Abstract
NMDA receptors (NMDARs) are a major class of excitatory neurotransmitter receptors in the central nervous system. They form glutamate-gated ion channels highly permeable to calcium that mediate activity-dependent synaptic plasticity1. NMDAR dysfunction is implicated in multiple brain disorders, including stroke, chronic pain and schizophrenia2. NMDARs exist as multiple subtypes with distinct pharmacological and biophysical properties largely determined by the type of NR2 subunit (NR2A-NR2D) incorporated in the heteromeric NR1/NR2 complex1,3,4. A fundamental difference between NMDAR subtypes is their channel maximal open probability (Po), which spans a 50-fold range from ~0.5 for NR2A-containing receptors to ~0.01 for NR2C- and NR2D-containing receptors; NR2B-containing receptors having an intermediate value (~0.1)5–9. These differences in Po confer unique charge transfer capacities and signaling properties on each receptor subtype4,6,10,11. The molecular basis for this profound difference in activity between NMDAR subtypes is unknown. Here we demonstrate that the subunit-specific gating of NMDARs is controlled by the region formed by the NR2 N-terminal domain (NTD), an extracellular clamshell-like domain previously shown to bind allosteric inhibitors12–15, and the short linker connecting the NTD to the agonist-binding domain (ABD). Subtype specificity of NMDAR Po largely reflects differences in the spontaneous (ligand-independent) equilibrium between open-cleft and closed-cleft conformations of the NR2-NTD. This NTD-driven gating control also impacts pharmacological properties, by setting the sensitivity to the endogenous inhibitors zinc and protons. Our results provide a proof-of-concept for a drug-based bidirectional control of NMDAR activity using molecules acting either as NR2-NTD “closers” or “openers” promoting receptor inhibition or potentiation, respectively.
Keywords: glutamate receptors, NMDA, gating, channel, synapse, allosteric modulation
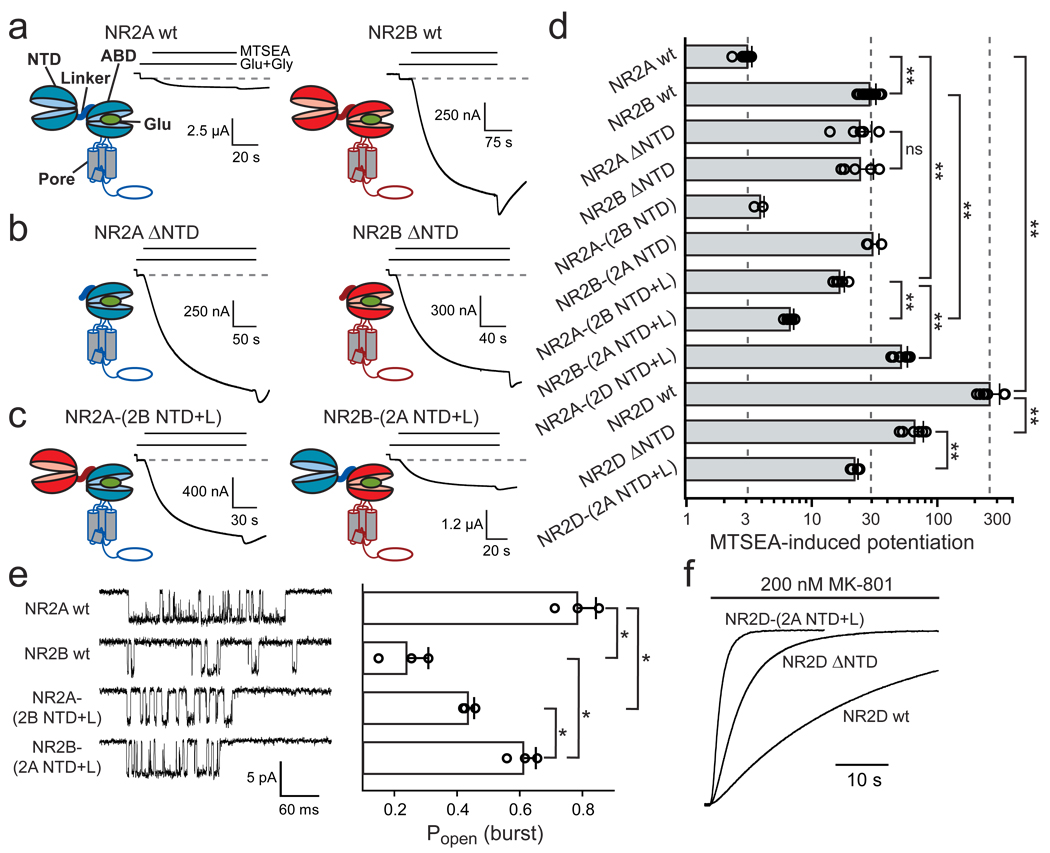
We first explored the role of the NR2-NTD in the difference of Po between NR1/NR2A and NR1/NR2B receptors by evaluating the effect of deleting the entire NR2-NTD on receptor activity. We estimated Po using a method based on the covalent modification of a cysteine introduced in the NR1 subunit (NR1-A652C), which locks open the NMDAR channel16. Although this method does not give access to the absolute Po of receptors containing the wild-type (wt) NR1 subunit, it can report relative differences in channel activity17. Indeed, the extent to which the sulfhydryl-modifying reagent MTSEA potentiates NMDAR currents is inversely related to the channel Po17. MTSEA potentiated currents carried by NR1-A652C/NR2B receptors to a much greater extent than currents of NR1-A652C/NR2A receptors (Fig. 1a&d), consistent with the much lower Po of NR2B-containing receptors compared to NR2A-containing receptors5,6,17. In contrast, MTSEA-induced potentiations of NR1-A652C/NR2A-ΔNTD and NR1-A652C/NR2B-ΔNTD receptors were indistinguishable (Fig. 1b&d) indicating equal receptor activities. However, receptors incorporating chimeric NR2A-(2B NTD) or NR2B-(2A NTD) subunits displayed MTSEA-induced potentiations similar to those of the parental NR2 subunits, indicating that swapping the NTDs alone did not exchange the Po (Fig. 1d). We therefore swapped both the NTD and the highly divergent short (14 residues) linker segment that connects the NTD to the ABD (Fig. S1). Remarkably, NR1-A652C/NR2A-(2B NTD+L) and NR1-A652C/NR2B-(2A NTD+L) responses supported levels of MTSEA potentiation closer to those of NR2Bwt-containing and NR2Awt-containing receptors, respectively (Fig. 1c&d). Direct measurement of channel activity using single-channel recordings confirmed this exchange of Po (Fig. 1e and Fig S2).
Figure 1. The NR2 NTD+linker region controls NMDAR Po.
a–c Potentiation by MTSEA of receptors incorporating NR1-A652C and the indicated NR2 subunits. NTD, N-terminal domain; ABD, agonist-binding domain. d Pooled data (mean +/− s.d.), from top to bottom: 3.2 +/− 0.3 (n=12), 30 +/− 4 (n=14), 25 +/− 6 (n=6), 25 +/− 7 (n=5), 4.0 +/− 0.3 (n=3), 32 +/− 4 (n=3), 17 +/− 2 (n=6), 6.9 +/− 0.5 (n=5), 53 +/− 7 (n=9), 270 +/− 60 (n=7), 68 +/− 12 (n=6) and 23 +/− 2 (n=5) (**p<0.001). e Po within bursts of openings for receptors incorporating NR1wt and the indicated NR2 subunit. Left: representative traces of bursts. Right (from top to bottom): 0.78 +/− 0.06 (n=3), 0.24 +/− 0.07 (n=3), 0.43 +/− 0.02 (n=3) and 0.61 +/− 0.04 (n=3) (*p<0.05, Student’s t-test). f Kinetics of inhibition by MK-801 at receptors incorporating NR1wt and NR2Dwt (τon = 32 s), NR2D-ΔNTD (5.7 s) or NR2D-(2A NTD+L) (1.6 s). Error bars represent s.d.
We next extended the analysis to the NR2D subunit. MTSEA-induced potentiations of NR2D-containing receptors were considerable (~300-fold), reflecting the very low Po of NR1/NR2D receptors (Fig. 1d). Deleting the NR2D-NTD resulted in a 4-fold decrease in MTSEA potentiation, indicative of a markedly increased Po (Fig. 1d). This gain-of-function phenotype could be reinforced by grafting on NR2D-ΔNTD the NTD+linker region of the high-Po subunit NR2A. Reversibly, receptors containing the chimeric NR2A-(2D NTD+L) subunit displayed 17-fold higher MTSEA-potentiation than NR2Awt-containing receptors, suggestive of a much lower Po (Fig. 1d). Thus, the low Po of the NR2D-containing receptors is also set by the NR2-NTD.
Since Po estimation using MTSEA relies on a mutated NR1 subunit (NR1-A652C), we checked that the effects observed did not depend on this mutation. We used the time constant of inhibition by MK-801, an NMDAR open-channel blocker, as an alternative method to assess Po5,18. Consistent with the higher Po of NR2A- vs NR2B-containing receptors, MK-801 inhibited wt NR1/NR2A receptors significantly faster than wt NR1/NR2B receptors (Fig. S3a&b). Deleting the NR2-NTDs abolished this difference (Fig. S3b). While swapping the NR2-NTD alone did not exchange MK-801 time constants, incorporating the NTD-ABD linker achieved almost complete transfer (Fig. S3a&b). As expected, the onset of MK-801 inhibition at wt NR1/NR2D receptors was much slower than at NR2A- or NR2B-containing receptors. Deleting the NR2D-NTD or replacing the NTD+linker region of NR2D by that of NR2A strongly accelerated MK-801 inhibition, indicative of a much increased Po (Fig. 1f and Fig S3c). Conversely, MK-801 inhibition of receptors incorporating NR2A-(2D NTD+L) was 15-fold slower than at NR2Awt-containing receptors (Fig. S3b). Together with the MTSEA experiments, these results demonstrate that the NR2-NTD+linker region is a major determinant of the NR2 subunit-specific activity of NMDARs.
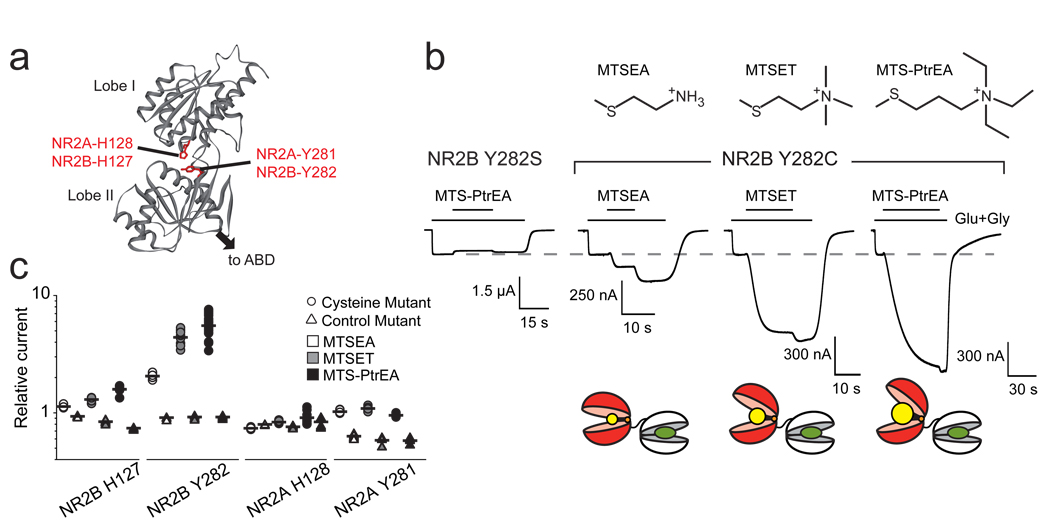
We next investigated the mechanism by which a distal domain, the NR2-NTD, influences channel activity. Previous studies on allosteric inhibition of NMDARs by NR2-NTD ligands, such as zinc and ifenprodil, suggested that these ligands bind the NTD cleft and promote its closure12,15,19. This in turn leads to receptor inhibition through disruption of the NR1/NR2 ABD dimer interface, resembling the mechanism underlying AMPAR desensitization20–22. Since the NTD can adopt at least two conformations, a ligand-free open state and a ligand-bound closed state, we hypothesized that the NTD-driven control of Po might result from spontaneous oscillations of the NR2-NTD between an open-cleft conformation, favoring channel opening, and a closed-cleft conformation, favoring pore closure. Such ligand-independent oscillations have been observed in several clamshell-like proteins, including the bacterial maltose-binding-protein23 (MBP) and the GABA-B receptor24. To test this hypothesis, we introduced cysteines in the NR2-NTD cleft to lock open the NR2-NTDs using thiol-reactive MTS reagents. Based on 3D models, we first introduced a cysteine deep in the cleft of the NR2B-NTD by mutating the hinge residue NR2B-Y282, whose side chain points toward the cleft entrance (Fig. 2a and ref25). Application of the positively charged MTSEA potentiated NR1wt/NR2B-Y282C receptors but not control NR1wt/NR2B-Y282S receptors (Fig. 2b). Strikingly, using MTS compounds of same valence but different sizes, we observed that the larger the MTS, the greater the potentiation (Fig. 2b&c). Comparison of the rates of MK-801 inhibition before and after MTS treatment together with direct measurement of single-channel activity revealed that current potentiations reflected an increase in Po (Fig. S4 and S5). Sensitivity to glycine (binding the NR1-ABD) was unaltered by MTS treatment, while sensitivity to glutamate (binding the NR2-ABD) was slightly decreased (Fig. S6), as expected from the known allosteric interaction between the NR2 NTD and ABD26. Interestingly, MTS action was significantly faster on resting than activated receptors (Fig. S7), further arguing for a facilitated opening of the NR2-NTD when the ABD is open. Altogether, these results show that trapping open the NR2-NTD enhances receptor activity. They also indicate that the NTD of NR2B-Y282C is not permanently open (since there was a potentiating effect of the MTS compounds) nor closed (since the introduced cysteine was accessible to MTS), but rather alternates between open and closed conformations, the latter favoring pore closure.
Figure 2. Locking open the NR2-NTD increases NMDAR activity.
a 3D model of NR2B-NTD. b Top: chemical formula of the transferable moiety of MTSEA, MTSET and MTS-PtrEA. Middle: Recordings from NR1wt/NR2B-Y282C and control NR1wt/NR2B-Y282S receptors during MTS treatment. The potentiation upon MTS wash likely reflects the washout of a reversible pore-blocking effect of the positively charged MTS. Bottom: Schematic representations of the NTD-ABD tandem of NR2B-Y282C after MTS-modification (MTS head group in yellow). c Relative currents after application of MTSEA, MTSET and MTS-PtrEA to receptors incorporating NR1wt and the indicated NR2 subunit. See Table S1 for values.
Because NR2B-Y282 is a large residue, we considered the possibility that its mutation into a small residue (cysteine or serine) may have artificially increased the flexibility of the NTD hinge, favoring NTD closure. Indeed, such mutations strongly reduced receptor activity (Fig. S8). This effect highlights the unsuspected role of the NR2-NTD hinge in shaping NMDAR Po, reminiscent of the critical role of the MBP hinge in controlling the apparent maltose affinity27. To extend our conclusion of spontaneous NR2-NTD oscillations to receptors with unaltered gating properties, we targeted NR2B-NTD H127, since its mutation into cysteine minimally affects receptor activity (Fig. S8). MTS compounds still potentiated NR1wt/NR2B-H127C receptors (but not control NR1wt/NR2B-H127A receptors) in a size-dependent manner. However, potentiations were considerably smaller than with NR1wt/NR2B-Y282C receptors (Fig. 2c and Fig S9a). Two reasons may explain this difference: higher basal Po of NR1wt/NR2B-H127C receptors, and wider opening of the NTD at MTS-modified NR2B-Y282C subunits because of the deeper location in the cleft of Y282. Overall, these results provide the important new information that spontaneous oscillations of the NR2B-NTD contribute to the low Po of wt NR1/NR2B receptors.
We then tested the prediction that the high Po of NR2A-containing receptors results from the NR2A-NTD preferring the open conformation. As for NR2B, we found the Po of NR2A-containing receptors to be significantly reduced by the mutation of NR2A-Y281 into small residues (Fig. S8). A potentiating component was also observed at receptors containing NR2A-Y281C during treatment by MTS compounds, but not at control NR2A-Y281A receptors. However, MTS-induced potentiations were much smaller than at NR2B-Y282C receptors and were independent of MTS size (Fig. 2c and Fig S9b), suggesting that the NR2A-NTD is much less sensitive to steric hindrance than the NR2B-NTD. In addition, no potentiation was observed at NR2A-H128C receptors even with the large MTS-PtrEA (Fig. 2c and Fig S9b). This is consistent with the NR2A-NTD spending most of its time in an open-cleft conformation, thus contributing to the relatively high Po of NR2A-containing receptors.
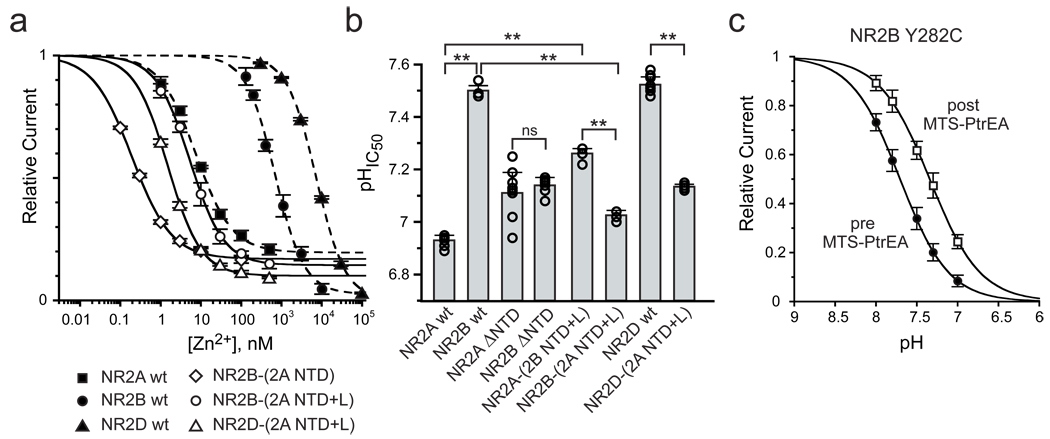
Our results on chimeric NR2 subunits, showing that the NTD-ABD linker is required for the differential influence of the NR2-NTD on receptor Po, raised the possibility that this element is also crucial during allosteric modulation of NMDARs by NTD-ligands. NR2A-NTD forms a high affinity zinc inhibitory site12–14; accordingly, NR1wt/NR2D-(2A NTD+L) receptors were highly sensitive to zinc (Fig. 3a). NR1wt/NR2B-(2A NTD) receptors are also highly sensitive to zinc. Surprisingly, zinc appears much more potent at these receptors than at wt NR1/NR2A receptors (Fig. 3a), suggesting that the NR2B NTD-ABD linker facilitates NTD-cleft closure. Increasing the chimera length to incorporate the NR2A NTD-ABD linker almost completely restored NR2Awt-like zinc sensitivity (Fig. 3a). This highlights again the importance of the NTD-ABD linker for the communication between the NTD and the gating machinery.
Figure 3. The NR2 NTD+linker region controls zinc and proton sensitivities of NMDARs.
a Zinc sensitivity of receptors incorporating NR1wt and (Inhibmax, IC50): NR2Awt (81%, 7.5 nM [n=6]), NR2Bwt (98%, 720 nM [n = 13]), NR2Dwt (100%, 7.8 µM [n=3]), NR2B-(2A NTD) (83%, 0.20 nM [n=4]), NR2B-(2A NTD+L) (86%, 5.4 nM [n=4]) or NR2D-(2A NTD+L) (90%, 1.5 nM [n=5]). nH in the 0.9–1.2 range. b pHIC50 of receptors incorporating NR1wt and the indicated NR2 subunit. See Table S2 for values. (**p<0.001). c Proton sensitivity of NR1wt/NR2B-Y282C receptors before (pHIC50 = 7.70, nH = 1.5 [n=3]) and after (pHIC50 = 7.34, nH = 1.4 [n=3]) MTS-PtrEA modification. Error bars represent s.d.
Proton is another allosteric modulator that differentially inhibits NMDAR subtypes1. In contrast with the zinc sensor, the proton sensor is thought to be closely associated with the channel gate28. Unexpectedly, deleting the NR2-NTDs fully abolished the difference in pH sensitivity between wt NR1/NR2A and NR1/NR2B receptors (Fig. 3b). Moreover, swapping the NTD+linker region between NR2A and NR2B reversed their pH sensitivities, while grafting the NR2A NTD+linker region onto NR2D decreased its proton sensitivity close to that of NR2Awt-containing receptors (Fig. 3b). Proton sensitivity was also decreased when locking open the NR2B-Y282C NTD with MTS-PtrEA (Fig. 3c). Hence, the NR2 dependence of pH sensitivity is unlikely to result from an intrinsic difference in the proton sensor between NR1/NR2 receptor subtypes, but rather from differential access to the proton binding-site owing to the NR2-NTD influence on channel activity.
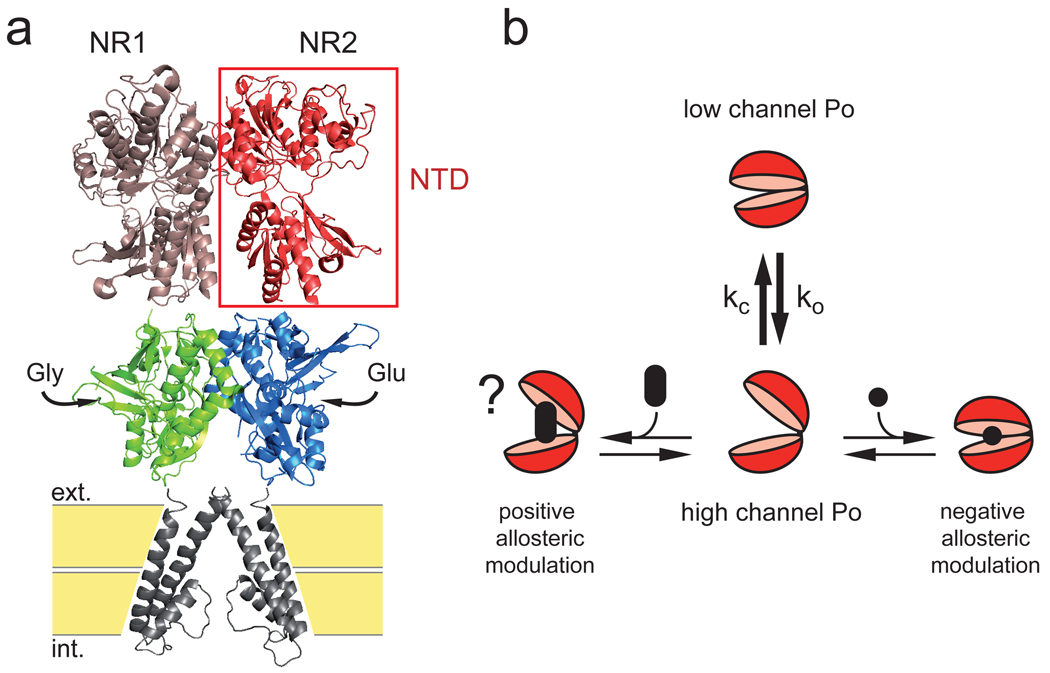
Our study reveals that the large differences in channel activity conferred by the various NR2 NMDAR subunits originate from a region remote from the agonist-binding/channel gating core. This region comprises the large NR2-NTD and the short linker connecting the NR2-NTD to the ABD. The bilobate NR2-NTD oscillates spontaneously between open-cleft and closed-cleft conformations (Fig. 4), the latter triggering disruption of the ABD dimer interface and subsequent channel closure20. The NTD-ABD linker could exert its key influence by tuning the equilibrium between the different conformations of the NR2-NTD. The identity of the NR2-NTD+linker region also determines the sensitivity to zinc and protons, two endogenous allosteric inhibitors of NMDARs that are likely to be critical in the regulation of NMDAR activity under physiological and pathological conditions1,3. Through its dynamic conformational equilibrium, the NR2-NTD could serve as a target for either negative or positive subunit-specific allosteric modulators (Fig. 4). Compounds like ifenprodil, which bind the NTD cleft and promote its closure (NTD “closers”), behave as subunit-specific NMDAR inhibitors and show good efficacy as neuroprotectants2. We propose that molecules that bind the same cleft but impede its closure (NTD “openers”) would behave as NMDAR potentiators (Fig. 4). Such molecules may prove of significant therapeutic benefit, given the accumulating evidence that major human psychoses, including schizophrenia, are associated with a deficit of NMDAR activity2,29.
Figure 4. Model for the control of NMDAR activity by the NR2 N-terminal domain.
a Structural depiction of an NMDAR. The full receptor is a tetramer but only a NR1/NR2 dimer is shown30. b In its ligand-free state, the NR2-NTD alternates between open- and closed-cleft conformations, the latter favoring pore closure. In the model, this equilibrium determines the subtype-specificity of NMDAR Po (ko/kc[NR2B] < ko/kc[NR2A]). The NTD is also the target of subunit-specific allosteric inhibitors such as zinc12–14,19 or ifenprodil15,25, which bind the NTD central cleft and promote domain closure. We hypothesize that a molecule binding in the same cleft, but preventing its closure, behaves as a positive allosteric modulator, enhancing receptor activity.
METHODS SUMMARY
cDNA constructs and site-directed mutagenesis
The pcDNA3-based expression plasmids, mutagenesis and sequencing procedure have been described previously19. Chimeras were obtained by classical PCR amplification and subsequent subcloning into the parental clone.
Electrophysiology
Recombinant NMDARs were expressed in Xenopus laevi oocytes after co-injection of cDNAs (at 10 ng/µl; nuclear injection) coding for the various NR1 and NR2 subunits (ratio 1:1). Oocytes were prepared, injected, voltage-clamped and superfused as described previously12. Single-channels were recorded from HEK cell outside-out patches.
Methods
Two electrode voltage-clamp recordings and analysis
For all experiments, except for those aimed at measuring pH sensitivity, the standard external solution contained (in mM): 100 NaCl, 2.5 KCl, 0.3 BaCl2, 5 HEPES (pH 7.3). To chelate trace amounts of contaminant zinc, DTPA (10 µM) was added in all the “0” zinc solutions31. For free zinc concentrations in the 1 nM-1 µM range, tricine (10 mM) was used to buffer zinc, while ADA (1 mM) was used to buffer zinc in the 0.1–100 nM range20. For the pH sensitivity experiments, an enriched HEPES external solution was used to insure proper pH buffering20. Currents were elicited by co-application of saturating concentrations of glutamate and glycine (100 µM each), and measured at a holding potential of −60 mV. MTS compounds were used at 0.2 mM (except for Fig. S7). Experiments were done at room temperature. Data collection and analysis of pH and zinc dose-response curves were performed according to ref20. MK-801 time constants of inhibition were obtained by fitting currents with a monoexponential component within a window corresponding to 10%–90% of the maximal inhibition. Data points used for statistical tests were supposed log-normally distributed prior to a Student’s t-test (unless otherwise indicated).
Single-channel recordings and analysis
HEK cells were transfected with 2 µg of cDNAs mixed at a ratio of 1 NR1:3 NR2:3 GFP using calcium phosphate precipitation or FuGENE Transfection Reagent (Roche). Positive cells were visualized by GFP epi-fluorescence. Patch pipettes of 5–10 MΩ were filled with a solution containing (in mM): 115 CsF, 10 CsCl, 10 HEPES, 10 EGTA (pH 7.15 with CsOH). Osmolality was 270 mosm/kg. The standard external solution contained (in mM): 140 NaCl, 2.8 KCl, 0.5 CaCl2, 10 HEPES, 0.01 EDTA (pH 7.3 with NaOH). Osmolality was adjusted to 290 mosm/kg with sucrose. EDTA was added to chelate trace amounts of contaminant zinc31. Channel openings were activated by 100 µM glycine, with 0.05 or 0.01 µM glutamate in most experiments, or with 100 µM glutamate in some patches (included only if no double openings were observed). The holding potential (after correction for junction potential) was −80 to −90 mV. Experiments were performed at room temperature. Currents were recorded with an Axopatch 200B amplifier (Molecular Devices), sampled at 20 to 50 kHz, low-pass filtered (8-pole Bessel) at 5 to 10 kHz. Prior to analysis of Po within a burst, data were digitally refiltered to give a cascaded low-pass filter cutoff frequency of 2 kHz. pClamp 9 or 10 (Molecular Devices) was used to acquire and analyze the data.
The principal goal of single-channel analysis was to measure the open probability (Popen) within bursts of channel openings, which provides a good estimate of the Popen within an NMDAR activation6,32,33. To idealize single-channel data, transitions were detected using a 50% threshold criterion34. Events of ≤200 µs duration were excluded from analysis. Missing and ignoring brief events can significantly influence dwell-time histograms. However, such brief events contribute only a tiny fraction of the total time that a channel spends open or closed. Thus, missed events should not have significantly affected measurements of Popen. Histograms are presented as square root vs. log time plots35. Shut-time histograms were fitted with 3 or 4 exponential components. A burst was defined as a series of openings separated by closures of duration less than a critical duration, Tcrit. Bursts with two levels of openings were discarded. We calculated Tcrit between the two longest components of the shut-time histograms so that total number of event misclassifications is minimized34,36. For NR1wt/NR2Awt and NR1wt/NR2B-(2A NTD+L) receptors, the two longest components of the shut-time distribution differed by a mean factor > 390, while these components were less separated for NR1wt/NR2Bwt and NR1wt/NR2A-(2B NTD+L) (23-fold and 54-fold separation, respectively). For the latter two constructs, the separation between shut-time components results in a greater than desired number of misclassification of shut times34. This may have lead to an overestimation of the Popen within a burst. However, for wild-type receptors, our data are overall consistent with previous results6,33, suggesting that our Popen estimates are reliable.
Chemicals
HEPES, L-glutamate, glycine, DTPA, EDTA, tricine and ADA were obtained from Sigma, D-APV from Ascent Scientific, 2-aminoethylmethanethiosulfonatehydrobromide (MTSEA), [2-(trimethylammonium)ethyl]methanethiosulfonatebromide (MTSET) and 3-(triethylammonium)propylmethanethiosulfonatebromide (MTS-PtrEA) from Toronto Research Chemicals, (+)MK-801 from Tocris. MTS compounds were prepared as 40 mM stock solutions in bi-distilled water, aliquoted in small volumes (50 µL) and stored at −20°C; aliquots were thawed just before use.
Construction of Figure 4
The molecular architecture shown in figure 4a was illustrated by the crystal structure of the mGluR1 ligand-binding domain dimer (pdb 1ewv, ref37) at the level of the NTD, the NMDAR NR1/NR2A agonist-binding domain dimer (pdb 2a5T, ref30) and two subunits of the KcsA tetramer (pdb 1bl8, ref38) as the transmembrane region of the receptor. The fourth transmembrane segment and the C-terminal cytoplasmic region are lacking in this structural depiction.
Supplementary Material
Acknowledgements
This work was supported by Ministère de la Recherche (MG, LM), UPMC and FRM (MG), NIH grant R01 MH045817 (JWJ), INSERM, ANR, GlaxoSmithKline and Equipe FRM grant (PP). We thank Boris Barbour, Pierre-Jean Corringer, Jacques Neyton and David Stroebel for comments on the manuscript; Stéphanie Carvalho, Mariano Casado and Marie Gendrel for experimental help.
References
- 1.Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- 2.Kemp JA, McKernan RM. NMDA receptor pathways as drug targets. Nat Neurosci. 2002;5(Suppl):1039–1042. doi: 10.1038/nn936. [DOI] [PubMed] [Google Scholar]
- 3.Paoletti P, Neyton J. NMDA receptor subunits: function and pharmacology. Curr Opin Pharmacol. 2007;7:39–47. doi: 10.1016/j.coph.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 4.Cull-Candy SG, Leszkiewicz DN. Role of distinct NMDA receptor subtypes at central synapses. Sci STKE. 2004;2004:re16. doi: 10.1126/stke.2552004re16. [DOI] [PubMed] [Google Scholar]
- 5.Chen N, Luo T, Raymond LA. Subtype-dependence of NMDA receptor channel open probability. J Neurosci. 1999;19:6844–6854. doi: 10.1523/JNEUROSCI.19-16-06844.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erreger K, Dravid SM, Banke TG, Wyllie DJ, Traynelis SF. Subunit-specific gating controls rat NR1/NR2A and NR1/NR2B NMDA channel kinetics and synaptic signalling profiles. J Physiol. 2005;563:345–358. doi: 10.1113/jphysiol.2004.080028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wyllie DJ, Behe P, Colquhoun D. Single-channel activations and concentration jumps: comparison of recombinant NR1a/NR2A and NR1a/NR2D NMDA receptors. J Physiol. 1998;510(Pt 1):1–18. doi: 10.1111/j.1469-7793.1998.001bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dravid SM, Prakash A, Traynelis SF. Activation of recombinant NR1/NR2C NMDA receptors. J Physiol. 2008;586:4425–4439. doi: 10.1113/jphysiol.2008.158634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Popescu G, Robert A, Howe JR, Auerbach A. Reaction mechanism determines NMDA receptor response to repetitive stimulation. Nature. 2004;430:790–793. doi: 10.1038/nature02775. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y, et al. NMDA receptor subunits have differential roles in mediating excitotoxic neuronal death both in vitro and in vivo. J Neurosci. 2007;27:2846–2857. doi: 10.1523/JNEUROSCI.0116-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu L, et al. Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science. 2004;304:1021–1024. doi: 10.1126/science.1096615. [DOI] [PubMed] [Google Scholar]
- 12.Paoletti P, et al. Molecular organization of a zinc binding N-terminal modulatory domain in a NMDA receptor subunit. Neuron. 2000;28:911–925. doi: 10.1016/s0896-6273(00)00163-x. [DOI] [PubMed] [Google Scholar]
- 13.Low CM, Zheng F, Lyuboslavsky P, Traynelis SF. Molecular determinants of coordinated proton and zinc inhibition of N-methyl-D-aspartate NR1/NR2A receptors. Proc Natl Acad Sci U S A. 2000;97:11062–11067. doi: 10.1073/pnas.180307497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi YB, Lipton SA. Identification and mechanism of action of two histidine residues underlying high-affinity Zn2+ inhibition of the NMDA receptor. Neuron. 1999;23:171–180. doi: 10.1016/s0896-6273(00)80763-1. [DOI] [PubMed] [Google Scholar]
- 15.Perin-Dureau F, Rachline J, Neyton J, Paoletti P. Mapping the binding site of the neuroprotectant ifenprodil on NMDA receptors. J Neurosci. 2002;22:5955–5965. doi: 10.1523/JNEUROSCI.22-14-05955.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones KS, VanDongen HM, VanDongen AM. The NMDA receptor M3 segment is a conserved transduction element coupling ligand binding to channel opening. J Neurosci. 2002;22:2044–2053. doi: 10.1523/JNEUROSCI.22-06-02044.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuan H, Erreger K, Dravid SM, Traynelis SF. Conserved structural and functional control of N-methyl-D-aspartate receptor gating by transmembrane domain M3. J Biol Chem. 2005;280:29708–29716. doi: 10.1074/jbc.M414215200. [DOI] [PubMed] [Google Scholar]
- 18.Blanke ML, VanDongen AM. Constitutive activation of the N-methyl-D-aspartate receptor via cleft-spanning disulfide bonds. J Biol Chem. 2008;283:21519–21529. doi: 10.1074/jbc.M709190200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rachline J, Perin-Dureau F, Le Goff A, Neyton J, Paoletti P. The micromolar zinc-binding domain on the NMDA receptor subunit NR2B. J Neurosci. 2005;25:308–317. doi: 10.1523/JNEUROSCI.3967-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gielen M, et al. Structural rearrangements of NR1/NR2A NMDA receptors during allosteric inhibition. Neuron. 2008;57:80–93. doi: 10.1016/j.neuron.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun Y, et al. Mechanism of glutamate receptor desensitization. Nature. 2002;417:245–253. doi: 10.1038/417245a. [DOI] [PubMed] [Google Scholar]
- 22.Mayer ML. Glutamate receptors at atomic resolution. Nature. 2006;440:456–462. doi: 10.1038/nature04709. [DOI] [PubMed] [Google Scholar]
- 23.Tang C, Schwieters CD, Clore GM. Open-to-closed transition in apo maltose-binding protein observed by paramagnetic NMR. Nature. 2007;449:1078–1082. doi: 10.1038/nature06232. [DOI] [PubMed] [Google Scholar]
- 24.Kniazeff J, et al. Locking the dimeric GABA(B) G-protein-coupled receptor in its active state. J Neurosci. 2004;24:370–377. doi: 10.1523/JNEUROSCI.3141-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mony L, et al. Structural basis of NR2B-selective antagonist recognition by N-methyl-D-aspartate receptors. Mol Pharmacol. 2009;75:60–74. doi: 10.1124/mol.108.050971. [DOI] [PubMed] [Google Scholar]
- 26.Zheng F, et al. Allosteric interaction between the amino terminal domain and the ligand binding domain of NR2A. Nat Neurosci. 2001;4:894–901. doi: 10.1038/nn0901-894. [DOI] [PubMed] [Google Scholar]
- 27.Marvin JS, Hellinga HW. Manipulation of ligand binding affinity by exploitation of conformational coupling. Nat Struct Biol. 2001;8:795–798. doi: 10.1038/nsb0901-795. [DOI] [PubMed] [Google Scholar]
- 28.Low CM, et al. Molecular determinants of proton-sensitive N-methyl-D-aspartate receptor gating. Mol Pharmacol. 2003;63:1212–1222. doi: 10.1124/mol.63.6.1212. [DOI] [PubMed] [Google Scholar]
- 29.Lisman JE, et al. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31:234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Furukawa H, Singh SK, Mancusso R, Gouaux E. Subunit arrangement and function in NMDA receptors. Nature. 2005;438:185–192. doi: 10.1038/nature04089. [DOI] [PubMed] [Google Scholar]
- 31.Paoletti P, Ascher P, Neyton J. High-affinity zinc inhibition of NMDA NR1-NR2A receptors. J Neurosci. 1997;17:5711–5725. doi: 10.1523/JNEUROSCI.17-15-05711.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Erreger K, Traynelis SF. Zinc inhibition of rat NR1/NR2A N-methyl-D-aspartate receptors. J Physiol. 2008;586:763–778. doi: 10.1113/jphysiol.2007.143941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schorge S, Elenes S, Colquhoun D. Maximum likelihood fitting of single channel NMDA activity with a mechanism composed of independent dimers of subunits. J Physiol. 2005;569:395–418. doi: 10.1113/jphysiol.2005.095349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Colquhoun D, Sigworth FJ. In: Single-Channel Recording. Sakmann B, Neher E, editors. New York: Plenum Press; 1995. pp. 483–587. [Google Scholar]
- 35.Sigworth FJ, Sine SM. Data transformations for improved display and fitting of single-channel dwell time histograms. Biophys J. 1987;52:1047–1054. doi: 10.1016/S0006-3495(87)83298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jackson MB, Wong BS, Morris CE, Lecar H, Christian CN. Successive openings of the same acetylcholine receptor channel are correlated in open time. Biophys J. 1983;42:109–114. doi: 10.1016/S0006-3495(83)84375-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kunishima N, et al. Structural basis of glutamate recognition by a dimeric metabotropic glutamate receptor. Nature. 2000;407:971–977. doi: 10.1038/35039564. [DOI] [PubMed] [Google Scholar]
- 38.Doyle DA, et al. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.