented in Table 3. No significant differences were observed between implants and teeth regarding GCF ICTP levels normalized by time of collection (pg/site/10 s) or concentrations of ICTP (ng/ml). The probing depth measurements around dental implants were significantly greater than around natural teeth (P=0.02). No significant differences were noted among the other clinical parameters measured. Fig. 1 demonstrates that relatively few subjects exhibited any ICTP around implant sites compared to their natural dentition. Of the 40 subgingival species monitored only Actinomyces naeslundii genospecies 2 (Actinomyces viscosus) and Campylobacter curvus were found at significantly greater levels around teeth compared to implant sites (P<0.05), while the remaining 38 species revealed no significant differences between sites (data not shown).
Table 3.
Mean (±SD) clinical measurements and GCF ICTP level around implants and teeth
| Implants | Teeth | |
|---|---|---|
| Number of subjects | 22 | 20 |
| Number of sites | 71 | 370 |
| Mean pocket depth (mm) | 3.3±0.9 | 2.7±0.5* |
| Mean attachment level (mm) | 2.7±0.9 | 2.5±0.8 |
| % Sites with: | ||
| Bleeding on probing | 34±38 | 30±25 |
| Plaque | 34±41 | 39±31 |
| Gingival redness | 30±41 | 35±35 |
| ICTP (pg/10 s) | 8.23±5.12 | 9.01±2.90 |
| ICTP (ng/ml) | 18.48±10.17 | 18.35±4.94 |
P<0.05.
Fig. 1.
Mean ICTP levels associated with teeth and implants. The left panel presents the mean ICTP (± SD) for teeth and implant sites. In the right panel, each circle represents the mean ICTP value at teeth and implants in each subject. The horizontal line and whiskers represent the mean ICTP (± SD) levels for the 2 groups.
Comparison of subgingival species at ICTP positive sites and ICTP negative sites
No significant differences in mean levels of subgingival species were observed between ICTP positive sites and ICTP negative sites around dental implants. However, Table 4 demonstrates that a number of organisms were significantly elevated at ICTP positive sites around natural teeth compared with ICTP negative sites (P<0.05). Elevated subgingival species included: B. forsythus, Peptostreptococcus micros, Prevotella nigrescens, F. nucleatum ss vincentii, Fusobacterium nucleatum ss nucleatum, Campylobacter rectus, Streptococcus intermedius, and Selenomonas noxia.
Table 4.
Means level (× 105, ± SD) of subgingival species that differed significantly (P<0.05) at ICTP positive sites and ICTP negative sites around natural teeth
| Species | ICTP positive site | ICTP negative site |
|---|---|---|
| B. forsythus | 0.39±0.12 | 0.10±0.03 |
| C. rectus | 0.30±0.10 | 0.11±0.02 |
| F. nucleatum ss nucleatum | 1.03±0.24 | 0.56±0.07 |
| F. nucleatum ss vincentii | 1.37±0.37 | 0.57±0.07 |
| P. micros | 0.98±0.28 | 0.28±0.07 |
| P. nigrescens | 0.53±0.18 | 0.25±0.07 |
| S. Intermedius | 0.67±0.15 | 0.33±0.05 |
| S. noxia | 0.34±0.10 | 0.15±0.04 |
Relationship of ICTP levels to mean clinical parameters and subgingival species
The relationship of ICTP levels with clinical parameters and subgingival species was examined by regression analysis using both subject and site data. Around dental implants, no significant relationships between ICTP levels (pg/site/10 s), clinical parameters, and subgingival species were seen on a subject level. On a site level, a weak positive association was observed between GCF ICTP levels and Campylobacter gracilis (r=0.17, P<0.05). In contrast, both Streptococcus constellatus (r=−0.30, P<0.05) and Campylobacter sputorum (r=−0.33, P<0.05) exhibited significant inverse relationships with ICTP levels around implant sites.
Stronger associations were identified between ICTP levels and subgingival species levels around natural teeth compared to dental implants on a subject level. Both Streptococcus oralis (r=0.54, P<0.01) and C. gracilis (r=0.43, P<0.05) exhibited a significant positive relationship with ICTP levels and C. sputorum (r=−0.76, P<0.01) exhibited a strong inverse relationship. On a site level, F. nucleatum ss vincentii (r=0.09, P<0.05), S. oralis (r=0.14, P<0.01), and S. intermedius (r=0.15, P<0.01) all demonstrated weak, but significant associations with ICTP levels. An inverse relationship was detected among GCF ICTP levels with Actinomyces naeslundii genospecies 2 (A. viscosus) (r=−0.1, P<0.05) and C. sputorum (r=−0.23, P<0.01). Significant, but weak associations were also identified at the site level between traditional clinical parameters and GCF ICTP levels (P<0.05). A positive relationship was shown between ICTP and plaque, gingival redness, attachment level, and probing depth (r=0.10, r=0.17, r=0.2l, r=0.10, respectively) by the Spearman Rank Correlation Coefficient. Similar results were found utilizing GCF ICTP concentration levels (ng/ml) (data not shown).
Relationship between the presence of ICTP and elevated levels of subgingival species
To determine the likelihood of detecting ICTP (i.e., ICTP positive site vs. ICTP negative site) around implants and teeth given the presence of a particular subgingival species, the sensitivities, specificities, odds ratios, and adjusted odds ratios (Mantel-Haenszel) were determined. As presented in Table 5 and Table 6 the presence of ICTP around both dental implants and natural teeth was related to the detection of specific subgingival species above threshold levels. At implant sites, site-based analysis demonstrated a significant relationship between 4 subgingival species and ICTP levels although subject-based analysis failed to demonstrate significant relationships (Table 5). Interestingly F. nucleatum, ss. vincentii related to ICTP at both tooth and implant sites, while no other species were found in common at teeth and implants. The subgingival species that exhibited strong associations with ICTP also demonstrated modest sensitivities and high specificities suggesting a relationship between the subgingival plaque composition and presence of ICTP.
Table 5.
Association of ICTP with subgingival species above threshold values at dental implant sites
| Site-based |
Subject-based | ||||
|---|---|---|---|---|---|
| Species | Threshold | Odds ratio | Sensitivity | Specificity | Mantel–Haenszel odds ratio |
| P. intermedia | ≥105 | 12.4a | 0.30 | 0.97 | 3.5b |
| C. gingivalis | ≥105 | 9.3a | 0.40 | 0.93 | 6.3b |
| F. nucleatum ss vincentii | >105 | 8.1a | 0.30 | 0.95 | 4.2b |
| S. gordonii | ≥105 | 6.7a | 0.60 | 0.82 | 4.3b |
P<0.01
Not Significant.
Table 6.
Association of ICTP with subgingival species over threshold values at natural teeth sites
| Site-based |
Subject-based | ||||
|---|---|---|---|---|---|
| Species | Threshold | Odds ratio | Sensitivity | Specificity | Mantel–Haenszel odds ratio |
| P micros | >105 | 10.0a | 0.10 | 0.99 | 25.6c |
| F. nucleatum ss vincentii | >105 | 6.1a | 0.24 | 0.95 | 3.7b |
| F. periodonticum | >105 | 6.1a | 0.08 | 0.99 | 4.5b |
| F. nucleatum ss nucleatum | >105 | 5.0a | 0.12 | 0.97 | 3.7a |
| P. nigrescens | >105 | 3.2a | 0.22 | 0.92 | 4.6b |
| F. nucleatum ss polymorphum | >105 | 3.1a | 0.28 | 0.89 | 2.3a |
P<0.01
P<0.001;
P<0.0001.
Discussion
Probing and radiography, the traditional methods utilized to detect peri-implantitis and periodontitis, provide information regarding the presence of attachment and bone loss. However, these techniques do not identify sites actively undergoing disease progression or those sites at risk of deterioration (Haffajee et al. 1991). Host factors in the GCF associated with the anatomic events of periodontitis and peri-implantitis may be useful as markers for identifying and predicting future disease progression (Page 1992). A biochemical marker specific for bone degradation may be useful to differentiate the presence of gingival inflammation from active periodontal and peri-implant bone destruction (Giannobile 1997). The results of the present study demonstrate that C-telopeptide pyridinoline cross-links (ICTP) can be identified in the GCF around dental implants. In addition, a positive relationship was observed between elevated levels of ICTP at implant sites and organisms associated with disease progression.
In the present study, probing depth measurements around dental implants were significantly greater than around natural teeth. A report which evaluated probing around implants and teeth in an animal model (Ericsson & Lindhe, 1993) demonstrated that probing depth measurements around implants were significantly deeper compared to probing depth measurements around teeth. Histological examination revealed that the probe tip was coronal to the apical extension of the junctional epithelium around teeth. However, around implants the probe tip was apical to the junctional epithelium and close to the alveolar bone crest. The authors concluded that the soft tissue/implant interface is less resistant to probing forces compared to the soft tissue attachment which exists around natural teeth.
No significant differences were noted at endosseous implants and natural teeth regarding GCF ICTP levels normalized by time of collection (pg/ site/10 s) or for concentrations of ICTP (ng/ml). The ICTP levels observed in this maintenance population were similar to ICTP levels seen in a previously studied gingivitis population (Palys et al. 1998). Overall, no differences in the subgingival plaque composition were noted between implants and teeth. Previous studies have reported similar findings (Apse et al. 1989; Quirynen & Listgarten, 1990; Mombelli et al. 1995). One report compared subgingival plaque samples from tooth and implant sites in 15 partially edentulous and 6 totally edentulous subjects (Apse et al. 1989). Microbiological assessment included darkfield microscopy and anaerobic culturing. Similar bacterial species were reported around implants and teeth in the partially edentulous subjects. A higher percentage of Bacteroides and Capnocytophaga species was observed at partially edentulous implant sites when compared with edentulous implant sites. Quirynen & Listgarten (1990) examined bacterial morphotypes in subgingival plaque samples around teeth and implants from 24 partially edentulous subjects. Again, no significant differences were reported. Mombelli et al. (1995) demonstrated a high prevalence of putative periodontal pathogens around 2-stage implants after being exposed for 3 and 6 months to the oral environment in patients previously treated for periodontal disease. Recently, Gouvoussis et al. (1997) have revealed cross-infection from periodontitis sites to failing implant sites in the same mouth. The results of these studies support the concept that teeth may serve as a reservoir for bacterial colonization around implants in partially edentulous individuals (reviewed in Mombelli (1997)).
A comparison of subgingival species at ICTP positive sites and ICTP negative sites around natural teeth with a history of periodontal disease revealed that a number of putative periodontal pathogens were significantly elevated at ICTP positive sites. A recent study demonstrated that GCF ICTP levels were significantly elevated in patients with periodontitis and these levels related strongly with mean subgingival levels of periodontal pathogens including B. forsythus, T. denticola, and P. gingivalis (Palys et al. 1998). At implant sites no significant difference in mean levels of subgingival species was noted between ICTP positive sites and ICTP negative sites. Perhaps no relationship was observed in this population around implants because few implant sites demonstrated detectable GCF ICTP levels as shown in Fig. 1. The lack of a significant relationship at implant sites between ICTP status and subgingival plaque composition may also be due to colonization of healthy and diseased sites with similar microorganisms.
No strong associations were demonstrated utilizing regression analysis to examine the relationship of ICTP levels to mean levels of clinical parameters and subgingival species. To further explore the relationship between GCF ICTP and subgingival species the sites were subset into those that were either ICTP positive or ICTP negative. The data revealed significant associations between the presence of ICTP and the level of P. intermedia, C. gingivalis, F. nucleatum ss vincentii, and S. gordonii and exhibited site-based odds ratios of 12.4, 9.3, 8.1, and 6.7, respectively, indicating an increased likelihood of detecting ICTP at implant sites colonized by these species (Table 5). An association between elevated ICTP levels and the presence of putative periodontal pathogens has been demonstrated around teeth in a periodontitis population (Palys et al. 1998). The subgingival species that exhibited significant associations with ICTP also demonstrated modest sensitivities and high specificities suggesting a relationship between subgingival plaque composition and presence of ICTP (Table 6). Elevated levels of P. intermedia and Fusobacterium spp. have been observed at failing implant sites (Mombelli et al. 1987; Saadoun 1993). Recently, it was reported that F. nucleatum ss vincentii was more prevalent in mouths with failing implant sites as compared to mouths with healthy “stable” implants (Salcetti et al. 1997).
The results of the present study suggest a relationship between elevated ICTP levels at implant sites and some species associated with disease progression. Future studies should examine levels of ICTP and other putative pyridinoline crosslinks at implants exhibiting clinical signs of failure. Finally, longitudinal studies are necessary to examine the predictive ability of GCF ICTP to identify the development of peri-implant bone loss.
Acknowledgements
The authors would like to thank Ms Claire Smith for her excellent technical assistance. This study was supported by NIH DE 04881, P30 DE11814, and the Oral & Maxillofacial Surgery Foundation.
References
- Apse P, Ellen RP, Overall CM, Zarb GA. Micro-biota and crevicular fluid collagenase activity in the osseointegrated dental implant sulcus: a comparison of sites in edentulous and partially edentulous patients. Journal of Periodontal Research. 1989;24:96–105. doi: 10.1111/j.1600-0765.1989.tb00863.x. [DOI] [PubMed] [Google Scholar]
- Engler-Blum G, Meier M, Frank J, Muller GA. Reduction of background problems in nonradioactive Northern and Southern blot analyses enables higher sensitivity than 32P-based hybridizations. Annals of Biochemistry. 1993;210:235–244. doi: 10.1006/abio.1993.1189. [DOI] [PubMed] [Google Scholar]
- Ericsson I, Lindhe J. Probing depth at implants and teeth. Journal of Clinical Periodontology. 1993;20:623–627. doi: 10.1111/j.1600-051x.1993.tb00706.x. [DOI] [PubMed] [Google Scholar]
- Eriksen EF, Charles P, Melsen F, Mosekilde L, Risteli L, Risteli J. Serum markers of type I collagen formation and degradation in metabolic bone disease: correlation with bone histomorphometry. Journal of Bone and Mineral Research. 1993;8:127–132. doi: 10.1002/jbmr.5650080202. [DOI] [PubMed] [Google Scholar]
- Feinberg AP, Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Annals of Biochemistry. 1983;132:6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fiorellini JP, Martuscelli G, Weber HP. Longitudinal studies on implant systems. Periodontology 2000. 1998;17:125–131. doi: 10.1111/j.1600-0757.1998.tb00130.x. [DOI] [PubMed] [Google Scholar]
- Giannobile WV, Lynch SE, Denmark RG, Paquette DW, Fiorellini JP, Williams RC. Crevicular fluid osteocalcin and pyridinoline cross-linked carboxyterminal telopeptide of type I collagen (ICTP) as markers of rapid bone turnover in periodontitis: a pilot study in beagle dogs. Journal of Clinical Periodontology. 1995;22:903–910. doi: 10.1111/j.1600-051x.1995.tb01793.x. [DOI] [PubMed] [Google Scholar]
- Giannobile WV, Palys MD, Howell TH, Haffajee AD, Socransky SS. Crevicular fluid ICTP in patients with periodontitis. Journal of Dental Research. 1996;75:157. [Google Scholar]
- Giannobile WV, Socransky SS, Palys MD, Haffajee AD. Relationship between crevicular fluid ICTP and putative pathogens. Journal of Dental Research. 1997;76:268. [Google Scholar]
- Giannobile WV. Crevicular fluid biomarkers of oral bone loss. Current Opinion in Periodontology. 1997;4:11–20. [PubMed] [Google Scholar]
- Golub LM, Lee HM, Greenwald RA, Ryan ME, Sorsa T, Salo T, Giannobile WV. A matrix metalloproteinase inhibitor reduces bone-type collagen degradative fragments and specific collagenases in gingival crevicular fluid during adult periodontitis. Inflammation Research. 1997;46:310–319. doi: 10.1007/s000110050193. [DOI] [PubMed] [Google Scholar]
- Goodson JM. Diagnosis of periodontitis by physical measurement: interpretation from episodic disease hypothesis. Journal of Periodontology. 1992;63:373–382. doi: 10.1902/jop.1992.63.4s.373. [DOI] [PubMed] [Google Scholar]
- Gouvoussis J, Sindhusake D, Yeung Y. Cross-infection from periodontitis sites to failing implant sites in the same mouth. International Journal of Oral and Maxillofacial Implants. 1997;12:666–673. [PubMed] [Google Scholar]
- Haffajee AD, Socransky SS, Lindhe J, Kent RL, Okamoto H, Yoneyama T. Clinical risk indicators for periodontal attachment loss. Journal of Clinical Periodontology. 1991;18:117–125. doi: 10.1111/j.1600-051x.1991.tb01700.x. [DOI] [PubMed] [Google Scholar]
- Hassager C, Jensen LT, Pødenphant J, Thomsen K, Christiansen C. The carboxy-terminal pyridinoline cross-linked telopeptide of type I collagen in serum as a marker of bone resorption: the effect of nadrolone decanoate and hormone replacement therapy. Calcified Tissue International. 1994;54:30–33. doi: 10.1007/BF00316286. [DOI] [PubMed] [Google Scholar]
- Mombelli A, Van Oosten MAC, Schürch E, Lang NP. The microbiota associated with successful and failing osseointegrated titanium implants. Oral Microbiology and Immunology. 1987;2:145–151. doi: 10.1111/j.1399-302x.1987.tb00298.x. [DOI] [PubMed] [Google Scholar]
- Mombelli A, Marxer M, Gaberthüel T, Grunder U, Lang NP. The microbiota of osseointegrated implants in patients with a history of periodontal disease. Journal of Clinical Periodontology. 1995;22:124–130. doi: 10.1111/j.1600-051x.1995.tb00123.x. [DOI] [PubMed] [Google Scholar]
- Mombelli A. Etiology, diagnosis, and treatment considerations in peri-implantitis. Current Opinion in Periodontology. 1997;4:127–136. [PubMed] [Google Scholar]
- Page RC. Host response tests for diagnosing periodontal diseases. Journal of Periodontology. 1992;63:356–366. doi: 10.1902/jop.1992.63.4s.356. [DOI] [PubMed] [Google Scholar]
- Palys MD, Haffajee AD, Socransky SS, Giannobile WV. Relationship between C-telopeptide cross-links (ICTP) and putative periodontal pathogens in periodontitis. Journal of Clinical Periodontology. 1998;25 doi: 10.1111/j.1600-051x.1998.tb02383.x. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirynen M, Listgarten MA. The distribution of bacterial morphotypes around natural teeth and titanium implants ad modum Brånemark. Clinical Oral Implants Research. 1990;1:8–12. doi: 10.1034/j.1600-0501.1990.010102.x. [DOI] [PubMed] [Google Scholar]
- Risteli J, Elomaa I, Niemi S, Novamo A, Risteli L. Radioimmunoassay for the pyridinoline cross-linked carboxyterminal telopeptide of type I collagen: a new serum marker of bone collagen degradation. Clinical Chemistry. 1993;39:635–640. [PubMed] [Google Scholar]
- Saadoun AP. Microbial infections and occlusal overload: causes of failure in osseointegrated implants. Practical Periodontics and Aesthetic Dentistry. 1993;5:11–20. [PubMed] [Google Scholar]
- Salcetti JM, Moriaty JD, Cooper LF, Smith FW, Collins JG, Socransky SS, Offenbacher S. The clinical, microbial, and host response characteristics of the failing implant. International Journal of Oral and Maxillofacial Implants. 1997;12:32–42. [PubMed] [Google Scholar]
- Talonpoika JT, Hämäläinen MM. Type I collagen carboxyterminal in human gingival crevicular fluid in different clinical conditions and after periodontal treatment. Journal of Clinical Periodontology. 1994;21:320–326. doi: 10.1111/j.1600-051x.1994.tb00720.x. [DOI] [PubMed] [Google Scholar]